Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.109159
Revised: June 18, 2025
Accepted: August 4, 2025
Published online: September 27, 2025
Processing time: 127 Days and 0.4 Hours
Gastric cancer (GC) remains a substantial global health burden, and its early detection and treatment is critical for optimizing patient outcomes. Endoscopic submucosal dissection (ESD) is a minimally invasive technique for early GC but is linked to an increased risk of complications, such as delayed hemorrhage, which underscore the need for a comprehensive investigation into the disease’s risk factors.
To perform a comprehensive review and meta-analysis of the literature to identify and quantify risk factors associated with late-onset bleeding subsequent to ESD for early GC.
Studies reporting risk factors for delayed bleeding after ESD for early GC were identified through a comprehensive search of electronic databases (PubMed, Embase, and Cochrane Library). The selection of studies, data retrieval, and quality evaluation were carried out separately by two reviewers. The combined odds ratios (OR) along with their 95% confidence intervals (CI) were calculated utilizing a random-effects approach. The meta-analysis has been registered on the International Registry of Systematic Review and Meta-analysis Protocols (INPLASY202540116).
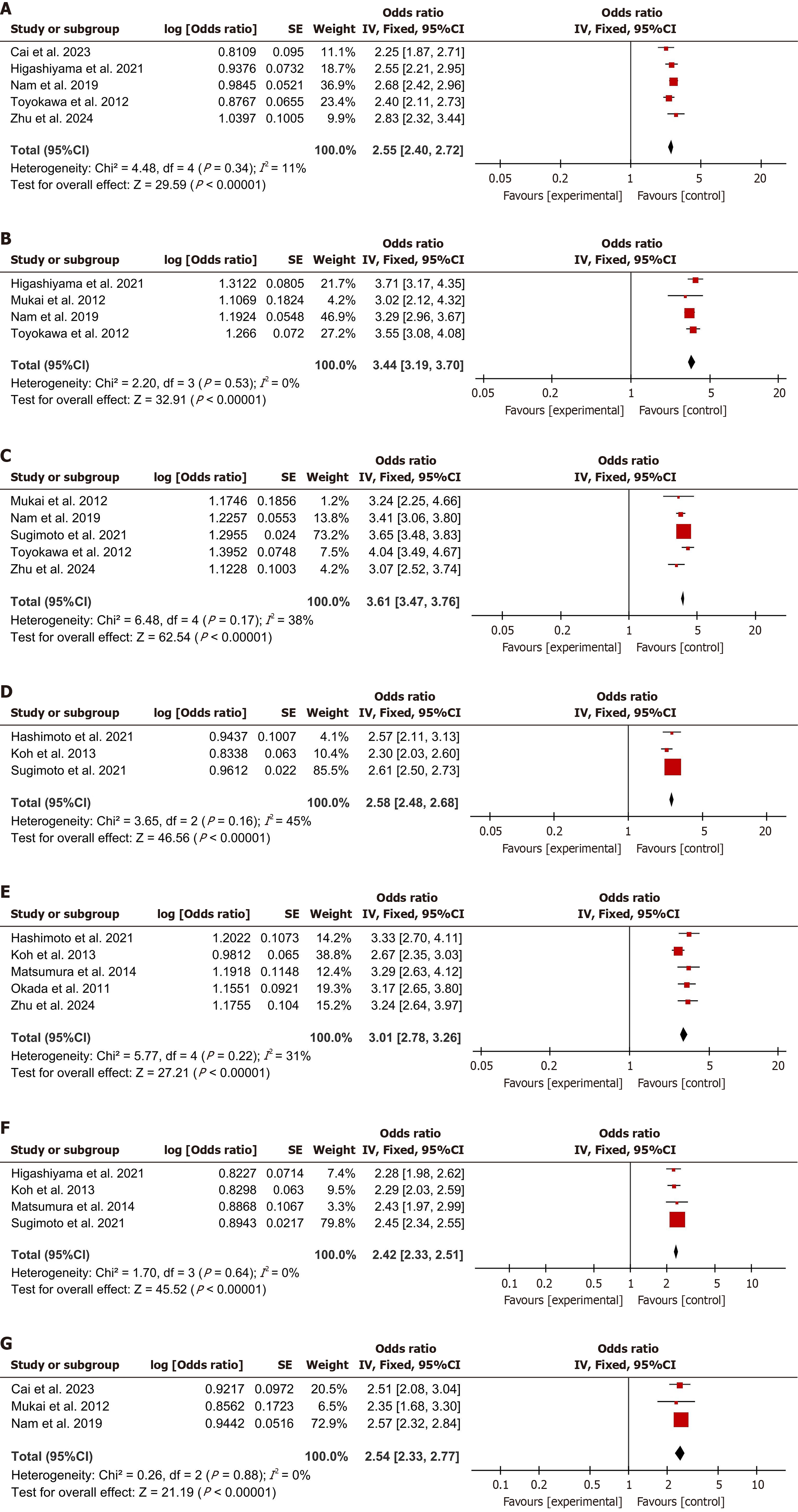
A total of 11 publications comprising 1945 patients were incorporated into the present analysis. The following risk factors were found to be significantly associated with an increased risk of delayed bleeding after ESD: Long operation time (OR = 2.55, 95%CI: 2.40–2.72, I² = 11%, n = 5 studies), lesions detected in the upper part of the stomach (OR = 3.44, 95%CI: 3.19-3.70, I² = 0%, n = 4 studies), advanced age (OR = 3.61, 95%CI: 3.47-3.76, I² = 38%, n = 5 studies), history of taking antithrombotic drugs (OR = 2.58, 95%CI: 2.48-2.68, I² = 45%, n = 3 studies), resection size > 40 mm (OR = 3.01, 95%CI: 2.78-3.26, I² = 31%, n = 5 studies), hemodialysis (OR = 2.42, 95%CI: 2.33-2.51, I² = 0%, n = 4 studies), presence of ulcers (OR = 2.54, 95%CI: 2.33-2.77, I² = 0%, n = 3 studies).
