Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.109980
Revised: June 25, 2025
Accepted: July 28, 2025
Published online: September 27, 2025
Processing time: 120 Days and 17.1 Hours
Large number of decompensated liver cirrhosis patients in China have been diagnosed with hepatitis B virus (HBV). Human umbilical cord-derived mesen
To explore the safety and effect of treating liver cirrhosis with HBV by hUC-MSCs.
Twenty-four participants were recruited, divided into 3 groups, and injected with different amounts of hUC-MSCs via the peripheral vein. Therapy was administered 3 times. A 24-week follow-up examination of each patient’s liver function, coagulation function, general condition, and immune system was performed. Adverse events were also recorded. A 2-year survival assessment was subsequently performed.
Infusion therapy rapidly improved liver function. Serum albumin transiently increased on days 57 and 85 but returned to baseline by day 169, while prothrombin time activity demonstrated sustained improvement from day 29 through day 169. Interleukin-8 levels decreased persistently throughout treatment. All dosage groups achieved 100% 6-month survival; 2-year survival rates were 66.7% (low-dose), 100% (medium-dose), and 87.5% (high-dose). The interaction between dosage and efficacy was weak. Notably, the improvement in liver function was statistically significant and sustained for almost 3 months, suggesting clinically meaningful therapeutic durability.
hUC-MSCs can be considered a safe treatment for patients with decompensated liver cirrhosis associated with HBV. However, larger-scale randomized controlled trials are needed to prove its therapeutic effect.
Core Tip: This pioneering trial demonstrates that peripheral vein infusion of human umbilical cord-derived mesenchymal stem cells provides rapid and durable liver function improvement in HBV-related decompensated cirrhosis. Key results include: (1) Improvement in liver function sustained for almost 3 months; (2) Coagulation improvement and interleukin-8 reduction sustained > 3 months; (3) 100% 6-month survival across all doses and exceptional 2-year survival (66.7%-100%); (4) Weak dose-efficacy correlation suggesting even low-dose efficacy; and (5) Promising safety profile. human umbilical cord-derived mesenchymal stem cells represent a novel therapy for end-stage liver disease, warranting large-scale validation.
- Citation: Qin X, Chen J, Zhang HN, Du L, Ma Y, Li Y, Lu Y, Wang YT, Wu LF, Yu ZH, Hu MJ, Li LJ, Liao B, Li Z, Yang ZY, Li K, Yuan YF. Treatment of human umbilical cord-derived mesenchymal stem cells for hepatitis B virus-associated decompensated liver cirrhosis: A clinical trial. World J Gastrointest Surg 2025; 17(9): 109980
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/109980.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.109980
Stem cells are immature cells capable of self-differentiation, self-renewal, and self-replication[1]. Mesenchymal stem cells (MSCs) are a common family of stem cells that originate from the mesoderm and ectoderm in the early stage of embryonic development[2]. Initially discovered in bone marrow, MSCs have garnered increasing attention from researchers because of their multidirectional differentiation potential, beneficial effects on the hematopoietic system, stem cell implantation, immune regulation, self-renewal and replication[3]. Recent global studies have revealed that MSCs could differentiate into bone, muscle, fat, cartilage, tendon, liver, nervous system, myocardium, endothelium and other tissues, participating in the repairment and regeneration process under the corresponding induction conditions in vivo or in vitro[4-7]. Even after continuous subculture and cryopreservation, MSCs still have the potential for multidirectional differentiation, making them ideal seed cells for repairing organ or tissue damage caused by ageing and disease[8]. Human umbilical cord-derived MSCs (hUC-MSCs) are a type of adult stem cell that are convenient to sample, have stable biological performance, are ethical to use and have low immunogenicity. Because of these characteristics, hUC-MSCs are widely applied in research on tissue engineering.
The liver, one of the most important and complex organs in the human body, is susceptible to damage from drugs, alcohol, viruses and other factor, resulting in hepatitis, fatty liver disease, cirrhosis and other liver diseases. The effect of conventional therapy is not satisfactory in most patients with hepatitis B virus (HBV)-related decompensated cirrhosis. As the disease progresses, patients may suffer from hepatic encephalopathy, oesophageal variceal bleeding, liver failure, hepatorenal syndrome and other critical conditions. Because of their multidirectional differentiation potential, high proliferative capacity, and ability to secrete cytokines and growth factors, MSCs exhibit hematopoietic, immunomodulatory, and anti-inflammatory properties, as demonstrated in animal experiments and clinical trials. We believe that MSCs might be beneficial in the treatment of HBV-related decompensated cirrhosis.
Therefore, we designed this clinical trial to explore a new therapy for decompensated liver cirrhosis associated with HBV. The main objectives of this study were to investigate the safety and tolerability of hUC-MSC infusions in patients with decompensated hepatitis B cirrhosis, as well as investigate their survival rates, liver function, and overall health improvement following infusion therapy. We expect that the findings of this trial will benefit decompensated liver cirrhosis patients.
Our study was a single-arm, dose-escalation, single-center study. A total of 24 patients with HBV-related decompensated cirrhosis were recruited from September 2019 to December 2021 at Zhongnan Hospital of Wuhan University and treated with hUC-MSCs via venous transfusion. The participants were divided into 3 groups: A low-dose group, which received an infusion of 2.5 × 107 cells; a medium-dose group, which received an infusion of 5.0 × 107 cells; and a high-dose group, which received an infusion of 1.0 × 108 cells. Each group contained 8 patients. The low-, medium-, and high-dose groups were treated in turn. All patients underwent baseline examinations, including vital sign assessment, laboratory testing, electrocardiograph, computerized tomography, magnetic resonance imaging, and ultrasound. hUC-MSC infusions were performed on the 1st, 8th and 15th days. Liver function, coagulation function, clinical features and immune status were evaluated on day 1 (baseline) and on days 14, 29, 57, and 169. Survival was then assessed via telephone interviews and during clinic visits at 1 year, 1.5 years, and 2 years.
We recruited participants according to strict inclusion and exclusion criteria. The inclusion criteria were as follows: Diagnosis of decompensated cirrhosis caused by HBV, aged between 18 years and 65 years; refractory to conventional medical treatment; serum albumin (ALB) < 35 g/L; total bilirubin (T-Bil) < 170 μmol/L; prothrombin activity (PTTA) > 30%; Child-Pugh score ≥ 7; Model for End-Stage Liver Disease (MELD) score ≤ 15; haemoglobin > 70 g/L; blood platelet count > 3 × 109/L; unsuitable for liver transplantation, now and in the future; and voluntary participation and willingness to provide informed consent.
The exclusion criteria were as follows: Spontaneous peritonitis or other serious infections; hepatorenal syndrome; severe hepatic encephalopathy; massive haemorrhage of varicose veins in the upper digestive tract in the past month; portal vein thrombosis; serious diseases of the heart, lung, kidney, blood or endocrine system; human immunodeficiency virus positivity; positive for autoantibodies related to autoimmune liver disease; malignancy of the liver or any other organ; pregnancy or lactation; a recent plan for pregnancy; a history of alcohol/drug abuse with inability to abstain; and participation in other clinical trials within 3 months prior to enrolment or any other clinical research on stem cells.
