INTRODUCTION
In recent years, with advances in medical technology and growing public awareness of health, the diagnosis and treatment of rare diseases have attracted increasing attention. Pancreatic neuroendocrine tumors (PNETs), a rare type of tumor originating from the endocrine system, have exhibited a rising incidence over the past few decades, despite their overall low occurrence rate[1-3]. PNETs comprise well-differentiated neuroendocrine tumors and poorly differentiated neuroendocrine carcinomas[4], and are classified into functional and non-functional pancreatic neuroendocrine neoplasms based on hormonal activity[5]. Due to the heterogeneity in tumor type and differentiation[6], pancreatic neuroendocrine neoplasms often present with non-specific symptoms and highly variable clinical manifestations[7]. Treatment strategies for patients with PNETs are diverse, including surgical resection, chemotherapy, and radiotherapy. Among these, surgical resection is commonly regarded as one of the most effective therapeutic options[2,8-11]. However, for patients with resectable primary tumors but unresectable liver metastases (LM), treatment selection remains particularly complex. In clinical practice, there has been ongoing controversy regarding whether primary tumor resection (PTR) should be performed in such cases[12].
PTR’s role in treating PNETs has long been a focus of oncological research[13]. On the one hand, several studies have supported that surgical resection of the primary tumor can significantly improve patient survival, particularly in early-stage or locally resectable tumors[14]. On the other hand, the benefits of surgery become less clear in PNET patients with unresectable LM[15,16]. In such cases, systemic treatment becomes particularly critical, and the role and timing of PTR must be carefully evaluated[17]. Therefore, it is necessary to systematically synthesize and analyze existing research findings to provide more explicit guidance on this clinical dilemma.
As a statistical method, meta-analysis combines results from multiple studies and can offer more accurate and reliable conclusions[18,19]. When faced with conflicting evidence regarding the role of PTR in treating PNET patients with LM, meta-analysis allows for integrating current data to quantify treatment effects, thereby offering more scientifically grounded clinical recommendations[20]. Through meta-analysis, we evaluated and compared prognostic indicators, such as overall survival (OS) and tumor staging, between patients whose primary tumors were resectable but had unresectable LM and those who underwent PTR in order to further clarify the therapeutic value of surgery in this specific patient population.
As a key resource of the United States National Cancer Institute, the Surveillance, Epidemiology, and End Results (SEER) database has provided extensive population-based data for oncological research. The SEER database covers approximately one-third of the United States population and offers detailed information on cancer incidence, treatment methods, and survival outcomes[21,22]. Studies based on the SEER database have enabled researchers to understand better the epidemiological characteristics and treatment efficacy of various cancers. Its value is particularly evident in studying rare tumors such as PNETs[23,24]. By analyzing SEER data, researchers have investigated the prognostic impact of different treatment strategies on patients with unresectable LM, thereby providing stronger evidence to support clinical decision-making.
Therefore, this study aimed to comprehensively evaluate the prognostic value of PTR in patients with PNETs and unresectable LM through a synthesis of evidence from a systematic review, meta-analysis, and population-based SEER database analysis. By integrating current literature with large-scale real-world data, this work provides a more robust foundation for guiding treatment strategies in this challenging clinical setting. The findings may inform evidence-based surgical decision-making and contribute to improved prognostic outcomes and quality of life in this patient population.
MATERIALS AND METHODS
Search strategy
To evaluate the prognostic impact of PTR in patients with PNETs and unresectable LM, a dual-source data integration strategy was adopted, comprising a systematic review with meta-analysis and an independent analysis of the SEER database. The literature search was conducted in accordance with the PRISMA guidelines, covering five databases: PubMed, Web of Science, EMBASE, Cochrane Library, and CNKI, from inception to February 2025. The search strategy combined Medical Subject Headings (MeSH) or Emtree terms with relevant free-text keywords using Boolean operators (AND/OR) and wildcards. For example, the PubMed search string was: (“pancreatic neuroendocrine tumors”[MeSH] OR “PNETs”[Title/Abstract]) AND (“primary tumor resection”[MeSH] OR “pancreatectomy”[Title/Abstract]) AND (“unresectable liver metastases”[Title/Abstract] OR “liver metastasis”[MeSH]) AND (“prognosis”[MeSH] OR “overall survival”[Title/Abstract] OR “progression-free survival”[Title/Abstract]).
All search results were deduplicated using EndNote X9. Two independent reviewers screened titles and abstracts to exclude irrelevant studies, animal experiments, reviews, and non-English publications. Full-text articles were then assessed based on predefined inclusion criteria: Pathologically confirmed PNETs with unresectable LM and comparative analysis of PTR vs non-surgical management in relation to survival outcomes. Discrepancies were resolved by consensus or third-party adjudication. Additional sources, including references from eligible studies, conference proceedings, and unpublished data obtained by author contact, were reviewed to ensure data completeness.
The SEER database was analyzed separately and not included in the meta-analysis. Patients with pathologically confirmed PNETs and LM were identified, excluding those with missing surgical records or zero survival time. Data were extracted using SEER*Stat software, including demographic characteristics, tumor grade, surgical status, and survival outcomes. Survival analysis and subgroup comparisons were conducted using SPSS software. The SEER findings served to externally validate the meta-analysis results; no pooled statistical combination was performed between the two datasets.
Study selection and eligibility criteria
Studies were selected according to predefined inclusion and exclusion criteria. Eligible studies met the following requirements: (1) Included patients with pathologically confirmed PNETs and radiologically or intraoperatively confirmed unresectable LM, defined as those not amenable to R0 resection or local control; (2) Compared outcomes between PTR, with or without additional therapies, and non-surgical treatments such as systemic or supportive care; (3) Reported hazard ratios (HRs) for OS with 95% confidence intervals (CIs), or provided data from which these could be estimated; (4) Were designed as prospective or retrospective cohort studies, case-control studies, or clinical trials; and (5) Were peer-reviewed original articles published in English-language SCI-indexed journals.