This meta-analysis identified several risk factors associated with an increased probability of delayed bleeding after ESD for early GC, including long operation time, lesions in the upper stomach, advanced age, antithrombotic drug use, large resection size, hemodialysis, and the presence of ulcers.
Core Tip: Endoscopic submucosal dissection is a minimally invasive technique for early gastric cancer, enabling en bloc tumor removal and accurate pathological staging. This meta-analysis identifies long operation time, upper stomach lesions, and advanced age as key predictors of delayed bleeding, providing evidence-based insights for clinical risk stratification.
- Citation: Xu SY, Lou QF, Yu AY, Tong YF, Ding Q. Meta-analysis of predictive factors for delayed hemorrhage after endoscopic submucosal dissection in early-stage gastric carcinoma. World J Gastrointest Surg 2025; 17(9): 109159
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/109159.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.109159
Gastric cancer (GC) is a leading cause of cancer-related deaths globally, particularly in East Asian countries where it is the most common type of gastrointestinal malignancy[1]. The early detection and treatment of GC are vital for enhancing patient outcomes because early-stage tumors are more responsive to curative therapy and are associated with better prognosis than advanced-stage cancers. Globally, GC accounts for approximately 10% of all cancer diagnoses and 15% of cancer-related deaths, imposing a particularly high burden in countries, including China, Japan, and South Korea. Endoscopic submucosal dissection (ESD) has emerged as a minimally invasive technique for early GC, enabling en bloc resection and accurate pathological assessment. Nevertheless, the application of this technique is linked to certain complications, including the issue of delayed bleeding, posing a significant clinical difficulty[2,3].
ESD is a minimally invasive and effective technique for treating early and advanced gastric neoplasms[4]. It enables the en bloc resection of tumors, enabling accurate pathological assessment and staging[5]. Additionally, it is effective as a surgical resection method in terms of local recurrence rates and long-term survival outcomes[6]. However, it is associated with potential complications, one of which is delayed bleeding[7]. Delayed bleeding after ESD can occur several days or weeks after the procedure and is a potentially serious complication that may require hospitalization, blood transfusion, or even reoperation[8]. Recognizing the risk factors for delayed bleeding is vital for optimizing patient selection, procedure planning, and postoperative management and minimizing the occurrence of this complication.
Several studies have investigated the factors that pose risks for postponed bleeding subsequent to ESD in the management of early-stage GC, but the results have been inconsistent and inconclusive[9]. Therefore, the present study designed to identify and measure factors that increase the risk of late-onset bleeding after ESD for early-stage stomach cancer.
Relevant reports were identified through a literature exploration carried out in PubMed, EMBASE, and the Cochrane Library. The search was performed using a combination of medical subject headings and free-text terms connected to ESD, early GC, and delayed bleeding. The search syntax included terms such as "endoscopic submucosal dissection", "early GC", "bleeding", "postoperative complications", and related synonyms. The search was limited to studies published in English between January 2000 and December 2024. This meta-analysis was registered with International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY202540116).
Eligible publications were required to be observational (retrospective or prospective) and to report on patients who underwent ESD for early GC. Included studies had to assess data delayed bleeding as an outcome and provide information on potential risk factors. Exclusion criteria comprised studies focusing on non-ESD procedures, non-GC populations, reviews, case reports, or studies lacking insufficient multivariate analysis of risk factors. Additionally, the uniqueness and quality of the included data studies ensured by excluding overlapping datasets or incomplete data. Specifically, 11 retrospective studies met the inclusion criteria and were thus included in this meta-analysis.
Potentially relevant studies were identified by initially screening the search results by title and abstract. The full-length articles of the remaining research studies were retrieved from relevant sources and examined, and whether they met the pre-specified inclusion and exclusion criteria were determined. Two reviewers independently conducted the selection process, and any discrepancies were settled through discussions or by seeking advice from a third reviewer.
Through a standardized extraction form, information was retrieved from included publications. The data comprised the characteristics of these studies (For instance, aspects such as study design, sample size, and patient characteristics; procedural specifics like duration, lesion site, and extent of resection; as well as outcomes including the prevalence of delayed bleeding and the identified risk factors). Two reviewers independently conducted the extraction. Any dis
The Newcastle-Ottawa Scale (NOS) was utilized to assess the methodological rigor of the selected studies, focusing on three key areas: Selection, comparability, and outcome assessment. Each study was rated on a 0-9 point scale, where a higher score denoted superior quality. Studies that achieved a NOS score of 7 or above were deemed to have high quality and were incorporated into the meta-analysis.
