Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.110034
Revised: June 30, 2025
Accepted: August 4, 2025
Published online: September 27, 2025
Processing time: 119 Days and 16.8 Hours
In the field of anesthesia for procedure for prolapse and hemorrhoids (PPH) surgery, combined spinal-epidural (CSE) anesthesia has been a common app
To compare remimazolam-CSE vs CSE alone on State-Trait Anxiety Inventory-State scale (STAI-S) scores, sedation, and hemodynamics in PPH surgery.
This study is a single-center, prospective, randomized controlled trial. Between November 23, 2022, and August 6, 2024, 60 eligible patients were randomly as
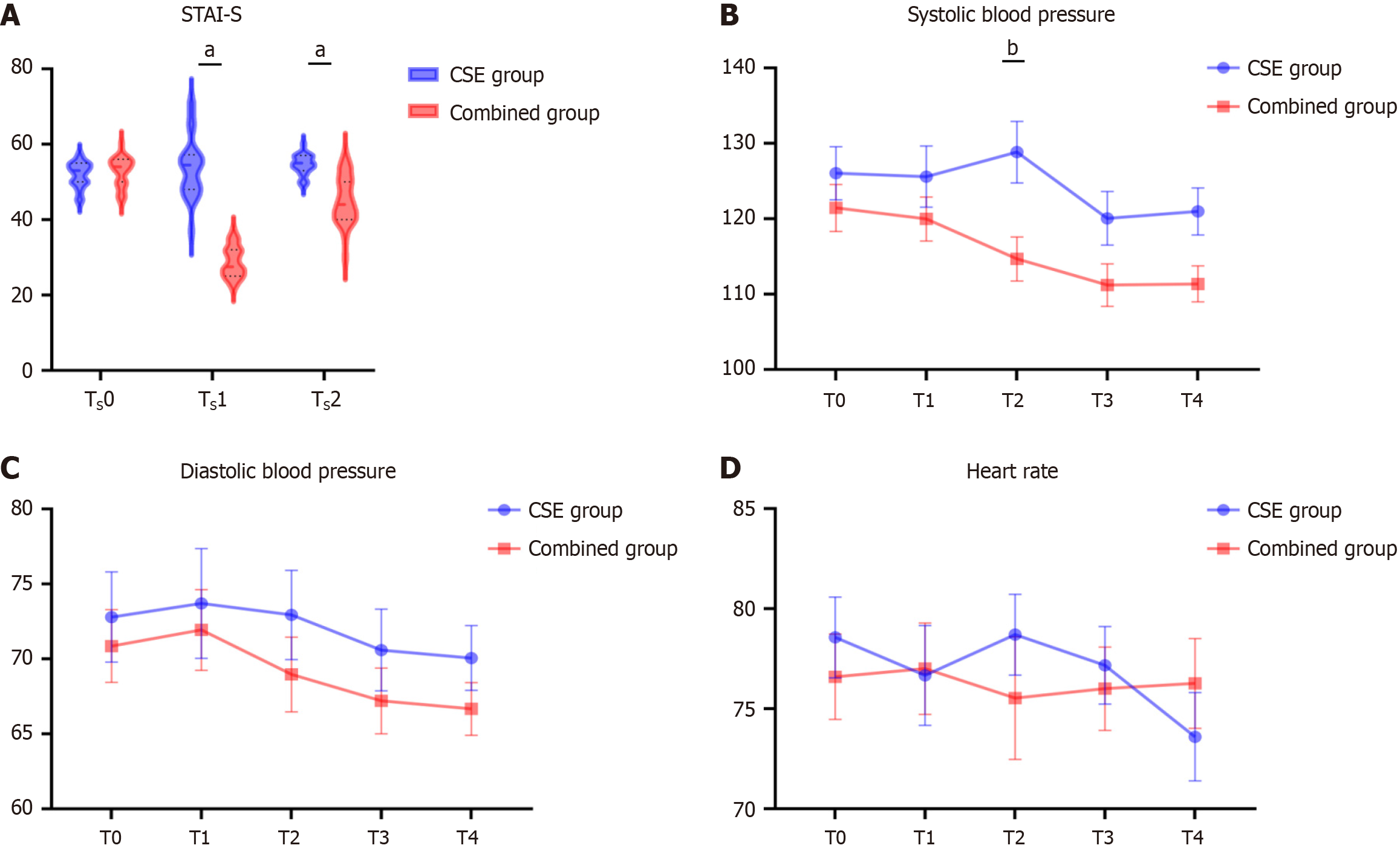
The Combined group demonstrated significantly lower STAI-S scores before leaving the operating room [mean: 28.80 vs 54.03, mean difference (95%CI): 25.23 (21.24-29.23), P < 0.001] and 24 hours post-operation [mean: 45.07 vs 54.53, mean difference (95%CI): 9.47 (6.29-12.64), P < 0.001] than the CSE group. Moreover, the Combined group achieved a deeper sedation level during intraoperative maintenance [median: 5.00 (IQR: 5.00-5.00) vs 2.00 (IQR: 2.00-2.00); median difference (95%CI): 3.00 (3.00-3.00), P < 0.001]. Regarding hemodynamics, a significant inter-group difference in systolic blood pressure was observed at the start of the surgery [mean: 128.8 vs 114.7 for the Combined and CSE groups, mean difference (95%CI): 14.17 (0.77-27.57), adjusted P = 0.033].
