Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.110064
Revised: June 16, 2025
Accepted: August 1, 2025
Published online: September 27, 2025
Processing time: 119 Days and 0.7 Hours
Lymphadenectomy of the infrapyloric region remains technically demanding in laparoscopic radical gastrectomy. Traditional vessel-guided approaches often result in incomplete dissection and higher complication rates, especially at station No. 6.
To propose a mesentery-based strategy for infrapyloric lymphadenectomy and evaluate its safety, feasibility, and efficacy.
By identifying key anatomical landmarks and defining the inferior mesenteric boundary of the pyloric region (right gastro-omental mesentery), this approach enables full exposure and en bloc resection of anterior and posterior mesenteric planes, with proximal ligation at the root of feeding vessels. A retrospective cohort study was conducted on 330 gastric cancer patients who underwent D2 lymphadenectomy (D2) from January 2020 to December 2021. Outcomes were compared between 165 patients treated with D2 plus complete mesogastric ex
The D2 + CME group demonstrated significantly improved surgical outcomes, including shorter total operative time (279.19 ± 45.50 minutes vs 301.25 ± 52.30 minutes, P < 0.001), reduced infrapyloric dissection time (22.24 ± 3.80 minutes vs 27.58 ± 4.20 minutes, P < 0.001), and lower blood loss (4.71 ± 1.12 mL vs 24.83 ± 6.35 mL, P < 0.001). More lymph nodes were retrieved overall (43.80 ± 10.05 vs 37.25 ± 8.80, P < 0.001), particularly at station No. 6 (5.26 ± 0.87 vs 4.14 ± 0.41, P < 0.001). Postoperative recovery indicators and hospital stay were comparable between groups, while the complication rate was significantly lower in the D2 + CME group (20% vs 30.3%, P = 0.042).
The mesentery-based approach enables safe pyloric lymphadenectomy. Systematic mesogastric excision improves operative efficiency and lymph node yield, especially at station No. 6, offering potential oncological benefits in gastric cancer surgery.
Core Tip: This study introduces a mesentery-guided approach to pyloric lymphadenectomy in laparoscopic gastrectomy, centered on defining anatomical landmarks within the right gastro-omental mesentery. By integrating complete mesogastric excision into the standard D2 dissection, this technique enhances the retrieval of No. 6 lymph nodes, reduces operative time and blood loss, and significantly lowers postoperative complication rates. The standardized procedure provides both surgical precision and oncologic potential, offering a valuable strategy for improving outcomes in minimally invasive gastric cancer surgery.
- Citation: Pan GF, Zhang WH, Cai ZM, Chen J, Wu JH, Weng JB, Zhu ZP, Guo ZX, Lin JJ, Li ZX, Xu YC. Mesenteric-guided approach to pyloric lymphadenectomy in laparoscopic radical gastrectomy. World J Gastrointest Surg 2025; 17(9): 110064
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/110064.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.110064
Although the incidence of gastric cancer has declined, it remains one of the most prevalent malignancies worldwide. According to reports, gastric cancer ranks fifth in incidence and fourth in mortality globally[1]. Gastrectomy with regional lymphadenectomy is considered the standard surgical approach for gastric cancer[2]. However, multiple studies have reported that more than 30% of patients experience local recurrence after D2 Lymphadenectomy (D2) radical gas
The infrapyloric region is a common site of lymph node metastasis in gastric cancer, with a metastasis rate of up to 40% in advanced cases, making it a key target for lymphadenectomy[13-15]. However, due to mesenteric fusion and changes in fascial planes during embryonic development, complete resection of the right gastroepiploic mesentery, which contains the infrapyloric lymph nodes, is technically challenging[16,17]. Although complete removal of the right gastroepiploic mesentery enables more thorough clearance of infrapyloric lymph nodes, no standardized procedure for right gastroepiploic mesentery resection has been established. Previously, we proposed an infrapyloric lymphadenectomy technique based on embryological planes, which involves identifying and separating fused fascial spaces to achieve complete dissection of the right gastroepiploic mesentery[18]. To assist young surgeons in accurately and efficiently identifying fusion planes between mesenteries and to reduce the difficulty of complete right gastroepiploic mesentery resection, we refined our previous surgical approach by localizing key anatomical landmarks and delineating the resection boundaries of the right gastroepiploic mesentery. Based on these refinements, we propose a standardized, reproducible surgical technique and evaluate its safety and feasibility in lymphadenectomy. To highlight the novelty and practical difference between the D2 + CME and conventional D2 procedures, schematic comparisons of the dissection planes and vascular ligation sites are illustrated, emphasizing the mesentery-based anatomical boundaries that distinguish our approach.
A retrospective analysis was conducted on 165 patients who underwent laparoscopic D2 + CME at Putian First Hospital between January 2020 and December 2021. An additional 165 patients who underwent laparoscopic D2 alone during the same period were included for comparison. The inclusion criteria were as follows: (1) Histopathologically confirmed gastric adenocarcinoma; (2) Clinically staged as locally advanced gastric cancer (cT2-4a, N-/+, M0); and (3) No evidence of distant metastasis on preoperative evaluation. The exclusion criteria were: (1) History of upper abdominal surgery (excluding laparoscopic cholecystectomy); (2) Intraoperative findings of peritoneal dissemination or tumor invasion of adjacent organs (T4b); (3) Concurrent malignancies; and (4) Prior neoadjuvant therapy. D2 Lymphadenectomy was performed in strict accordance with the 5th edition of the Japanese Gastric Cancer Treatment Guidelines 2018[19]. Tumor-related characteristics were defined based on the 3rd edition of the Japanese Classification of Gastric Carcinoma[20]. Laparoscopic D2 + CME involved complete mesogastric excision within the D2 range. Operative time and blood loss specific to the infrapyloric region were recorded by the lead surgeon and scrub nurse in real time using intraoperative anesthesia records and surgical video timestamps.
