INTRODUCTION
The heart is a vital organ of physiological integration, playing a crucial role in circulating oxygenated blood, essential nutrients, and minerals throughout the body, thereby contributing to the sustenance of active life[1]. It also facilitates the transport of carbon dioxide and catabolic leftovers to the respective organs for elimination and excretion, ensuring homeostasis[2]. The medulla oblongata of the brain transmits neural signals responsible for the regulation of cardiac function through the spinal cord[3]. Moreover, the hypothalamus-mediated production of pituitary hormones appears to influence cardiac function[4]. The efficiency of cardiac functions relies on the coordinated activity of various cell types present in the heart that include cardiomyocytes, endothelial cells, cardiac fibroblasts, immune cells, and a recently recognized population, cardiac nexus glial cells[5,6]. Among various cell types, cardiac nexus glial cells located in the cardiac ganglia have gained increasing scientific attention due to their possible role in the modulation of neurocardiac pathways. Evidence from the molecular studies conducted in experimental animal models indicates that cardiac nexus glial cells express glial fibrillary acidic protein (GFAP), a potential marker for astrocytes in the brain, and are capable of secreting gliotransmitters[7]. While neurotransmitters have long been the primary focus of intense research, the physiological significance of gliotransmitters in modulating both the brain and other organs, including the heart, remains largely unexplored[8]. Though the cerebrovascular system and cardiac nerve roots are important, functional synchrony between the heart and brain necessitates reciprocal communication through molecular exchange. Among various molecules, gliotransmitters such as glutamate, adenosine triphosphate (ATP), D-serine, gamma-aminobutyric acid (GABA), atrial natriuretic peptide, and brain-derived neurotrophic factor (BDNF) have been identified as potential mediators, ensuring the functional significance of the heart–brain axis (HBA)[9].
The heart and brain undergo dynamic changes in response to mechanical, metabolic, and electrical stimuli driven by physiological changes, environmental factors, and medical interventions. Notably, aging, cardiovascular diseases, and neurological disorders are characterized by progressive cytoarchitectural deterioration in both organs, resulting in physiological decline and behavioral impairments[10]. A growing body of evidence indicates a strong overlap between comorbid conditions such as diabetes, hypertension, obesity, and gastrointestinal disorders with cardiovascular and neurodegenerative diseases, including Parkinson's disease (PD) and Alzheimer's disease (AD)[11]. While current research has primarily focused on organ-specific degenerative mechanisms, effective treatment options remain limited. Given the presence of glial cells in both the heart and brain, these cells may secrete key mediators of inter-organ communication and undergo collective alterations during disease progression. Thus, exploring the regulatory roles and involvement of gliotransmitters and their cellular origin in these pathological processes may help in expanding our understanding of HBA mechanisms. Furthermore, both the brain and heart are traditionally considered to have limited regenerative capacity. While considerable progress has been made in understanding the neuroregenerative mechanisms of the brain, conclusive evidence for the regenerative capacity of the heart remains limited. Therefore, elucidating the functional characteristics of cardiac nexus cells and their potential connection to neuroregenerative pathways could pave the way for novel therapeutic strategies that synergistically support both cardioprotection and neuroprotection.
REVIEW OF LITERATURE
We conducted extensive searches in two major electronic databases: PubMed and Google Scholar, and selected current scientific studies, reviews, and experimental reports related to the HBA, gliotransmitters, and cardiac nexus glia up to April 2025. The different combinations of the following keywords were employed to ensure the topic was widely covered: “concept of neuroplasticity” "neurocardiac communication", "cardiac glial cells" "cardiac nexus glia" "astroglia in the heart", "gliotransmitters" "glial signaling" "cardiovascular", "intrinsic cardiac nervous system", "astrocytes" "neurodegeneration" "cardiac function", "neurogenesis" "peripheral nervous system" "cardiac ganglia", "cardiovascular diseases" "central nervous system", "glial dysfunction" "stroke", "Parkinson's Disease", "Alzheimer's Disease", "multiple sclerosis".
Inclusion criteria
Preference was given to studies on the morphological, molecular, and functional description of cardiac glial cells, gliotransmitter release, astrocytes, glial cells and neurogenesis connecting neurodegenerative disorders with cardiovascular diseases. Highly relevant references were incorporated into this review and presented as an integrative overview of historical, experimental, and clinical perspectives related to the emerging roles of cardiac nexus glia and their potential for regeneration.