Remimazolam-combined anesthesia outperformed CSE anesthesia in reducing STAI-S scores, enhancing intraoperative sedation, and stabilizing systolic blood pressure at a critical stage, indicating its superiority in perioperative management.
Core Tip: In procedure for prolapse and hemorrhoids (PPH) surgery, traditional combined spinal-epidural (CSE) anesthesia commonly encounters challenges of insufficient intraoperative sedation and hemodynamic fluctuations. This study confirms that a remimazolam-based CSE anesthesia protocol significantly reduces patient anxiety before leaving the operating room and at 24 hours post-operation, while achieving deep sedation and stabilizing systolic blood pressure during critical surgical phases. The rapid onset of remimazolam's sedative effect and its anxiolytic properties offer an optimized approach to perioperative management of CSE anesthesia in PPH surgery.
- Citation: Hu T, Huang Q, Wei L, Zhong S, Wang J. Remimazolam reduces State-Trait Anxiety Inventory-State Scale scores in hemorrhoid surgery with spinal-epidural anesthesia: A randomized trial. World J Gastrointest Surg 2025; 17(9): 110034
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/110034.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.110034
The perioperative psychological stress associated with surgical procedures, particularly those involving sensitive anatomical regions, remains a critical concern in modern anesthesia practice[1]. Procedure for prolapse and hemorrhoids (PPH) surgery, a minimally invasive technique for treating severe hemorrhoids, is no exception. Despite its efficacy, PPH surgery often induces heightened anxiety due to its perianal location, postoperative pain concerns, and perceived social stigma. Psychological distress in these patients manifests through elevated State-Trait Anxiety Inventory-State scale (STAI-S) scores, reflecting acute preoperative anxiety that can exacerbate sympathetic nervous system activation, leading to hemodynamic instability and delayed recovery[2]. Current anesthesia protocols, such as standard Combined Spinal-Epidural (CSE) anesthesia, provide effective analgesia but fail to address psychological stress adequately, leaving patients vulnerable to intraoperative awareness and postoperative anxiety-related complications.
Remimazolam, a novel ultra-short-acting benzodiazepine, offers a promising solution to this unmet need. As a selective agonist of γ-aminobutyric acid type A (GABAA) receptors, remimazolam enhances chloride ion influx into neurons, hyperpolarizing membranes and inhibiting neuronal excitability. This mechanism not only produces rapid-onset sedation and anxiolysis but also stabilizes hemodynamics by reducing sympathetic tone[3]. Preclinical and clinical studies in non-PPH settings, including endoscopic procedures and pediatric surgeries, demonstrate remimazolam’s ability to mitigate anxiety and maintain cardiovascular stability with minimal respiratory depression[4,5]. However, its application in PPH surgery remains under investigated. Existing research primarily focuses on general surgical contexts, leaving a critical gap in understanding its effects on anxiety, hemodynamics, and postoperative outcomes specific to PPH patients.
Against this backdrop, this randomized, double-blind, single-center trial aims to evaluate the efficacy of remimazolam-combined CSE anesthesia compared to standard CSE anesthesia in PPH surgery. The study will assess preoperative and postoperative anxiety levels using STAI-S scores and monitor hemodynamic parameters [e.g., heart rate (HR), blood pressure] to determine stability during surgery. We hypothesize that patients in the remimazolam-combined CSE group will exhibit significantly lower STAI-S scores and more stable hemodynamics than those in the standard CSE group, driven by remimazolam’s dual action on anxiety reduction and sympathetic modulation. This research will address the current lack of evidence on remimazolam’s role in PPH surgery, potentially optimizing anesthesia protocols to enhance patient comfort and perioperative outcomes.
This prospective, single-center, randomized controlled trial was carried out at the Hunan Provincial People's Hospital. The First Affiliated Hospital of Hunan Normal University granted ethical approval to perform the study within its facilities, Ethical Application No.[2022]-51, ethical approval was obtained for protocol version 3.0, dated 2022-05-21. Prior to the initiation of the study, detailed information about the trial procedures, potential risks, benefits, and patients’ rights was provided to all eligible patients or their legal representatives. Informed consent forms were obtained from the individuals themselves or their authorized representatives. After trial commencement, no important changes to the study methods (including eligibility criteria, intervention delivery, and outcome assessments) were made. All procedures were strictly followed as outlined in the pre-registered protocol.
Between November 23, 2022 and August 6, 2024, patients aged 18-60 years classified as American Society of Anesthesiologists physical status I-II, who were scheduled to undergo procedure for PPH and provided informed consent, were enrolled in this study. Exclusion criteria included: (1) Patients who declined participation; (2) Those with contraindications to spinal anesthesia; (3) individuals with known allergies to local anesthetics; (4) Patients with significant airway anatomical abnormalities (including the nose, mouth, pharynx, and larynx) or acute and chronic respiratory diseases, as well as those with moderate to severe obstructive sleep apnea syndrome; (5) Individuals with psychiatric disorders or cognitive impairments that hindered cooperation; and (6) Any other conditions deemed unsuitable for study parti
After obtaining informed consent, participants were randomly allocated in a 1:1 ratio to either the standard CSE Anesthesia group (CSE group) or the remimazolam-combined CSE anesthesia group (Combined group) using a computer-generated randomization process, with the allocation details stored in sealed, opaque envelopes. For the double-blind design, remimazolam and the control anesthetic were placed in identical-looking syringes labeled only with unique codes. When patients entered the operating room, the designated anesthesiologist opened the envelope and prepared the corresponding anesthetic without revealing the group information to the patients, ensuring that both the medical staff administering the anesthesia and the patients remained unaware of the group assignments throughout the study. Assessors responsible for evaluating anxiety levels and hemodynamic parameters were also blinded to the group allocations.