The study was approved by the Medical Ethics Committee. After obtaining fully informed consent from donor mothers, healthy fetal umbilical cord tissues were collected. These tissues were transported to the laboratory via a 2-8 °C cold chain. The umbilical cord tissues were washed with sodium chloride to remove blood stains, epidermis and blood vessels. Wharton’s jelly was collected and minced into 1 mm³ tissue blocks. Using the tissue explant method, the tissue blocks were evenly distributed into cell culture flasks, and an appropriate amount of α-minimum essential medium complete culture medium containing 10% fetal bovine serum was added. The cultures were incubated at 37 °C in a 5% CO2 incubator, and the medium was replaced twice a week. When the cell confluence reached approximately 90%, the cells were digested with 0.25% trypsin, washed twice with sodium chloride, and the primary cells were collected. The cells were then expanded and passaged at a ratio of 1:5 until the 5th generation.
In all the groups, the hUC-MSCs were suspended in 100 mL of 0.9% sodium chloride solution and injected intravenously 3 times (day 1, day 8 and day 15). The cell densities were 2.5 × 107, 5.0 × 107, and 1.0 × 108 in the low-dose, medium-dose, and high-dose groups, respectively. The total infusion time was limited to 1 hour or less.
The 6-month and 2-year survival rates after the first infusion of hUC-MSCs in each treatment group were documented. Liver function, coagulation function, clinical features and immune status were evaluated on day 1 (baseline), 14, 29, 57, 85, and 169. For liver function, the serum ALB, alanine aminotransferase (ALT), aspartate aminotransferase (AST), cholinesterase (CHE), T-Bil, direct bilirubin (D-Bil) and total cholesterol (TC) levels were measured. For coagulation function, PTTA and antithrombin-III (AT-III) were tested. Symptoms, including ascites, oedema of the legs, haematemesis, jaundice and poor appetite, were also recorded at every follow-up. To evaluate the general condition and quality of life of the participants, their body weight, Child-Pugh score, MELD score and 36-item Short Form Health Survey score were measured. To describe the immune status, the counts of CD4+ and CD8+ T cells; the proportions of Th1, Th2, natural killer and natural killer-T cells; and the serum contents of several interleukins (ILs), including IL-1β, IL-4, IL-6, IL-8, IL-12, IL-15 and IL-17A, were tested. Adverse events (AEs) were defined as infusion reactions, anaphylaxis, haemolysis, acute liver failure, acute kidney failure and other obvious variations in examinations. AEs were recorded during the first 2 weeks and on days 29, 57, 85, and 169. A severe AE (SAE) is defined as an AE causing the following: Death, threat to life, admission to the hospital, delay of discharge from the hospital, or loss of function (permanent or severe).