POTENTIAL ROLES OF CARDIAC NEXUS GLIA IN THE FUNCTIONS OF THE HEART AND NEUROCARDIAC CONNECTIONS
The brain is considered the primary organ of cognitive processes; however, since ancient times, it has been widely believed that memory is an engram, distributed throughout the body. Initially, Aristotelian philosophy strongly advocated the cardiocentric hypothesis, proposing that the heart is a psychophysiological system, responsible for intelligence, kinetics, and emotions[12]. Aristotle believed that the heart mediates neuroplasticity through the fluidic nature of blood, as it is bidirectionally connected to all organs and tissues via the circulatory system. The cephalocentric theory, supported by renowned philosophers and scientists including Pythagoras, Plato, Hippocrates, and Galen, posited that the brain is the central organ of thought processes and sensation[12]. Eventually, numerous neurobiological experiments and neurobehavioral studies have unequivocally demonstrated that key biochemical substrates and underlying mechanisms of memory are localized exclusively within the brain. After a long lapse of centuries, mounting evidence now provides hints to the possibility that various peripheral organs and tissues could be involved in the consolidation and retention of memory, due to the presence of extra-cephalic nervous systems[13]. Accordingly, the notion that peripheral organs contribute to the regulation of neuroplasticity has become an increasingly recognized and evolving area of research. The enteric nervous system (ENS) of the gut is most commonly referred to as the ‘second brain’ because it contains a complex network of neurons and glial cells that is capable of functioning independently of the central nervous system (CNS). While the ENS can autonomously regulate gut physiology, it simultaneously communicates with the brain via the gut–brain axis[14]. In addition, neurocardiology, psychophysiology, and systems neuroscience approaches have identified the presence of diverse neuronal populations contributing to the complexity of the intrinsic cardiac nervous system (ICNS) in the heart. Considering the ability to process and send information semi-independently, and its dynamic influence on brain function, emotional states, especially anxiety, and overall psychophysiological balance, the heart has been metaphorically considered as a “third brain”[15]. The ICNS comprises multiple intracardiac ganglia, which are neuronal clusters embedded within the heart. These ganglia play a crucial role in modulating cardiac function, including the regulation of pacemaker activity[16,17]. The existence of intrinsic neuronal networks in the heart represents a substantial gap in our understanding of whether, and to what extent, the heart may be associated with neuroplasticity underlying the integration of cognitive functions, either independently or in concert with the brain. In general, the proper functioning of neuronal networks requires the support of auxiliary cells such as glia, which are essential for maintaining neuronal homeostasis and facilitating synaptic plasticity[18]. While the functional significance of neural clusters in the heart remains a topic of intensive investigation, studies on the crucial roles of glial cells in the regulation of the neurophysiology of the cardiac system are limited.
The concept of neuroglia was first introduced by Rudolf Virchow in the mid-19th century, referring to different types of non-neuronal cells in the brain and spinal cord that support the homeostasis of the CNS and contribute to neuroplasticity[19]. Among different glial cells, astrocytes have been extensively explored for their functional significance in the physiological state, and their activation has been implicated in the pathomechanisms of various disease conditions that affect neuroplasticity[20]. Given their shared expression of markers and functional analogy to brain-resident astrocytes, cardiac nexus glia in the heart may be important for supporting the neuroplasticity of the heart[18]. They could also play a crucial role in supporting ICNS, modulating synaptic activity, maintaining homeostasis, and potentially influencing cardiac rhythm, autonomic regulation, and neurocardiac integration[6]. However, the characteristics and functional significance of this astroglial counterpart in the heart are not fully determined. In 2018, Skelly et al[21] were the first to characterize a population of non-myocyte lineage in the mouse heart using single-cell resolution techniques. Tedoldi et al[18] further reported that these peripheral glial cells are involved in the modulation of neuronal excitability and autonomic tone in the heart. Further, they also demonstrated that S100 calcium-binding protein B (S100B), a glial protein commonly secreted by brain astrocytes but also found in cardiac glia, could influence ganglionated plexus neurons. Similarly, another study by Scherschel et al[22] validated the expression of S100B in cells located in the ICNS of subjects with atrial fibrillation, suggesting a link to neuronal injury in the heart. Further, a prominent study by Kikel-Coury et al[6] identified neural crest-derived cardiac nexus glia in a zebrafish model, which are found to express astrocytic markers such as GFAP, glutamate-aspartate transporter, and glutamine synthetase. The loss of cardiac nexus glia, both in a clustered regularly interspaced short palindromic repeats/CRISPR-associated protein (CRISPR/Cas)-9-based knockout model of meteorin (MetRn-/-), a glial cell differentiation regulator, and in a model of laser-based ablation targeting GFAP-driven nuclear-localized green fluorescent protein positive cells, disrupted the cardiac rhythm, thereby highlighting their role in maintaining heart rate and rhythm stability as they interact with the ICNS. A study by Xie et al[23] demonstrated that selective activation of peripheral GFAP-expressing cells influences cardiac output, thereby indicating their role in activating the cardiac ganglia. Collectively, these results support the notion that the cardiac nexus glia are essential for the development and neurophysiological function of the heart, whereas their activation or dysfunction can result in pathogenic changes in response to cardiac defects. Besides, studies demonstrate that extracellular ATP can activate cardiac nexus glia, triggering intracellular calcium (Ca2+) signaling, a response also observed in glia across the CNS, peripheral nervous system (PNS), and ENS of the gut[24]. This influx of Ca2+ can promote astrocytic gliotransmission, enabling glia to influence the excitability of neighboring neurons. Thus, the secretion of these astrocytic gliotransmitters appears to be essential for communication with specific subsets of neural populations within the heart, and they also influence the brain through circulation, potentially contributing to the establishment and regulation of the neurocardiac pathways.