Exclusion criteria were as follows: Case reports, reviews, conference abstracts, animal studies, and other non-original articles; studies that did not distinguish between PTR and non-PTR groups or lacked extractable survival data; studies including patients who received liver metastasis resection or liver-directed therapies (e.g., ablation, embolization); duplicate publications (only the most complete version was retained); and studies published in languages other than English. Two reviewers independently screened titles and abstracts using EndNote X9, followed by full-text review of potentially eligible articles. Disagreements were resolved through discussion or adjudication by a third reviewer. Reference lists of included studies were manually searched to identify additional eligible studies.
Risk of bias assessment and data extraction
Risk of bias in the included non-randomized studies was assessed using the ROBINS-I tool developed by the Cochrane Collaboration, which evaluates seven domains: Confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results. Two reviewers independently conducted the assessments, with discrepancies resolved by discussion or third-party adjudication. Summary judgments were visualized using Review Manager (RevMan) 5.3. Data extracted from each study included author, year, country, study design, and sample size; survival outcomes such as HRs for OS and PFS; and clinical characteristics including tumor grade, liver metastasis burden, primary tumor location, and combined treatments.
Meta-analysis
Meta-analysis was performed using Review Manager (RevMan) version 5.3, which was also used to generate forest plots and assess heterogeneity. Only studies identified through the PRISMA-based systematic review were included in the meta-analysis; data from the SEER database were analyzed separately without statistical pooling. Effect sizes were reported as HRs or ORs with 95%CIs. When HRs were not directly available, they were reconstructed from survival curves or Kaplan-Meier plots using the Guyton method. Heterogeneity was evaluated using the I2 statistic: A fixed-effects model was applied when I2 ≤ 50%, while a random-effects model (DerSimonian-Laird method) was used when I2 > 50%, indicating substantial heterogeneity. For example, in the OS analysis, I2 = 92.2%, suggesting considerable heterogeneity, and therefore a random-effects model was applied. To assess the robustness of the results, sensitivity analysis was conducted by sequentially omitting each study. Publication bias was examined using funnel plots combined with the trim-and-fill method. All statistical tests were two-sided, with a significance threshold set at P < 0.05.
SEER database-based analysis
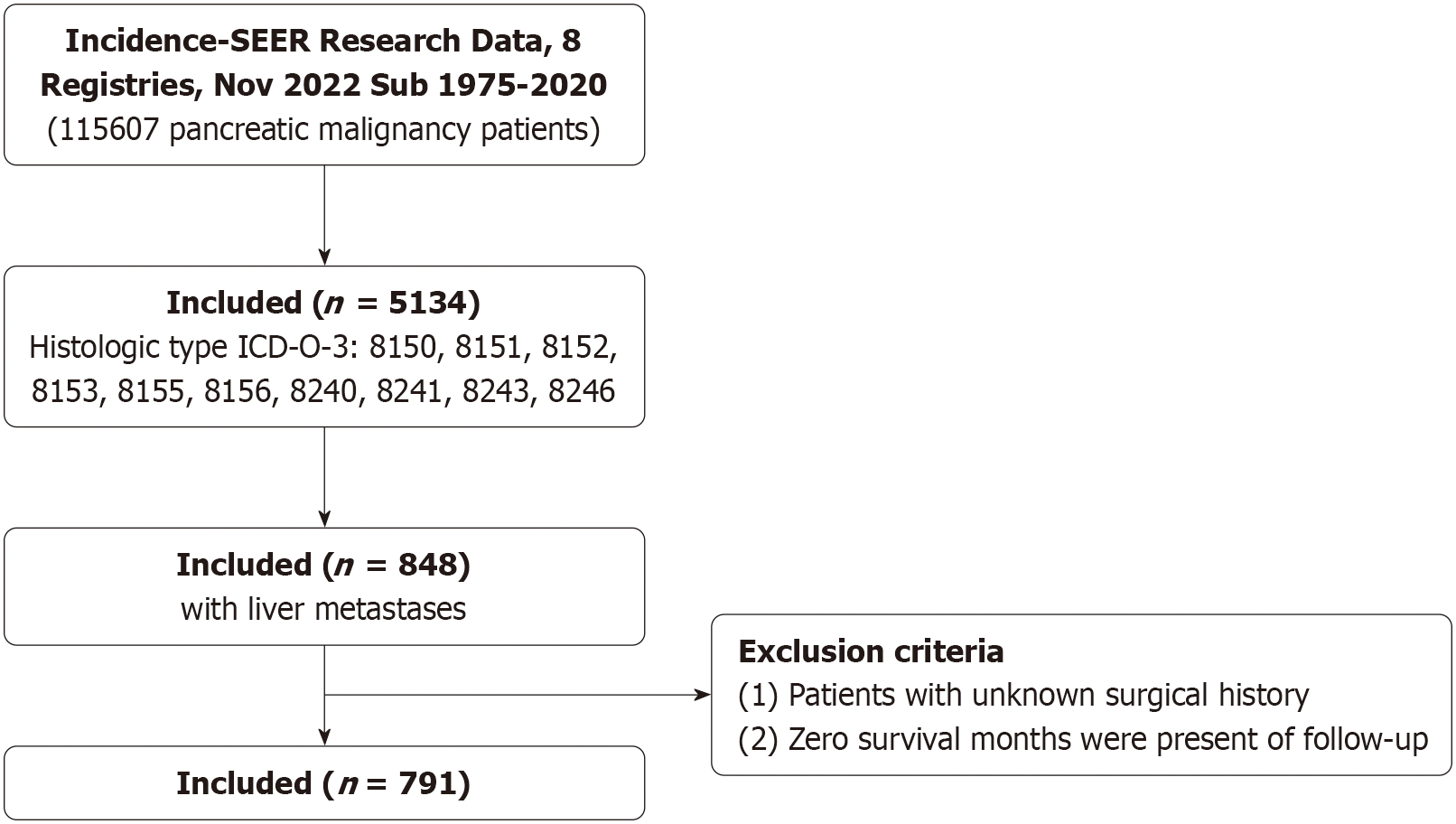
This study conducted an independent analysis of the SEER database, which served as an external validation for the findings of the PRISMA-based meta-analysis. The SEER and meta-analysis datasets were entirely separate, with no statistical pooling between them, and the two analyses were performed using distinct data sources and methodologies. Case information was collected from the SEER database (Incidence-SEER Research Data, 8 Registries, Nov 2022 Sub, 1975-2020) using SEER*Stat software (version 8.4.3; Cancer Statistics Branch, NCI, Bethesda, MD, United States). The inclusion criteria were as follows: (1) Site recode International Classification of Diseases for Oncology, third edition/World Health Organization 2008: “Pancreas”; (2) Histologic Type International Classification of Diseases for Oncology, third edition codes: Islet-cell adenocarcinoma (8150), malignant beta-cell tumor (8151), malignant alpha-cell tumor (8152), G-cell tumor (8153), VIPoma (8155), malignant somatostatinoma (8156), carcinoid tumors (8240), argentaffin carcinoid tumor (8241), mucocarcinoid tumor (8243), and neuroendocrine carcinoids (8246); and (3) Patients with LM. The exclusion criteria included: (1) Patients with unknown surgical history; and (2) Patients with a survival time of zero. The variables included in the analysis were sex, race, age, tumor grade, tumor site, year of diagnosis, surgery on the primary site, survival time, and vital status.