For continuous variables, descriptive statistics will include the number of participants (n), number of missing visitors (missing), mean ± SD, and quartile (Q1, Q3). We used the Shapiro-Wilk test to assess the normality of the samples. The signed-rank test was used for comparisons within groups (any visit compared with baseline). For comparisons among the low-, medium- and high-dose groups, we used the Fisher test for normally distributed data and the Kruskal-Wallis test for nonparametric data. For categorical variables, the frequency and component ratio (%) were used for statistical description, and the χ2 test was used for analysis. Kaplan-Meier curves were used for survival analysis, and statistical analysis was performed via log-rank analysis. For all analyses, a P value < 0.05 was considered to indicate statistical significance. If missing data affected ≥ 5 participants within any treatment group, we reported the raw values in Supplementary material but excluded that indicator from statistical analysis.
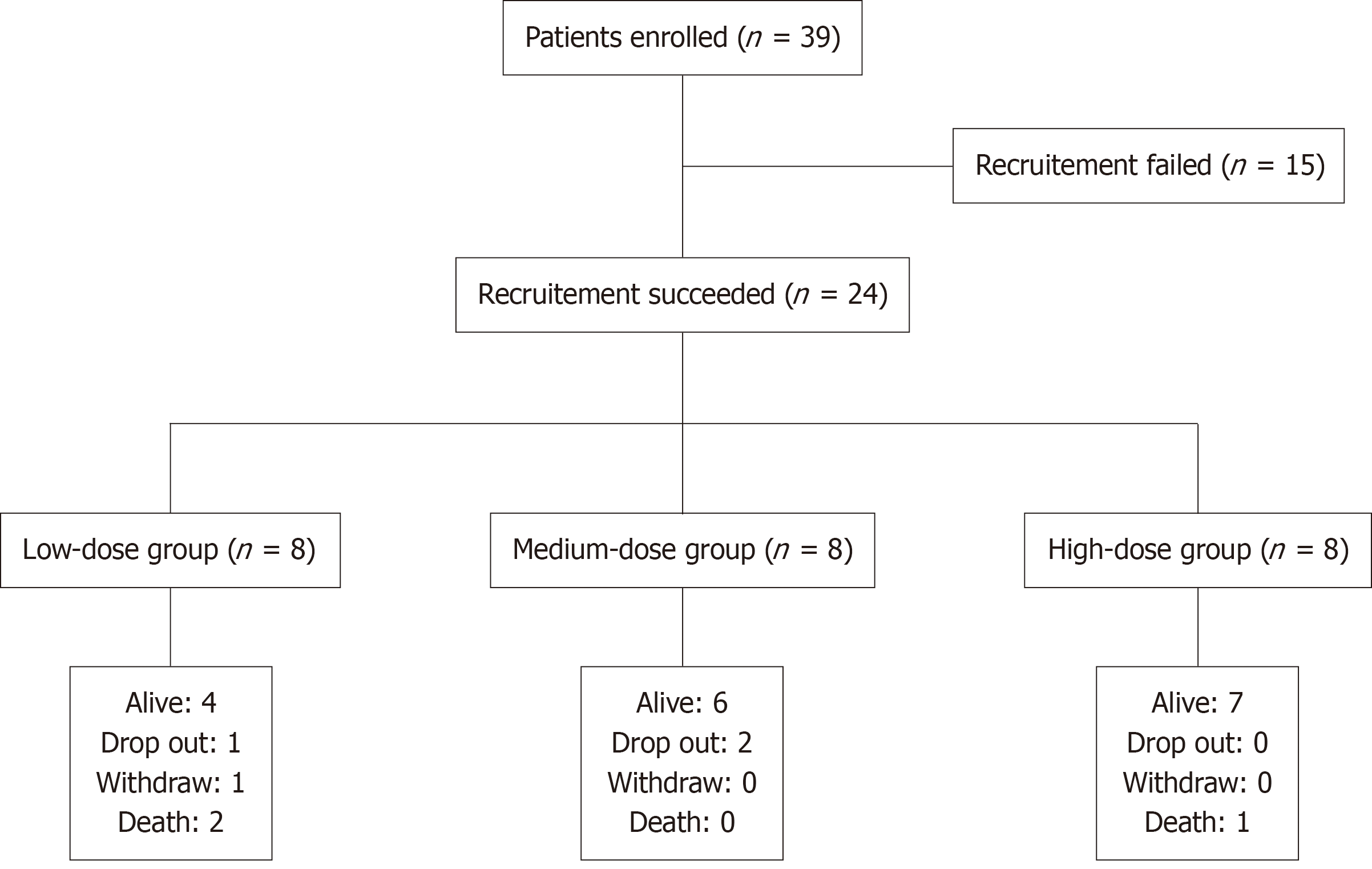
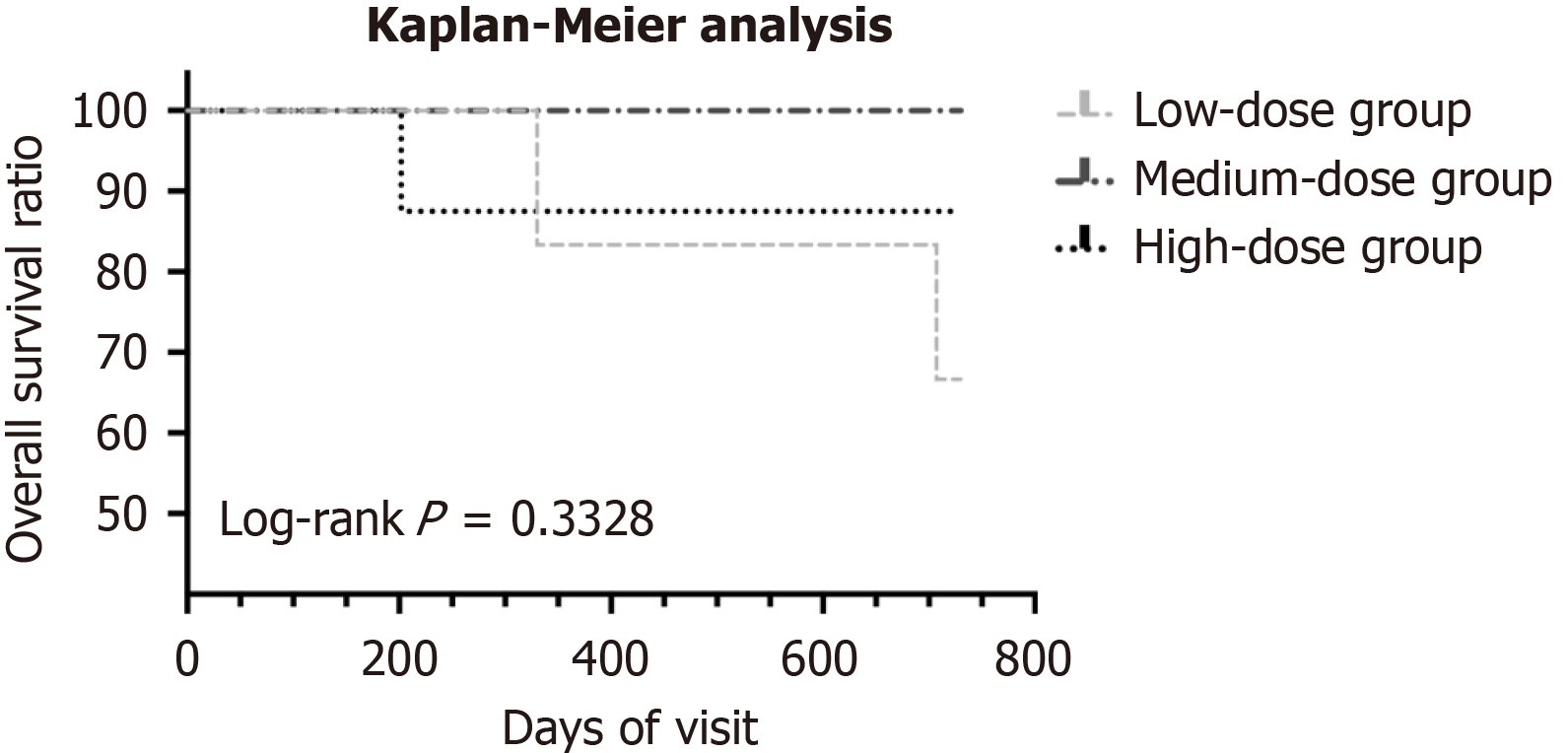
A total of 39 patients were enrolled, of whom15 did not meet the inclusion criteria. The remaining 24 participants completed 3 infusions of hUC-MSCs. All participants had already received and continued to receive antiviral treatment with entecavir (0.5 mg daily) or tenofovir disoproxil fumarate (300 mg daily). In the low-dose group, one participant withdrew from the follow-up because of newly diagnosed liver cancer at the 7th visit (day 85), and another participant lost to follow-up after the 7th visit. In the low-dose group, the 6-month survival rate was 100% (6 of 6) and the 2-year survival rate was 66.7% (4 of 6), with two deaths (traumatic cerebral hemorrhage at month 23; hepatic encephalopathy at month 11). Two patients in the medium-dose group lost to follow-up, and six participants completed all the follow-ups. The 6-month and 2-year survival rates were both 100% (6 of 6). For the high-dose group, the 6-month survival rate was 100% (8 of 8) and the 2-year survival rate was 87.5% (7 of 8) with one death caused by hepatic failure and hepatic encephalopathy 7 months after the first infusion. The flowchart for the whole study is shown in Figure 1. Kaplan-Meier survival analysis is displayed in Figure 2. There was no significant difference in the survival ratio among the low-, medium- and high-dose groups (log-rank P = 0.3328), suggesting that higher hUC-MSCs doses might not improve survival.