THE MOLECULAR PATHWAYS OF THE HEART IN HEALTH AND NEUROCARDIAC DISEASES
Insight into the reciprocal link between the heart and the brain has been a fascinating topic in biomedical research for centuries. Although early ideas about the interdependence between the heart and brain can be traced back to Egyptian and Greek civilizations, in 1628, Sir William Harvey provided the first description for the physiological framework of the circulatory system throughout the body, including the brain, which laid the literal foundation for the modern field of neurovascular biology[25]. During the 19th century, pivotal physiological experiments conducted by Claude Bernard and Carl Ludwig demonstrated that the autonomic nervous system (ANS), particularly its sympathetic and parasympathetic branches, plays a crucial role in regulating heart rate and cardiac contractility[26]. Later, Walter Cannon further validated the idea that emotional changes can drastically modulate the physiology of the heart[27]. This strengthened the idea that the brain could directly influence cardiac function, both during relaxed states and under stress. Moreover, Walter Cannon postulated that overactivation of the sympathetic nervous system could lead to massive adrenaline release, circulatory collapse, and heart failure[28]. This critical insight led to the emergence of neurocardiology, a field devoted to the exploration of the intricate interplay between the nervous and cardiovascular systems. In a healthy state, the heart is directly modulated by the brain through the sympathetic and parasympathetic branches of the ANS[29]. The afferent fibers of the vagus nerve transmit information from the heart and other visceral organs to different areas of the brain. Key brain regions involved in this bidirectional communication include the medulla oblongata, hypothalamus, thalamus, amygdala, and cerebral cortex[29]. The medulla oblongata of the brainstem controls heart rate, contractility, and vascular tone via the sympathetic and parasympathetic nervous systems. Chemoreceptors located in the carotid body monitor blood O2, CO2, pH, blood pressure, and volume status, and send signals to the brainstem to modulate respiration and cardiac output to maintain gas homeostasis. The brainstem ensures appropriate cardiac output based on rest, alertness, stress, and activity levels. Baroreceptors in the aortic arch and carotid sinus detect changes in blood pressure and send afferent signals to the brainstem, which adjusts sympathetic and parasympathetic output to stabilize pressure, preventing hypotension and hypertension[30]. This dynamic exchange ensures that cardiac output is continuously regulated to meet the metabolic demands of the body. This neurocardiac axis enables adaptive responses to exercise, emotional stimuli, and circadian rhythms, reflecting an evolutionarily conserved mechanism that optimizes survival and performance under challenging conditions. Recent research also highlights the influence of higher-order brain regions, such as the insular cortex and anterior cingulate gyrus, in shaping autonomic output, linking emotional processing with cardiac control[31]. Meanwhile, contemporary research has revealed a complex network of communication pathways involving the ANS, the hypothalamic-pituitary-adrenal (HPA) axis, neurohormonal signaling, neuroimmune modulation, and afferent neural feedback[4]. Notably, the HPA axis influences cardiac function through hormonal outputs, primarily the release of cortisol and adrenaline, in response to various experiences and stressors[32]. Despite significant scientific advancements, key gaps persist in our understanding of how emotional changes precisely trigger cardiac dysregulation and the long-term impacts associated with imbalances in brain and cardiac functions. Addressing these questions through interdisciplinary approaches is expected to deepen our understanding and pave the way for integrative strategies to manage cardiovascular function, neurological health, and mental well-being.