Statistical analysis
The χ2 test was used to compare differences in clinical characteristics between patients who underwent PTR and those who did not (non-resection). OS was analyzed using the Kaplan-Meier method with the log-rank test. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics version 22.0 (IBM, Chicago, IL, United States).
RESULTS
Study overview
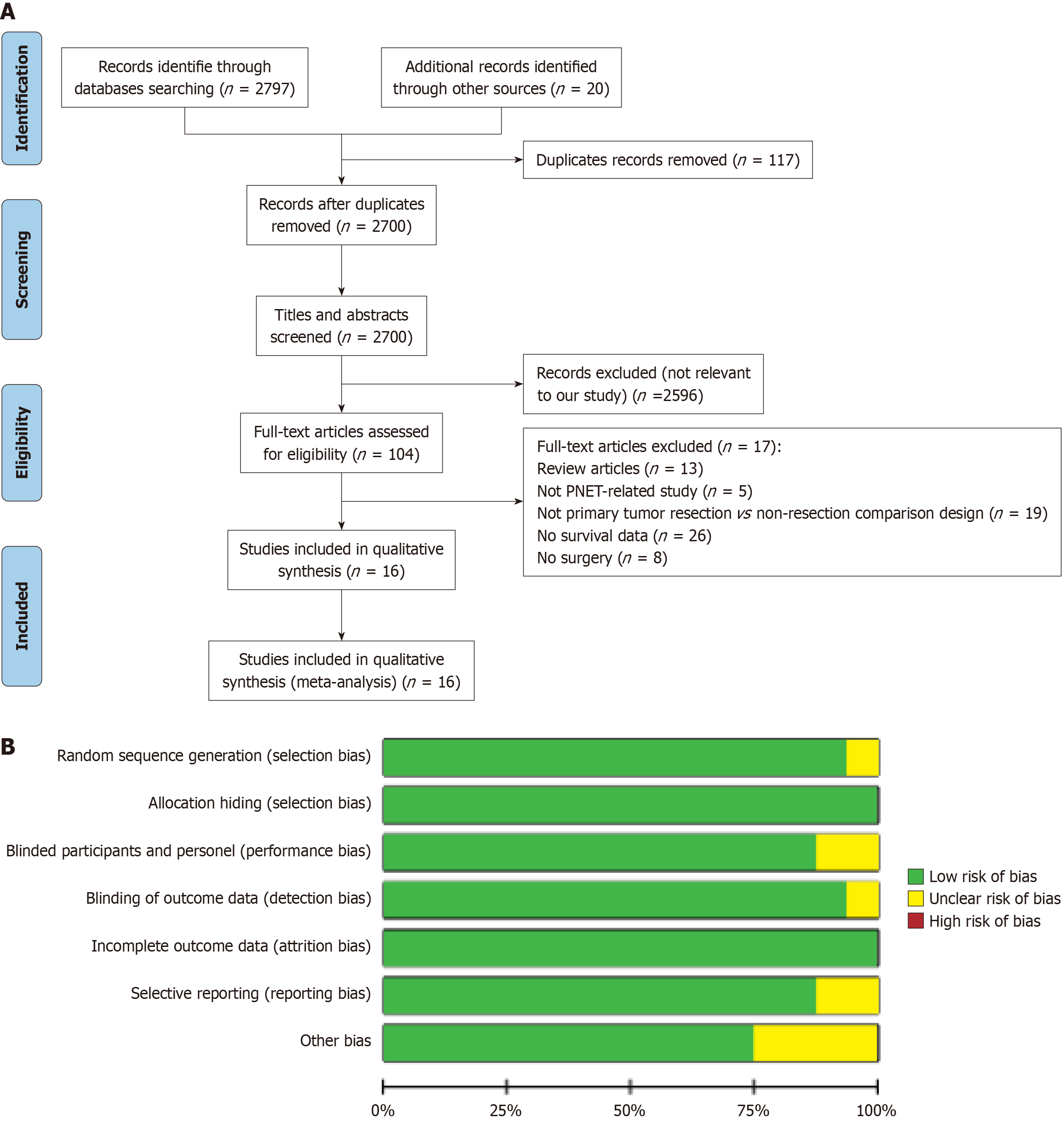
A systematic literature screening and evaluation were conducted in accordance with the PRISMA guidelines (Figure 1A) to assess the prognostic impact of PTR on OS and tumor grading in patients with PNETs and unresectable LM. A total of 2817 records were retrieved, with 117 duplicates removed. After title and abstract screening, 2596 studies were excluded as irrelevant. Full-text review was performed for 104 articles, and 16 studies met the inclusion criteria and were included in the qualitative and quantitative analyses[8,20,25-38]. Six of the 16 selected studies were conducted in the United States, two in Italy, and eight in China. One study was prospective, while the remaining fifteen were retrospective. A total of 8761 patients were included, comprising 3612 patients who underwent PTR and 5149 patients who received non-resection treatment. The essential characteristics of these studies are summarized in Supplementary Table 1.
Figure 1 Literature selection process and quality assessment of included studies.
A: Flow diagram of literature inclusion; B: Summary of risk-of-bias assessment for included studies. PNET: Pancreatic neuroendocrine tumor.
