Published online Oct 26, 2025. doi: 10.4330/wjc.v17.i10.109731
Revised: June 5, 2025
Accepted: September 4, 2025
Published online: October 26, 2025
Processing time: 157 Days and 22.2 Hours
The use of sodium-glucose cotransporter 2 (SGLT2) inhibitor in heart failure (HF) patients is increasing significantly, regardless of whether they have a history of diabetes. The effects of SGLT2 inhibitor on HF are likely mediated through mul
To evaluate SGLT2 inhibitor effects on HF, focusing on hospitalization for HF (HHF), cardiovascular (CV) deaths, and all-cause mortality.
A comprehensive search was conducted in PubMed for randomized controlled trials (RCTs) evaluating the effects of SGLT2 inhibitor in HF patients compared to placebo, covering the period from January 1, 2014, to January 1, 2025. The primary outcomes assessed were HHF, CV deaths, and all-cause mortality. RevMan Web 5.4.1 was used to assess the risk of bias heterogeneity and to perform the statistical analyses. A random-effects model was employed for all statistical evaluations.
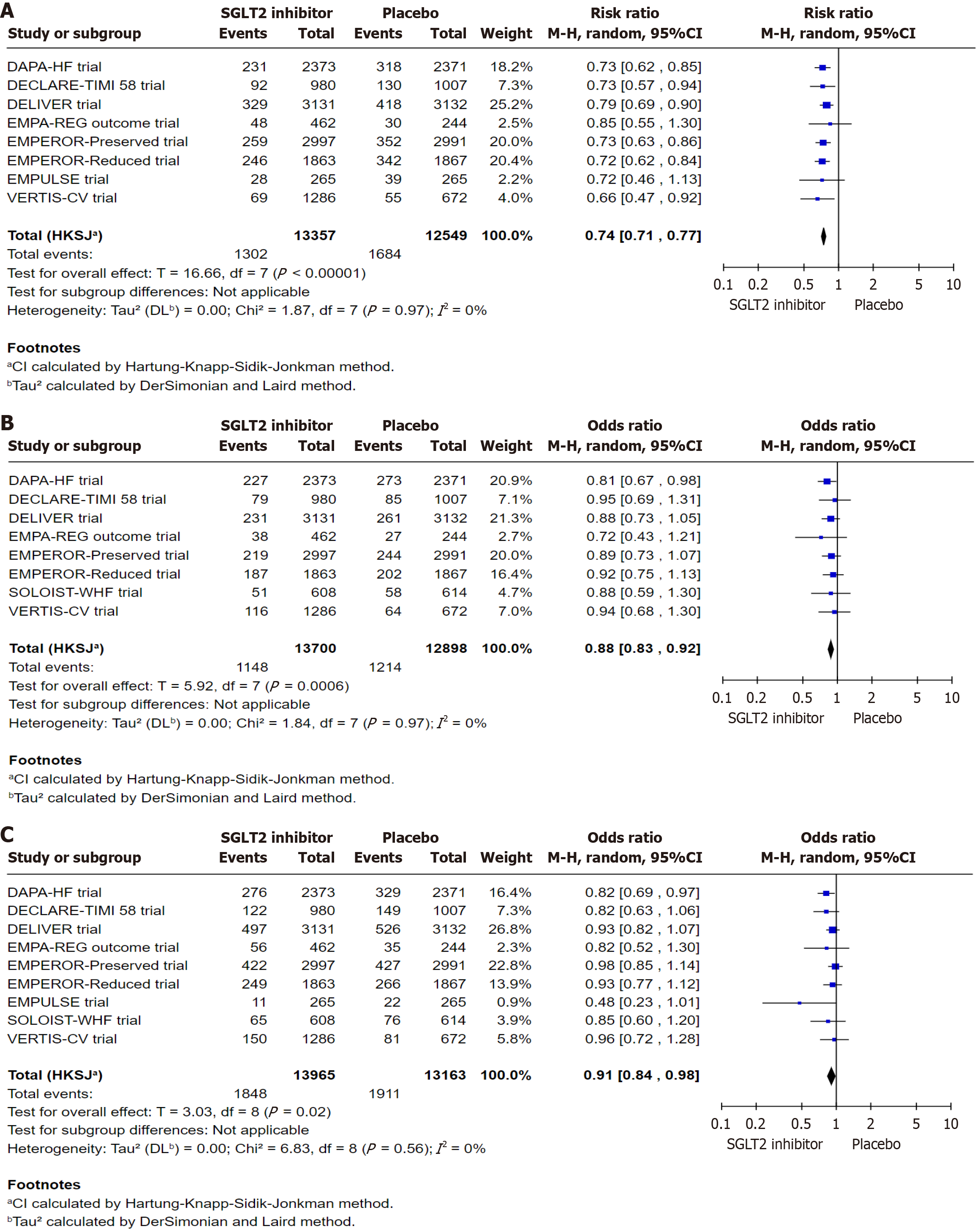
A total of nine RCTs were included in this analysis: DELIVER, DECLARE-TIMI 58, DAPA-HF, EMPA-REG OUTCOME, EMPEROR-Reduced, EMPEROR-Preserved, SOLOIST-WHF, EMPULSE, and VERTIS-CV. For HHF, eight trials (excluding the SOLOIST-WHF; n = 25906) were pooled, while CV deaths were assessed using data from eight trials (excluding the EMPULSE; n = 26598). Compared to placebo, SGLT2 inhibitor significantly reduced the risk of HHF (relative risk: 0.74; 95%CI: 0.71-0.77; P < 0.00001) and CV death (odds ratio: 0.88; 95%CI: 0.83-0.92; P = 0.0006). All nine trials (n = 27128) were included in the analysis of all-cause mortality. SGLT2 inhibitor were associated with a statistically significant reduction in all-cause mortality compared to placebo (OR: 0.91; 95%CI: 0.84-0.98; P = 0.02).