All data were meticulously recorded in the electronic medical records system (EMR), and access to the EMR was restricted to authorized personnel overseeing data collection and analysis to maintain confidentiality regarding the group assignments. To ensure quality control, all research staff received standardized training on study procedures, data collection, and patient management prior to the trial, and regular data audits were scheduled every month to check for data integrity and compliance with the study protocol.
Upon arrival in the operating room, all patients were standardly monitored with HR, 5-lead continuous electrocardiogram, non-invasive blood pressure, and oxygen saturation. Peripheral intravenous access was established, and oxygen was administered via nasal cannula at 2 L/minute to maintain adequate oxygen supply. Considering the anatomical characteristics and requirements of PPH surgery, standard CSE anesthesia was performed at the L3-4 interspace, which is a commonly used and relatively safe approach for achieving effective anesthesia in this type of surgery.
For the CSE group, an epidural needle was inserted, and once the epidural space was reached, a spinal needle was used to puncture the subarachnoid space. After confirming the free flow of cerebrospinal fluid, 3 mL of 0.5% ropivacaine was slowly injected over approximately 20 seconds. Following the injection, an epidural catheter was placed. Once anesthesia was administered, the patient was instructed to assume the supine position, and the anesthetic level was tested, aiming to achieve a sensory block at the T10 Level. This level of anesthesia is sufficient to meet the requirements of PPH surgery while minimizing potential adverse effects on other physiological functions. After confirming the anesthesia level, the patient was repositioned to the prone position to begin the surgery.
For the Combined group, the standard CSE procedure was followed as described above. After positioning, re
The surgical approach was determined preoperatively by the surgeon and explained to the patient. All procedures were performed by the same surgical team. For the Procedure for PPH, a circular stapler was used to excise and anastomose the prolapsed hemorrhoidal tissue. After positioning the patient, a digital rectal examination was performed to confirm the location of the prolapsed hemorrhoids. A purse-string suture was placed around the mucosa, and the circular stapler was inserted to excise the tissue and perform the anastomosis. After deploying the stapler, the prolapsed tissue was removed, and the mucosal defect was closed. The surgical site was irrigated, and the incisions were closed. The patient was monitored for bleeding and complications and observed during recovery.
The State-Trait Anxiety Inventory (STAI) was developed by Spielberger et al[6] in collaboration with other prominent researchers. Due to its good reliability and validity, it has been widely used in psychological and medical research fields globally. It can measure the anxiety levels of individuals in specific situations accurately and reliably. The STAI is divided into two parts: The STAI-S and the STAI-Trait scale. In this study, the STAI-S was mainly used to assess the immediate anxiety feelings and emotional experiences of patients at different timepoints during the perioperative period, such as nervousness, worry, and unease. The STAI-S consists of 20 items, and each item adopts four options ranging from 1 to 4. Assessment for each item: 1: Completely without, 2: Some, 3: Middle degree, 4: Obvious. These items assist in surveying the frequency of such symptoms, like “I feel extremely anxious” or “I feel content”. Of the 20 items, 10 are negatively worded. When calculating scores, these negatively worded items require reverse scoring. The total score ranges from 20 to 80 points. The higher the score, the more intense the anxiety an individual experiences at the moment.
The primary outcome was to assess patients’ perioperative anxiety levels, achieved by recording STAI-S scores at three specific time points: TS0 (upon entry into the operating room), TS1 (prior to leaving the operating room), and TS2 (24 hours postoperatively). TS0 serves as the pre-operative anxiety baseline - patients’ anxiety often heightens due to unfamiliar surroundings and surgical concerns, making it a critical starting point for tracking anxiety changes. TS1 measures immediate postoperative anxiety: Patients may experience relief or increased post-surgery, and comparing TS1 to TS0 scores reveals the intervention’s impact on intraoperative anxiety. TS2, 24 hours postoperatively, evaluates early recovery anxiety, when patients face pain and mobility challenges. Comparing TS2 scores with TS0 and TS1 clarifies anxiety trajectories from pre-operation to early recovery, indicating whether the intervention alleviates anxiety during this stage.
For the secondary outcome, the Numeric Pain Rating Scale scores were measured 24 hours postoperatively. Additional outcome measures included the monitoring of non-invasive blood pressure and HR at five different time points. These time points were T0 (upon patients' entry into the operating room), T1 (prior to positioning), T2 (at the commencement of surgery), T3 (10 minutes after the start of the surgical procedure), and T4 (when patients were discharged from the operating room). No changes to the trial outcomes were made after commencement.
Based on preliminary data, the STAI-S scores at the end of surgery were 30 for the Combined group and 30 for the CSE group (n = 10, pre-experiment data). A sample size calculation was performed using GPower software, with a power of 0.8 and a significance level of 0.05. The calculation determined that a minimum of 30 participants per group would be required, resulting in a total sample size of 60 participants.
Data analysis was carried out using GraphPad Prism 9.5.0 software. Initially, the Shapiro-Wilk test was used to evaluate the normality of sample data. For data conforming to a normal distribution, independent samples t-tests were applied for comparisons, and the outcomes were presented as mean ± SD. In cases where data were non-normally distributed, the Mann-Whitney U test was utilized, with results reported as the median or interquartile range (Q1, Q3). For categorical data, we assessed whether the conditions for the χ2 test were met. If so, the χ2 test was used to verify the null hypothesis of no association between variables; if not, Fisher’s exact test was adopted. The results are presented as ratios and percentages. The significance level was set at P < 0.05.