Risk of bias assessment
The methodological quality of the included studies was evaluated using the Cochrane risk-of-bias framework (Figure 1B). The ROBINS-I tool was applied to assess bias across seven domains: Classification of interventions, control of confounding, deviations from intended interventions, missing data, outcome measurement, selective reporting, and other sources of bias. Detailed assessments for each study are provided in Supplementary Table 2, supporting the overall reliability of the findings. Each potential bias, including random sequence generation, allocation concealment, blinding, and outcome assessment, was graded as low, high, or unclear risk. Studies with insufficient randomization or allocation concealment were classified as high risk of selection bias, while incomplete or missing information was marked as unclear. Studies with adequate randomization, concealment, and blinding were considered at low risk of bias. This systematic evaluation ensured that all included studies underwent a rigorous and balanced quality assessment.
PTR significantly improved OS in PNET patients
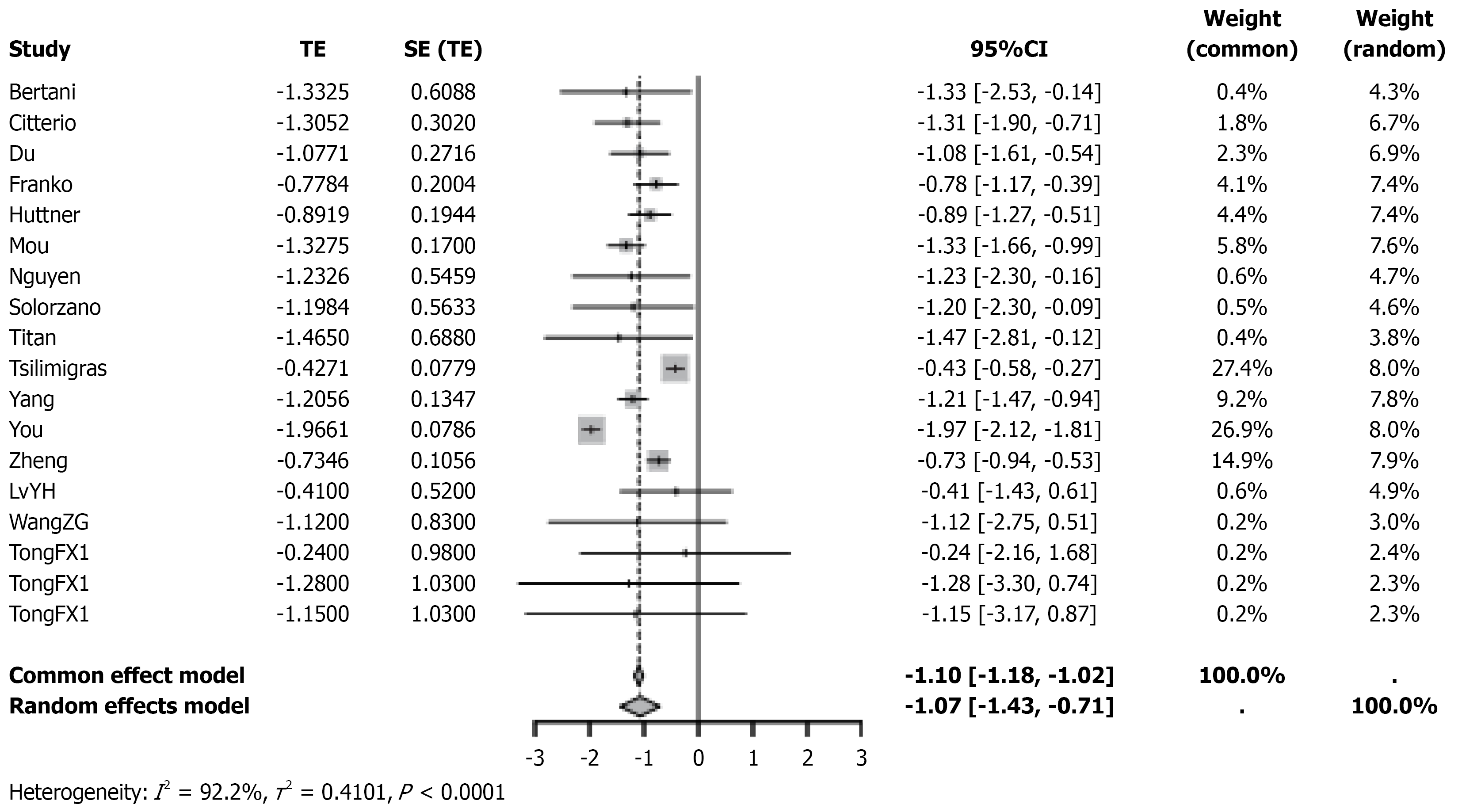
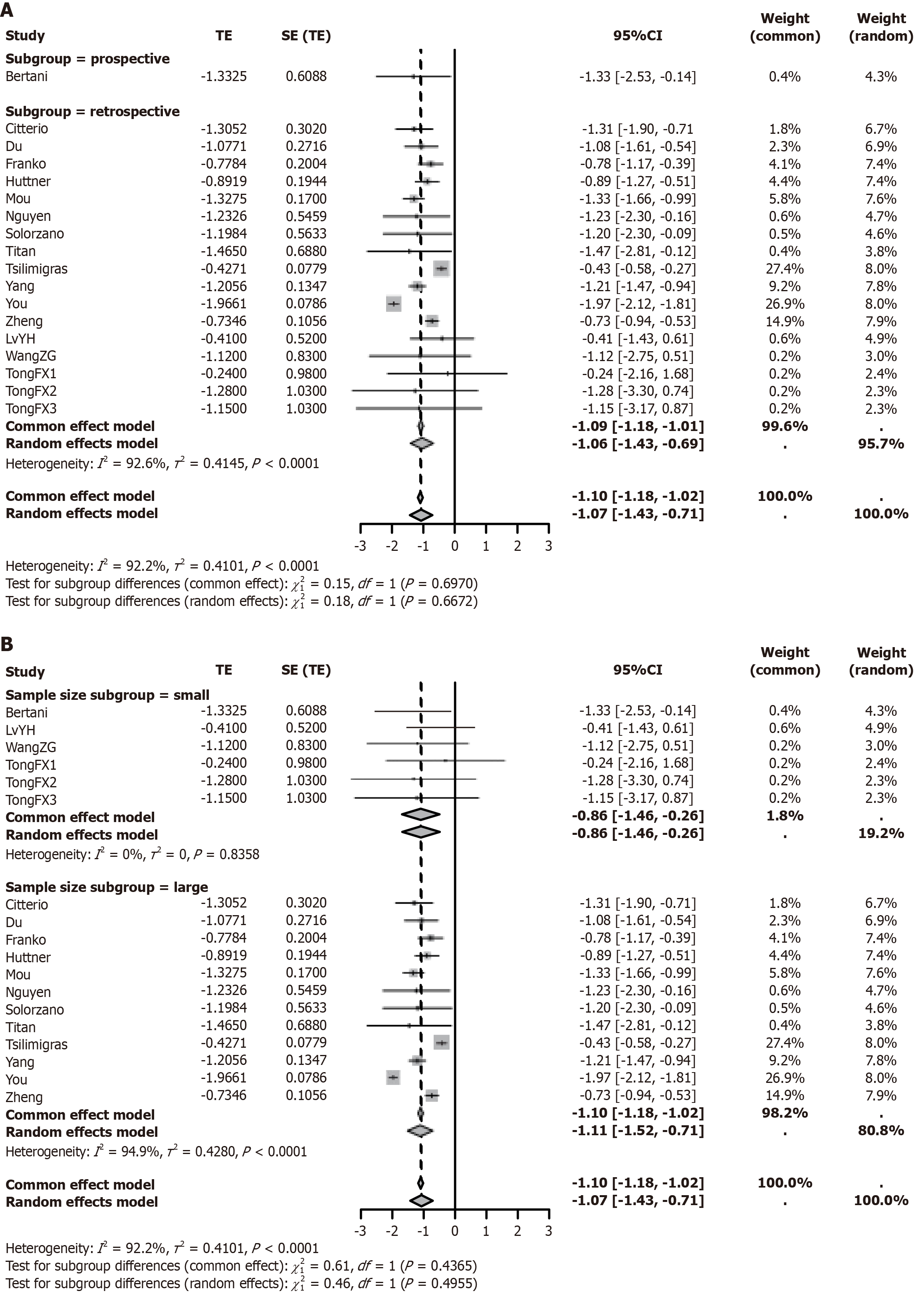
A meta-analysis was performed to assess the effect of PTR on OS in patients with resectable PNETs and unresectable LM. Significant heterogeneity was observed across studies (I2 = 92.2%), and a random-effects model was applied. The pooled effect estimate was total effect (TE) = -1.10 (95%CI: -1.43 to -0.71, P < 0.0001), with all 16 studies showing consistent directional trends (Figure 2). Subgroup analyses showed no significant difference in effect size between prospective (TE = -1.10, 95%CI: -1.18 to -1.02, I2 = 92.2%) and retrospective studies (TE = -1.07, 95%CI: -1.18 to -1.01, I2 = 92.6%) (interaction P = 0.6672; Figure 3A). Similarly, findings were consistent across studies with smaller (< 400; TE = -0.86, 95%CI: -1.46 to -0.26,I² = 0%) and larger sample sizes (≥ 400; TE = -1.11, 95%CI: -1.52 to -0.71, I2 = 94.9%; interaction P = 0.4955; Figure 3B). Sensitivity analysis by sequential exclusion of individual studies demonstrated robustness of the findings (pooled TE range: -1.18 to -0.87; all 95%CIs excluding 0; I2 = 86.2%-92.6%; Supplementary Figure 1). Funnel plot symmetry and Egger’s test (P = 0.153) did not suggest significant publication bias (Supplementary Figure 2).
Figure 2 Forest plot of hazard ratios for survival comparing primary tumor resection with non-resection treatment in patients with pancreatic neuroendocrine tumor.
TE: Total effect; SE: Standard error; CI: Confidence interval.
Figure 3 Subgroup analysis of overall survival in pancreatic neuroendocrine tumor patients undergoing primary tumor resection vs non-resection treatment.
A: Impact of different study designs (prospective vs retrospective) on overall survival following primary tumor resection vs non-resection treatment; B: Impact of different sample sizes (< 400 vs ≥ 400) on overall survival following primary tumor resection vs non-resection treatment. TE: Total effect; SE: Standard error; CI: Confidence interval.
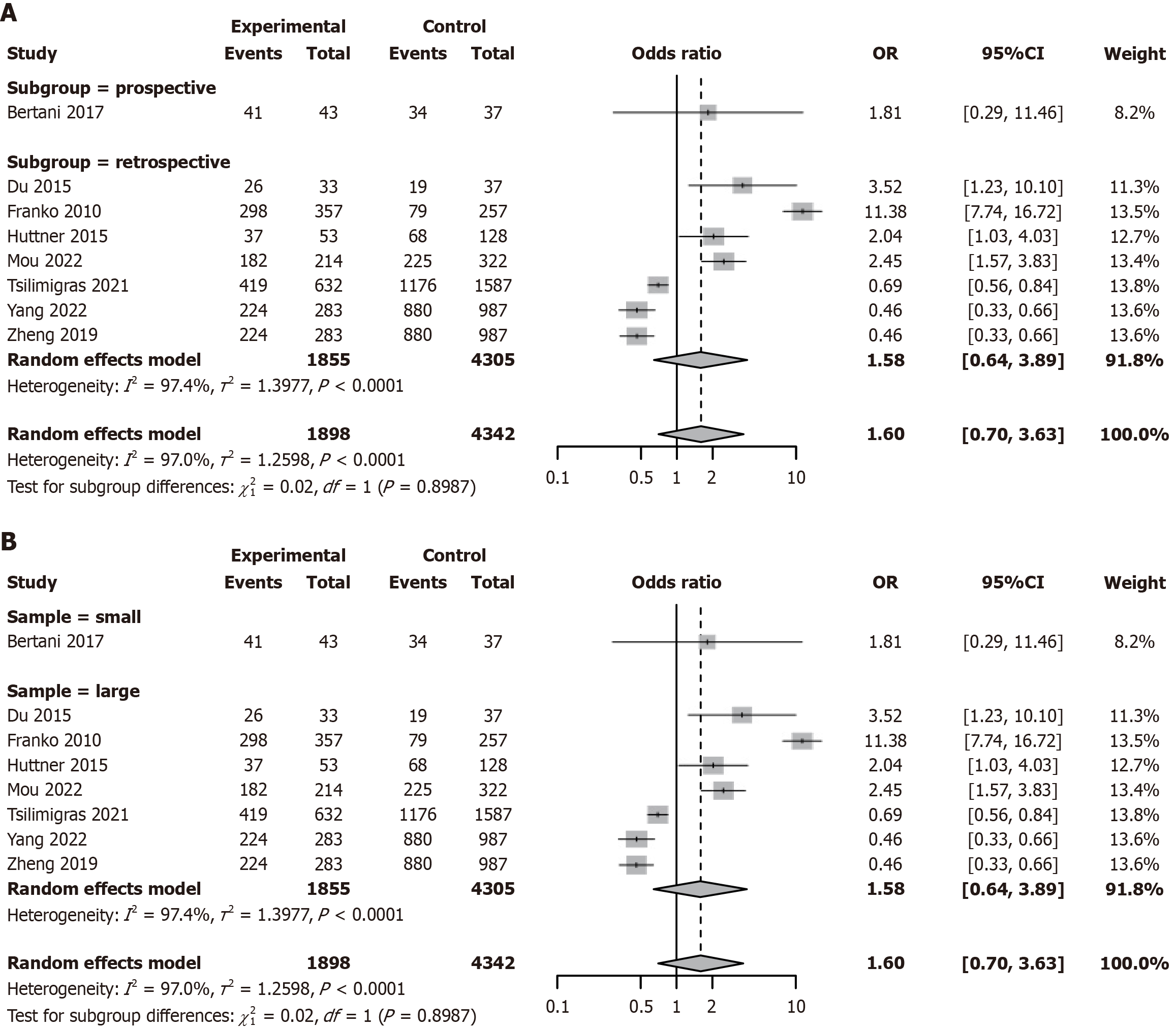
PTR did not affect tumor grading in PNET patients
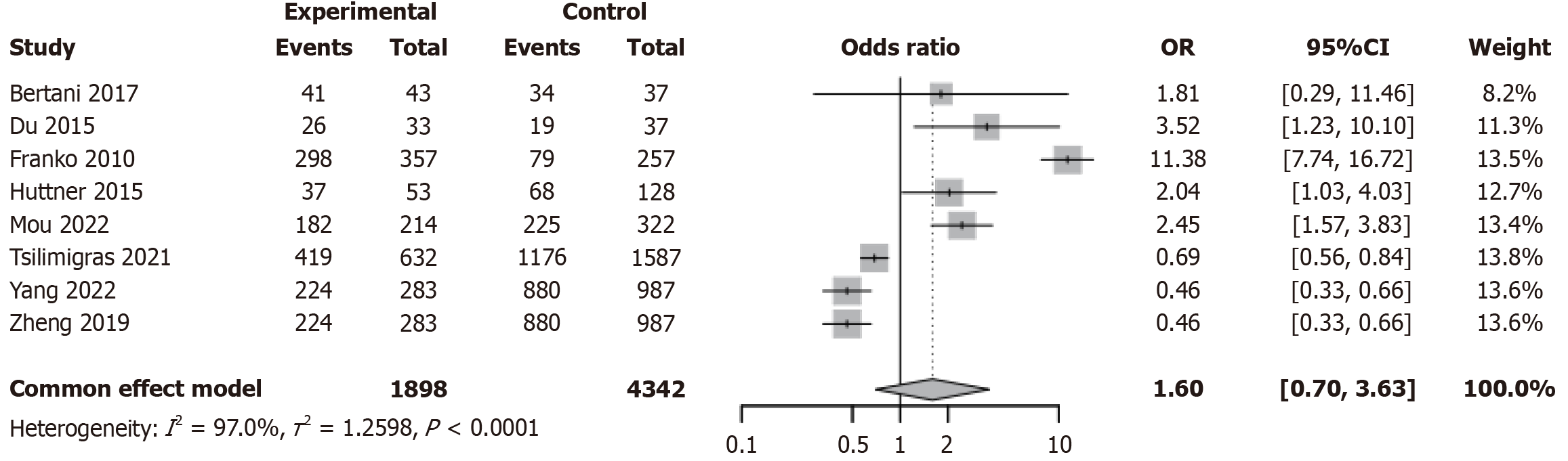