These results suggest that SGLT2 inhibitor significantly reduce the risk of hospitalization for HF, CV deaths, and all-cause mortality.
Core Tip: Sodium-glucose cotransporter 2 (SGLT2) inhibitor have been incorporated into heart failure (HF) treatment guidelines due to their established benefits in patients with HF. They can be initiated in HF patients irrespective of their diabetes history. These medications significantly lower the risk of hospitalization for HF. SGLT2 inhibitor may also reduce cardiovascular deaths and all-cause mortality in HF patients, though the statistical significance of these outcomes has not been consistently demonstrated across all studies.
- Citation: Parsi S, Shirsat PD, Mahali LP, Surani S, Kashyap R. Sodium-glucose cotransporter 2 inhibitor in heart failure patients and their outcomes: A meta-analysis of randomized controlled trials. World J Cardiol 2025; 17(10): 109731
- URL: https://www.wjgnet.com/1949-8462/full/v17/i10/109731.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i10.109731
Heart failure (HF) is among the most rapidly increasing cardiovascular (CV) disease and is currently the second most prevalent condition, after stroke. In the United States, its prevalence is projected to rise by 33.4% between 2025 and 2060 (9.7 million to 12.9 million)[1]. HF is also a leading cause of recurrent hospitalizations following sepsis[2], contributing significantly to both all-cause and CV mortality[3].
HF management includes beta blockers, angiotensin receptor neprilysin inhibitors (ARNi), and mineralocorticoid receptor antagonists (MRAs)[4]. In 2022, SGLT2 inhibitor were added to guideline-directed medical therapy (GDMT) for patients with HF, receiving a Class IIa recommendation for both HF with reduced and preserved ejection fraction (HFrEF and HFpEF, respectively)[5].
SGLT2 protein is located in the membrane of tubular epithelial cells of proximal convoluted tubules, where it co-transports glucose and sodium across the membrane[6]. SGLT2 inhibitor target this co-transporter, blocking glucose reabsorption and promoting its excretion in the urine, thereby exerting an anti-glycemic effect[7]. By inhibiting sodium reabsorption in the proximal tubule, SGLT2 inhibitor increase sodium delivery to the distal convoluted tubules. This suppresses activation of the renin-angiotensin-aldosterone system (RAAS), a key mechanism underlying the improvement in HF outcomes.[8,9] Additionally, SGLT2 inhibitor help prevent cardiac remodeling by reducing myocardial fibrosis, in part through the downregulation of alpha-smooth muscle actin expression in human atrial myofibroblast[10].
SGLT2 inhibitors also promote ketogenesis and lipolysis by increasing glucagon secretion from pancreatic alpha cells and reducing circulating glucose levels through urinary glucose loss[11]. Ketones serve as an efficient energy substrate, producing more energy per unit of oxygen consumed compared to glucose and fatty acids, thereby reducing oxidative stress at the cellular level[12]. In HF, the myocardium utilizes ketones as an energy source due to dysfunction in glucose and fatty acid metabolism, which generates an energy source that, in turn, decreases oxidative stress and improves heart efficiency[13].
We aim to conduct a systemic review and meta-analysis to study the CV outcomes of SGLT2 inhibitor, when added to GDMT in patients with HF. Outcomes of interest include hospitalization for HF (HHF), CV deaths, and all-cause mortality.
This systematic review and meta-analysis focus on randomized controlled trials (RCTs) evaluating the effects of SGLT2 inhibitor in patients with HF compared to the placebo group. Studies meeting our inclusion criteria were included in the analysis. A detailed description of the study design is mentioned below.