To minimize selection bias in this retrospective cohort, a 1:1 patient matching strategy was used. Patients in the D2 group were consecutively selected to match the D2 + CME group based on age, sex, American Society of Anesthesiologists (ASA) score, body mass index (BMI), tumor location, and clinical stage. The choice of surgical approach was determined by the availability of experienced surgeons qualified to perform D2 + CME, as well as the intraoperative evaluation of mesenteric involvement and patient preference after detailed preoperative counseling.
Moreover, during the study period, the adoption of the D2 + CME technique was progressively integrated into institutional surgical practice. Patients were not randomized, and allocation to either group was partially influenced by the availability of specialized surgeons trained in mesenteric excision, surgical scheduling, and evolving institutional protocols. When such expertise was unavailable, or when patient-related factors (e.g., comorbidities, intraoperative complexity) contraindicated extended dissection, conventional D2 was preferred. These considerations, while clinically pragmatic, introduce inherent limitations that warrant further evaluation in a randomized prospective setting.
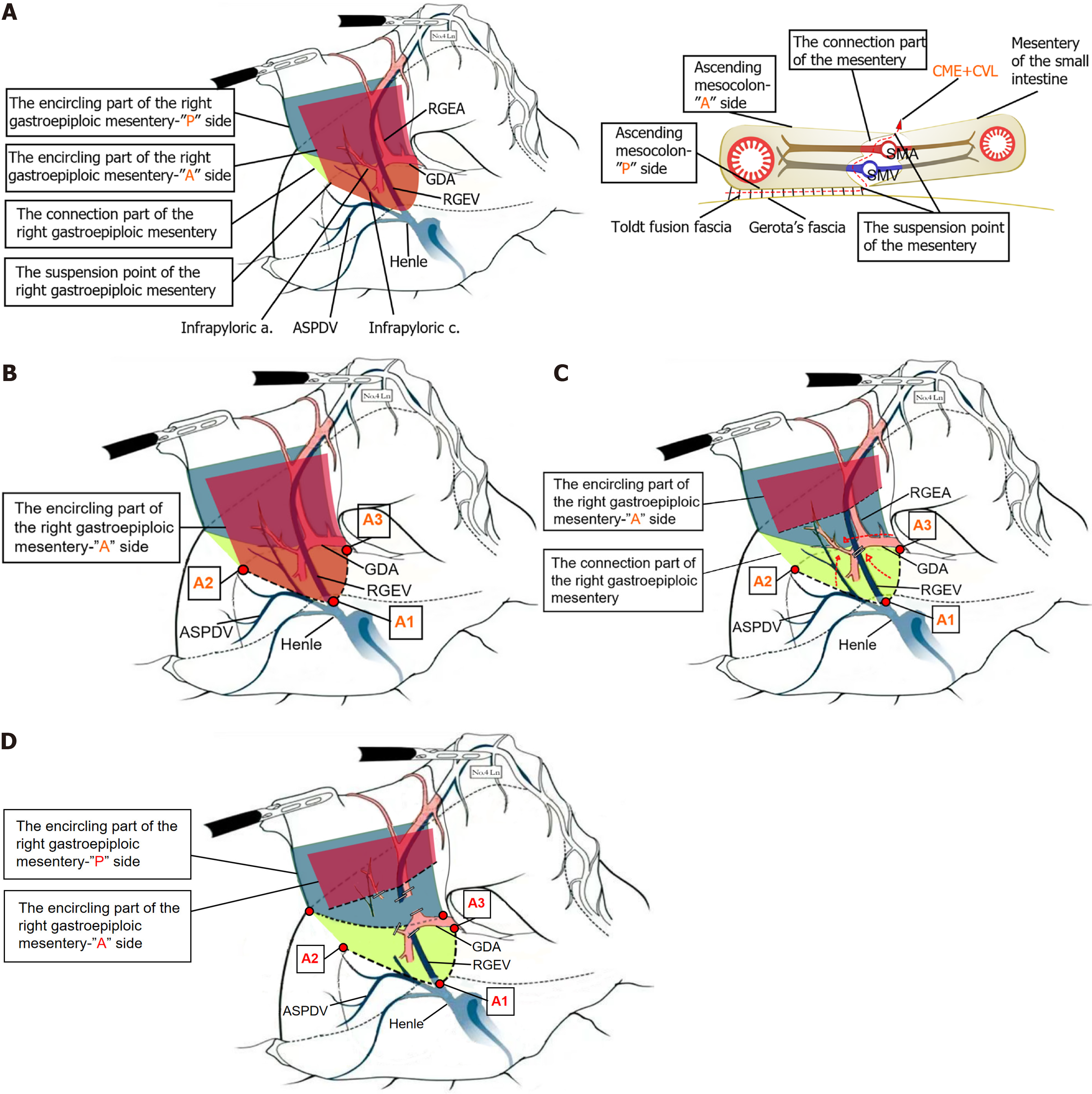
Lymphadenectomy of the infrapyloric region, specifically the dissection of No. 6 Lymph nodes, requires a shift in perspective from the traditional vessel-guided lymphadenectomy approach - where lymph nodes are cleared along the right gastroepiploic artery, including those near its first branch and proximal trunk, extending distally to the confluence of the anterior (A) superior pancreaticoduodenal vein and the right gastroepiploic vein[16] - to a mesentery-guided lymphadenectomy approach. A precise intraoperative understanding of the three key elements of the mesentery is essential: (1) The enveloping structure, resembling an envelope, with an A and posterior (P) surface; (2) The anchoring points, which are the basal attachment sites of the mesentery’s A and P surfaces; and (3) The connecting structures, which contain the vasculature within the mesentery. In our anatomical approach, we identified and marked the basal at
Complete separation of the right omental mesentery from the mesentery of the transverse colon and identification of the three suspended points on the “A” surface of the right omental mesentery: After detaching the greater omentum above the transverse colon, the membrane bridge between the right omental mesentery and the mesentery of the transverse colon is located in the area beneath the pylorus. The membrane bridge is incised to access the fused fascial plane, and dissection is performed bluntly in a rightward and downward direction along the superficial layer of the fused fascia. Lateral dissection proceeds to the descending part of the duodenum, and inferior dissection extends to the pre-fascial plane of the A mesentery of the right colon vessels, the A mesentery of the gastric-colonic vein trunk, and the A mesentery of the superior mesenteric vein at the lower margin of the pancreatic neck, achieving complete separation of the right omental mesentery from the mesentery of the transverse colon. The junction of the right gastroepiploic vein and the A vein of the pancreaticoduodenal vein is designated as the first suspended point (A1) on the lower edge of the “A” surface of the right omental mesentery. The A1 point, along with the continuing gastric-colonic vein trunk, marks the boundary and fusion point of the right omental mesentery with the surrounding mesenteries, including the mesentery of the transverse colon and the pancreatic mesentery. The identification of A1 aids in distinguishing and accurately entering the fascial gap between the right omental mesentery and the surrounding mesenteries. The dissection is then extended rightward to expose the lateral wall of the descending duodenum, which serves as the second suspended point on the lower edge of the “A” surface, representing the point of mesenteric regression as the stomach transitions into the small intestine. Finally, the left side of the gastroduodenal artery (GDA) is exposed P to the pylorus and superior to the pancreatic neck, marking the third suspended point on the lower edge of the “A” surface. The line connecting these three suspended points constitutes the lower boundary of the “A” surface and serves as the landmark for dissection (Figure 1B).