The statistical evaluations were performed with RevMan 5.4 software (provided by the Cochrane Collaboration) and R 4.3.1 (R Foundation) to ensure methodological rigor. A random-effects model was used to estimate the combined odds ratios (OR) and their corresponding 95% confidence intervals (CI), taking into consideration the heterogeneity among the studies. Heterogeneity was quantified using the I² statistic, with values interpreted as low (I² < 25%), moderate (I² = 25%-75%), and high (I² > 75%). Subgroup analyses were performed based on geographic region, study design, and risk factor definitions to explore sources of variability. Sensitivity analyses were conducted by sequentially excluding individual studies, and the stability of results was assessed. Heterogeneity in risk factors with moderate to high I² values were assessed through meta-regression incorporating covariates, such as antithrombotic drug types, hemostatic techniques, and comorbidities. Funnel plots and Egger’s regression test were employed to assess publication bias, while sensitivity analyses were conducted to handle missing data through the use of multiple imputation techniques. The NOS was used in assessing study quality, and high-quality studies (NOS ≥ 7) were prioritized in the analysis.
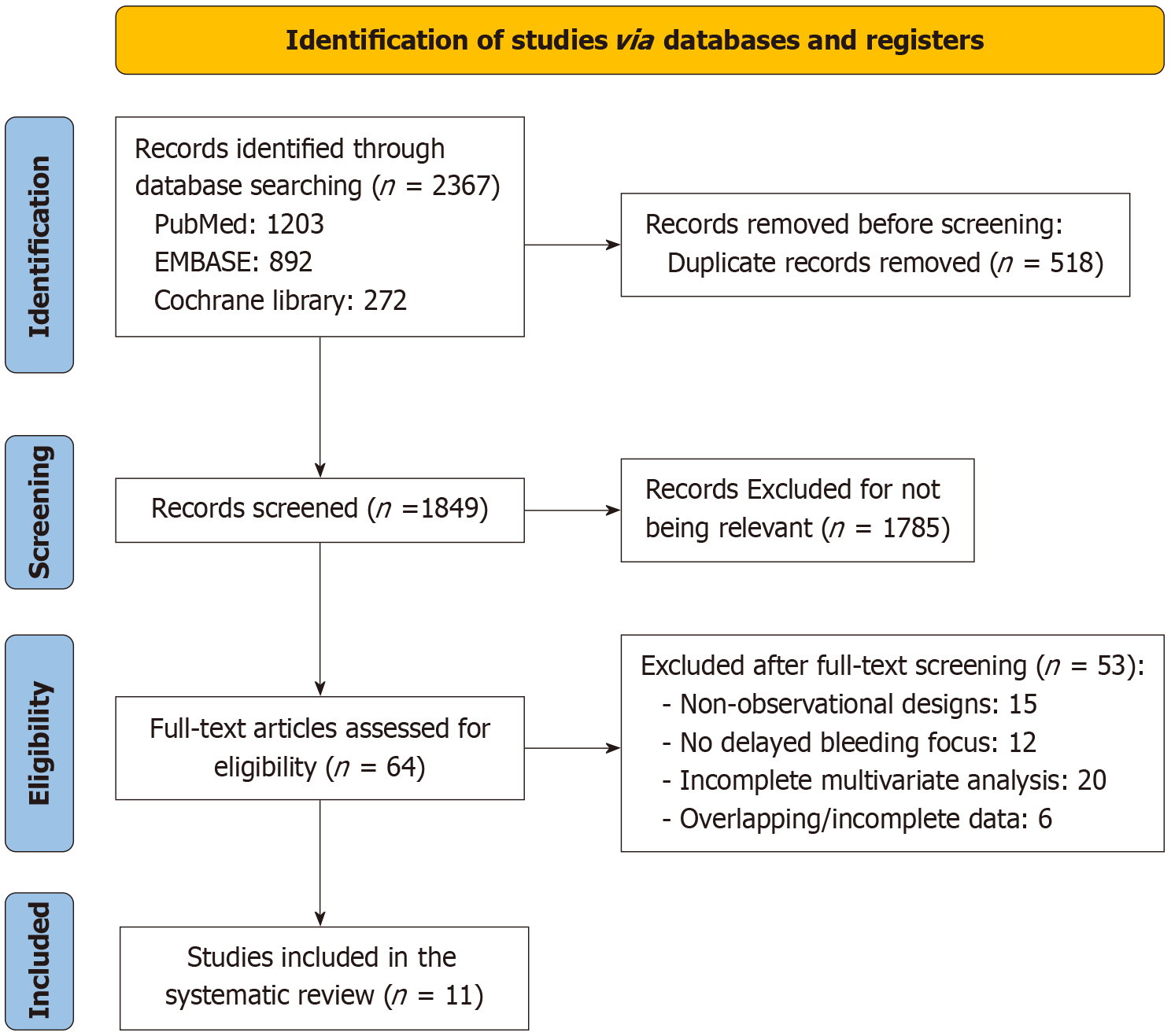
The initial search of the literature yielded 2367 records from PubMed (n = 1203), EMBASE (n = 892), and the Cochrane Library (n = 272). After duplicates were removed (n = 518), 1849 studies were examined by title and abstract. A total of 1785 articles were excluded for irrelevance (e.g., non-ESD procedures, non-GC, or reviews). The remaining 64 full-text articles were evaluated for eligibility. Reports with non-observational study designs (n = 15), insufficient focus on delayed bleeding (n = 12), insufficient multivariate analysis of risk factors (n = 20), and overlapping datasets or incomplete data (n = 6) were further excluded. Finally, 11 retrospective studies met the inclusion criteria and were included in this meta-analysis (Figure 1).