In adverse situations and pathological contexts, the breakdown in communication between the heart and brain is closely linked to the emergence of neurocardiac disorders, wherein neurological deficits can impair cardiac function, while cardiac diseases may disrupt neuroplasticity and negatively affect mental health[33]. Mounting evidence suggests that disruptions in the nervous system can lead to significant cardiovascular consequences. Conditions such as stroke, epilepsy, and neurodegenerative disorders exhibit particularly compelling patterns of disrupted neurocardiac interplay[33]. Myocardial injury following acute ischemic stroke is increasingly recognized as a consequence not only of systemic stress and reduced perfusion but also of direct alteration of neurogenic mechanisms[34]. Elevated serum troponin levels, a biochemical signature of myocardial damage, often occur independently of coronary artery disease, pointing to CNS-driven cardiac dysfunction[35]. Notably, myocardial injury is frequently characterized by necrosis and microinfarcts in association with elevated catecholamine levels released during activation of the ANS[36]. Moreover, the occurrence of ischemic stroke in the insular cortex has been reported to induce cardiac arrhythmias[37]. The peak incidence of tachyarrhythmias within the first 24 to 72 hours post-stroke further underscores the acute failure of the neurocardiac interaction. Supporting these clinical findings, animal studies have demonstrated that stroke-induced insular damage can promote inflammatory changes in the left atrium, thereby heightening vulnerability to arrhythmogenesis[38]. Consequently, cardiovascular complications now represent the second leading cause of mortality in stroke patients, surpassed only by neurological injury itself. Indeed, Borovac et al[39] described the potential for sudden cardiac death (SCD) triggered by heightened sympathetic nervous system activation. As a study by Tahsili-Fahadan and Geocadin[40] pointed out, autonomic imbalance alone may not fully explain SCD in patients without prior cardiac pathology, suggesting the involvement of multifactorial contributors. Particularly in sudden unexpected death in epilepsy (SUDEP), the role of neurocardiac dysfunction may serve as an underlying cause in a particularly poignant way[41]. Fatal post-ictal arrhythmias, ranging from bradycardia to ventricular fibrillation, have been reported following seizures and are considered potential contributors to SUDEP[42]. Intriguingly, genetic studies have revealed shared pathogenic mutations affecting both cardiac and neuronal excitability, reinforcing the concept of SUDEP as a complex, genetically intertwined phenomenon[43].
AD, traditionally viewed through a neurodegenerative perspective, is now being explored for its cardiac impairments[44]. Diastolic dysfunction has been observed in early AD, potentially linked to intramyocardial deposition of amyloid-beta, although the causal mechanisms remain unclear[45]. Similarly, a significant portion of PD patients exhibit cardiac autonomic dysfunction, reflecting long-standing alterations in neural control of cardiovascular homeostasis[46]. In Huntington's disease (HD), heart failure has emerged as a potential cause of mortality independent of the classic motor and cognitive symptoms, suggesting that cardiac dysfunction is an important but often less explored aspect of the disease[47]. Studies in both human patients and animal models of HD have reported ventricular remodeling, arrhythmias, and signs of early heart failure[48,49]. Similarly, individuals with multiple sclerosis (MS) are associated with an increased risk for cardiovascular complications such as myocardial infarction, heart failure, and arrhythmias[50]. In MS, acute cardiac presentations, such as paroxysmal atrial fibrillation and cardiogenic shock, have been reported throughout disease progression[51]. Adding another layer, psychiatric conditions such as major depression, anxiety disorders, post-traumatic stress disorder, schizophrenia, and chronic sleep disturbances are associated with an increased risk of cardiovascular failure[52]. Notably, many of these neurological disorders have been characterized by pathogenic changes driven by reactive astroglia in the brain[53,54]. Although multiple neuropathomechanisms have been implicated in neurological and psychiatric disorders linked to cardiac dysfunction, the role of astroglial dysfunction, both in the brain and the heart, and their disrupted communication contributing to neurocardiac defects remains largely unexplored. Moreover, astroglial dysfunction is a common feature, a direct causal relationship between glial abnormalities and cardiac pathology across these comorbid conditions has yet to be clearly established. Notably, the recent discovery of cardiac nexus glia raises the possibility that astroglia-like cells may play a crucial role in contributing to both cardiac regeneration and pathogenesis, depending on the physiological or pathological context. Considering the pivotal role of the heart, insight into the cardiac nexus glia in neurocardiac functions has become increasingly important.