Exposure and dissection of the right omental mesentery junction, with high ligature of the corresponding central vessel: The “A” surface is incised with A1 as the center, and the right gastroepiploic vein is freed along its cephalad side to an appropriate distance. The vein is divided at the point where it separates from the pancreas, and the dissection point is a certain distance from A1. The “A” surface of the right omental mesentery is then incised on both sides, starting on the right, followed by the left. The right mesentery lies on the surface of the pancreaticoduodenal A vein and the head of the pancreas. From A1, the dissection extends dorsally to the mesenteric junction, reaching toward second suspended point. During this step, attention should be given to the 1-2 branches of the pancreaticoduodenal A vein that arise toward the pylorus. These branches are carefully transected using an ultrasonic scalpel with slow coagulation settings. The dissection proceeds to the lateral wall of the duodenal bulb, exposing the right side of the lower edge of the right omental mesentery “P” surface. On the left side, beginning from third suspended point, the mesentery is freed from left to right around the GDA, exposing the left side of the “P” surface at the pylorus. Next, dissection along the surface of the GDA is extended from proximal to distal, exposing the inferior pyloric artery and right gastroepiploic artery. This dissection is completed by joining and dividing the right side (Figure 1C).
Exposure and dissection of the right omental mesentery “P” surface, achieving pyloric denudation: Following the completion of the previous two steps, the “P” surface is naturally exposed and subsequently divided and separated in an upward direction, achieving the denudation of the pylorus. At this point, the right omental mesentery is completely excised as a whole (Figure 1D).
Statistical analysis was performed using SPSS software (version 26.0, Armonk, NY, United States). Continuous data were presented based on the results of normality tests: Normally distributed data are expressed as mean ± SD, and comparisons between two groups were performed using the independent samples t-test; skewed data are presented as median (25th percentile; 75th percentile), with comparisons between two groups made using the Mann-Whitney U test. Categorical data are presented as frequency (percentage) and compared between two groups using the Pearson χ2 test. If the expected frequency in any cell was < 1 or the proportion of cells with expected counts < 5 exceeded 20%, Fisher’s exact test was used instead. All hypothesis tests were two-sided, and a P value < 0.05 was considered statistically significant. Moreover, the sample size was determined retrospectively based on clinical feasibility and previous studies using similar surgical comparisons. Adjustments for multiple comparisons were performed using the Bonferroni correction to control for type I error inflation across multiple outcome comparisons. This correction was applied primarily to secondary outcome measures (e.g., postoperative recovery indicators and complication subtypes), while primary outcomes (operative time, blood loss, lymph node yield) were pre-specified and analyzed without adjustment. After correction, all P values remained consistent with their respective statistical significances. A post-hoc power analysis was conducted based on the primary surgical outcomes (blood loss and operative time). Assuming a two-tailed α of 0.05 and an effect size (Cohen’s d) ranging from 0.5 to 0.8, the study achieved a statistical power of 0.91, indicating adequate sensitivity to detect meaningful intergroup differences.