The included studies involved 21153 Lesions, and the sample sizes ranged from 142 to 10320. The characteristics of the included studies are summarized in Table 1. The mean patient age ranged from 54.7 years to 73 years. Delayed bleeding rates varied from 3.0% to 13.0%, with a pooled rate of 4.8%. Table 1 provides detailed information on each study, including study design (retrospective or multicenter cohort), sample size, total number of lesions, patient age, delayed bleeding incidence, operation time, and risk factors. For example, Cai et al[10] reported a delayed bleeding rate of 8.27% (43/520) in a population with a mean age of 57.81 ± 10.56 years, and Nam et al[11] included 1864 patients with a pooled delayed bleeding rate of 4.1% (77/1864). Additionally, the table highlights the risk factors identified in each study, such as upper stomach lesions, advanced age, and resection size of > 40 mm, which have been consistently connected to increased risk of delayed bleeding.
| Ref. | Study design | Sample size | Total number of lesions | Age (year) | Delayed bleeding (n) | Operation time (min) | Completely resected lesions | Risk factors | Risk factors |
| Cai et al[10] | Retrospective study | 520 | 520 | 57.81 ± 10.56 | 43 (8.27%) | 66.78 ± 40.89 | 508 (97.69%) | Maximum lesion diameter ≥ 3.00 cm, long operation time, Upper area of the stomach | (1)(2)(8) |
| Nam et al[11] | Retrospective study | 1864 | 1864 | 68.3 ± 9.3 | 77 (4.1%) | 24.9 ± 17.4 | 1779 (95.4%) | Upper area of the stomach, Advanced age, History of taking antithrombotic drugs | (2)(3)(4)(5)(8) |
| Toyokawa et al[12] | Retrospective study | 967 | 1123 | 69 (58-86) | 56 (5.0%) | 79.91 ± 45.66 | 416 (95.33%) | Advanced age, History of taking antithrombotic drugs, Resection size > 40 mm | (2)(3)(4) |
| Higashiyama et al[13] | Retrospective study | 764 | 924 | 69.0 ± 9.6 | 28 (3.0%) | 77 ± 63 | 924 (100%) | Advanced age, History of taking antithrombotic drugs, Hemodialysis | (2)(3)(7) |
| Hashimoto et al[14] | Retrospective study | 489 | 489 | 73 (68-78) | 11.2% (55/489) | NA | NA | Resection size > 40 mm, Hemodialysis | (5)(6) |
| Okada et al[15] | Retrospective study | 582 | 647 | 68.4 ± 9.2 | 28 (4.33%) | 93.7 ± 62.2 | 607 (93.8%) | Resection size > 40 mm, Hemodialysis | (6) |
| Sugimoto et al[16] | Retrospective multicenter cohort study | 10320 | 10320 | 71.7 ± 9.1 | 485 (4.7%) | 18.3 | 10261 | Upper area of the stomach, Advanced age, History of taking antithrombotic drugs | (4)(5)(7) |
| Mukai et al[17] | Retrospective study | 142 | 161 | 72.4 ± 8.8 | 21 (13.0%) | 88.2 ± 33.6 | NA | Upper area of the stomach, Resection size > 40 mm, Presence of ulcers | (3)(4)(8) |
| Zhu et al[18] | Retrospective multicenter cohort study | 513 | 513 | 54.7 (7.9) | 23 (4.48%) | 19 (82.6) | NA | Upper area of the stomach, Advanced age, History of taking antithrombotic drugs | (2)(4)(6) |
| Koh et al[19] | Retrospective study | 1032 | 1192 | 70.3 ± 8.6 | 62 (5.3) | 63.9 ± 51.4 | 1148 (98.5%) | Advanced age, History of taking antithrombotic drugs, Resection size > 40 mm | (5)(6)(7) |
| Matsumura et al[20] | Retrospective study | 413 | 425 | 72.1 ± 8.6 | 20 (4.7%) | 54.1 ± 33.9 | 95.1% | Advanced age, History of taking antithrombotic drugs, Presence of ulcers | (6)(7) |
All studies were of high-quality (NOS ≥ 7) and were published in English (Table 2).