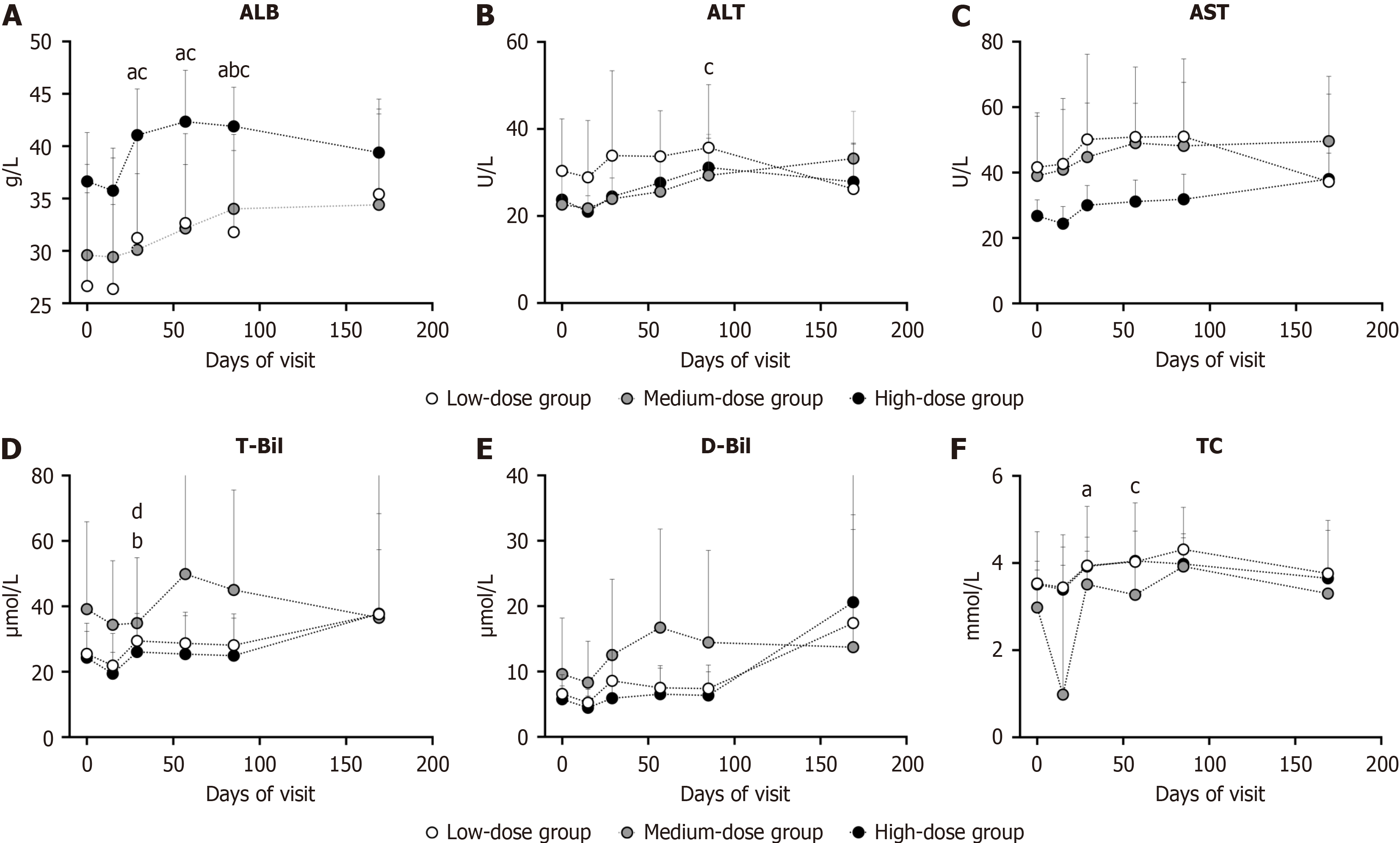
The serum levels of ALB, ALT, AST, CHE, T-Bil, D-Bil, and TC were monitored during the trial and longitudinally assessed vs baseline. The serum ALB level was significantly increased in the low- and high-dose groups on days 29 (P = 0.0156, 0.0156) and 57 (P = 0.0156, 0.0078) and in all 3 groups on day 85 (P = 0.0156, P = 0.0313, P = 0.0313) (Figure 3A). The ALT level exhibited minor elevation only in the high-dose group on day 85 (P = 0.0469), remaining below the upper limit of normal (45 U/L) (Figure 3B). The AST, T-Bil, and D-Bil levels showed no significant changes vs baseline at each follow-up across all groups (Figure 3C-E). The CHE levels were slightly increased in the high-dose group on day 29 and in the low-dose group on day 85, both within the upper limit of normal (12500 U/L) (Supplementary Table 1). On day 29, the T-Bil level decreased in the medium-dose group (P = 0.0156) (Figure 3D). The serum TC levels increased in the low-dose group on day 29 and the high-dose group on day 57 (Figure 3F) within the normal range. Despite slight changes in the intermediate follow-ups mentioned above, all these parameters returned to baseline level on day 169 (Figure 3A-E and Supplementary Table 1).
The variation in each index between every follow-up and baseline was also calculated and compared among different dose groups. The T-Bil variation was significant on day 29 among the groups (P = 0.0108) without dose-effect rela
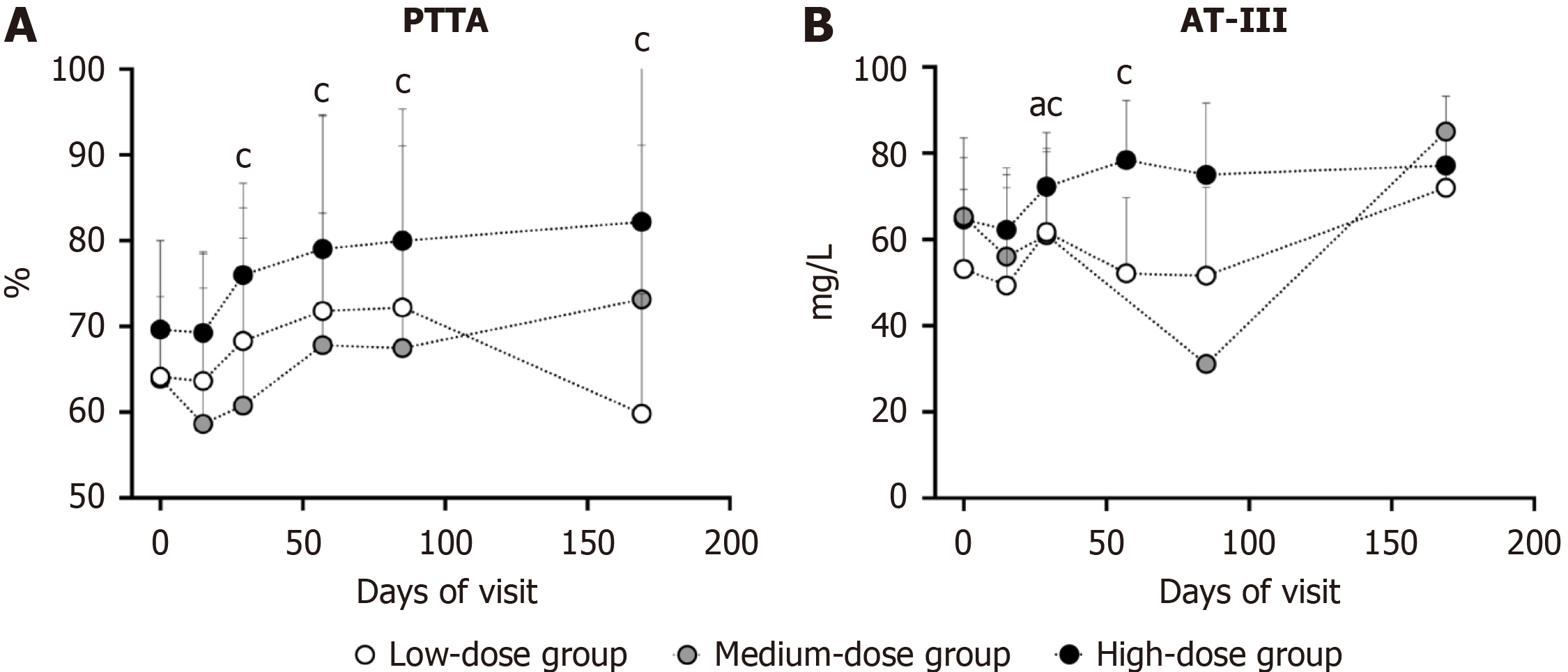
Compared with the baseline level, high-dose hUC-MSC infusion demonstrated sustained PTTA elevation from day 29 to the final visit (day 29, P = 0.0078; day 57, P = 0.0391; day 85, P = 0.0156; and day 169, P = 0.0156) (Figure 4A). On day 29, the AT-III level was markedly increased in the low- (P = 0.0156) and high-dose groups (P = 0.0156) and remained above baseline level on day 57 (P = 0.0156) (Figure 4B). There were no statistically significant differences in the variations in PTTA or AT-III between each follow-up and baseline among the different dose groups (Supplementary Table 2). Al
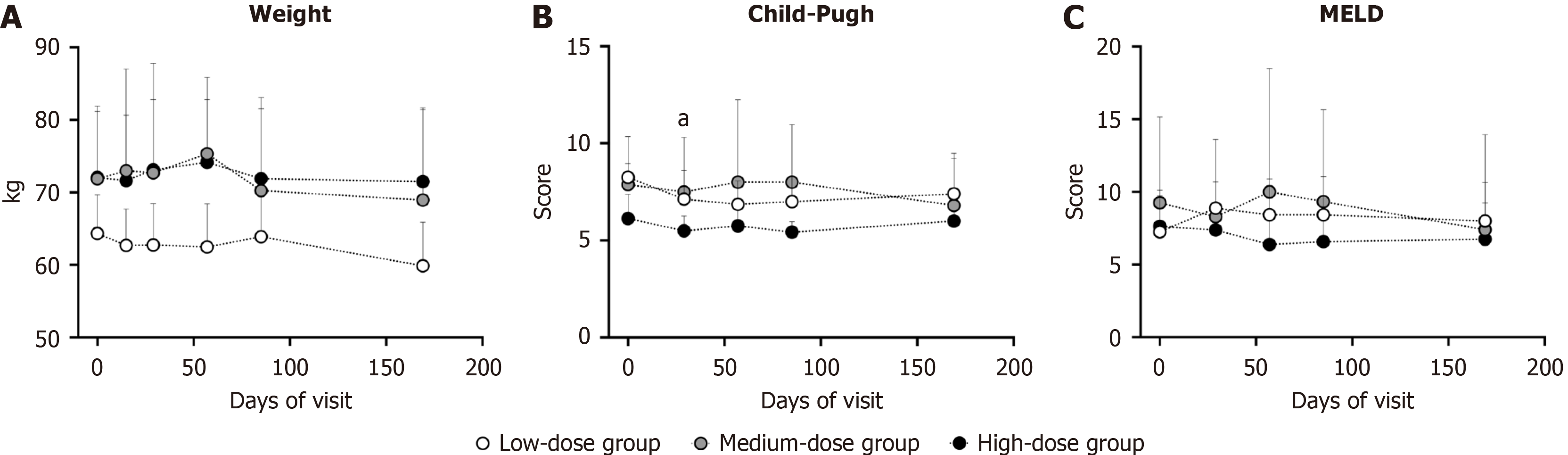