In addition, for the repeated measurement data of the two groups at multiple time points (e.g., systolic blood pressure, diastolic blood pressure, HR, and STAI-S scores), considering the possible violation of the sphericity assumption, a two-way analysis of variance (mixed model) with Geisser-Greenhouse correction was conducted in GraphPad Prism. Subsequently, Bonferroni’s multiple comparison test was performed to determine the intergroup differences at each time point. Bonferroni correction (α = 0.01) was applied for multiple comparisons to control the type I error rate.
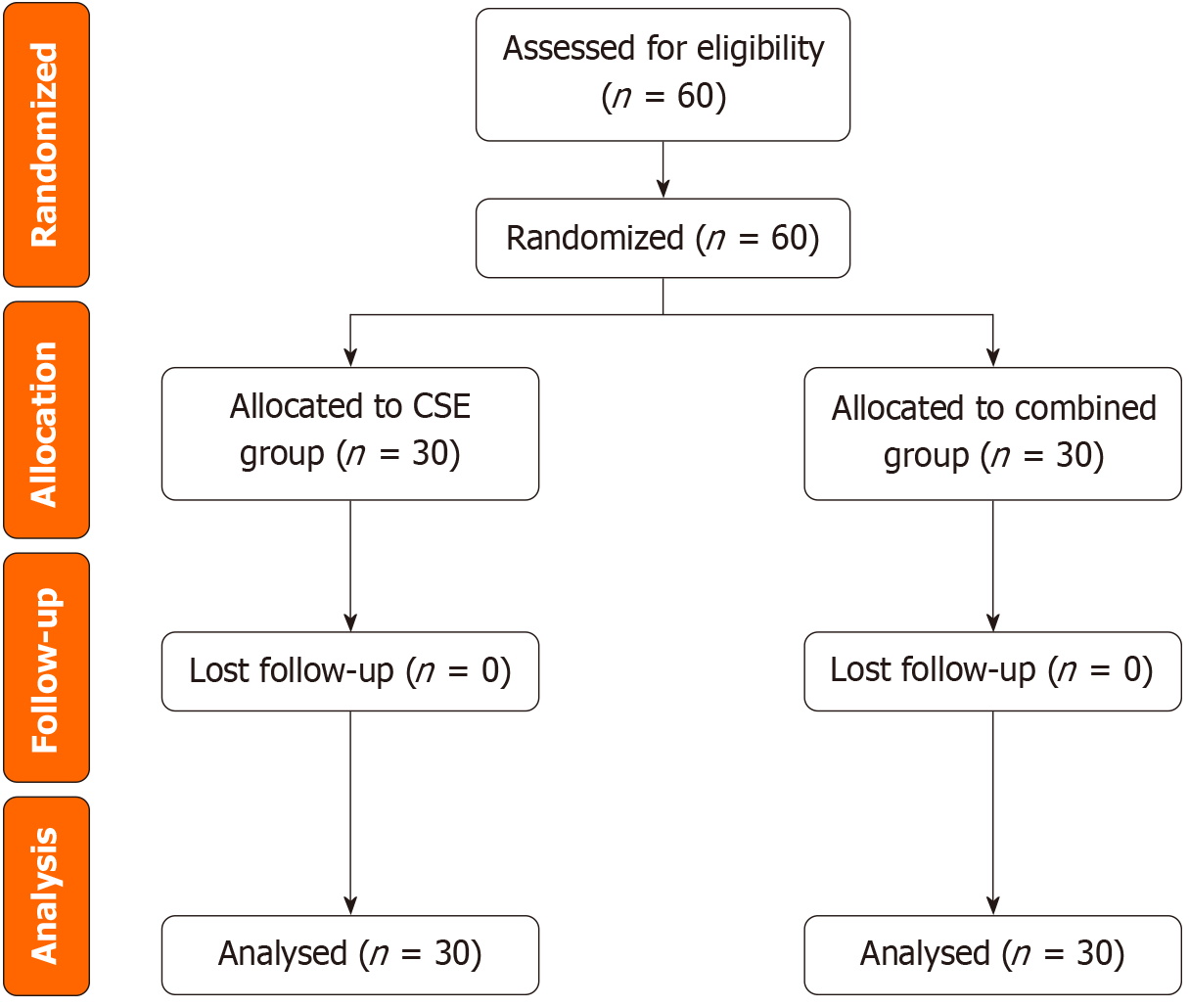
Between November 23, 2022, and August 6, 2024, a total of 60 patients were screened for eligibility. Notably, no patients were excluded during the screening process, there was no loss to follow-up, and no serious adverse events occurred. All patients were enrolled and included in the analysis. The patients who provided informed consent were then randomly allocated into two groups using a computer - generated random sequence, with 30 patients in each group. The Consolidated Standards of Reporting Trials diagram, which provides a visual representation of the flow of participants at each stage of the trial, is shown in Figure 1. Patient demographics and surgical characteristics, summarized in Table 1 to facilitate a clear comparison between the two groups, revealed no significant differences between the two groups.