Studies have indicated that patients undergoing surgical treatment may be more likely to present with advanced disease or a high tumor burden[39,40]. We further compared the tumor grading between PTR and non-resection groups in patients with PNETs. A meta-analysis was conducted to evaluate the impact of PTR vs non-resection on tumor grade (G1/G2 vs G3/G4) in patients with PNETs. Substantial heterogeneity was observed across studies (I2 = 97.0%, τ2 = 1.2598, P < 0.0001; Figure 4). The pooled OR was 1.60 (95%CI: 0.70-3.63, P = 0.26), indicating no statistically significant association. Although certain studies, such as Franko et al[28] 2010 [odds ratio (OR) = 11.38, 95%CI: 7.74-16.72] and Mou et al[20] 2022 (OR = 2.04, 95%CI: 1.03-4.03), reported a higher proportion of high-grade tumors in the surgical group, the overall direction of effect was inconsistent (Figure 4). Subgroup analysis showed no significant difference in effect size between prospective (OR = 1.60, 95%CI: 0.70-3.63, I2 = 97.0%) and retrospective studies (OR = 1.58, 95%CI: 0.64-3.89, I2 = 97.4%; interaction P = 0.8987; Figure 5A). Similarly, results were consistent across smaller (< 400) and larger (≥ 400) studies, with no significant interaction effect (interaction P = 0.8987; Figure 5B). Sensitivity analysis demonstrated robustness, with pooled ORs ranging from 1.58 to 1.62 and all 95%CIs crossing 1; heterogeneity remained stable (I2 = 97.0%-97.4%; Supplementary Figure 3). Funnel plot symmetry and Egger’s test (P = 0.213) did not indicate significant publication bias (Supplementary Figure 4).
Figure 4 Forest plot from meta-analysis comparing tumor grading between primary tumor resection and non-resection treatment groups in patients with pancreatic neuroendocrine tumor.