During the entire trial, the body weights of the participants remained stable (Figure 5A). Compared with baseline, the low-dose group had a significantly lower Child-Pugh score on day 57 (P = 0.0313) (Figure 5B). During the 169-day follow-up, neither the Child-Pugh nor the MELD score baseline score deteriorated vs baseline (Figure 5B and C). The variations in body weight, Child-Pugh score and MELD score among the different dose groups were not statistically significant. These data demonstrated that hUC-MSC infusion might have potential hepatoprotective protective effects.
Although 36-item Short Form Health Survey scale scores were partially missing in the low- and medium-dose groups, available results, especially in the high-dose group, revealed that all participants maintained a good quality of life until the last follow-up (Supplementary Table 3).
Symptoms (Supplementary Tables 4-8) were recorded from recruitment to the last follow-up. Most participants in the low-dose group had ascites at baseline, and 5 of 8 (62.5%) became ascites-free on day 29, whereas 1 of 4 (25%) remained ascites-free on day 169. In the medium-dose group, 3 of 5 (60%) patients underwent treatment to cure the ascites. In the high-dose group, 2 of 8 patients (25%) became ascites-free, whereas 2 of 8 patients (25%) who were negative for ascites at the beginning of the study developed de novo ascites. Most low-dose group participants had baseline leg oedema. On days 57 and 85, 5 of 7 patients (71.4%) reported improvement of oedema, with 1 of 3 patients (33.3%) maintaining im
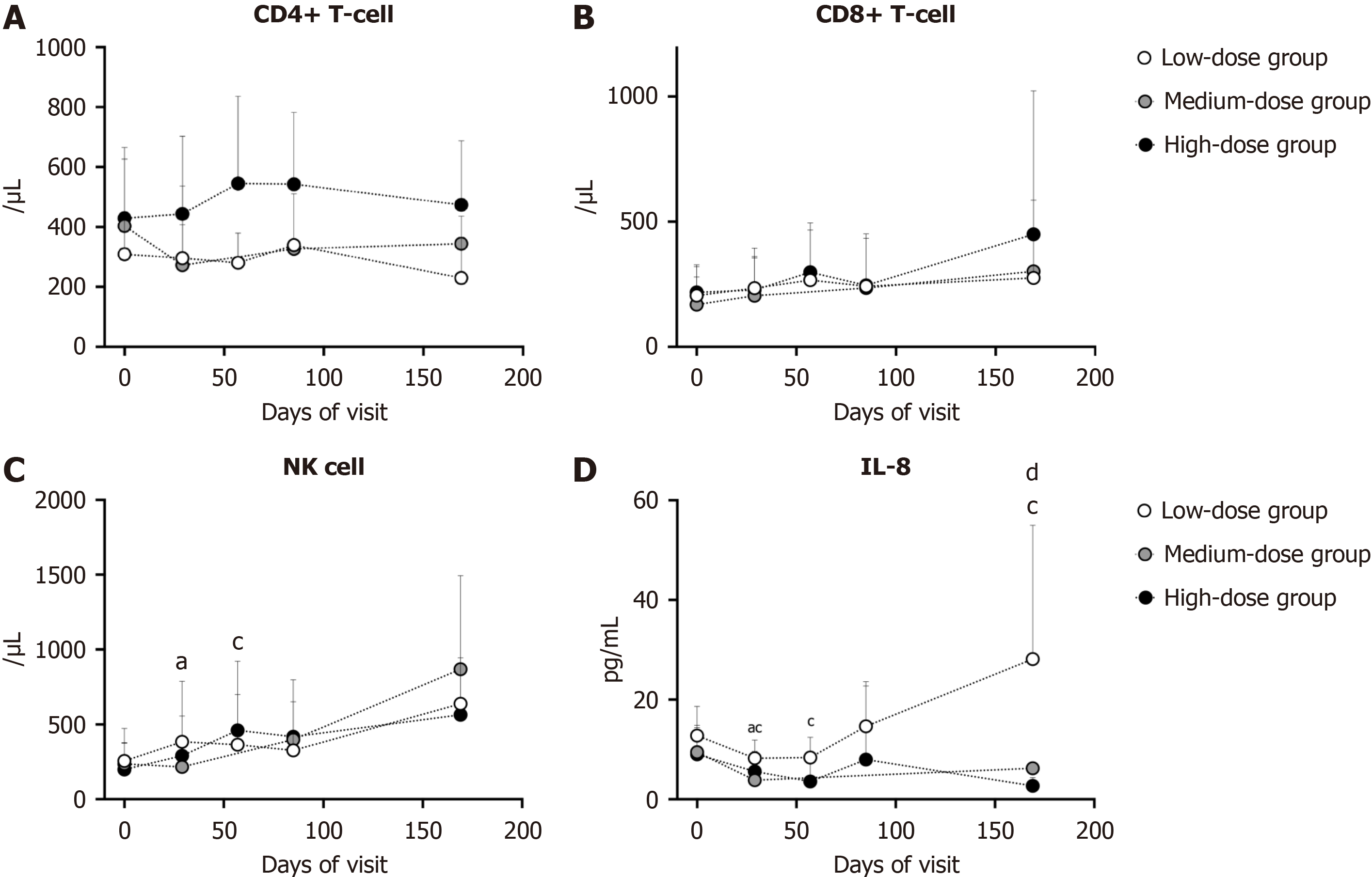
Immune profiling demonstrated no significant alterations in CD4+ T cell, CD8+ T cell, Th1 cell, Th2 cell counts post-hUC-MSC infusion across groups vs baseline (Figure 6A and B, Supplementary Table 9). The number of natural killer cells temporarily increased on day 29 in the low-dose group (P = 0.0469) and on day 57 in the high-dose group (P = 0.0391), reverting to baseline thereafter (Figure 6C). In addition, the variation in lymphocyte subsets between every follow-up and baseline was not significant among the different dose groups (Figure 6A and B, Supplementary Table 9).