| CSE group, n = 30 | Combined group, n = 30 | P value | |
| Age (years) | 43.07 ± 12.84 | 41.03 ± 12.02 | 0.511 |
| Gender | 0.439 | ||
| Female | 16 (53.33) | 13 (43.33) | |
| Male | 14 (46.67) | 17 (56.67) | |
| Weight (kg) | 62.73 ± 12.68 | 64.00 ± 13.52 | 0.699 |
| Height (cm) | 164.13 ± 7.92 | 166.07 ± 9.34 | 0.348 |
| ASA classification | 0.818 | ||
| I | 50 (90.9) | 47 (92.1) | |
| II | 5 (9.1) | 4 (7.9) | |
| Anesthesia induction to incision time (minutes) | 15.00 (15-20) | 15.00 (15-20) | 0.756 |
| Surgical duration (minutes) | 49.23 ± 20.15 | 53.67 ± 23.88 | 0.312 |
| Post-surgical exit time from operating room (minutes) | 10.00 (5-15) | 10.00 (10-10) | 0.234 |
| Hospital stay (days) | 5.93 ± 2.26 | 5.67 ± 2.96 | 0.149 |
A two-way mixed-effects ANOVA was employed to explore the impacts of time (TS0: Upon patients’ entry into the operating room, TS1: Prior to leaving the operating room, and TS2: 24 hours postoperatively), group (CSE group vs Combined group), and their interaction on the STAI-S scores.
The analysis revealed significant main effects of time [F (1.707, 98.99) = 83.56, P < 0.001] and group [F (1, 58) = 201.4,
| Variable | Effect | Fdf1, df2 value | P value | Geisser-Greenhouse's epsilon |
| STAI-S | Time | F1.71, 98.99 = 83.56 | < 0.001a | 0.8534 |
| Group | F1, 58 = 201.4 | < 0.001a | ||
| Time × group | F2, 116 = 110.0 | < 0.001a | ||
| SBP | Time | F3.28, 190.5 = 10.04 | < 0.001a | 0.8210 |
| Group | F1, 58 = 4.34 | 0.042a | ||
| Time × group | F4, 232 = 2.44 | 0.048a | ||
| DBP | Time | F2.87, 166.5 = 3.96 | 0.010a | 0.7176 |
| Group | F1, 58 = 0.75 | 0.391 | ||
| Time × group | F4, 232 = 0.26 | 0.901 | ||
| HR | Time | F2.72, 157.7 = 0.85 | 0.462 | 0.6796 |
| Group | F1, 58 = 0.07 | 0.796 | ||
| Time × group | F4, 232 = 1.06 | 0.380 |
| Variable | Time point | CSE group, n = 30 | Combined group, n = 30 | Mean difference (95%CI) | Adjusted P value |
| STAI-S | TS0 | 51.93 | 52.80 | -0.87 (-3.143-1.409) | > 0.999 |
| TS1 | 54.03 | 28.80 | 25.23 (21.24-29.23) | < 0.001a | |
| TS2 | 54.53 | 45.07 | 9.47 (6.29-12.64) | < 0.001a | |
| SBP | T0 | 126.0 | 121.4 | 4.60 (-7.92-17.12) | > 0.999 |
| T1 | 125.6 | 120.0 | 5.63 (-7.71-18.97) | > 0.999 | |
| T2 | 128.8 | 114.7 | 14.17 (0.77-27.57) | 0.033a | |
| T3 | 120.1 | 111.2 | 8.87 (-3.18-20.91) | 0.273 | |
| T4 | 121.0 | 111.4 | 9.60 (-0.89-20.09) | 0.089 | |
| DBP | T0 | 72.80 | 70.87 | 1.93 (-8.38-12.25) | > 0.999 |
| T1 | 73.70 | 71.93 | 1.77 (-10.39-13.92) | > 0.999 | |
| T2 | 72.93 | 68.97 | 3.97 (-6.38-14.31) | > 0.999 | |
| T3 | 70.60 | 67.20 | 3.40 (-5.92-12.71) | > 0.999 | |
| T4 | 70.07 | 66.67 | 3.40 (-4.02-10.82) | > 0.999 | |
| HR | T0 | 76.94 | 76.28 | 1.967 (-5.86-9.79) | > 0.999 |
| T1 | 76.67 | 77.00 | -0.33 (-9.33-8.66) | > 0.999 | |
| T2 | 78.70 | 75.53 | 3.17 (-6.65-12.98) | > 0.999 | |
| T3 | 77.17 | 76.00 | 1.17 (-6.41-8.75) | > 0.999 | |
| T4 | 73.60 | 76.27 | -2.67 (-11.05-5.72) | > 0.999 |
In the analysis of sedation depth (Table 4), the Combined group demonstrated significantly higher Ramsay sedation scores at T2 and T3 compared to the CSE group [median: 5.00 (IQR: 5.00-5.00) vs 2.00 (IQR: 2.00-2.00); median difference = 3.00, 95%CI: 3.00-3.00, P < 0.001]. This finding indicates that the Combined group achieved deeper sedation during the intraoperative maintenance phase (T2-T3) likely attributable to the pharmacological profile of remimazolam. As a novel benzodiazepine, remimazolam offers rapid onset, predictable recovery, and titratable sedation depth. Its integration into the combined anesthesia protocol played a pivotal role in enhancing sedation efficacy at these critical stages, enabling patients to attain profound sedation levels. No statistically significant differences were observed at T0, T1, or T4 (median scores: 2.00 for both groups; P ≥ 0.492), suggesting comparable sedation levels between groups during preoperative preparation (T0-T1) and late recovery (T4). The gradual decline in remimazolam’s effect by T4 Likely contributed to the convergence of sedation states between groups, mirroring their similar preoperative profiles. This implies that neither protocol conferred distinct advantages in sedation management during early or late perioperative phases.