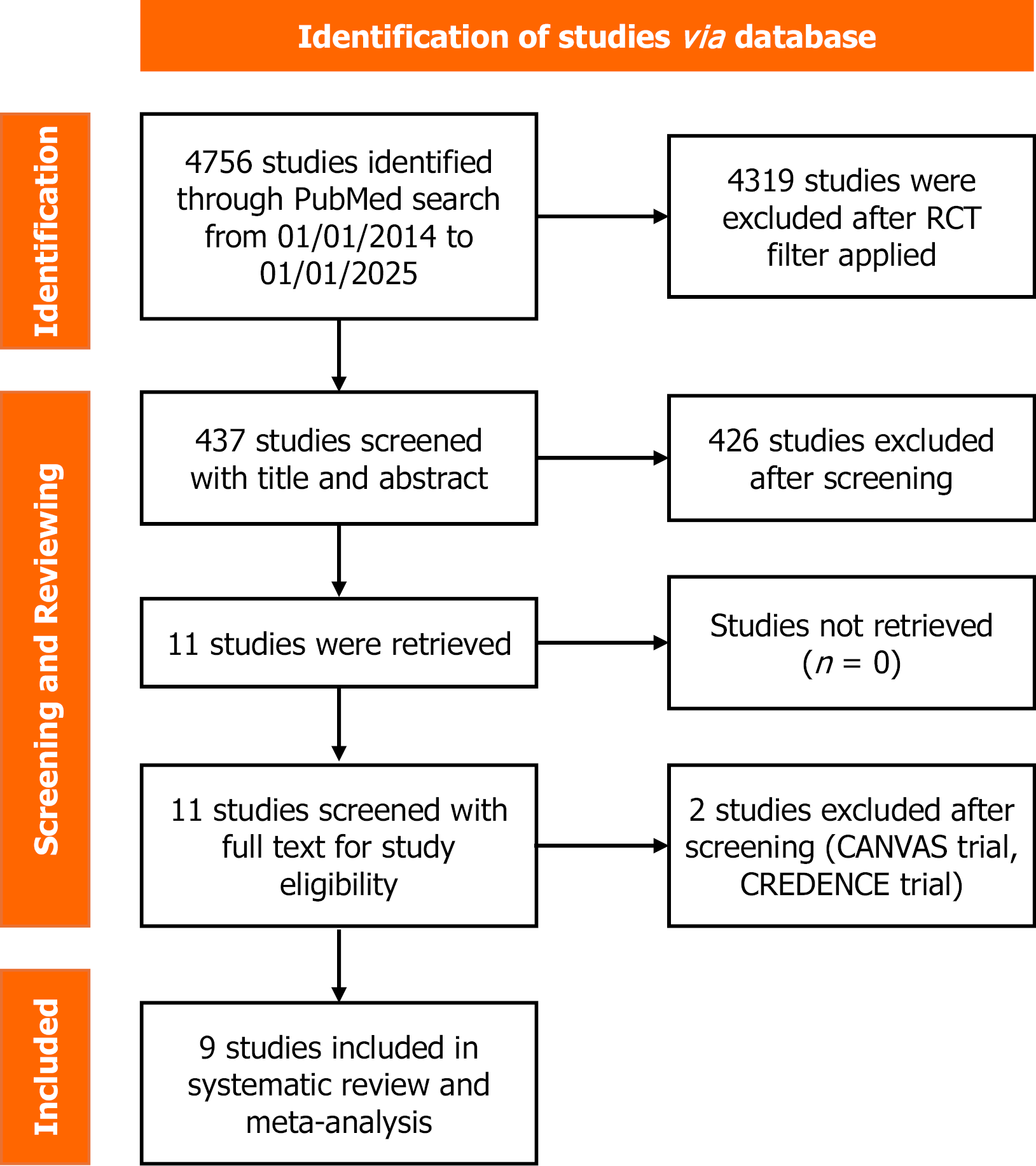
A comprehensive systematic literature search was conducted on PubMed using the following search terms: (SGLT 2 inhibitor OR SGLT2i OR sodium-glucose cotransporter-2 inhibitor OR dapagliflozin OR empagliflozin OR sotagliflozin OR ertugliflozin OR tofogliflozin OR bexagliflozin OR canagliflozin) AND (Heart failure OR HF). Two filters were applied: (1) Custom duration (studies published from January 1, 2014, to January 1, 2025; and (2) Study type was limited to RCTs. Initial search yielded a total of 4756 studies, of which 437 were identified as RCTs.
The eligibility criteria for inclusion in this study were: (1) Patients with a documented history of HF; (2) HF patients not previously treated with SGLT2 inhibitor; (3) HF patients receiving GDMT except SGLT2 inhibitor, such as loop diuretics, beta-blocker, angiotensin receptor blockers (ARBs), angiotensin-converting enzyme inhibitors, ARNi, MRA; and (4) Studies reporting at least two of the predefined clinical outcomes of interest.