OR: Odds ratio; CI: Confidence interval.
Figure 5 Subgroup analysis of tumor grading in patients with pancreatic neuroendocrine tumor undergoing primary tumor resection vs non-resection treatment.
A: Subgroup analysis by study design (prospective vs retrospective); B: Subgroup analysis by sample size (< 400 vs ≥ 400). OR: Odds ratio; CI: Confidence interval.
PTR and survival outcomes in PNETs: Insights from the SEER database
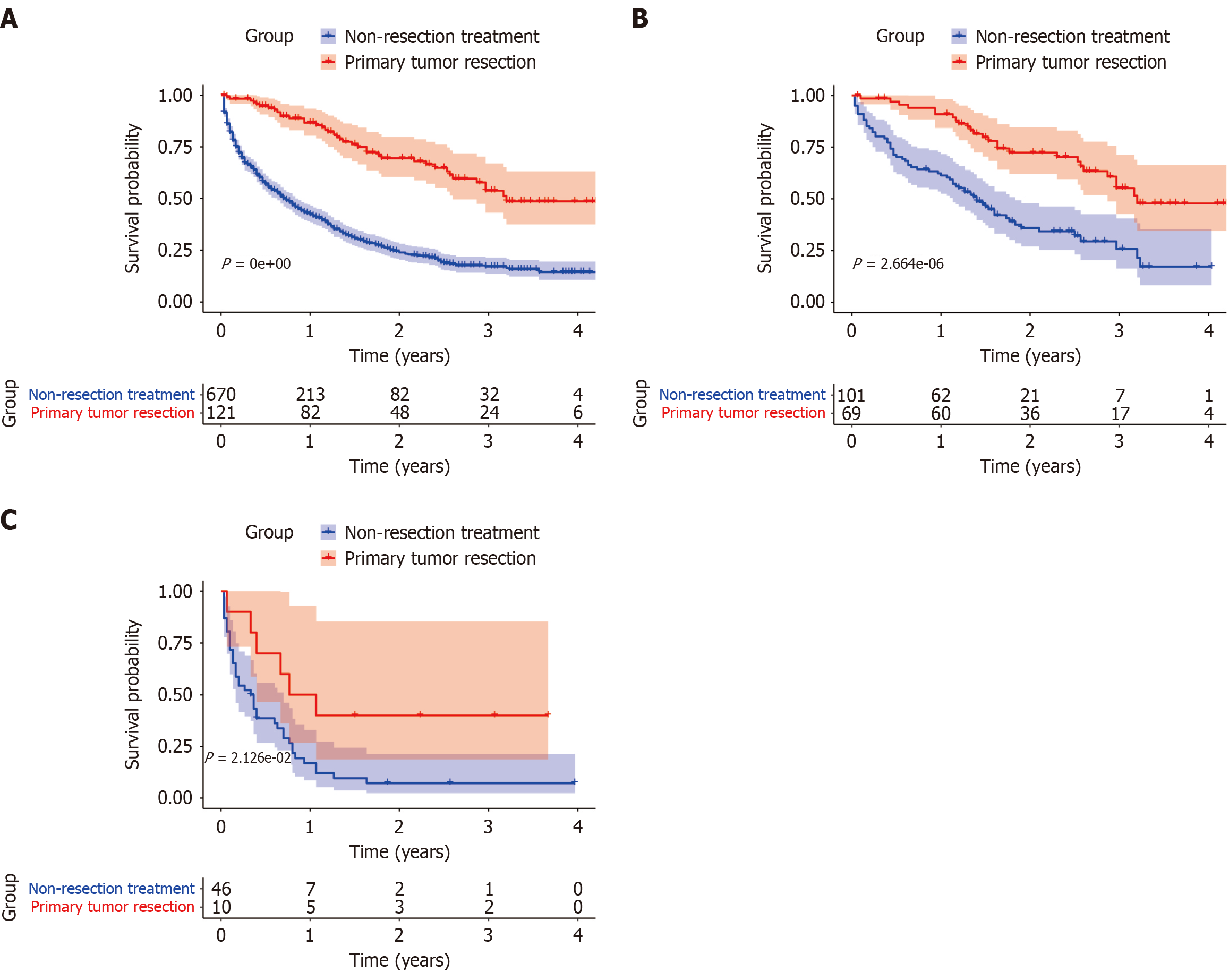
The SEER program, established in 1973, provides comprehensive population-level data on rare tumors. A total of 791 patients diagnosed with PNETs and LM were identified for this analysis (Figure 6). Baseline characteristics are summarized in Supplementary Table 3. The cohort included 465 males and 326 females, with a median age of 64 years. Among them, 121 patients underwent PTR, while 670 received non-resection treatment. Compared with the non-resection group, the PTR group had a higher proportion of patients aged < 65 years (65.3% vs 53.1%, P = 0.013), White ethnicity (89.2% vs 78.7%, P = 0.02), tumors located in the pancreatic body or tail (64.5% vs 44.8%, P < 0.001), and G1 tumors (42.1% vs 11.0%, P < 0.001). Sex distribution did not differ significantly between groups. Kaplan-Meier analysis showed significantly improved OS in the PTR group compared to the non-resection group (Figure 7A). Stratified analyses by tumor differentiation revealed that PTR was associated with prolonged OS in both well-differentiated (G1/G2) and poorly/undifferentiated (G3/G4) subgroups (Figure 7B and C).
Figure 6 Study design with inclusion and exclusion criteria.
SEER: Surveillance, Epidemiology, and End Results; ICD-O-3: International Classification of Diseases for Oncology, third edition.
Figure 7 Survival analysis of primary tumor resection based on Surveillance, Epidemiology, and End Results database in patients with pancreatic neuroendocrine tumor.
A: Impact of primary tumor resection (PTR) on survival in patients with pancreatic neuroendocrine tumor (PNET); B: Impact of PTR on survival in patients with well-differentiated PNET; C: Impact of PTR on survival in patients with poorly/undifferentiated PNET.