| Time point | CSE group, n = 30 | Combined group, n = 30 | Median difference (95%CI) | P value |
| T0 | 2.00 (2.00-2.00) | 2.00 (2.00-2.00) | 0.00 (0.00-0.00) | 0.492 |
| T1 | 2.00 (2.00-2.00) | 2.00 (2.00-2.00) | 0.00 (0.00-0.00) | 0.492 |
| T2 | 2.00 (2.00-2.00) | 5.00 (5.00-5.00) | 3.00 (3.00-3.00) | < 0.001a |
| T3 | 2.00 (2.00-2.00) | 5.00 (5.00-5.00) | 3.00 (3.00-3.00) | < 0.001a |
| T4 | 2.00 (2.00-2.00) | 2.00 (2.00-2.00) | 0.00 (0.00-0.00) | > 0.999 |
A two-way ANOVA (mixed-effects model) was conducted to analyze systolic blood pressure, diastolic blood pressure, and HR data from 60 patients in the CSE and Combined groups across five time points (T0 to T4), with Bonferroni correction applied for multiple comparisons. The results revealed significant effects of time on systolic blood pressure
Post-hoc analysis using Bonferroni correction showed a significant difference in systolic blood pressure between the CSE and Combined groups only at the T2 time point (mean difference = 14.17, adjusted P = 0.0333), with no significant differences at T0, T1, T3, or T4 (adjusted P > 0.05 for all). As shown in Table 3, no significant differences in diastolic blood pressure or HR were observed at any time point (all adjusted P > 0.05). Trend analysis indicated that the CSE group exhibited an initial increase in systolic blood pressure from T0 to T2, followed by a decline, while the Combined group demonstrated an overall decreasing trend. Diastolic blood pressure and HR remained relatively stable over time in both groups, with no notable trends (Figure 2B-D).
This study investigated the differential effects of CSE with remimazolam vs CSE anesthesia alone on perioperative anxiety, sedation depth, and hemodynamic stability. Remimazolam, a novel ultra-short-acting benzodiazepine, de
The primary outcome revealed significantly reduced anxiety levels in the Combined group during the immediate postoperative recovery phase (TS1) compared to the CSE group, with this anxiolytic advantage persisting partially into the 24-hour postoperative period (TS2). Secondary outcomes demonstrated that the remimazolam-based protocol achieved superior intraoperative sedation depth (Ramsay score = 5) alongside decreased blood pressure, evidenced by a 14.17 mmHg reduction in mean intraoperative systolic blood pressure relative to the CSE group. Below, we integrate these findings with pharmacological mechanisms and current evidence to provide a comprehensive interpretation.
Compared to the CSE group, the Combined group showed significantly reduced STAI-S scores at TS1 (prior to OR discharge; mean difference, 25.23; 95%CI: 21.24-29.23). This anxiolysis is attributed to remimazolam’s pharmacological properties: Residual drug concentrations reduced anxiety despite meeting operating room discharge criteria, while unconsciousness during surgery coupled with gradual emergence further alleviated anxiety. In contrast, CSE patients’ intraoperative awareness heightened sensitivity to environmental stressors (e.g., procedural noises), surgical stress, and anticipatory discomfort, triggering progressive STAI-S elevation. By 24 hours postoperatively (TS2), both groups entered recovery where physiological stressors (e.g., wound pain, restricted mobility) overshadowed anesthesia-related anxiety differences. Crucially, although the Combined group's scores increased from TS1 to TS2, their absolute anxiety levels remained significantly lower than those of the CSE group (mean difference: 9.467; 95%CI: 6.289-12.64). This may reflect CSE patients’ adaptation to postoperative status and active psychological adjustment motivated by high TS1 anxiety; meanwhile sustained smooth emergence transition with low anxiety in the Combined group.
The STAI-S, recognized as one of the "gold standard" tools for assessing preoperative anxiety, has its reliability and validity verified by numerous studies (Cronbach’s α > 0.85)[10]. Clinically, a score of ≥ 44 (out of 80) is universally regarded as the threshold for clinically significant anxiety[10,11]. Studies have shown that a 10-point reduction in STAI-S score is considered a clinically significant improvement, which is significantly associated with a decrease in perioperative adverse events[12]. From the perspective of enhanced recovery after surgery (ERAS), preoperative anxiety management has become a critical component in optimizing postoperative rehabilitation[13]. Changes in STAI-S scores are significantly correlated with anesthesia recovery quality, analgesic requirements, and early rehabilitation efficiency. In clinical practice, screening high-anxiety-risk patients using STAI-S and implementing personalized interventions can effectively reduce postoperative pain sensitivity and opioid consumption, providing important support for the smooth imple
These findings align with established evidence that benzodiazepines are among the most used medications for treating anxiety, insomnia, and as pre-anesthetic agents[15]. Gamma-aminobutyric acid (GABA), as the primary inhibitory neurotransmitter in the central nervous system, plays a crucial role in regulating neuronal transmission in the brain[16]. Currently, drugs targeting GABAergic transmission have been widely used in the clinical treatment of anxiety disorders. GABA receptors are mainly divided into ionotropic GABAA receptors and metabotropic GABAB receptors[17]. Research has found that acting on the α2 subunit of the GABAA receptor exhibits significant anxiolytic-like effects. Meanwhile, mice with a deficiency in the B1 subunit of the GABAB receptor display anxiety and panic-like behaviors[18]. These findings fully confirm that GABAergic transmission and alterations in GABA receptors play a vital role in the occurrence and development of anxiety states.