All 437 studies identified through PubMed search were screened and reviewed, with no duplicates found. Initially, all 437 were screened by the first reviewer. Of these, a second reviewer also screened 324 and 113 by a third reviewer, based on titles and abstracts, and assessed for eligibility. Any conflicts among these three reviewers were resolved through an independent review by a fourth reviewer. Eligible studies were subsequently evaluated using the full text. The studies with different outcomes, no results, and no peer review were excluded. The final set of included studies was reviewed a second time prior to inclusion in the analysis. The study selection process is summarized in the PRISMA flowchart in Figure 1[14]. After comprehensive screening and evaluation, a total of nine studies were included in the meta-analysis[15-23].
Data on study characteristics, baseline demographics, interventions, and outcomes was collected from full text, supplements, and related studies derived from our included studies[15-30]. Tables 1 and 2 summarizes the baseline demographics of subjects in each study, including those in the drug and placebo groups.
| Type of SGLT2 inhibitor | DAPA-HF | DECLARE-TIMI 58 | DELIVER trial | EMPA-REG outcome | ||||
| Dapagliflozin | Placebo | Dapagliflozin | Placebo | Dapagliflozin | Placebo | Empagliflozin | Placebo | |
| HF patients | 4744 | 1987 | 6263 | 706 | ||||
| Drug group | 2373 | 2371 | 980 | 1007 | 3131 | 3132 | 462 | 244 |
| Age (years) | 66.2 (11) | 66.5 (10.8) | 64 (59-69) | 71.8 (9.6) | 71.5 (9.5) | 64.5 (8.8) | 64.5 (8.9) | |
| Female (%) | 23.8 | 23 | 33.7 | 43.6 | 44.2 | 30.7 | 28.3 | |
| BMI (kg/m2) | 28.2 (6) | 28.1 (5.9) | 32.6 (29.1-37.1) | 29.8 (6.2) | 29.9 (6.1) | 31.9 (5.6) | 32.3 (5.4) | |
| White (%) | 70 | 70.5 | 70.7 | 71 | 81.4 | 82 | ||
| Black (%) | 5.1 | 4.4 | 2.6 | 2.5 | 7.1 | 7 | ||
| Asian (%) | 23.3 | 23.8 | 20.1 | 20.6 | 11.3 | 10.2 | ||
| other (%) | 1.6 | 1.3 | 6.6 | 5.9 | 0.2 | 0.8 | ||
| NYHA II (%) | 67.7 | 67.4 | 56.1 | 73.9 | 76.6 | |||
| NYHA III (%) | 31.5 | 31.7 | 7.8 | 25.8 | 23.1 | |||
| NYHA IV (%) | 0.8 | 1 | 0.5 | 0.3 | 0.3 | |||
| LVEF (%) | 31.2 (6.7) | 30.9 (6.9) | 49 (43-54) | 54 (8.6) | 54.3 (8.9) | |||
| T2DM (%) | 41.8 | 41.8 | 100 | 44.7 | 44.9 | 100 | 100 | |
| HTN (%) | 72.6 | 72.2 | 92.9 | 88 | 89.3 | 98.7 | 99.2 | |
| CAD (%) | 42.2 | 43.4 | 89.8 | 29.8 | 30.8 | 83.3 | 85.7 | |
| AF/flutter (%) | 38.6 | 38 | 56.1 | 57.3 | 16.5 | 17.6 | ||
| Loop diuretic (%) | 93.4 | 93.5 | 38.8 | 76.7 | 76.9 | 48.5 | 45.1 | |
| Beta blocker (%) | 96 | 96.2 | 80.8 | 82.8 | 82.5 | 77.9 | 81.6 | |
| ACEi (%) | 56.1 | 56.1 | 36.5 | 36.7 | ||||
| ARB (%) | 28.4 | 26.7 | 36.2 | 36.4 | ||||
| ACEi or ARB (%) | 86.2 | 87.9 | 84.4 | |||||
| ARNI (%) | 10.5 | 10.9 | 5.3 | 4.3 | 0.6 | 0.4 | ||
| MRA (%) | 71.5 | 70.6 | 19.4 | 42.8 | 42.4 | 25.1 | 21.7 | |
| Type of SGLT2 inhibitor | EMPEROR-preserved | EMPEROR-reduced | EMPULSE | SOLOIST-WHF | VERTIS-CV | |||||
| Empagliflozin | Placebo | Empagliflozin | Placebo | Empagliflozin | Placebo | Sotagliflozin | Placebo | Ertugliflozin | Placebo | |
| HF patients | 5988 | 3730 | 530 | 1222 | 1958 | |||||
| Drug group | 2997 | 2991 | 1863 | 1867 | 265 | 265 | 608 | 614 | 1286 | 672 |
