Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.107831
Revised: May 11, 2025
Accepted: July 11, 2025
Published online: September 27, 2025
Processing time: 179 Days and 14.6 Hours
The treatment of locally advanced rectal cancer (LARC) has evolved significantly over the past century, driven by a deeper understanding of tumor biology, tech
Core Tip: The management of locally advanced rectal cancer has undergone major advancements, shifting toward more personalized and less invasive strategies. This review explores key innovations, including the watch and wait approach for clinical complete responders, the total neoadjuvant therapy paradigm, and the expansion of minimally invasive surgical techniques. Additionally, targeted therapies and immunotherapy are emerging as promising options. These advancements aim to improve survival while preserving organ function and minimizing morbidity. Future research should focus on optimizing patient selection and long-term outcomes, ensuring that novel therapeutic strategies continue to enhance cancer care.
- Citation: García-Fernández A, del Pozo-Elso P, Villadóniga-Sánchez A, Martínez R, Suárez M. Watch and wait in locally advanced rectal cancer: Evolution, current evidence, and future directions. World J Gastrointest Surg 2025; 17(9): 107831
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/107831.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.107831
Colorectal cancer (CRC) ranks among the most prevalent non-cutaneous neoplastic diseases, exerting a significant impact on both life expectancy and quality of life. Globally, it constitutes a substantial proportion of cancer-related mortality. While historically treated as a single entity, colon cancer (CC) and rectal cancer (RC) are now recognized as distinct conditions due to differences in their anatomy, physiology, biology, and therapeutic approaches. RC’s fixed anatomical position within the pelvis facilitates access through endoscopy and radiotherapy (RT). However, its proximity to critical structures, including the bladder, vagina, prostate, pelvic nerve plexuses, and anus, increases the risk of direct in
The standard treatment for locally advanced RC (LARC) includes radical surgery with total mesorectal excision (TME) and adequate resection margins. Neoadjuvant therapy, comprising RT and chemotherapy (CT), is recommended in the presence of high-risk features, such as lymph node involvement or deep tissue invasion. In cases with poor histological features, adjuvant CT may also be indicated. Despite general consensus on this approach, variability persists, particularly regarding neoadjuvant and adjuvant treatment protocols. Preoperative RT has demonstrated benefits, including improved surgical margins and enhanced survival, with better tolerance compared to postoperative RT. Notably, a significant subset of patients achieves complete tumor regression following neoadjuvant therapy, prompting discussions about the feasibility of managing RC without the need of surgery or, as it is known, organ-preserving therapy (OPT). These findings have catalyzed the development of alternative strategies, such as watch and wait (WW) and minimally invasive conservative surgeries. Studies indicate that up to 30% of patients achieve complete pathological response[1,2], suggesting the potential for overtreatment in certain cases.
While these innovative approaches have yielded promising oncological outcomes in selected patients, their adoption requires caution. Variability in treatment definitions and protocols complicates standardization and limits the ability to make definitive recommendations. As patient populations age and preferences increasingly shift towards prioritizing quality of life, the emphasis on less invasive treatments with reduced long-term sequelae grows. Strategies like WW, despite their limitations, offer substantial quality-of-life benefits and merit thoughtful integration into future treatment protocols. Continued clinical trials and rigorous research are essential to delineate their role in standard practice.
For this review, we conducted a literature search using PubMed/MEDLINE and Embase databases, applying keywords such as “rectal cancer”, “total mesorectal excision”, “watch and wait”, “non-operative treatment”, “total neoadjuvant therapy”, “transanal surgery”, and “clinical complete response”. We prioritized original studies published around the time of each historical milestone discussed, as well as systematic reviews and meta-analyses published between 2018 and 2025 according to its overall relevance.
CRC is a commonly used term to refer to all adenocarcinomas located within the intestinal segment between the Bauhin valve and the anus, that is, within the large intestine[3]. Although it has historically been widely used and continues to be in use today, the terminological unification of what are, in fact, distinct diseases originating in different organs has led to the development of a series of diagnostic and therapeutic protocols that are now being questioned or abandoned. This simplification originates from the perception of the large intestine as a single organ divided into segments of purely anatomical interest, with a common histological structure and homogeneous functions distributed throughout its length. According to this concept, all adenocarcinomas located in the large intestine, that is, the colon and rectum, are uniformly classified and treated as CRC. This overly simplified approach has shaped the way these diseases are diagnosed and managed.