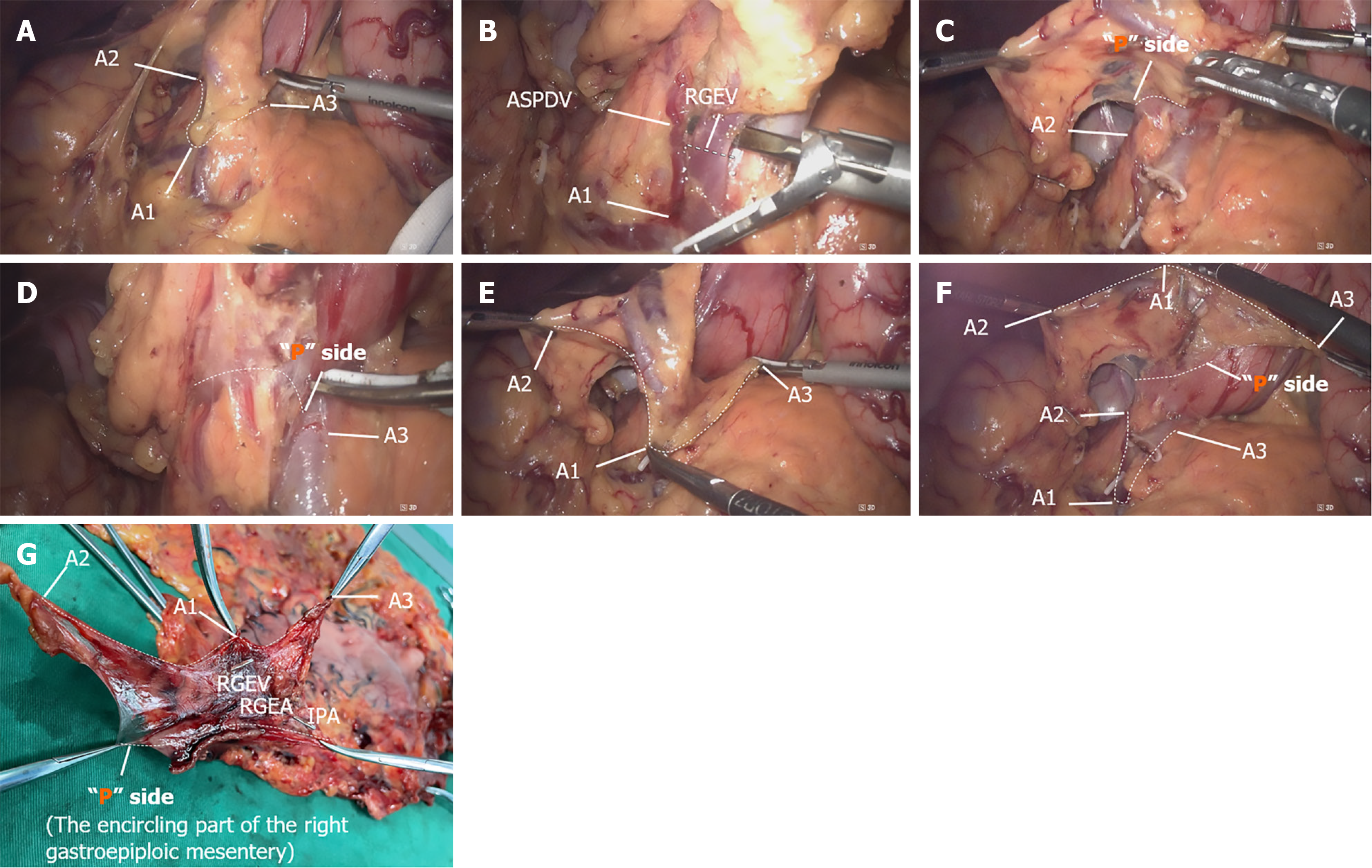
Firstly, we present the surgical quality control effect of D2 + CME, with detailed quality control checkpoints shown in Figure 2. Figure 2A shows the exposure of the “A” surface of the right omental mesentery, with the line connecting the three suspended points (denoted by white dashed lines) representing the lower edge of the “A” surface, which also serves as the dissection landmark. Figure 2B illustrates the point of division of the right gastroepiploic vein (indicated by the white dashed line), which is a certain distance from point A1. Figure 2C shows the right lateral wall of the duodenal bulb as the lower edge of the “P” surface of the right omental mesentery. Figure 2D shows the right side of the GDA as the left lateral edge of the “P” surface of the right omental mesentery. Figure 2E shows the cleaned right omental mesentery “A” surface after dissection. Figure 2F presents the result after complete excision of the right omental mesentery, with dashed lines indicating the suspended points of both the “A” and “P” surfaces of the omentum, repre
In the D2 + CME group (n = 165), 69.1% (114/165) were male and 30.9% (51/165) were female, with an average age of 66.12 ± 5.59 years and a BMI of 22.14 ± 1.04 kg/m2. According to the ASA classification, 5 patients (3.0%) were classified as grade I, 115 patients (69.7%) as grade II, and 45 patients (27.3%) as grade III. Tumor location distribution was as follows: Upper third of the stomach, 49.1% (81/165); middle third, 24.8% (41/165); lower third, 14.0% (23/165); multiple sites involved, 12.1% (20/165). Preoperative comorbidities were present in 84 patients (50.9%). In the D2 group (n = 165), the baseline characteristics were as follows: 67.9% (112/165) were male and 32.1% (53/165) were female, with an average age of 65.87 ± 5.72 years and a BMI of 22.15 ± 1.12 kg/m2. ASA classification showed 6 patients (3.6%) in grade I, 113 patients (68.5%) in grade II, and 46 patients (27.9%) in grade III. Tumor location distribution was: Upper third of the stomach, 50.3% (83/165); middle third, 24.2% (40/165); lower third, 14.5% (24/165); multiple sites involved, 11.0% (18/165). Preoperative comorbidities were present in 82 patients (49.7%). A comparison of the baseline data between the two groups is detailed in Table 1.