In the neural circuit of the brain that regulates anxiety, the amygdala-prefrontal cortex (PFC) pathway is of great significance[19]. The basolateral amygdala complex (BLA) receives threatening information and then activates the central nucleus (CeA), triggering the somatic manifestations of anxiety. The medial PFC and the anterior cingulate cortex are also involved. The PFC can regulate the activity of BLA neurons to control anxiety, and the activity of the PFC is abnormal in some patients with anxiety disorders. The GABAergic interneurons in the amygdala not only regulate the activation of the CeA by the BLA but also serve as the main targets of excitatory projections from the PFC. They play a key regulatory role in the transmission of anxiety-related information and affect the formation of anxiety.
Remimazolam, as a selective agonist of GABAA receptors, has shown certain efficacy in anti-anxiety. However, the metabolite of remimazolam, CNS7054, has no significant agonistic effect on GABAA receptors[8,20]. The results of this study show that 24 hours postoperatively, the STAI-S scores of patients in the Combined group were still lower than those in the CSE group. The difference in surgical experience provides another reasonable explanation for this phe
The study demonstrated that the Combined group achieved deep sedation (Ramsay score = 5) at T2 and T3 time points, attributable to remimazolam’s rapid onset and sustained intraoperative efficacy. Its short half-life facilitated a rapid return to baseline consciousness by time point T4 (median sedation score = 2.00 in both groups). Comparative trials by Zhou et al[23] highlighted remimazolam’s unique pharmacokinetic profile, enabling profound procedural sedation while circumventing post-anesthetic recovery delays - a critical advantage in ambulatory surgery settings with rising caseloads. Pharmacokinetic analysis revealed that following a 4-hour administration, the calculated context-sensitive half-time was 6.8 ± 2.4 minutes. The onset of unconsciousness occurred at 5 ± 1 minutes post-initiation, while restoration of complete wakefulness occurred 19 ± 7 minutes after terminating the infusion[24]. A meta-analysis of relevant studies revealed that during endoscopic surgery, remimazolam was associated with a significantly lower risk of respiratory depression compared to propofol[25]. This clearly indicates that remimazolam holds an advantage in respiratory depression safety. These characteristics solve two key perioperative problems: Accelerating surgical turnover and reducing sedation-related complications.
In this study, remimazolam had an impact on hemodynamic stability. The systolic blood pressure of the CSE group at the time point was significantly higher than that of the Combined group, with a difference of 14.17 mmHg (P = 0.033). This discrepancy likely reflects anxiety-induced sympathetic hyperactivity in conscious CSE patients, compounded by incomplete spinal sympathetic blockade sustaining catecholamine release. Remimazolam has been demonstrated in multiple clinical studies to induce blood pressure reduction through central GABAergic suppression and peripheral vasodilation[3]. However, its incidence of hypotension remains significantly lower than propofol, with studies reporting rates of 20% and 24% in the 6 and 12 mg/kg/hour dosing groups for remimazolam, compared to 49.3% in the propofol group[26]. Crucially, despite marked SBP elevation in the CSE group during skin incision (acute nociceptive stimulus), both regimens-maintained pressures below the critical threshold of 160 mmHg - a level associated with increased myocardial infarction risk[27]. Based on these findings, the CSE group’s transient hypertension during early intra
In summary, this study pioneers the integration of CSE anesthesia with remimazolam for PPH surgery. Beyond optimizing perioperative hemodynamic stability and sedation efficacy, the protocol introduces a novel psychological evaluation dimension via serial STAI-S assessments, marking a distinct advancement over conventional studies focusing solely on physiological parameters. Remimazolam exhibits dual effects: It alleviates perioperative anxiety, suppresses sympathetic overactivation, and reduces catecholamine release to stabilize the psychosomatic state, while modulating cardiovascular activity, reducing sympathetic tension, and maintaining hemodynamic stability. These characteristics enable patients under the remimazolam-integrated CSE protocol to experience improved surgical comfort. Clinically, the findings highlight the need for proactive, personalized psychological support measures to alleviate patient anxiety and optimize treatment outcomes and rehabilitation quality.
This study has inherent limitations. The sample size was relatively small, potentially limiting the generalizability of results to diverse patient populations receiving remimazolam-combined anesthesia. Additionally, the study focused on short-term outcomes and specific indicators, with long-term effects of remimazolam and its efficacy in special po
In conclusion, in Procedure for PPH surgery, compared with standard CSE alone, the anesthesia protocol combined with remimazolam significantly reduced patient STAI-S scores, particularly before leaving the operating room and 24 hours postoperatively. The protocol also achieved deeper sedation and better-controlled systolic blood pressure at key intraoperative time points. The findings indicate that remimazolam-combined CSE anesthesia demonstrates advantages in optimizing perioperative outcomes, although further research is warranted to validate this conclusion.