There were 7 kinds of interleukins tested during the visit, among which the level of IL-8 was significantly affected by hUC-MCS infusion. Compared with the baseline level, the serum IL-8 Level was evidently lower on day 29 in both the low- (P = 0.0156) and high-dose groups (P = 0.0234), and the tendency persisted until day 169 in the high-dose group (days 57 and 169, both P = 0.0156) (Figure 6D). Moreover, the variation in the IL-8 Level between day 169 and baseline was significant among the 3 groups (P = 0.0491). High-dose hUC-MSC infusions markedly reduced IL-8 Levels (Figure 6D). Given IL-8’s role in inflammatory injury, these findings suggest hUC-MSCs may promote hepatocyte repair and attenuate hepatic inflammation.
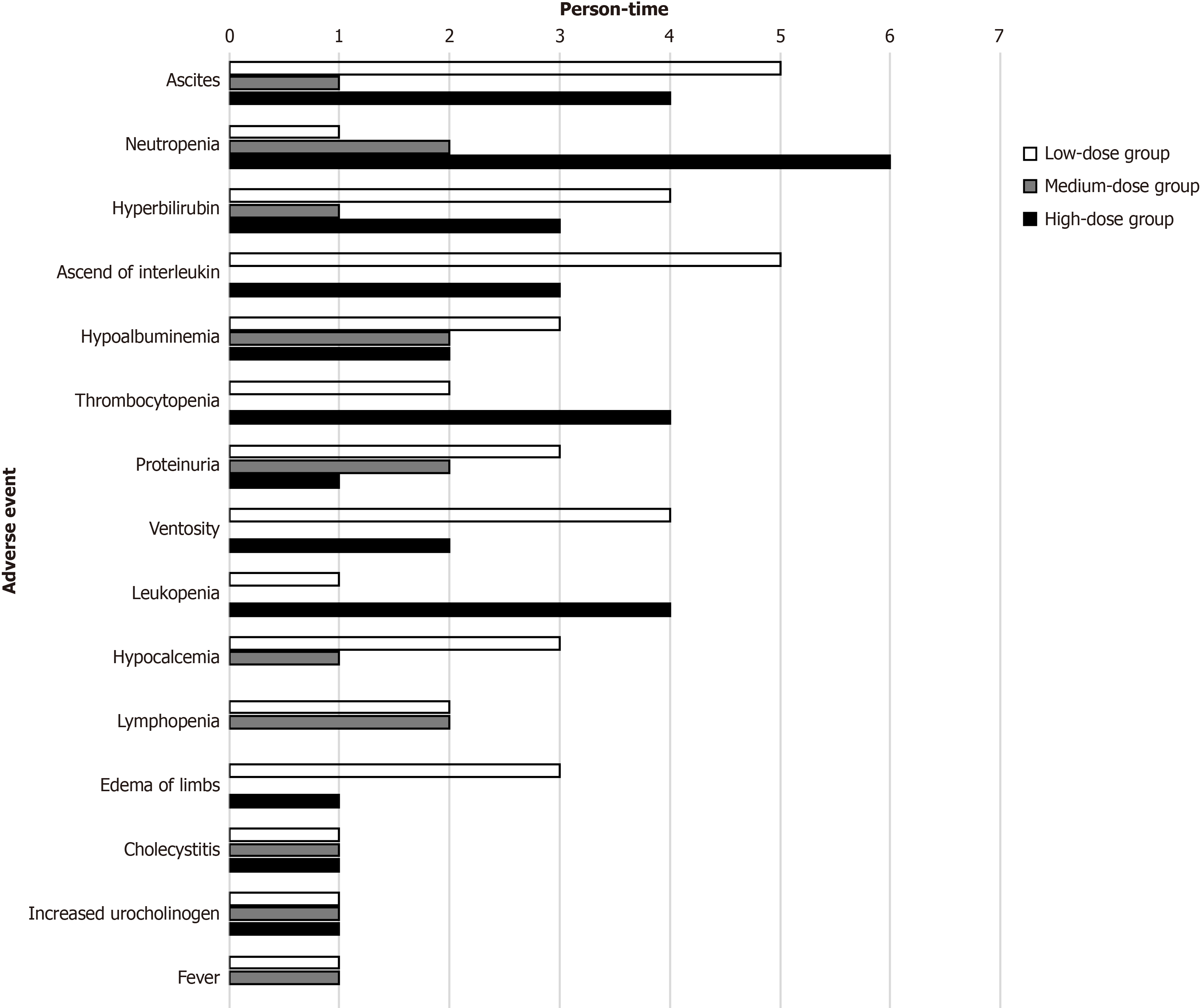
During the entire trial, AEs were rigorously recorded and handled. Sixty-three, 34 and 54 AEs occurred in the low-, medium- and high-dose groups, respectively, most of which were mild and classified as grade 1 or grade 2 (Table 1). The most commonly reported AEs included temporary gastrointestinal symptoms (ascites, ventosity) and laboratory abnormalities (neutropenia, thrombocytopenia) (Figure 7). No AE required dose reduction or treatment discontinuation. There were 3 SAEs reported, one in each group. A patient in the low-dose group was diagnosed with hepatocellular carcinoma (HCC) 3 months after enrolment and then withdrew from the trial. After percutaneous ethanol injection and radiofrequency ablation, the participant survived without HCC recurrence at the end of our trial. Upper gastrointestinal haemorrhage occurred once in a participant in the medium-dose group 5 months after the first hUC-MSC infusion and was cured after medication and endoscopy treatment. Unfortunately, another participant in the high-dose group suffered hepatic encephalopathy 5 months after the first infusion and died within 2 months. No differences in the occurrence of AEs or SAEs were found among the different dose groups.