| Age (years) | 71.8 (9.3) | 71.9 (9.6) | 67.2 (10.8) | 66.5 (11.2) | 71 (62-78) | 70 (59-78) | 69 (63-76) | 70 (64-76) | 64.2 (7.9) | 64.7 (7.8) |
| Female (%) | 44.6 | 44.7 | 23.5 | 24.4 | 32.5 | 35.1 | 32.6 | 34.9 | 30.7 | 34.1 |
| BMI (kg/m2) | 29.77 (5.8) | 29.9 (5.9) | 28 (5.5) | 27.8 (5.3) | 28.3 (24.5-32.5) | 29.1 (24.7-33.6) | 30.4 (26.3-34.3) | 31.1 (27.3-34.5) | 32.5 (5.5) | 32.7 (5.2) |
| White (%) | 76.3 | 75.4 | 71.1 | 69.8 | 79.6 | 76.2 | 93.3 | 93.2 | ||
| Black (%) | 4.4 | 4.2 | 6.6 | 7.2 | 7.9 | 12.5 | 4.1 | 4.1 | ||
| Asian (%) | 13.8 | 13.7 | 18.1 | 17.9 | 12.1 | 9.4 | 1.3 | 1.1 | ||
| Other (%) | 5.5 | 6.7 | 4.2 | 5 | 0.4 | 1.5 | 1.3 | 1.7 | ||
| NYHA II (%) | 81.1 | 81.9 | 75.1 | 75 | 35.8 | 34.3 | 45.2 | 65.2 | 67.1 | |
| NYHA III (%) | 18.4 | 17.8 | 24.4 | 24.4 | 50.6 | 54.7 | 45.8 | 7.9 | 5.5 | |
| NYHA IV (%) | 0.3 | 0.3 | 0.5 | 0.6 | 9.8 | 8.7 | 4.4 | 0.1 | 0 | |
| LVEF (%) | 54.3 (8.8) | 54.3 (8.8) | 27.7 (6) | 27.2 (6.1) | 31 (23-45) | 32 (22.5-49) | 35 (28-47) | 35 (28-45) | ||
| T2DM (%) | 48.9 | 49.2 | 49.8 | 49.8 | 46.8 | 43.8 | 100 | 100 | ||
| HTN (%) | 90.8 | 90.4 | 72.4 | 72.3 | 77.4 | 83.4 | 93.2 | 93.9 | ||
| CAD (%) | 35 | 45.0 | 41.9 | 29.4 | 29.4 | 83.7 | 84.1 | |||
| AF/flutter (%) | 51.5 | 50.6 | 37.7 | 39.5 | 50.6 | 48.3 | ||||
| Loop diuretic (%) | 77.4 | 77.5 | 84.8 | 85.4 | 87.9 | 77 | 95.4 | 94.6 | 28.9 | 30.8 |
| Beta blocker (%) | 86.7 | 85.9 | 94.7 | 94.7 | 80.4 | 78.5 | 92.8 | 91.4 | 78.7 | 79.9 |
| ACEi (%) | 40.2 | 33.2 | 33.6 | 41.8 | 39.3 | |||||
| ARB (%) | 38.7 | 24.2 | 19.6 | 40.3 | 44 | |||||
| ACEi or ARB (%) | 70.5 | 68.9 | ||||||||
| ARNI (%) | 2.2 | 2.3 | 18.3 | 20.7 | 13.6 | 17 | 15.3 | 18.2 | ||
| MRA (%) | 37.3 | 37.6 | 70.1 | 72.6 | 57 | 47.2 | 66.3 | 62.7 | 19.7 | 16.8 |
Risk ratio (RR) was used to calculate the statistical analysis of HHF, while odds ratio (OR) was used to evaluate CV deaths and all-cause mortality. Study heterogeneity was evaluated using Tau2, χ², and I2 statistics[31]. A random effects model with Mantel-Haenszel weighting was used to calculate the RR and OR, accounting for expected heterogeneity among studies[31]. All statistical analyses were conducted using RevMan 5.4.1. A P-value of < 0.05 is considered as statistically significant.
The studies included in this meta-analysis are randomized, double-blind, placebo-controlled clinical trials. Two reviewers individually assessed all studies in this meta-analysis as having a low risk of bias for all fields included in the Cochrane tool, with the exception of two fields in the SOLOIST-WHF study (Supplementary Figure 1)[32]. These two fields were rated as high risk due to loss of funding and a change in the primary endpoint. The risk of bias is assessed using the Cochrane collaboration tool, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The risk of bias in individual studies was categorized as either low risk or high risk.
Publication bias was assessed using Egger’s regression test, conducted with MedCalc® statistical software version 23.2.1 (MedCalc software Ltd, Ostend, Belgium). Funnel plots were generated in accordance with the Cochrane Collaboration’s methodology.
This systematic review and meta-analysis was not registered in PROSPERO or any other protocol registry.