FUNCTIONAL SIGNIFICANCE OF ASTROCYTES IN NEUROCARDIAC DISORDERS
Within the brain, astroglial activation resulting in neuroinflammation is considered a key pathomechanism in various mood, movement, and neuropsychiatric disorders. Astrocyte dysfunction plays a crucial role in the pathogenesis of various neurodegenerative diseases[55]. In AD, impaired astrocytic clearance of amyloid-β and disrupted calcium signaling contribute to neuronal toxicity[56]. In PD, reactive astrocytes undergo a phenotypic shift that results in the loss of their neuroprotective and synaptic roles, leading to impaired antioxidant defense mechanisms and ineffective regulation of oxidative stress, thereby contributing to dopaminergic neurodegeneration[57]. In HD, the mutant huntingtin protein impairs astrocytic glutamate transporters, leading to excitotoxic neuronal damage in the striatum[58]. In motor neuron disorders, such as amyotrophic lateral sclerosis, astrocytes acquire a toxic phenotype that promotes the degeneration of motor neurons by withdrawing metabolic support and releasing proinflammatory factors[59]. Collectively, these findings highlight astrocyte pathology as a central mechanism driving disease progression in a range of neurodegenerative conditions. Moreover, non-neural autonomous mechanisms originating from astrocytes have been speculated to contribute to neurodegeneration-mediated behavioral deficits in many disorders, including HD[60]. Also, astrocytes have been increasingly recognized as the main contributors to the pathophysiology of neurological disorders such as stroke, epilepsy, and viral infections. In stroke, astrocytes respond to ischemic injury by undergoing reactive astrogliosis, releasing proinflammatory cytokines, and disrupting the blood-brain barrier, thereby exacerbating neuronal damage[61]. In epilepsy, astrocytic dysfunction in glutamate uptake and defects in potassium buffering lead to neuronal hyperexcitability and the onset of seizure[62]. During viral infections, astrocytes act both as reservoirs for viral replication and as modulators of neuroinflammation, thereby impairing neuronal survival and exacerbating disease progression[63]. Emerging evidence suggests that the aberrant activation of astrocytes plays a detrimental role in the pathophysiology of depression, anxiety disorders, and schizophrenia. In major depressive disorder, astrocyte dysfunction is associated with impaired glutamate homeostasis and reduced neurotrophic support, leading to disrupted synaptic connectivity[64]. In anxiety, astrocytic regulation of neurotransmitter balance, particularly between GABA and glutamate, as well as neuroinflammation, has been implicated in the underlying pathomechanisms of disease[65]. In schizophrenia, reduced astrocytic density and defective astrocyte-neuron interactions have been linked to disrupted synaptic pruning, glutamate dysregulation, and cognitive dysfunctions[66]. Considering these facts, dysfunction of astrocytes and dysregulation in the release of astrocytic gliotransmitters appear to be key pathogenic determinants underlying neurodegenerative, mood, and psychiatric disorders. Notably, BDNF serves as a key regulator not only of neuronal survival but also of neurogenic processes in the brain[67]. Therefore, reduced levels of astrocytic gliotransmitters such as BDNF may underlie not only defects in synaptic plasticity but also impair neurogenesis, ultimately leading to functional deficits, including cognitive decline and dementia. While the negative impact of neurological disorders on cardiovascular health is well-recognized, the reverse relationship, such as how cardiovascular dysfunction contributes to brain pathology, has attracted growing scientific attention. Given the presence of cardiac nexus cells in the heart, which are analogous to astrocytes, neurocardiac pathogenesis could be attributed to the activation or dysfunction of these glial-like cells. Recent work by Ziegler et al[68] revealed extensive glial remodeling and inflammation in the superior cervical ganglia of patients with Takotsubo syndrome (TTS), a transient, stress-induced cardiomyopathy often mimicking acute myocardial infarction. While TTS was first described in the early 1990s, this syndrome is now increasingly recognized in post-stroke patients with insular cortex involvement, reflecting an acute catecholamine-induced myocardial dysfunction triggered by excessive sympathetic stimulation. Moreover, compelling evidence demonstrates that myocardial injury can activate astrocytes in the brain and trigger neuroinflammation[7]. Despite these few insights, the potential contribution of glial dysfunction to cardiac pathogenesis remains poorly explored. Since astrocytes share certain features with neural stem cells (NSCs) in the brain, accounting for the origin and course of neurogenesis, defects in astrocytes can lead to impaired neurogenesis. Considering the presence of cardiac nexus cells, it is plausible that a neurogenic niche exists within the heart, which may also be altered during neurocardiac disease.