| Characteristics | Low-dose group | Medium-dose group | High-dose group | F value | P value | |
| Total adverse event | 63 | 34 | 54 | - | - | |
| Severity of AE | Class 1 | 33 | 18 | 24 | 2.341 | 0.2101 |
| Class 2 | 24 | 10 | 16 | |||
| Class 3 | 6 | 5 | 13 | |||
| Class 4 | 0 | 1 | 1 | |||
| Association with hUC-MSCs | Absolutely relevant | 0 | 0 | 0 | 1.262 | 0.3435 |
| Possibly relevant | 4 | 0 | 0 | |||
| Possibly irrelevant | 1 | 1 | 0 | |||
| Absolutely irrelevant | 58 | 33 | 54 | |||
| AE led to reduce dosage | 0 | 0 | 0 | - | - | |
| AE led to terminate therapy | 0 | 0 | 0 | - | - | |
| AE led to death | 0 | 0 | 0 | - | - | |
The major concerns for HBV patients are liver cirrhosis and HCC. Liver transplantation is the only curative option for decompensated cirrhosis[9]. However, high cost of hospitalization, high risk of surgery and lifelong postoperative immunosuppressive therapy limit the widespread use of liver transplantation. The current therapies for decompensated cirrhosis are unsatisfactory. Most patients die from liver failure, hepatic encephalopathy, esophagogastric variceal hemorrhage, hepatorenal syndrome or other complications[10,11]. Therefore, we aimed to develop a new, safe and effective therapy for patients who cannot undergo liver transplantation or who will have a long wait before liver transplantation to improve survival and quality of life.
Moreover, whether stem cell therapy increases the risk of tumour development remains a major source of concern. A 75-month follow-up analysis of a prospective, open-label, randomized controlled study of umbilical cord-derived MSCs (UC-MSCs) in decompensated cirrhosis patients revealed no difference in the HCC-free survival ratio between the UC-MSC group and the control group[12]. Another retrospective cohort study of patients with decompensated cirrhosis revealed that UC-MSCs improved 3-year and 5-year survival without increasing the risk of HCC[13]. In this cohort, the overall incidence of HCC was 33.3% (67/201), 41.7% (15/36) of patients received UC-MSC treatment, and 31.5% (52/165) of patients did not (P = 0.242). In our trial, the overall incidence of HCC was 4.16% (1/24). Owing to the limitations of small-sample, single-arm studies, we were unable to directly determine whether this case of HCC was related to UC-MSC injection. However, our observed HCC incidence rate (4.16%) aligned with the natural disease progression reported in Li et al’s cohort[13], suggesting UC-MSC treatment did not significantly increase hepatocarcinogenic risk in this trial.
Nevertheless, it remains unclear whether MSCs promote or inhibit tumour development, since they are able to differentiate into a wide variety of cell types[14]. A meta-analysis revealed that MSCs can either suppress or promote tumour development, according to their derivation, modification and cell subtype[15]. MSCs suppress tumour development by inhibiting RADiation sensitive 51 paralog B via the phosphatidylinositol 3-kinase/protein kinase B pathway, yet paradoxically promote tumour progression by upregulating cyclin D through the WNT pathway[16]. Thus, both laboratory and clinical studies are essential to elucidate the effects of MSCs on the pathogenesis of HCC.
Most AEs in our trial were classified as class 1 or 2 (total proportion over 70%), suggesting that the toxicity and side effects of hUC-MSCs were acceptable, consistent with a prior phase I/II clinical trial showing no SAEs, permanent disability, neoplasia, or septic arthritis in MSC-treated osteoarthritis patients[17]. Another trial of the effects of hUC-MSCs on relapsing-remitting multiple sclerosis revealed that no patients developed tumours or organ disorders throughout the 10-year follow-up, despite partial demyelination lesions were detected[18]. At the 2-year follow-up, we noted that 3 of 24 patients had died - one due to trauma and two due to hepatic failure and encephalopathy (7 months and 11 months after the first infusion, respectively). Given the 47.2% 3-year survival rate with conventional therapy[13] and long intervals between MSC infusion and fatalities in our trial, these outcomes might reflect disease progression rather than treatment toxicity, supporting the clinical safety of MSC-based interventions.
Multiple studies have attempted to elucidate the theoretical basis for treating liver cirrhosis with hUC-MSCs. Liu et al[14] summarized the multiple roles of MSCs in repairing liver injury, such as direct differentiation into hepatocytes, fusion with hepatocytes, inducing the paracrine effects of cytokines and growth factors for hepatocyte regeneration, modulation of T cells (CD4+ T cells, CD8+ T cells, natural killer cells, etc.), and inhibition of hepatocellular apoptosis by MSC-conditioned medium. Yin et al[19] reported that exosomes derived from hUC-MSCs might alleviate liver fibrosis. Injection of hUC-MSCs exosomes into CCl4-induced injured mouse livers alleviated liver fibrosis by repressing the expression of transforming growth factor beta 1 and collagen types I and III, reducing the phosphorylation of mothers against decapentaplegic homolog 2, and promoting the expression of E-cadherin[20]. In addition to hepatocytes, hepatic stellate cells play crucial roles in liver fibrosis. There is also evidence that hUC-MSCs inhibit the proliferation of hepatic stellate cells via the transforming growth factor beta/mothers against decapentaplegic homolog 2 signalling pathway[21]. A novel antifibrotic factor, milk fat globule-EGF factor 8, which is secreted by MSCs, has been shown to alleviate liver fibrosis in mice[22]. Moreover, MSCs attenuate liver collagen deposition through the secretion of monocyte chemoattractant protein-1, hepatocyte growth factor and other factors[23].
On the basis of these many theories, we believe that hUC-MSCs may improve the liver function of patients with decompensated liver cirrhosis. Our data showed that hUC-MSC infusions improved liver function within 2-3 months, evidenced by increased serum ALB and decreased T-Bil, though the effect attenuated over time. This aligns with an randomized controlled trial (RCT) showing no significant improvement in Child-Pughs score, MELD score, ALB, international normalized ratio, serum transaminase level or liver volume at 12 months with peripheral vein delivery of MSCs vs placebo[24]. The author suggested that instead of infusion via the peripheral vein, infusion via the hepatic artery or portal vein might be better for treating liver cirrhosis. A meta-analysis revealed that intra-arterial injections were better than peripheral vein injections were, as they were associated with improvements in the MELD score, ALB and T-BiL levels[25], likely due to enhanced MSC homing to injured tissues[26]. When infused via the peripheral vein, the MSCs concentrate in the lung, meaning that fewer MSCs reach the liver[27]. Consequently, the 3-month liver functional stabilization in our research is an inspired result for future clinical work. The therapeutic benefit duration may potentially be extended through hepatic artery or portal vein delivery of hUC-MSCs, though these approaches carry higher procedural complexity and complication risks.