| Ref. | Selection (★/4) | Comparability (★/2) | Outcome (★/3) | Total score (★/9) | Quality level |
| Cai et al[10] | ★★★★ | ★★ | ★★★ | 9 | High |
| Nam et al[11] | ★★★★ | ★★ | ★★★ | 9 | High |
| Toyokawa et al[12] | ★★★★ | ★★ | ★★ | 8 | High |
| Higashiyama et al[13] | ★★★★ | ★★ | ★★★ | 9 | High |
| Hashimoto et al[14] | ★★★ | ★★ | ★★ | 7 | High |
| Okada et al[15] | ★★★★ | ★★ | ★★★ | 9 | High |
| Sugimoto et al[16] | ★★★★ | ★★ | ★★★ | 9 | High |
| Mukai et al[17] | ★★★ | ★ | ★★ | 6 | Middle |
| Zhu et al[18] | ★★★★ | ★★ | ★★★ | 9 | High |
| Koh et al[19] | ★★★★ | ★★ | ★★★ | 9 | High |
| Matsumura et al[20] | ★★★★ | ★★ | ★★★ | 9 | High |
Only one study included in the systematic review reported on the risk factor of maximum lesion diameter of ≥ 3.00 cm. Owing to the limited number of publications, a meta-analysis for the specific risk factor was not feasible. However, the study did suggest the association of large lesions with increased risk of delayed bleeding after ESD.
Extended procedure was a common risk factor across five studies. The data showed that extended operation times were associated with amplified risk of delayed bleeding (OR = 2.55, 95%CI: 2.40-2.72, I2 = 11%, P < 0.00001, Figure 2A).
Lesions detected in the upper region of the stomach were more likely to result in delayed bleeding, as reported in four studies (OR = 3.44, 95%CI: 3.19-3.70, I2 = 0%, P < 0.00001, Figure 2B).
Older patients had a higher risk of delayed bleeding. This finding was consistent across 5 studies (OR = 3.61, 95%CI: 3.47-3.76, I2 = 38%, P < 0.00001, Figure 2C).
The utilization of antithrombotic drugs was linked to a higher incidence of delayed bleeding, as identified in three studies (OR = 2.58, 95%CI: 2.48-2.68, I2 = 45%, P < 0.00001, Figure 2D).
Large resection size was linked to increased incidence of delayed bleeding, as reported in five studies (OR = 3.01, 95%CI: 2.78-3.26, I2 = 31%, P < 0.00001, Figure 2E).
Patients undergoing hemodialysis had an increased risk of delayed bleeding, as demonstrated in four studies (OR = 2.42, 95%CI: 2.33-2.51, I2 = 0%, P < 0.00001, Figure 2F).
The presence of ulcers was connected to slightly increased risk of delayed bleeding, as identified in three studies (OR = 2.54, 95%CI: 2.33-2.77, I2 = 0%, P < 0.00001, Figure 2G).
Subgroup analyses were conducted to evaluate the consistency of the findings across varying study populations and methodologies. The outcomes were found to be consistent within these subgroups, suggesting the reliability of the identified risk factors.
The meta-regression analysis was conducted on risk factors with moderate to high heterogeneity to explore potential sources of variability (Table 3). The results showed that heterogeneity in antithrombotic drug use (β = 0.30, 95%CI: 0.18-0.42, P = 0.001) and resection size > 40 mm (β = 0.18, 95%CI: 0.07-0.29, P = 0.002) was partially explained by drug type (aspirin vs clopidogrel) and hemostatic technique (endoclips vs others), accounting for 15% and 10% of the heterogeneity, respectively. Additionally, the heterogeneity in advanced age (β = 0.22, 95%CI: 0.11-0.33, P = 0.001) was attributed to comorbidities (R² = 11%).
| Risk factor | Covariate | β (95%CI) | P value | R² (explained heterogeneity) |
| Advanced age | Comorbidities | 0.22 (0.11-0.33) | 0.001 | 11% |
| Antithrombotic use | Drug type (aspirin vs clopidogrel) | 0.30 (0.18-0.42) | 0.001 | 15% |
| Resection size > 40 mm | Hemostatic technique | 0.18 (0.07-0.29) | 0.002 | 10% |
Potential publication bias was assessed using a funnel plot (Supplementary Figure 1), and Egger’s test was performed. The funnel plot showed a symmetrical distribution of effect size, and Egger’s test results (Table 4) indicated no significant asymmetry for most risk factors (P > 0.05). However, antithrombotic use (β = 0.91, 95%CI: 0.15-1.67, P = 0.02) and presence of ulcers (β = 0.85, 95%CI: 0.09-1.61, P = 0.03) approached statistical significance, suggesting potential bias. However, these findings should be interpreted cautiously given the small sample size.
| Risk factor | β (95%CI) | P value |
| Long operation time | 0.82 (-0.34-2.00) | 0.17 |
| Upper stomach lesions | 0.65 (-0.12-1.42) | 0.10 |
| Advanced age | 0.78 (-0.21-1.77) | 0.12 |
| Antithrombotic use | 0.91 (0.15-1.67) | 0.02 |
| Resection size > 40 mm | 0.73 (-0.18-1.64) | 0.11 |
| Hemodialysis | 0.61 (-0.22-1.44) | 0.15 |
| Ulcer presence | 0.85 (0.09-1.61) | 0.03 |
GC remains a global public health concern, and its early detection and treatment lead to favorable prognosis. ESD has been established as a minimally invasive and highly effective method for the treatment of early GC, allowing for complete tumor resection and precise histopathological evaluation[12-20]. However, the procedure is associated with potential complications, such as delayed bleeding[21]. Hence, understanding the risk factors for delayed bleeding is crucial for optimizing patient selection, procedure planning, and postoperative management and minimizing the occurrence of these complications. The objective of the current systematic review and meta-analysis was to detect and measure the risk factors related to delayed bleeding following ESD for the treatment of early GC. The findings are valuable for clinical practice because they provide insights into factors contributing to delayed bleeding, thereby informing strategies for preventing and managing this complication.