A comprehensive PubMed search using the specified criteria yielded 4756 studies published between January 1st, 2014, to January 1st, 2025. Applying the RCT filter narrowed the results to 437. Following thorough screening and evaluation, nine studies met the eligibility criteria for inclusion in this meta-analysis. These include the DELIVER trial, DECLARE-TIMI 58 Trial, DAPA-HF trial, EMPA-REG outcome trial, EMPEROR-Reduced trial, EMPEROR-Preserved trial, SOLOIST-WHF trial, EMPULSE trial, and VERTIS-CV trial[15-23]. The outcomes analyzed in this meta-analysis are: (1) HHF; (2) CV deaths; and (3) All-cause mortality.
Collective data from eight RCTs (except the SOLOIST trial) were used to evaluate HHF, comprising a total of 25906 subjects, 13357 in the SGLT2 inhibitor group and 12549 in the placebo group. For CV deaths, a collective data from eight RCTs (except the EMPULSE trial) were analyzed, involving 26598 subjects, 13700 in the SGLT2 inhibitor group, and 12898 in the placebo group. Collective data from all nine RCTs with a total of 27128 subjects, 13965 in the SGLT2 inhibitor group and 13163 in the placebo group, data was used for assessing the all-cause mortality.
A total of 13357 subjects were included in the SGLT2 inhibitor group, and 12549 subjects were included in the placebo group. HHF occurred in 1302 subjects in the SGLT2 inhibitor group and 1684 subjects in the placebo group, which demonstrates a 26% decrease in the SGLT2 inhibitor group compared to the placebo group (RR: 0.74, 95%CI: 0.71 to 0.77, I2: 0%, P < 0.00001, Figure 2A).
A total of 13700 subjects were included in the SGLT2 inhibitor, and 12898 subjects were included in the placebo group. CV deaths occurred in 1148 subjects in the SGLT2 inhibitor group and 1214 subjects in the placebo group, which demonstrates a 12% decrease in the SGLT2 inhibitor group compared to the placebo group (OR: 0.88, 95%CI: 0.83 to 0.92, I2: 0%, P value: 0.0006, Figure 2B). Additionally, a sensitivity analysis excluding the SOLOIST-WHF trial, which was identified as having a high risk of bias in outcome assessment, revealed no substantial change in the overall effect estimate (OR: 0.88, 95%CI: 0.83 to 0.93, I2: 0%, P = 0.002, Supplementary Figure 2-5).
A total of 13965 subjects were included in the SGLT2 inhibitor, and 13163 subjects were included in the placebo group. All-cause mortality in 1848 subjects in the SGLT2 inhibitor group and 1911 subjects in the placebo group, which demonstrates a 9% decrease in the SGLT2 inhibitor group compared to the placebo group (OR: 0.91, 95%CI: 0.84 to 0.98, I2: 0%, P = 0.002, Figure 2C). Additionally, a sensitivity analysis excluding the SOLOIST-WHF trial, which was identified as having a high risk of bias in outcome assessment, revealed no substantial change in the overall effect estimate (OR: 0.91, 95%CI: 0.84 to 0.99, I2: 0%, P = 0.03, Supplementary Figure 6).
Egger’s regression test revealed no significant evidence of publication bias across the three outcomes, as summarized in Table 3. Specifically, the results for HHF (95%CI: -1.43-1.07; P = 0.74), CV deaths (95%CI: -1.53 to 1.47; P value = 0.96), and all-cause mortality (95%CI: -2.77 to 0.06; P = 0.057) all exceeded the conventional threshold for statistical significance (P > 0.05), indicating no evidence of small-study effects. Furthermore, visual inspection of the corresponding funnel plots (Supplementary Figures 2-4) demonstrated symmetrical distributions, providing additional support for the absence of substantial publication bias.
| Outcomes | Hospitalization for HF | Cardiovascular deaths | All-cause mortality |
| Intercept | -0.1782 | -0.03214 | -1.3577 |
| 95%CI | -1.4307 to 1.0742 | -1.5346 to 1.4704 | -2.7718 to 0.05639 |
| Significance level | P = 0.7396 | P = 0.96 | P = 0.0575 |
In this systematic review, we included nine studies with about 25892 subjects. Our analysis showed a decrease in HHF, CV deaths, and all-cause mortality in the SGLT2 inhibitor group compared to the placebo group, which was statistically significant.
The beneficial effects of SGLT2 inhibitor on HF patients may be attributed to their underlying mechanism of action. These agents help prevent cardiac remodeling by inhibiting the RAAS pathway, achieved through increased sodium concentration in the distal tubule, which in turn reduces fibrosis by suppressing myofibroblast activity[33]. Additionally, SGLT2 inhibitor enhance cardiac muscle efficiency by promoting ketone oxidation, a process that requires less oxygen than glucose metabolism to an equivalent amount of ATP[34].