HEARTBEAT-DRIVEN REGULATION OF HIPPOCAMPAL NEUROGENESIS, THE POSSIBLE ROLES OF NEXUS GLIA IN CARDIAC FUNCTION, AND THE CONCEPT OF PERIPHERAL NEUROGENESIS
The fully grown brain is now considered to possess neuroregenerative potential due to the presence of NSCs in the hippocampus and the subventricular zone. Notably, NSCs with radial glial morphology have been identified as bona fide neuronal progenitors, capable of self-renewal and differentiating into glial cells and neuroblasts. Neuroblasts are considered immature neurons that subsequently mature into neurons, thereby accounting for neurogenesis[69]. Ongoing neurogenesis is crucial for cognitive function and the regulation of emotions. However, conditions such as stress, depression, anxiety disorders, neurodegenerative diseases, and psychiatric disorders are known to disrupt the neurogenic process in the hippocampus[70]. Neurogenesis, especially in the hippocampus, is modulated by various intrinsic and extrinsic inputs generated through motor and sensory activities[71]. The vagus nerve plays a crucial role in bidirectional communication, transmitting signals between the heart and the brain. The vagus nerve is a major component of the parasympathetic nervous system, and it exerts a cardioinhibitory effect by releasing acetylcholine, which acts on muscarinic receptors in the heart to decrease heart rate, known as bradycardia, and reduce contraction force[72]. Through this mechanism, it ensures cardiovascular homeostasis by counterbalancing the sympathetic 'fight-or-flight' responses. Notably, stimulation of the vagus nerve has been shown to enhance neurogenesis in the brain, leading to improvements in cognitive functions[73]. Therefore, it can be speculated that the heartbeat-synchronized modulation of the vagus nerve activity could be associated with an increase in hippocampal neurogenesis, linking cardiac rhythms to brain plasticity. Notably, the neurogenic process in the hippocampus has been linked not only to cognitive functions but also to the regulation of mood and emotion in physiological states. Stress, depression, and anxiety are characterized by impaired neurogenesis, conditions in which abnormal cardiac function is also clearly established[74]. In conditions such as anxiety, stress, depression, and other neuropsychiatric disorders, alterations in heart rate variability can lead to dysfunction in vagus nerve transmission, which may, in turn, impair neurogenesis in the brain[75].
Unlike other organs, the developed heart has traditionally been viewed as a terminally differentiated organ with limited regenerative capacity. However, recent experimental evidence suggests the presence of heart-resident stem/progenitor cells that may contribute to cardiac physiology and repair. These cardiac stem cells, identified by markers such as cellular-KIT proto-oncogene receptor tyrosine kinase, stem cell antigen-1, and Islet-1 gene (Isl1), are believed to possess the ability to differentiate into cardiomyocytes, endothelial cells, and smooth muscle cells[76]. Though the existence of stem cells in the heart remains a topic of debate, some studies support possibilities for cardiac regeneration, yet ambiguities persist regarding the ability of cardiac resident cells to restore heart tissue after cardiac insult. Notably, a significant portion of NSCs with radial glial morphology in the brain express GFAP, an astroglial marker that is also detected in cardiac nexus glia[6]. This shared marker expression suggests the possibility that cardiac nexus glia could represent a distal counterpart of NSCs, as both cell types originate from the neural crest[6]. Considering the presence and maintenance of a local cardiac neuronal network, it can be hypothesized that the heart may possess the potential to generate not only new cardiac cells but also neurons. As proof of concept, the proposed neurogenic potential could be further supported by the presence of the Isl1-positive cells in the heart. These cells, known to contribute to both cardiac and neuronal development, serve as markers of multipotent progenitors capable of differentiating into cardiac, endothelial, and smooth muscle cells, and have also been shown to give rise to a newborn subneuronal population, including cholinergic neurons[77,78].