| Variables | D2 group (n = 165) | D2 + CME group (n = 165) | t/χ2/Z | P value |
| Clinicopathological feature | ||||
| Male/female | 112 (67.9)/53 (32.1) | 114 (69.1)/51 (30.9) | 0.056 | 0.813 |
| Age (years), mean ± SD | 65.87 ± 5.72 | 66.12 ± 5.59 | -0.402 | 0.688 |
| BMI (kg/m2), mean ± SD | 22.15 ± 1.12 | 22.14 ± 1.04 | 0.084 | 0.933 |
| ASA sore (I/II/III) | 6 (3.6)/113 (68.5)/46 (27.9) | 5 (3.0)/115 (69.7)/45 (27.3) | 0.119 | 0.942 |
| Comorbidities | 82 (49.7) | 84 (50.9) | 0.012 | 0.912 |
| Tumor position (upper/middle/lower/mix) | 83 (50.3)/40 (24.2)/24 (14.5)/18 (11.0) | 81 (49.1)/41 (24.9)/23 (13.9)/20 (12.1) | 0.163 | 0.983 |
| Surgical outcomes | ||||
| Gastrectomy (total/distal) | 137 (83.0)/28 (17.0) | 141 (85.4)/24 (14.6) | 0.205 | 0.650 |
| Total operation time (minute), mean ± SD | 301.25 ± 52.30 | 279.19 ± 45.50 | 4.088 | < 0.001 |
| Infrapyloric operative time (minute), mean ± SD | 27.58 ± 4.20 | 22.24 ± 3.80 | 12.111 | < 0.001 |
| Infrapyloric bleeding loss (mL), mean ± SD | 24.83 ± 6.35 | 4.71 ± 1.12 | 40.081 | < 0.001 |
| Postoperative recovery | ||||
| Time of first postoperative flatus (days), Median (P25, P75) | 4 (3, 5) | 3 (3, 3) | 0.251 | 0.874 |
| Time of first liquid diet intake (days), Median (P25, P75) | 8 (6, 10) | 7 (5, 8) | 0.107 | 0.809 |
| Postoperative hospital stay (days), Median (P25, P75) | 12 (11, 14) | 11 (10, 12) | 0.057 | 0.935 |
| Pathologic results | ||||
| Tumor size (cm), mean ± SD | 4.82 ± 1.45 | 4.76 ± 1.38 | 0.668 | 0.505 |
| Number of total LNs dissected, mean ± SD | 37.25 ± 8.80 | 43.80 ± 10.05 | -6.298 | < 0.001 |
| Number of No. 6 LNs dissected, mean ± SD | 4.14 ± 0.41 | 5.26 ± 0.87 | -14.959 | < 0.001 |
| pTNM stage1, (I/II/III) | 19 (11.5)/55 (33.3)/91 (55.2) | 21 (12.7)/61 (37.0)/83 (50.3) | 0.778 | 0.678 |
In the D2 + CME group (n = 165), 141 patients underwent total gastrectomy and 24 patients underwent distal gastrectomy. The mean total surgical time was 279.19 ± 45.50 minutes, with a mean time for the pyloric antrum procedure of 22.24 ± 3.80 minutes, and a mean blood loss in the pyloric antrum region of 4.71 ± 1.12 mL. The median time to first flatus was 3 (3, 3) days, the median time to first liquid diet was 7 (5, 8) days, and the median postoperative hospital stay was 11 (10, 12) days. No perioperative deaths occurred among the 165 patients. Postoperative complications were observed in 33 patients (20%), including 1 case of anastomotic leakage, 2 cases of lymphatic fistula, 1 case of pancreatic fistula, 1 case of abdominal cavity infection, 4 cases of abdominal bleeding, 1 case of intestinal obstruction, and 23 cases of pulmonary infection. According to the Clavien-Dindo classification of postoperative complications, no patients were classified as grade III-IV. The mean tumor diameter was 4.76 ± 1.38 cm. The mean number of retrieved total lymph nodes was 43.80 ± 10.05, and the mean number of retrieved No. 6 group lymph nodes was 5.26 ± 0.87. According to the American Joint Committee on Cancer 8th edition gastric cancer staging system, there were 21 cases of stage I, 61 cases of stage II, and 83 cases of stage III.