Our systematic review and meta-analysis demonstrated that, compared to placebo, SGLT2 inhibitor were associated with a 26% reduction in risk of HHF. This finding is consistent with results from Zannad et al[35], who reported a 25% reduction in the composite outcome of recurrent HHF or CV deaths and from Vaduganathan et al[36] and Cao et al[37] who observed 28% and 31% reduction in risk of first HHF, respectively. Likewise, our analysis showed a 12% decrease in CV deaths, comparable to the 14%, 13%, and 12% reductions reported by Zannad et al[35], Vaduganathan et al[36], and Cao et al[37], respectively. Furthermore, we observed a 9% decrease in all-cause mortality, while Zannad et al[35], Vaduganathan et al[36], and Cao et al[37] reported decreases of 13%, 8%, and 10%, respectively.
As noted, our study has several strengths. The pooled patient population from various studies included in our systematic review and meta-analysis is diverse in terms of age, race, and multiple comorbidities. Moreover, all participants were HF patients already receiving other GDMTs, closely resembling the real-world clinical population. Only one outcome, all-cause mortality, demonstrated some publication bias, but it did not reach statistical significance, as confirmed by visual inspection.
However, our study also has some limitations. Our study is not registered on PROSPERO or other protocol registry due to unawareness of the registration requirement at the time of protocol development. Due to limited access to other databases, our study was confined to a single database, which impacted the comprehensiveness of our search. Our cohort included HF patients regardless of type of EF and diabetes history, due to insufficient data, we were unable to perform subgroup analyses comparing their outcomes. Additionally, our review incorporated data from a study by Bhatt et al[21], which experienced a loss of funding and subsequent change in its primary outcome during the trial period, potentially introducing bias. Lastly, our analysis was limited in its ability to assess outcomes bases on specific drug dosages.
In conclusion, this study demonstrates that the initiation of SGLT2 inhibitor in patients with HF is associated with a meaningful reduction in the risk of hospitalization for HF, CV deaths, and all-cause mortality, reinforcing their roles as an essential component of contemporary HF management.
| 1. | Mohebi R, Chen C, Ibrahim NE, McCarthy CP, Gaggin HK, Singer DE, Hyle EP, Wasfy JH, Januzzi JL Jr. Cardiovascular Disease Projections in the United States Based on the 2020 Census Estimates. J Am Coll Cardiol. 2022;80:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 215] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 2. | Salah HM, Minhas AMK, Khan MS, Pandey A, Michos ED, Mentz RJ, Fudim M. Causes of hospitalization in the USA between 2005 and 2018. Eur Heart J Open. 2021;1:oeab001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Lahoz R, Fagan A, McSharry M, Proudfoot C, Corda S, Studer R. Recurrent heart failure hospitalizations are associated with increased cardiovascular mortality in patients with heart failure in Clinical Practice Research Datalink. ESC Heart Fail. 2020;7:1688-1699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137-e161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1590] [Cited by in RCA: 2037] [Article Influence: 226.3] [Reference Citation Analysis (0)] |
| 5. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1618] [Cited by in RCA: 1633] [Article Influence: 408.3] [Reference Citation Analysis (0)] |
| 6. | Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 447] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 7. | Kitada K, Kidoguchi S, Nakano D, Nishiyama A. Sodium/glucose cotransporter 2 and renoprotection: From the perspective of energy regulation and water conservation. J Pharmacol Sci. 2021;147:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Dharia A, Khan A, Sridhar VS, Cherney DZI. SGLT2 Inhibitors: The Sweet Success for Kidneys. Annu Rev Med. 2023;74:369-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 9. | Yang F, Meng R, Zhu DL. Cardiovascular effects and mechanisms of sodium-glucose cotransporter-2 inhibitors. Chronic Dis Transl Med. 2020;6:239-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Kang S, Verma S, Hassanabad AF, Teng G, Belke DD, Dundas JA, Guzzardi DG, Svystonyuk DA, Pattar SS, Park DSJ, Turnbull JD, Duff HJ, Tibbles LA, Cunnington RH, Dyck JRB, Fedak PWM. Direct Effects of Empagliflozin on Extracellular Matrix Remodelling in Human Cardiac Myofibroblasts: Novel Translational Clues to Explain EMPA-REG OUTCOME Results. Can J Cardiol. 2020;36:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 11. | Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors May Predispose to Ketoacidosis. J Clin Endocrinol Metab. 2015;100:2849-2852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 383] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 12. | Papazafiropoulou AK, Georgopoulos MM, Katsilambros NL. Ketone bodies and the heart. Arch Med Sci Atheroscler Dis. 2021;6:e209-e214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 13. | Takahara S, Soni S, Maayah ZH, Ferdaoussi M, Dyck JRB. Ketone therapy for heart failure: current evidence for clinical use. Cardiovasc Res. 2022;118:977-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51401] [Article Influence: 10280.2] [Reference Citation Analysis (2)] |
| 15. | Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 3502] [Article Influence: 583.7] [Reference Citation Analysis (0)] |
| 16. | Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM; DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022;387:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 1771] [Article Influence: 442.8] [Reference Citation Analysis (0)] |