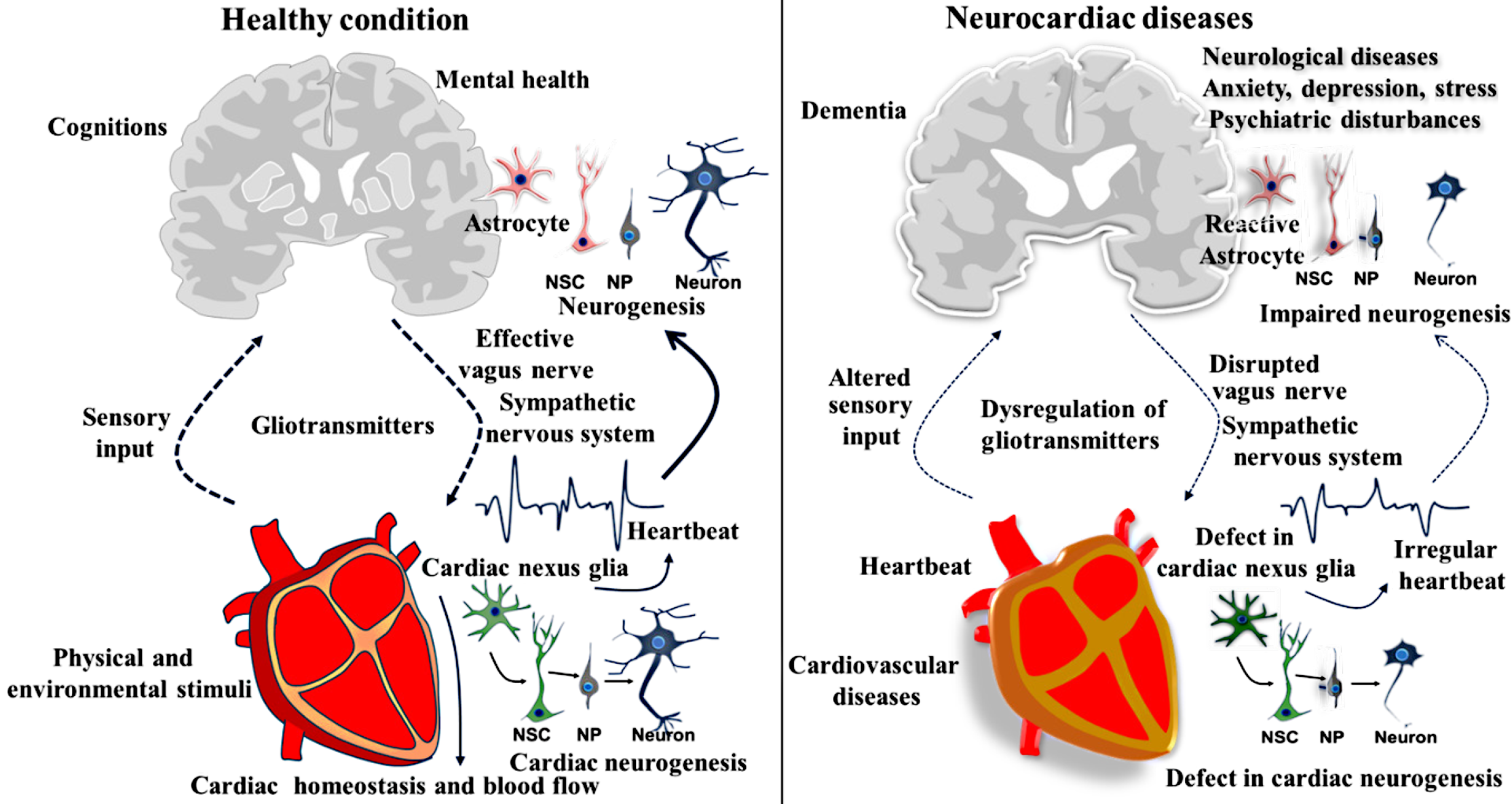
Likewise, many organs, such as the gut, pancreas, liver, kidneys, lungs, and spleen, have been shown to harbor astrocyte-like cells and a local neuronal population. These observations raise the intriguing possibility that neurogenic processes could occur outside the brain, although direct evidence remains elusive. While the identification of the neurogenic capacity of the PNS has been increasingly evident, the occurrence of neurogenesis in non-neural tissues and organs remains largely unexplored. Neurogenic activity in the gut is of particular interest, as it may contribute to maintaining organ function and facilitating repair; this concept could potentially be extended to the heart and other organs that possess an independent neural population and network. Considering the aforementioned facts, we propose a new term, “peripheral neurogenesis”, referring to the generation of new neurons in various peripheral tissues and organs, including the heart. This peripheral neurogenesis may arise from NSC-like cells, including astrocytes or neuroblasts present within the extracerebral organs. This peripheral neurogenic process could play a crucial role in maintaining the homeostasis of the organ, adapting to environmental changes, and supporting regeneration by facilitating the replacement and repair of damaged local neurons following injury or disease. Moreover, the newly formed neurons in the peripheral organs could play an important role in establishing communication with the brain. The newly generated neurons in peripheral organs may contribute to cognitive functions such as consolidation, storage, and retrieval of memory, either independently or in coordination with the brain through their respective bidirectional pathways. Eventually, during disease conditions, organ-specific pathogenesis may impair the local neurogenic process, potentially leading to miscommunication between the brain and peripheral organs, including the heart, thereby disrupting effective communication between the brain and peripheral organs. Therefore, the pathological state of peripheral organs could also contribute to disruptions in cognitive functions, including memory loss. Given its complex neurohumoral and bioelectrical characteristics, the proposed neurogenic capacity of the heart supports the idea that the cardiac system may directly or indirectly contribute to the neuroplasticity underlying memory processes. Considering that neurogenesis plays a key role in the consolidation and retrieval of memory, this article also supports the possibility that the neural basis of memory may exist beyond the brain. Eventually, this notion could support the ancient Aristotelian cardiocentric theory that memory is stored throughout the body. In disease states, the dysfunction of peripheral neurogenesis could overlap with comorbid conditions, alongside brain disorders. Therefore, cardiac disorders, along with neurological illnesses, could be strongly associated with defects in the development of nexus glia-derived progenitors, including neurons. This concept could also extend to other disorders where the respective organ of the defect is known to harbor signatures of NSCs and neural progenitors. Therefore, the concept proposed in this article may serve as a foundational reference for understanding peripheral neurogenesis; however, it requires further experimental validation (Figure 1).