In the D2 group (n = 165), 137 patients underwent total gastrectomy and 28 patients underwent distal gastrectomy. The mean total surgical time was 301.25 ± 52.30 minutes, with a mean time for the pyloric antrum procedure of 27.58 ± 4.20 minutes, and a mean blood loss in the pyloric antrum region of 24.83 ± 6.35 mL. The median time to first flatus was 4 (3, 5) days, the median time to first liquid diet was 8 (6, 10) days, and the median postoperative hospital stay was 12 (11, 14) days. No perioperative deaths occurred among the 165 patients. Postoperative complications were observed in 50 patients (30.3%), including 3 cases of anastomotic leakage, 5 cases of lymphatic fistula, 4 cases of pancreatic fistula, 6 cases of abdominal cavity infection, 7 cases of abdominal bleeding, 3 cases of intestinal obstruction, and 22 cases of pulmonary infection. According to the Clavien-Dindo classification of postoperative complications, 5 patients (3.0%) were classified as grade III-IV. The mean tumor diameter was 4.82 ± 1.45 cm. The mean number of retrieved total lymph nodes was 37.25 ± 8.80, and the mean number of retrieved No.6 group lymph nodes was 4.14 ± 0.41. According to the American Joint Committee on Cancer 8th edition gastric cancer staging system, there were 19 cases of stage I, 55 cases of stage II, and 91 cases of stage III.
Overall, compared with the D2 + CME group, the D2 group demonstrated significantly worse outcomes in multiple key metrics: Postoperative recovery (33% delayed flatus, 14% delayed liquid diet, 9% prolonged hospital stay), complication rates (51.5%), infrapyloric operative time (24.0%), infrapyloric blood loss (427.2%), retrieved total lymph nodes (-15.0%), and retrieved No.6 group lymph nodes (-27.1%). Table 1 and Table 2 provide a detailed comparison of the baseline data between the two groups.
| Variables | D2 group (n = 165) | D2 + CME group (n = 165) | t/χ2/Z | P value |
| Number of complications | 50 (30.3) | 33 (20.0) | 4.121 | 0.042 |
| Anastomotic leakage | 3 (1.8) | 1 (0.6) | - | - |
| Lymphatic fistula | 5 (3.0) | 2 (1.2) | - | - |
| Pancreatic fistula | 4 (2.4) | 1 (0.6) | - | - |
| Abdominal cavity infection | 6 (3.6) | 1 (0.6) | - | - |
| Abdominal bleeding | 7 (4.2) | 4 (2.4) | - | - |
| Intestinal obstruction | 3 (1.8) | 1 (0.6) | - | - |
| Pulmonary infection | 22 (13.3) | 23 (13.9) | - | - |
| Clavien-Dindo classification III-IV | 5 (3.0) | 0 (0.0) | 5.077 | 0.024 |
In recent years, an increasing number of studies have confirmed that cancerous nodules exist in the adipose connective tissue of the gastric cancer specimen, and this tissue containing cancerous nodules is surrounded by the same membrane as the stomach[21,22]. Traditional gastric cancer surgery emphasizes a vascular-guided lymph node dissection concept, focusing on ligating the root of the vessels. During surgery, the omentum is often intentionally disrupted, leading to the spread of cancerous nodules within the omental tissue, resulting in local recurrence and metastasis. Therefore, complete omentectomy is considered the ideal surgical approach for gastric cancer. This surgical concept has been applied in total mesorectal excision and CME, significantly improving patient survival rates[23,24]. However, unlike the barrel-shaped structure of the mesorectum and the flat structure of the mesocolon, the omental morphology is irregular. Particularly, the right omentum undergoes rotation and fixation during embryonic development and fuses with surrounding mesenteries (such as the transverse colon mesentery and pancreatic head mesentery), incorporating the pancreas into the mesentery. This makes it difficult to precisely identify the classic structural elements of the mesentery (the three mesenteric elements) during surgery. The essence of mesenteric dissection surgery is a reverse engineering of embryonic development. To achieve accurate omentectomy, it is essential to understand the embryonic development of the gastrointestinal tract and the anatomical structure of the mesentery. At our center, through continuous laparoscopic gastric cancer clinical practice, we have utilized the clearer anatomical view provided by laparoscopy to identify the classic three elements of the omentum in gastric cancer surgery, offering an anatomical theoretical basis for complete omentectomy. In this study, we applied this approach to the dissection of the subpyloric area and defined the boundary for right omental dissection, proposing a replicable, streamlined surgical method to shorten the learning curve for beginners in omentectomy.
To achieve precise and complete resection of the right omentum, the following key steps must be followed. In the first step of surgery, the identification and exposure of the three attachment points on the “A” surface of the right omentum are crucial, especially the A1 point. The omentum has a characteristic of fusion during development, and there is a loose connective tissue space between the fused fascial layers. To accurately dissect the space between the two layers of the membrane, it is necessary to first locate the point of fusion, known as the “membrane bridge”. We believe that the junction of the right gastric vein and the A superior pancreaticoduodenal vein (A1 point) is the starting point of the fusion between the right omentum and the surrounding organ mesenteries. By incising at the A1 point and utilizing the “cavitation” effect produced by the ultrasonic scalpel, it becomes easier to access the fused fascial space, which is an almost avascular embryological anatomical space, similar to the “dissectable layer” proposed by Shinohara et al[16]. In the second step of the surgery, we provide specific details on the exposure and dissection of the junction of the right omentum, which is of great significance for completing high-level ligation of the corresponding central vessels. After incising the mesentery on the “A” surface and appropriately exposing the right gastric vein toward the cranial side, the vein should only be dissected when it is sufficiently separated from the pancreas, as this section of the vessel, particularly on its P side, is believed to lie below the A pancreatic fascia plane of the pancreatic head. Care must be taken to avoid cutting the right gastric vein too low, which could lead to injury and bleeding by entering the P plane (i.e., within the pancreatic head parenchyma). Additionally, this vascular exposure can prevent the omission of some lymph nodes in the No.6v group. By first dissecting the right mesentery and then the left, one can preferentially sever 1-2 branches of the subpyloric vein originating from the A superior pancreaticoduodenal vein, thereby avoiding injury and bleeding during P arterial ligation from the left approach.