| 17. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4871] [Article Influence: 695.9] [Reference Citation Analysis (0)] |
| 18. | Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause-Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation. 2019;139:2528-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 407] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 19. | Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, Frederich R, Charbonnel B, Mancuso J, Shih WJ, Terra SG, Cater NB, Gantz I, McGuire DK; VERTIS CV Investigators. Efficacy of Ertugliflozin on Heart Failure-Related Events in Patients With Type 2 Diabetes Mellitus and Established Atherosclerotic Cardiovascular Disease: Results of the VERTIS CV Trial. Circulation. 2020;142:2205-2215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 20. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 3195] [Article Influence: 639.0] [Reference Citation Analysis (0)] |
| 21. | Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021;384:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 1351] [Article Influence: 270.2] [Reference Citation Analysis (0)] |
| 22. | Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, Ferreira JP, Nassif ME, Psotka MA, Tromp J, Borleffs CJW, Ma C, Comin-Colet J, Fu M, Janssens SP, Kiss RG, Mentz RJ, Sakata Y, Schirmer H, Schou M, Schulze PC, Spinarova L, Volterrani M, Wranicz JK, Zeymer U, Zieroth S, Brueckmann M, Blatchford JP, Salsali A, Ponikowski P. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 592] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 23. | Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME® trial investigators. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J. 2016;37:1526-1534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 669] [Cited by in RCA: 742] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 24. | Mc Causland FR, Claggett BL, Vaduganathan M, Desai AS, Jhund P, de Boer RA, Docherty K, Fang J, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Saraiva JFK, McGrath MM, Shah SJ, Verma S, Langkilde AM, Petersson M, McMurray JJV, Solomon SD. Dapagliflozin and Kidney Outcomes in Patients With Heart Failure With Mildly Reduced or Preserved Ejection Fraction: A Prespecified Analysis of the DELIVER Randomized Clinical Trial. JAMA Cardiol. 2023;8:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 25. | Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Brueckmann M, Jamal W, Zeller C, Schnaidt S, Zannad F. Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation. 2021;143:326-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 302] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 26. | Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Schnaidt S, Zeller C, Schnee JM, Anker SD. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation. 2021;144:1284-1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 260] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 27. | Dhingra NK, Verma S, Butler J, Anker SD, Ferreira JP, Filippatos G, Januzzi JL, Lam CSP, Sattar N, Zaremba-Pechmann L, Böhm M, Nordaby M, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Reduced Trial Committees and Investigators. Efficacy and Safety of Empagliflozin According to Background Diuretic Use in HFrEF: Post-Hoc Analysis of EMPEROR-Reduced. JACC Heart Fail. 2024;12:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Filippatos G, Butler J, Farmakis D, Zannad F, Ofstad AP, Ferreira JP, Green JB, Rosenstock J, Schnaidt S, Brueckmann M, Pocock SJ, Packer M, Anker SD; EMPEROR-Preserved Trial Committees and Investigators. Empagliflozin for Heart Failure With Preserved Left Ventricular Ejection Fraction With and Without Diabetes. Circulation. 2022;146:676-686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 29. | Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Sattar N, Brueckmann M, Jamal W, Cotton D, Iwata T, Zannad F; EMPEROR-Reduced Trial Committees and Investigators. Empagliflozin in Patients With Heart Failure, Reduced Ejection Fraction, and Volume Overload: EMPEROR-Reduced Trial. J Am Coll Cardiol. 2021;77:1381-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 30. | Adamson C, Docherty KF, Heerspink HJL, de Boer RA, Damman K, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Petrie MC, Ponikowski P, Sabatine MS, Schou M, Solomon SD, Verma S, Bengtsson O, Langkilde AM, Sjöstrand M, Vaduganathan M, Jhund PS, McMurray JJV. Initial Decline (Dip) in Estimated Glomerular Filtration Rate After Initiation of Dapagliflozin in Patients With Heart Failure and Reduced Ejection Fraction: Insights From DAPA-HF. Circulation. 2022;146:438-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 31. | In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley Sons, 2019. |
| 32. | Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Cochrane, 2024. |
| 33. | Zhang Y, Lin X, Chu Y, Chen X, Du H, Zhang H, Xu C, Xie H, Ruan Q, Lin J, Liu J, Zeng J, Ma K, Chai D. Dapagliflozin: a sodium-glucose cotransporter 2 inhibitor, attenuates angiotensin II-induced cardiac fibrotic remodeling by regulating TGFβ1/Smad signaling. Cardiovasc Diabetol. 2021;20:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 34. | Lopaschuk GD, Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. 2020;5:632-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 637] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 35. | Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 948] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 36. | Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, McMurray JJV, Solomon SD. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 588] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 37. | Cao Y, Li P, Li Y, Han Y. Sodium-glucose cotransporter-2 inhibitors in heart failure: an updated meta-analysis. ESC Heart Fail. 2022;9:1942-1953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/