Figure 1 Role of cardiac nexus cells in the regulation of neurogenesis and neurocardiac interaction in health and neurocardiac disease.
The left panel illustrates the role of cardiac nexus cells in regulating neurogenesis in the brain through heart dynamics, emphasizing the effective communication and reciprocal relationship between the heart and brain, along with the potential for neurogenesis within the heart itself. In contrast, the right panel depicts pathological alterations observed in neurocardiac disorders, characterized by a disrupted heart–brain axis and impaired neurogenic processes linked to cardiac dysfunction. NSC: Neural stem cells; NP: Neural progenitor.
In summary, this article emphasizes that astrocyte-like cells in the heart may function as NSC-like entities, potentially contributing to local neurogenic activity. Peripheral organs, including the heart, which possess independent neural networks, may harbor localized neurogenic processes referred to as peripheral neurogenesis. Through neural communication or humoral factors such as astrocytic gliotransmitters, the heart may also have the potential to modulate neurogenesis in the brain. Thus, the neural basis of cognitive functions may be partly supported by peripheral organs, reinforcing the emerging concept that memory could be processed and, to some extent, stored throughout the body. However, the proposed hypothesis requires further experimental validation with robust supporting evidence. For example, fate mapping and neurogenic assessments directed at NSCs in the brain should be extended to peripheral organs. Transgenic approaches using CRISPR-Cas9 to deplete specific cardiac nexus cells could help in investigating their role in memory. Additionally, the assessment of cognitive functions, compiling metadata on disease courses within peripheral organs exhibiting neurogenesis, may provide valuable insights into their contribution to the proposed concept of extracephalic memory. A deeper understanding of the pathophysiology and cognitive processes in neurocardiac diseases could further support and validate this idea. Moreover, neuroimaging, electrophysiological recordings, gene expression profiling, and biochemical studies targeting peripheral organ–related engrams may help establish or refute the existence of extracephalic memory. Therefore, the existence of extracephalic neurogenesis and memory cannot be completely ruled out at this moment.
CONCLUSION
Emerging evidence suggests that peripheral organs like the heart, through specialized glial-like cells such as cardiac nexus cells, may possess neurogenic potential analogous to NSCs in the brain. This raises the intriguing possibility of localized neurogenesis, termed peripheral neurogenesis, occurring extra-cephalically in non-classical neural organs. The bidirectional communication between peripheral neurogenic niches and the brain could have important implications for cognitive functions, including memory consolidation, storage, and retrieval. While current data remain mostly correlational, advances in genetic and neuroimaging techniques could offer a promising direction to explore the contributions of peripheral neurogenesis to the extracerebral neural basis of cognition. Ultimately, a deeper understanding of neurocardiac interactions and extracephalic memory may revolutionize the existing grasp of memory processing and neuroplasticity, expanding it beyond traditional brain-centered models and opening new possibilities for regeneration-based therapeutic interventions in neurocardiac diseases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country of origin: India
Peer-review report’s classification
Scientific Quality: Grade A, Grade A, Grade B, Grade C
Novelty: Grade A, Grade A, Grade B, Grade C
Creativity or Innovation: Grade A, Grade B, Grade B, Grade C
Scientific Significance: Grade A, Grade B, Grade C, Grade D
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Kanthlal S, Chief, Full Professor, Professor, India; Mburu SN, PhD, Academic Fellow, Associate Research Scientist, Chairman, Postdoc, Senior Researcher, Senior Scientist, Kenya; Roaquin L, Professor, Philippines S-Editor: Liu H L-Editor: A P-Editor: Wang WB