Our previous studies have confirmed that combined subpyloric lymph node dissection and right omentectomy offer advantages over traditional subpyloric lymph node dissection, including reduced intraoperative blood loss and an increased number of No. 6 Lymph nodes harvested[17]. The new approach to subpyloric lymph node dissection proposed in this study is an improvement on the previous technique, aimed at reducing the difficulty of right omentectomy and enhancing its applicability among junior surgeons. The overall postoperative complication rate in this study was 20%, with pulmonary infections being the most common (13.9%), and no severe postoperative complications occurred, demonstrating the safety of this technique. To better characterize the safety profile, we further analyzed the severity and clinical management of postoperative complications according to the Clavien-Dindo classification. Notably, while the overall complication rate was lower in the D2 + CME group (20.0% vs 30.3%), all complications were Clavien-Dindo grade I-II and were successfully managed conservatively, including antibiotics, supportive care, or drainage without reoperation. In contrast, 5 patients (3.0%) in the D2 group experienced grade III-IV complications requiring invasive interventions such as reoperation or image-guided drainage. The absence of severe complications (grade ≥ III) in the D2 + CME group highlights the reduced procedural trauma and enhanced perioperative safety associated with this approach. This surgical approach, based on anatomical landmarks, not only simplifies the procedural steps of right omentectomy but also retains the associated surgical advantages. Experienced surgeons often rely on intuition and sensory perception to identify the fascial fusion spaces, which presents a significant challenge for junior surgeons. By locating these key anatomical landmarks, this new surgical method may help surgeons more easily perform complete right omentectomy. We further conducted a multivariate logistic regression to explore risk factors associated with postoperative complications. Age > 70 years, ASA III classification, and conventional D2 dissection were identified as independent predictors (P < 0.05). These findings suggest that enhanced mesenteric excision may help mitigate complication risk, especially in high-risk subgroups.
Several studies have shown that CME-based techniques can significantly improve lymph node yield and reduce recurrence compared to conventional D2 resection[9-12,23,24]. Our findings are consistent with Xie et al’s report[9], which demonstrated improved short-term outcomes using CME principles in gastric cancer. However, unlike Xie et al’s study[9] which focused on distal gastrectomy, our cohort primarily involved total gastrectomy, expanding the applicability of this technique. Compared with previous approaches guided by vascular anatomy, the mesenteric landmark-based method aligns with modern oncological surgery principles, emphasizing complete compartmental resection - similar to total mesorectal excision in rectal cancer and CME in colonic cancer[23,24]. In our current study, due to the relatively short follow-up period and the retrospective design, long-term oncological outcomes such as disease-free survival and recurrence patterns were not evaluated. However, we recognize the oncological potential of D2 + CME in minimizing micrometastasis and local recurrence through en bloc mesogastric excision. To further investigate these long-term effects, we have initiated a prospective follow-up program within our institution, including survival analysis and recurrence tracking for all patients treated with D2 + CME since 2020. The results of this follow-up cohort are expected to provide valuable insights into the prognostic implications of this technique and will be reported in future studies.
Notwithstanding its innovative contributions, the present investigation is circumscribed by several notable constraints. First, the procedure was exclusively executed by a single, highly experienced surgeon, necessitating future validation of its reproducibility across varying levels of surgical expertise, particularly among junior practitioners. Second, no formal oncological efficacy assessment was undertaken. Hypothetically, this novel surgical methodology not only facilitates more meticulous subpyloric lymphadenectomy but also eradicates potential malignant cells or micrometastatic foci within the right omental adipose tissue, potentially augmenting long-term oncological outcomes. Therefore, prospective randomized trials evaluating both short-term morbidity and long-term survival outcomes are warranted. Moreover, although the majority of infrapyloric lymph node metastasis occurs in lower-third gastric cancer, studies have identified occasional No. 6 involvement even in upper-third tumors with serosal invasion or high nodal burden. Therefore, to preserve the integrity of D2 dissection and mesogastric excision principles, we included upper-third tumors in our cohort. Future subgroup analyses focusing on lower-third tumors may provide more focused oncologic insight. Additionally, the retrospective nature of our study imposes certain limitations on the strength of our conclusions. Although we employed a matched-pair design to minimize confounding variables, the absence of randomization may allow residual selection bias related to surgeon experience, patient health status, or other institutional factors. As such, the generalizability of our findings should be approached with caution. Prospective, multi-institutional randomized controlled trials will be essential to confirm the reproducibility, safety, and oncological benefits of the D2 + CME technique in broader clinical contexts.