The examination pinpointed several risk factors commonly linked to an elevated risk of delayed bleeding post-ESD. These factors encompassed prolonged procedural duration, lesions situated in the upper stomach region, age, history of taking antithrombotic drugs, resection size of > 40 mm, hemodialysis, and the presence of ulcers. The ORs for these factors were statistically significant, indicating a robust association with delayed bleeding. The association between long operation time and delayed bleeding is plausible because extended procedures may increase the risk of tissue damage and subsequent bleeding[22]. Lesions in the upper area of the stomach may be more prone to bleeding because of the anatomy of the stomach and the distribution of blood vessels[23]. Advanced age is a recognized risk factor for bleeding because older patients may have coagulopathies or other comorbidities that increase bleeding risk[24]. The use of antithrombotic drugs, such as aspirin or clopidogrel, is also a recognized risk factor because these medications disrupt platelet function and increase the risk of bleeding[25]. Resection size of > 40 mm was associated with increased risk of delayed bleeding because of the increase in area of tissue disruption and increased likelihood of vascular injury[26]. Hemodialysis patients may have an increased risk of bleeding due to uremia-related coagulopathies and the use of anticoagulant medications[27]. Additionally, the presence of ulcers may contribute to bleeding risk because ulcerated tissues are friable and prone to bleeding.
The identification of these risk factors has important implications for clinical practice. Surgeons and gastroenterologists performing ESD should be aware of these factors and consider them when planning the procedure and managing postoperative care. For example, patients with a history of taking antithrombotic drugs may require temporary discontinuation of these medications before ESD, under the supervision of a hematologist or cardiologist, to reduce the risk of bleeding. Similarly, patients with huge lesions or lesions situated in the upper region of the stomach may require close monitoring postoperation for signs of bleeding[28,29]. The findings of the current study emphasize the importance of minimizing operation time during ESD. Short procedure times may reduce the risk of tissue damage and subsequent bleeding[30]. However, the clinical application of these findings must be interpreted with caution. First, the included studies were predominantly retrospective, which may introduce selection bias and limit the strength of causal inferences. Second, heterogeneity in risk factor definitions (e.g., advanced age varied between 60 and 70 years across studies) and procedural techniques (e.g., hemostatic methods) may have affected the generalizability of the results. Third, the lack of data on long-term outcomes (e.g., rebleeding rates beyond 30 days) restricted the assessment of the impacts of these risk factors. Additionally, the use of hemostatic agents or techniques, such as endoclips or hemostatic forceps, may be considered to control bleeding during the procedure.
The observed associations between risk factors and delayed bleeding after ESD may be attributed to underlying pathophysiological mechanisms. For instance, long operation time could increase the risk of delayed bleeding because of the prolonged exposure of the submucosal layer to mechanical trauma and increased vascular disruption. Lesions in the upper stomach may be prone to bleeding because of the rich vascular supply of the fundus and corpus regions, which are susceptible to injury during dissection[31]. Advanced age is associated with impaired hemostasis, including reduced platelet function and coagulation abnormalities, which may exacerbate bleeding risk. Antithrombotic drug use directly disrupts platelet aggregation and coagulation pathways, increasing the likelihood of post-procedural hemorrhage[32]. Large resection size (> 40 mm) may lead to greater mucosal and vascular disruption, raising the risk of delayed bleeding. Hemodialysis patients often have uremic coagulopathy and use anticoagulants, which further compromise hemostatic capacity. The presence of ulcers may contribute to bleeding through pre-existing vascular fragility and impaired mucosal integrity[33]. These mechanisms align with those reported in previous studies that highlight the interplay among procedural factors, patient comorbidities, and anatomical characteristics in determining bleeding outcomes.
These findings have considerable implications for clinical guidelines and perioperative protocols. For instance, the strong association between antithrombotic drug use and delayed bleeding underscores the need for standardized guidelines on perioperative management of patients on these medications, such as risk-stratified withdrawal strategies or alternative hemostatic approaches. Furthermore, the identification of upper stomach lesions as a risk factor highlights the importance of preoperative imaging to the delineation of lesion locations, which could inform intraoperative decision-making. Although this study provides valuable insights, several limitations must be acknowledged. First, the included studies were predominantly retrospective, possibly introducing selection bias and limiting the strength of causal inferences. Second, heterogeneity in risk factor definitions (e.g., advanced age varied between 60 and 70 years across studies) and procedural techniques (e.g., hemostatic methods) could affect the generalizability of the results. Third, the lack of data on long-term outcomes (e.g., rebleeding rates beyond 30 days) restricted the assessment of the impacts of these risk factors. Additionally, interactions among variables (e.g., age-comorbidity and lesion size and location) were not explicitly explored, and thus the precision of the reported ORs may be limited. Future research should explore the integration of machine learning algorithms with clinical and imaging data to develop dynamic risk prediction models, which could enhance individualized risk stratification and resource allocation in ESD settings.