Heterogeneity and interpretation
Substantial heterogeneity was observed in both OS (I2 = 92.2%) and tumor grade analyses (I2 = 97.0%). Potential contributors include variability in baseline patient characteristics (e.g., age, liver tumor burden, comorbidities), treatment strategies (e.g., timing and use of chemotherapy or targeted agents), and study design (15 retrospective vs 1 prospective study), as well as differences in surgical indication criteria. Despite this, sensitivity analyses demonstrated consistent directionality of effect estimates (OS: TE range -1.18 to -0.87; tumor grade: OR range 1.58-1.62). Subgroup analyses showed that study design, sample size, and regional distribution (50% from China) did not materially alter the findings (all interaction P > 0.49). These results suggest that heterogeneity may affect the precision of effect estimates rather than the overall direction of association. Standardized definitions of unresectable LM, harmonized reporting of multimodal therapy, and future prospective multicenter studies are warranted to improve evidence consistency.
DISCUSSION
The treatment and prognosis of PNETs have been a major focus of medical research. This is particularly true for patients with unresectable PNETs who also have LM, where the role and value of PTR have attracted significant attention. This study, through systematic review and meta-analysis combined with SEER database analysis, comprehensively evaluated the prognostic impact of PTR in this specific patient population, offering new perspectives and evidence.
According to the European Neuroendocrine Tumor Society’s guidelines for PNET, PTR is not recommended for PNETs with metastases[41]. The guidelines only recommend palliative resection for patients with low-risk life-threatening conditions related to PNET[42]. However, our meta-analysis suggests that clinicians may consider PTR for patients with unresectable LM from PNET. Recent studies on synchronous metastatic PNETs support the positive role of PTR in improving OS[14,20,43,44]. Moreover, in elderly PNET patients, the effect of PTR on improving OS appears to be more pronounced, particularly for those over 80 years of age and those aged 70 and older with non-functional PNETs smaller than 2 cm[45,46]. For low-grade PNETs, PTR affects OS positively[47]. Another study showed that chemotherapy and tumors located in the tail of the pancreas might be independent positive prognostic factors, suggesting that the effect of PTR on improving OS should be considered independently of other treatments, such as chemotherapy[48].
The observed survival benefit associated with PTR in this study may be driven by multiple, synergistic biological mechanisms. Previous studies have shown that PTR can reduce circulating tumor cell burden and suppress the release of pro-metastatic factors, thereby delaying systemic disease progression and prolonging survival in patients with advanced PNETs[49,50]. In addition, PTR may alleviate the immunosuppressive tumor microenvironment associated with the primary lesion, restoring host immune surveillance, although this mechanism requires further validation through translational research. PTR can also relieve local complications caused by the primary tumor, such as biliary obstruction, bleeding, or mass effect, which may improve the patient’s general condition and increase tolerance and compliance with systemic therapies[26]. Furthermore, emerging evidence suggests that PTR may enhance the efficacy of targeted therapies, such as PRRT, by improving drug delivery, altering the tumor microenvironment, or modulating somatostatin receptor expression[35]. Taken together, PTR may exert a synergistic effect with modern systemic therapies and provide a survival advantage even in patients with poorly or undifferentiated (G3/G4) tumors. Prospective mechanistic studies are still needed to further clarify the underlying biological pathways and optimal timing for surgical intervention.
The findings of this study support the potential survival benefit of PTR in selected patients with unresectable LM from PNETs, particularly those who are younger than 65 years, of White ethnicity, with tumors located in the pancreatic body or tail, and with well-differentiated histology (G1/G2). Notably, a survival benefit was also observed in patients with poorly or undifferentiated tumors (G3/G4), suggesting that surgical indications may be broader than currently recommended by existing guidelines. In clinical practice, a multidisciplinary team approach is recommended to evaluate factors such as patient age, performance status, tumor grade, Ki-67 index, growth kinetics, extent of LM, and response to systemic treatment in order to identify those most likely to benefit from PTR. For example, patients with limited hepatic disease (e.g., fewer than five metastases and < 30% liver involvement) and tumors located in the body or tail of the pancreas may experience improved outcomes from PTR through reduction of tumor burden, alleviation of local symptoms, and potential immune activation, even in the presence of unresectable LM.
Moreover, a synergistic effect between PTR and novel systemic therapies (e.g., everolimus, 177 Lu-Dotatate) warrants attention. Neoadjuvant treatment may help downstage tumors and reduce surgical risk, while adjuvant systemic therapy may enhance long-term efficacy. Interestingly, the study found particularly pronounced survival benefits among elderly patients, which may reflect careful surgical selection and favorable tumor biology. These findings suggest that chronological age alone should not be considered an absolute contraindication to surgery and underscore the importance of comprehensive geriatric assessment for individualized decision-making. This study provides preliminary evidence for expanding surgical indications and highlights the critical role of precise patient stratification in optimizing treatment strategies for PNETs. Future multicenter prospective studies are needed to validate the ideal timing for intervention and define postoperative management pathways to maximize survival outcomes while ensuring patient safety.
Although this study provides important evidence on the potential survival benefit of PTR in patients with PNETs and unresectable LM, several limitations should be considered. First, all included studies were observational (mostly retrospective), with inherent risks of selection bias and residual confounding. Patients undergoing surgery were likely to be younger, with lower tumor burden and better performance status, which may have led to overestimation of the benefit. Second, substantial heterogeneity was observed (I2 = 92.2%), likely due to inconsistent definitions of unresectable LM, variations in treatment strategies (e.g., timing and use of chemotherapy or targeted therapy), and differences in follow-up intervals. Many studies did not clearly report systemic treatment details, limiting attribution of survival outcomes. Although funnel plots and Egger’s test showed no significant publication bias, underreporting of negative or small-sample studies cannot be ruled out. Moreover, most analyses focused solely on OS, without data on recurrence patterns, postoperative recovery, or quality of life. The absence of individual patient data restricted further stratification by metastasis load, tumor function, or surgical details. The SEER database, while population-based, lacks variables such as treatment adherence and complications, and its United States-based population may limit applicability to other regions.
Future research should focus on prospective, multicenter studies using standardized criteria for unresectability and comprehensive data collection. Advanced statistical methods such as propensity score matching and multivariable regression models are essential to reduce confounding. Moreover, integration of molecular profiling, radiomics, and circulating biomarkers (e.g., circulating tumor DNA) could enable the development of predictive models for identifying patients most likely to benefit from PTR, thereby supporting personalized treatment strategies with greater clinical relevance.