This study highlights a mesenteric anatomical approach for subpyloric lymphadenectomy, which provides both surgical precision and potential oncological benefits. This technique achieves complete right omentectomy with significantly reduced intraoperative blood loss and operative time while maintaining high-quality No. 6 Lymph node dissection. Importantly, the approach reduces postoperative complications by 51.5% compared to conventional D2 dissection, underscoring its safety profile. Although further multicenter trials are required to validate long-term survival outcomes, these findings suggest this method represents a significant advancement in minimally invasive gastric cancer surgery.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68648] [Article Influence: 13729.6] [Reference Citation Analysis (201)] |
| 2. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 842] [Article Influence: 280.7] [Reference Citation Analysis (2)] |
| 3. | Souza WP, Pereira MA, Cardili L, Zilberstein B, Ribeiro-Junior U, Ramos MFKP. Evaluation of the endoscopic cure criteria in patients undergoing surgery for early gastric cancer. J Surg Oncol. 2024;130:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Lee AY, Kim MC, Cho S, Yoo IK, Kim YM, Lee TH, Seo JY, Kim SH, Cho JY. Da Vinci robot-assisted endoscopic full-thickness gastric resection with regional lymph node dissection using a 3D near-infrared video system: a single-center 5-year clinical outcome. Surg Endosc. 2024;38:2124-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Irino T, Ohashi M, Hayami M, Makuuchi R, Ri M, Sano T, Yamaguchi T, Nunobe S. Updated Review of Proximal Gastrectomy for Gastric Cancer or Cancer of the Gastroesophageal Junction. J Gastric Cancer. 2025;25:228-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 6. | Park SH, Chung SY, Lee JH, Kim HK, Lee D, Kim H, Kim JH, Kim MS, Lee JH, Park JY, Yoon HM, Ryu KW, Kook MC. Feasibility of intraoperative pathologic examination for sentinel lymph nodes during sentinel node navigation surgery in early gastric cancer: results of pathologic protocol for SENORITA trial. Gastric Cancer. 2024;27:858-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Abate M, Drebin H, Shimada S, Fei T, McKinley S, Poruk K, Ferguson B, Neuwirth M, Tang LH, Vardhana S, Strong VE. Feasibility and Efficacy of Sentinel Lymph Node Mapping in Gastric Cancer. Ann Surg Oncol. 2024;31:6959-6969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Gong J. [D2 gastrectomy and complete mesentery excision based on metastasis IIIII( and membrane anatomy]. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18:121-122. [PubMed] [DOI] [Full Text] |
| 9. | Xie D, Shen J, Liu L, Cao B, Wang Y, Qin J, Wu J, Yan Q, Hu Y, Yang C, Cao Z, Hu J, Yin P, Gong J. Complete mesogastric excision for locally advanced gastric cancer: short-term outcomes of a randomized clinical trial. Cell Rep Med. 2021;2:100217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Zhao D, Deng J, Cao B, Shen J, Liu L, Xiao A, Yin P, Xie D, Gong J. Short-term outcomes of D2 lymphadenectomy plus complete mesogastric excision for gastric cancer: a propensity score matching analysis. Surg Endosc. 2022;36:5921-5929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Sun Y, Hou L, Zhao E. Short-term outcomes of laparoscopic D2 lymphadenectomy versus D2 lymphadenectomy plus complete mesogastric excision in distal gastric cancer patients with high body mass index. BMC Cancer. 2025;25:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Xie D, Wang Y, Shen J, Hu J, Yin P, Gong J. Detection of carcinoembryonic antigen in peritoneal fluid of patients undergoing laparoscopic distal gastrectomy with complete mesogastric excision. Br J Surg. 2018;105:1471-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Dehal A. Immunotherapy for gastric cancer and liver metastasis: Is it time to bid farewell. World J Gastrointest Surg. 2024;16:2365-2368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Mizuno A, Shinohara H, Haruta S, Tsunoda S, Kurahashi Y, Ohkura Y, Udagawa H, Sakai Y. Lymphadenectomy along the infrapyloric artery may be dispensable when performing pylorus-preserving gastrectomy for early middle-third gastric cancer. Gastric Cancer. 2017;20:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 15. | Liu CG, Lu P, Lu Y, Jin F, Xu HM, Wang SB, Chen JQ. Distribution of solitary lymph nodes in primary gastric cancer: a retrospective study and clinical implications. World J Gastroenterol. 2007;13:4776-4780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Shinohara H, Haruta S, Ohkura Y, Udagawa H, Sakai Y. Tracing Dissectable Layers of Mesenteries Overcomes Embryologic Restrictions when Performing Infrapyloric Lymphadenectomy in Laparoscopic Gastric Cancer Surgery. J Am Coll Surg. 2015;220:e81-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Shinohara H, Kurahashi Y, Kanaya S, Haruta S, Ueno M, Udagawa H, Sakai Y. Topographic anatomy and laparoscopic technique for dissection of no. 6 infrapyloric lymph nodes in gastric cancer surgery. Gastric Cancer. 2013;16:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Xu Y, Li Z, Pan G, Wu H, Li J, Lin W, Chen J, Cai Z. Anatomical Findings and Short-term Efficacy of Fascial Anatomy-guided Infrapyloric Lymphadenectomy in Laparoscopic Radical Gastrectomy for Gastric Cancer. Surg Laparosc Endosc Percutan Tech. 2021;31:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1415] [Article Influence: 283.0] [Reference Citation Analysis (2)] |
| 20. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2950] [Article Influence: 196.7] [Reference Citation Analysis (1)] |
| 21. | Etoh T, Sasako M, Ishikawa K, Katai H, Sano T, Shimoda T. Extranodal metastasis is an indicator of poor prognosis in patients with gastric carcinoma. Br J Surg. 2006;93:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Xie D, Osaiweran H, Liu L, Wang X, Yu C, Tong Y, Hu J, Gong J. Mesogastrium: a fifth route of metastasis in gastric cancer? Med Hypotheses. 2013;80:498-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1973] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 24. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-64; discussion 364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1150] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/