One of the first challenges encountered when studying RC is the difficulty in finding homogeneous data in the literature that allow for a consistent analysis of its epidemiology over the years. While recent trends have increasingly distinguished RC from CC, this differentiation has not been universally adopted at the same pace across all countries and scientific societies, it is still common to find references to the incidence of CRC without distinguishing between its distinct components[4]. Today, however, it is well established that tumors of the rectum and colon, and even those in different segments of the colon, have distinct prevalence and incidence rates. Recently published data report an annual incidence of 732000 new cases of RC, accounting for 3.8% of all newly diagnosed cancers worldwide. Separately, CC has been reported to have 1148000 new cases annually, representing 6.0% of all global cancer diagnoses. Combined, these figures rank CRC as the third most common malignancy worldwide, surpassed only by breast and lung cancer[5]. Comparing both incidence rates, one could conclude that the risk of developing CC vs RC worldwide, regardless of geographic origin or sex, is 1.5/1. However, this ratio changes drastically when adjusted for mucosal surface area, with previously reported estimates suggesting a relative risk per centimeter of at least one-fourth for colon vs rectum[6]. These data suggest that the differences between the colon and rectum are far more profound and complex than mere anatomical location. This becomes even more evident when analyzing how the incidence of both diseases has evolved in certain regions over the years. Specifically, a decline has been observed in the incidence of neoplasms in the distal colon (beyond the hepatic flexure) and the rectum. In contrast, the incidence in the proximal colon has remained stable or even increased, according to various studies conducted[6-9] in different regions[10,11]. Furthermore, various epidemiological studies have evaluated the main modifiable risk factors for CRC, revealing that identified preventive measures affect the risk of developing CC and RC differently[12-16]. The most well-documented example is physical activity, which has a dose-dependent effect in reducing the risk of CC but not RC[13,17-19]. The influence of other risk factors, such as sex, age, caloric intake, or body mass index, also varies, sometimes significantly, depending on different segments of the large intestine[20-23].
There are multiple arguments supporting the distinction between CC and RC. One of the most compelling is the different embryological origins of the proximal and distal colon, as well as the rectum. Using the splenic flexure as an approximate dividing point, the proximal colon, along with the distal duodenum, jejunum, and ileum, originates from the midgut (mesenteron). During the 6th to 10th week of development, it forms as a herniation of the endodermal yolk sac through the primitive umbilical ring. In contrast, the distal colon, rectum, and the upper third of the anal canal derive from the hindgut (proctodeum). Its most distal segment forms the urorectal cloaca, which is subsequently divided by the urorectal septum into an anterior portion, the urogenital sinus, and a posterior portion, the rectum and anus[24-27].
Anatomically, the colon and rectum are clearly distinct. The rectum, approximately 13-18 cm long, extends from the confluence of the taeniae coli to the anorectal ring[6]. However, these boundaries are not clearly defined. Though its limits are debated, modern definitions often rely on the “sigmoid take-off”, where the mesosigmoid transitions to the mesorectum[28,29]. Distally, the transition between the rectum and the anus is marked by the anorectal ring, the point where the puborectalis muscle merges with the external anal sphincter. The rectum lies within the pelvis and is divided into upper, middle, and lower thirds, each with unique relationships to the peritoneum and surrounding structures. The upper third is partially intraperitoneal, the middle third varies depending on the anterior peritoneal reflection, and the lower third is always extraperitoneal. These anatomical relationships, especially those involving the mesorectum, are key in surgical planning. The mesorectum is enclosed by the perirectal fascia[30], a critical surgical plane, which is dorsally fused to the presacral fascia, laterally continuous with the pararectal spaces and lateral rectal ligaments[31] and anteriorly related to Denonvilliers’ fascia[32]. Anteriorly, the rectum is adjacent to the prostate and seminal vesicles in males and to the vagina in females.
Rectal innervation involves sympathetic fibers from the aortic and superior hypogastric plexuses via the hypogastric nerves, and parasympathetic fibers from sacral nerves S3-S4. These nerves converge in the pelvic plexus, located on the lateral pelvic wall. From there, rectal nerves radiate toward the rectum, entering via the lateral rectal ligaments. The plexus also innervates nearby pelvic organs including the bladder, prostate, uterus, and vagina[33]. Arterial supply comes from the superior rectal artery (from the inferior mesenteric artery), the middle rectal arteries (from the internal iliac), and the inferior rectal arteries (from the internal pudendal), all of which form anastomoses in the submucosa. Venous drainage involves both portal (superior rectal vein) and systemic (middle and inferior rectal veins) pathways, connected via submucosal and extramuscular venous plexuses. Lymphatic drainage above the dentate line begins in perirectal nodes within the mesorectum and progresses to inferior mesenteric lymph nodes[34].
As a group of highly prevalent neoplasms, CC and RC have been extensively studied from histological, molecular, and genetic perspectives. When analyzing the distribution of these characteristics, a clear distinction emerges between tumors located in the proximal colon and those found in the distal colon and rectum. For example, tumors of the proximal colon tend to exhibit histological types with a poorer prognosis more frequently, such as mucinous adenocarcinoma or signet-ring cell carcinoma, in up to 45% of cases[35]. These tumors are also more likely to present certain genetic instability profiles, such as microsatellite instability (MSI), which can be either hereditary (Lynch syndrome) or sporadic, as well as the CpG island methylator phenotype. The incidence of B-Raf proto-oncogene mutations and MutL homolog 1 hypermethylation is also significantly higher[36].