While our data demonstrate transient functional improvement with hUC-MSC monotherapy, sustained reversal of fibrosis may require combinatorial strategies. Several antifibrotic agents and regenerative strategies have been proved potentially effective and safe in treating cirrhosis, such as the cAMP response element binding protein binding protein/β-catenin inhibitor PRI-724 (PubChem database CID: 71509318)[28] and liver-targeted small interfering RNA lipid na
There are still several limitations of our trial. First, it is not a randomized clinical trial, but an open-labelled trial, thus the evidence is of lower quality. Thus, the findings presented here can serve as the foundation for a RCT in the future. Second, the sample size was small. Thus, a larger sample of patients is needed in future studies. Third, the infusion frequency and path require further research. Ultimately, the application of hUC-MSCs to treat decompensated liver cirrhosis associated with HBV requires further investigation.
In our trial, we revealed that hUC-MSCs could improve liver function in a short time, as well as improve coagulation function. It is also safe enough for clinical application, as the risks of serious AEs and tumour development are low. hUC-MSCs have great potential in treating decompensated liver cirrhosis associated with HBV but require further validation in larger-scale RCTs.
| 1. | Steinert AF, Rackwitz L, Gilbert F, Nöth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Liu J, Gao J, Liang Z, Gao C, Niu Q, Wu F, Zhang L. Mesenchymal stem cells and their microenvironment. Stem Cell Res Ther. 2022;13:429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 147] [Reference Citation Analysis (0)] |
| 3. | Cui L, Wu Y, Cen L, Zhou H, Yin S, Liu G, Liu W, Cao Y. Repair of articular cartilage defect in non-weight bearing areas using adipose derived stem cells loaded polyglycolic acid mesh. Biomaterials. 2009;30:2683-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9:584-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 310] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 5. | Taghizadeh RR, Cetrulo KJ, Cetrulo CL. Wharton's Jelly stem cells: future clinical applications. Placenta. 2011;32 Suppl 4:S311-S315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 7. | Puglisi MA, Saulnier N, Piscaglia AC, Tondi P, Agnes S, Gasbarrini A. Adipose tissue-derived mesenchymal stem cells and hepatic differentiation: old concepts and future perspectives. Eur Rev Med Pharmacol Sci. 2011;15:355-364. [PubMed] |
| 8. | Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 9. | Villeret F, Dharancy S, Erard D, Abergel A, Barbier L, Besch C, Boillot O, Boudjema K, Coilly A, Conti F, Corpechot C, Duvoux C, Faitot F, Faure S, Francoz C, Giostra E, Gugenheim J, Hardwigsen J, Hilleret MN, Hiriart JB, Houssel-Debry P, Kamar N, Lassailly G, Latournerie M, Pageaux GP, Samuel D, Vanlemmens C, Saliba F, Dumortier J. Liver transplantation for NAFLD cirrhosis: Age and recent coronary angioplasty are major determinants of survival. Liver Int. 2022;42:2428-2441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 1052] [Article Influence: 210.4] [Reference Citation Analysis (2)] |
| 11. | Mindikoglu AL, Pappas SC. New Developments in Hepatorenal Syndrome. Clin Gastroenterol Hepatol. 2018;16:162-177.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Shi M, Li YY, Xu RN, Meng FP, Yu SJ, Fu JL, Hu JH, Li JX, Wang LF, Jin L, Wang FS. Mesenchymal stem cell therapy in decompensated liver cirrhosis: a long-term follow-up analysis of the randomized controlled clinical trial. Hepatol Int. 2021;15:1431-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 13. | Li Z, Zhou X, Han L, Shi M, Xiao H, Lin M, Chi X. Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell Transplantation for Patients with Decompensated Liver Cirrhosis. J Gastrointest Surg. 2023;27:926-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Liu WH, Song FQ, Ren LN, Guo WQ, Wang T, Feng YX, Tang LJ, Li K. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med. 2015;19:511-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Christodoulou I, Goulielmaki M, Devetzi M, Panagiotidis M, Koliakos G, Zoumpourlis V. Mesenchymal stem cells in preclinical cancer cytotherapy: a systematic review. Stem Cell Res Ther. 2018;9:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 17. | Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, Alcayaga-Miranda F, González PL, Muse E, Khoury M, Figueroa FE, Espinoza F. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl Med. 2019;8:215-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 279] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 18. | Lu Z, Zhu L, Liu Z, Wu J, Xu Y, Zhang CJ. IV/IT hUC-MSCs Infusion in RRMS and NMO: A 10-Year Follow-Up Study. Front Neurol. 2020;11:967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Yin F, Wang WY, Jiang WH. Human umbilical cord mesenchymal stem cells ameliorate liver fibrosis in vitro and in vivo: From biological characteristics to therapeutic mechanisms. World J Stem Cells. 2019;11:548-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 702] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 21. | Zhang LT, Peng XB, Fang XQ, Li JF, Chen H, Mao XR. Human umbilical cord mesenchymal stem cells inhibit proliferation of hepatic stellate cells in vitro. Int J Mol Med. 2018;41:2545-2552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | An SY, Jang YJ, Lim HJ, Han J, Lee J, Lee G, Park JY, Park SY, Kim JH, Do BR, Han C, Park HK, Kim OH, Song MJ, Kim SJ, Kim JH. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology. 2017;152:1174-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 23. | Chen L, Zhang C, Chen L, Wang X, Xiang B, Wu X, Guo Y, Mou X, Yuan L, Chen B, Wang J, Xiang C. Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem Cells Transl Med. 2017;6:272-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 24. | Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, Bashtar M, Ghavamzadeh A, Malekzadeh R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Zhao L, Chen S, Shi X, Cao H, Li L. A pooled analysis of mesenchymal stem cell-based therapy for liver disease. Stem Cell Res Ther. 2018;9:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, van Zijl PC, Huang J, Bulte JW. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 27. | Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M, Marini FC. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 545] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 28. | Kimura K, Kanto T, Shimoda S, Harada K, Kimura M, Nishikawa K, Imamura J, Ogawa E, Saio M, Ikura Y, Okusaka T, Inoue K, Ishikawa T, Ieiri I, Kishimoto J, Todaka K, Kamisawa T. Safety, tolerability, and anti-fibrotic efficacy of the CBP/β-catenin inhibitor PRI-724 in patients with hepatitis C and B virus-induced liver cirrhosis: An investigator-initiated, open-label, non-randomised, multicentre, phase 1/2a study. EBioMedicine. 2022;80:104069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Zhang J, Shen H, Xu J, Liu L, Tan J, Li M, Xu N, Luo S, Wang J, Yang F, Tang J, Li Q, Wang Y, Yu L, Yan Z. Liver-Targeted siRNA Lipid Nanoparticles Treat Hepatic Cirrhosis by Dual Antifibrotic and Anti-inflammatory Activities. ACS Nano. 2020;14:6305-6322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/