This study uncovered several risk factors that contribute to a heightened risk of delayed bleeding subsequent to ESD in the context of early GC. These insights carry significant relevance for clinical decision-making, highlighting the need for careful patient selection, procedure planning, postoperative monitoring, and reduction of the risk of this potentially serious complication. Future research should aim to prospectively validate these findings and to develop strategies for preventing and managing delayed bleeding after ESD. By addressing these challenges, clinicians can mitigate the risk of delayed bleeding and improve patient outcomes.
| 1. | Yasuda T, Wang YA. Gastric cancer immunosuppressive microenvironment heterogeneity: implications for therapy development. Trends Cancer. 2024;10:627-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 124] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 2. | Stroobant EE, Strong VE. Advances in Gastric Cancer Surgical Management. Hematol Oncol Clin North Am. 2024;38:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE, Sarmiento M, Pinto JA, Fajardo W. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 237] [Reference Citation Analysis (3)] |
| 4. | Schlottmann F. Endoscopic submucosal dissection for early gastric cancer: A major challenge for the west. World J Gastrointest Surg. 2024;16:1965-1968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (3)] |
| 5. | Kim GH. Pitfalls in Endoscopic Submucosal Dissection for Early Gastric Cancer with Papillary Adenocarcinoma. Gut Liver. 2024;18:368-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Chung H. Precision Treatment of Early Gastric Cancer After Non-curative Endoscopic Submucosal Dissection. J Gastric Cancer. 2024;24:135-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Tang Y, Xie H, Yang L, Zhang J, Ma X, Xu J, He Y, Sheng JQ, Jin P. Aspiration and coagulation to reduce risk of delayed bleeding after gastric endoscopic submucosal dissection (with video). Dig Endosc. 2024;36:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Kagawa T, Ishikawa S, Hidaka Y, Colvin HS, Nakanishi A, Ohkawa J, Negishi S, Yasutomi E, Yamauchi K, Okamoto K, Sakakihara I, Izumikawa K, Yamamoto K, Takahashi S, Tanaka S, Matsuura M, Wato M, Hasui T, Inaba T. Risk factors for postgastric endoscopic submucosal dissection bleeding in direct oral anticoagulant users. Dig Endosc. 2024;36:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Chen L, Jiang J, Li H, Yin X, Tang X, Zhu Y, Chen W, Lu Q, Shi R. Efficacy of alternate mucosa-submucosa clip closure in preventing postoperative adverse events for patients with gastric mucosal lesions after endoscopic submucosal dissection: a multicenter retrospective study. Therap Adv Gastroenterol. 2025;18:17562848251317145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Cai RS, Yang WZ, Cui GR. Associate factors for endoscopic submucosal dissection operation time and postoperative delayed hemorrhage of early gastric cancer. World J Gastrointest Surg. 2023;15:94-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Nam HS, Choi CW, Kim SJ, Kim HW, Kang DH, Park SB, Ryu DG. Risk factors for delayed bleeding by onset time after endoscopic submucosal dissection for gastric neoplasm. Sci Rep. 2019;9:2674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S, Morikawa T, Murakami T, Tomoda J. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Higashiyama M, Oka S, Tanaka S, Sanomura Y, Imagawa H, Shishido T, Yoshida S, Chayama K. Risk factors for bleeding after endoscopic submucosal dissection of gastric epithelial neoplasm. Dig Endosc. 2011;23:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Hashimoto M, Hatta W, Tsuji Y, Yoshio T, Yabuuchi Y, Hoteya S, Doyama H, Nagami Y, Hikichi T, Kobayashi M, Morita Y, Sumiyoshi T, Iguchi M, Tomida H, Inoue T, Mikami T, Hasatani K, Nishikawa J, Matsumura T, Nebiki H, Nakamatsu D, Ohnita K, Suzuki H, Ueyama H, Hayashi Y, Sugimoto M, Fujishiro M, Masamune A, Ohira H; Collaborators. Rebleeding in patients with delayed bleeding after endoscopic submucosal dissection for early gastric cancer. Dig Endosc. 2021;33:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Okada K, Yamamoto Y, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Chino A, Tsuchida T, Fujisaki J, Nakajima A, Hoshino E, Igarashi M. Risk factors for delayed bleeding after endoscopic submucosal dissection for gastric neoplasm. Surg Endosc. 2011;25:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Sugimoto M, Hatta W, Tsuji Y, Yoshio T, Yabuuchi Y, Hoteya S, Doyama H, Nagami Y, Hikichi T, Kobayashi M, Morita Y, Sumiyoshi T, Iguchi M, Tomida H, Inoue T, Mikami T, Hasatani K, Nishikawa J, Matsumura T, Nebiki H, Nakamatsu D, Ohnita K, Suzuki H, Ueyama H, Hayashi Y, Murata M, Yamaguchi S, Michida T, Yada T, Asahina Y, Narasaka T, Kuribayashi S, Kiyotoki S, Mabe K, Fujishiro M, Masamune A, Kawai T. Risk Factors for Bleeding After Endoscopic Submucosal Dissection for Gastric Cancer in Elderly Patients Older Than 80 Years in Japan. Clin Transl Gastroenterol. 