We express our sincere gratitude to Dr. Xing Huang from Hunan Provincial People's Hospital for his outstanding surgical expertise and meticulous clinical supervision throughout this study. Moreover, we would like to extend our heartfelt appreciation to the operating room nurses and ward nurses of Hunan Provincial People's Hospital. Their invaluable contributions have been instrumental in the successful implementation of this research.
| 1. | Arora S, Sevdalis N, Nestel D, Woloshynowych M, Darzi A, Kneebone R. The impact of stress on surgical performance: a systematic review of the literature. Surgery. 2010;147:318-330, 330.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 454] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 2. | Powell R, Scott NW, Manyande A, Bruce J, Vögele C, Byrne-Davis LM, Unsworth M, Osmer C, Johnston M. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database Syst Rev. 2016;2016:CD008646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 3. | Hu Q, Liu X, Wen C, Li D, Lei X. Remimazolam: An Updated Review of a New Sedative and Anaesthetic. Drug Des Devel Ther. 2022;16:3957-3974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 4. | Cai YH, Zhong JW, Ma HY, Szmuk P, Wang CY, Wang Z, Zhang XL, Dong LQ, Liu HC. Effect of Remimazolam on Emergence Delirium in Children Undergoing Laparoscopic Surgery: A Double-blinded Randomized Trial. Anesthesiology. 2024;141:500-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Zhu H, Su Z, Zhou H, Lu J, Wang X, Ji Z, Chen S, Wang X, Yao M, Lu Y, Yu W, Su D. Remimazolam Dosing for Gastroscopy: A Randomized Noninferiority Trial. Anesthesiology. 2024;140:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 6. | Spielberger C, Gorsuch R, Lushene R, Vagg PR, Jacobs G. Manual for the State-Trait Anxiety Inventory (Form Y1 - Y2). Palo Alto, CA, United States: Consulting Psychologists Press, 1983: IV. |
| 7. | Saari TI, Uusi-Oukari M, Ahonen J, Olkkola KT. Enhancement of GABAergic activity: neuropharmacological effects of benzodiazepines and therapeutic use in anesthesiology. Pharmacol Rev. 2011;63:243-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, Wiard RP, Feldman PL, Collins H, Waszczak BL, Tilbrook GS. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Rex DK, Bhandari R, Desta T, DeMicco MP, Schaeffer C, Etzkorn K, Barish CF, Pruitt R, Cash BD, Quirk D, Tiongco F, Sullivan S, Bernstein D. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88:427-437.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 10. | Ni K, Zhu J, Ma Z. Preoperative anxiety and postoperative adverse events: a narrative overview. Anesth Perioper Sci. 2023;1:23. [DOI] [Full Text] |
| 11. | Tadesse M, Ahmed S, Regassa T, Girma T, Hailu S, Mohammed A, Mohammed S. Effect of preoperative anxiety on postoperative pain on patients undergoing elective surgery: Prospective cohort study. Ann Med Surg (Lond). 2022;73:103190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Zemła AJ, Nowicka-Sauer K, Jarmoszewicz K, Wera K, Batkiewicz S, Pietrzykowska M. Measures of preoperative anxiety. Anaesthesiol Intensive Ther. 2019;51:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Brodersen F, Wagner J, Uzunoglu FG, Petersen-Ewert C. Impact of Preoperative Patient Education on Postoperative Recovery in Abdominal Surgery: A Systematic Review. World J Surg. 2023;47:937-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 14. | Stamenkovic DM, Rancic NK, Latas MB, Neskovic V, Rondovic GM, Wu JD, Cattano D. Preoperative anxiety and implications on postoperative recovery: what can we do to change our history. Minerva Anestesiol. 2018;84:1307-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Balon R, Starcevic V, Silberman E, Cosci F, Dubovsky S, Fava GA, Nardi AE, Rickels K, Salzman C, Shader RI, Sonino N. The rise and fall and rise of benzodiazepines: a return of the stigmatized and repressed. Braz J Psychiatry. 2020;42:243-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Piñol RA, Reitman ML. Cool(ing) brain stem GABA neurons. Cell Res. 2019;29:785-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Gomes FIF, Aragão MGB, Bezerra MM, Chaves HV. GABAergic transmission and modulation of anxiety: A review on molecular aspects. Braz J Biol Sci. 2019;6:e363. [DOI] [Full Text] |
| 18. | Cryan JF, Kaupmann K. Don't worry 'B' happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 321] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat. 2015;11:165-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (3)] |
| 20. | Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 21. | Chieng YJ, Chan WC, Klainin-Yobas P, He HG. Perioperative anxiety and postoperative pain in children and adolescents undergoing elective surgical procedures: a quantitative systematic review. J Adv Nurs. 2014;70:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Åhs F, Kragel PA, Zielinski DJ, Brady R, LaBar KS. Medial prefrontal pathways for the contextual regulation of extinguished fear in humans. Neuroimage. 2015;122:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Zhou J, Leonowens C, Ivaturi VD, Lohmer LL, Curd L, Ossig J, Schippers F, Petersen KU, Stoehr T, Schmith V. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66:109899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 24. | Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and Pharmacodynamics of Remimazolam (CNS 7056) after Continuous Infusion in Healthy Male Volunteers: Part I. Pharmacokinetics and Clinical Pharmacodynamics. Anesthesiology. 2020;132:636-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 25. | Zhao MJ, Hu HF, Li XL, Li XM, Wang DC, Kuang MJ. The safety and efficacy between remimazolam and propofol in intravenous anesthesia of endoscopy operation: a systematic review and meta-analysis. Int J Surg. 2023;109:3566-3577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 313] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 27. | Abbott TEF, Pearse RM, Archbold RA, Ahmad T, Niebrzegowska E, Wragg A, Rodseth RN, Devereaux PJ, Ackland GL. A Prospective International Multicentre Cohort Study of Intraoperative Heart Rate and Systolic Blood Pressure and Myocardial Injury After Noncardiac Surgery: Results of the VISION Study. Anesth Analg. 2018;126:1936-1945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/