2021;12:e00404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Mukai S, Cho S, Kotachi T, Shimizu A, Matuura G, Nonaka M, Hamada T, Hirata K, Nakanishi T. Analysis of delayed bleeding after endoscopic submucosal dissection for gastric epithelial neoplasms. Gastroenterol Res Pract. 2012;2012:875323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Zhu Y, Ji M, Yuan L, Yuan J, Shen L. A risk prediction model for delayed bleeding after ESD for gastric precancerous lesions. Surg Endosc. 2024;38:3967-3975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Koh R, Hirasawa K, Yahara S, Oka H, Sugimori K, Morimoto M, Numata K, Kokawa A, Sasaki T, Nozawa A, Taguri M, Morita S, Maeda S, Tanaka K. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Matsumura T, Arai M, Maruoka D, Okimoto K, Minemura S, Ishigami H, Saito K, Nakagawa T, Katsuno T, Yokosuka O. Risk factors for early and delayed post-operative bleeding after endoscopic submucosal dissection of gastric neoplasms, including patients with continued use of antithrombotic agents. BMC Gastroenterol. 2014;14:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Nagami Y, Hatta W, Tsuji Y, Yoshio T, Kakushima N, Hoteya S, Tsuji S, Fukunaga S, Hikichi T, Kobayashi M, Morita Y, Sumiyoshi T, Iguchi M, Tomida H, Inoue T, Mikami T, Hasatani K, Nishikawa J, Matsumura T, Nebiki H, Nakamatsu D, Ohnita K, Suzuki H, Ueyama H, Hayashi Y, Yoshida H, Fujishiro M, Masamune A, Fujiwara Y. Antithrombotics increase bleeding after endoscopic submucosal dissection for gastric cancer: Nationwide propensity score analysis. Dig Endosc. 2022;34:974-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Ono S, Ieko M, Tanaka I, Shimoda Y, Ono M, Yamamoto K, Sakamoto N. Bleeding After Gastric Endoscopic Submucosal Dissection Focused on Management of Xa Inhibitors. J Gastric Cancer. 2022;22:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Oh SJ, Kim JW, Oh CH, Jang JY. Ideal Timing of Discontinuation of Antiplatelet Agents Before Gastric Endoscopic Submucosal Dissection for Reducing Delayed Bleeding. Dig Dis Sci. 2023;68:3365-3373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Ikeda R, Hirasawa K, Sato C, Ozeki Y, Sawada A, Nishio M, Fukuchi T, Kobayashi R, Makazu M, Taguri M, Maeda S. Third-look endoscopy prevents delayed bleeding after endoscopic submucosal dissection under antithrombotic therapy. World J Gastroenterol. 2020;26:6475-6487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kono Y, Hirata I, Katayama T, Uemura H, Hirata T, Gotoda T, Miyahara K, Moritou Y, Nakagawa M. Current evidence and issues of endoscopic submucosal dissection for gastric neoplasms during antithrombotic therapy. Clin J Gastroenterol. 2020;13:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Shiotsuki K, Takizawa K, Notsu A, Kakushima N, Kawata N, Yoshida M, Yabuuchi Y, Kishida Y, Ito S, Imai K, Ishiwatari H, Hotta K, Matsubayashi H, Ono H. Endoloop closure following gastric endoscopic submucosal dissection to prevent delayed bleeding in patients receiving antithrombotic therapy. Scand J Gastroenterol. 2021;56:1117-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Horie Y, Horiuchi Y, Ishiyama A, Tsuchida T, Yoshimizu S, Hirasawa T, Fujisaki J, Maetani I, Yoshio T. The effect of antithrombotic drug use on delayed bleeding with esophageal endoscopic resection. J Gastroenterol Hepatol. 2022;37:1792-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Jun Oh D, Jung Na H, Hyung Nam J, Jeong Lim Y, Hak Kim J. Could immediate second-look endoscopy reduce post-endoscopic submucosal dissection bleeding? Arab J Gastroenterol. 2023;24:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Yamamoto Y, Yahagi N, Yamamoto H, Ono H, Inoue H. Innovative therapeutic endoscopy in the upper gastrointestinal tract: Review of Japan Gastroenterological Endoscopic Society Core Sessions. Dig Endosc. 2020;32:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Yu Y, Hu T, Kuai X, Liu X, Li R, Zhou C. Propensity score matching analysis to evaluate efficacy of polyethylene oxide adhesive on preventing delayed bleeding after gastric endoscopic submucosal dissection. Sci Rep. 2022;12:4538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 353] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 32. | Goto O, Kaise M, Iwakiri K. What's New with Endoscopic Treatments for Early Gastric Cancer in the "Post-ESD Era"? Digestion. 2022;103:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Nonaka S, Oda I, Sato C, Abe S, Suzuki H, Yoshinaga S, Hokamura N, Igaki H, Tachimori Y, Taniguchi H, Kushima R, Saito Y. Endoscopic submucosal dissection for gastric tube cancer after esophagectomy. Gastrointest Endosc. 2014;79:260-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/