In the rectum and distal colon beyond the splenic flexure, serrated adenocarcinomas are more commonly found, often associated with chromosomal instability, amplified human epidermal growth factor receptors 1 and 2, and epidermal growth factor receptor signaling. On the other hand, MSI is exceptional, as are B-Raf proto-oncogene mutations[37]. While hereditary non-polyposis CRC predominantly affects the proximal colon, familial adenomatous polyposis favors the development of tumors in the distal colon and rectum.
Another aspect where CC and RC (and distal colon) behave differently is in terms of prognosis and metastatic spread. Rectal neoplasms more frequently metastasize to extra-abdominal organs compared to proximal colon lesions, which have a higher preference for peritoneal dissemination. While CC has a better prognosis in the early stages, locally advanced and metastatic RC shows a better prognosis than proximal colon lesions, except for tumors in the proximal colon with MSI[37-39]. The differences between various epigenetic profiles are directly related to tumor aggressiveness and, above all, to the response to chemotherapeutic and immunotherapeutic agents used as adjuvant treatments. The fact that both RC and CC, whether locally advanced or metastatic, receive similar adjuvant therapies despite having different molecular profiles, may significantly contribute to explaining the documented variations in prognosis[38,40].
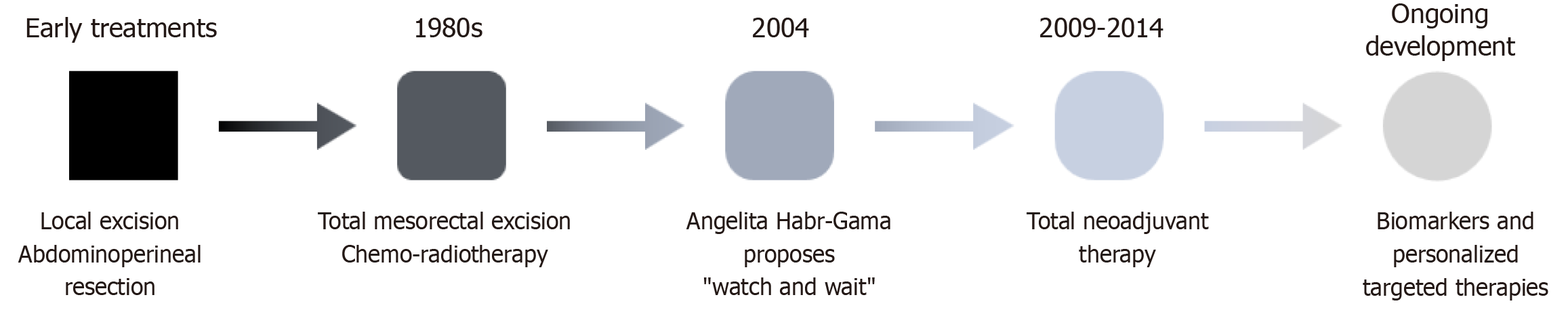
Treatment before Halsted: These differences, however, did not begin to attract significant attention until the late 20th and early 21st centuries. In the early 20th century (Figure 1), when treatment for RC started its development, it was based solely on the surgical resection of the entire rectum[41]. It was Miles in 1908 who first described a technique capable of completely eradicating the primary tumor through abdominoperineal resection with a permanent stoma[42]. At that time, the mechanisms of tumor spread were not well understood, and little attention was given to the resection of the entire lymphatic compartment formed by the mesorectum. The focus was instead on addressing the more immediate problem of local recurrence, which at that time was practically the norm. Efforts in the following decades concentrated on minimizing local recurrence and improving the quality of life for patients undergoing surgery. Between the 1920s and 1940s, the technique of low anterior resection was developed, improving patient experience by avoiding the need for a permanent stoma[43,44]. However, it did not result in better oncological outcomes, with death from either local or distant progression occurring almost systematically. In these procedures, the mesorectal dissection was done bluntly, sacrificing mesorectum that remained adhered to the walls of the lesser pelvis.
The present of RC treatment, TME, and adjuvant therapy: Two major milestones that define the current approach to treating RC are the description of the “Holy Plane” and TME by Heald, along with the introduction of both local RT and systemic CT adjuvant treatments. In 1987, Heald described the plane between the mesorectal fascia and the presacral and pelvic fascia, whose dissection allowed for the removal of the entire first lymphatic drainage station and the primary site of tumor dissemination of the rectum[45,46]. Beyond the surgical technique, Heald truly delved into the importance of completely removing both local and regional disease in the case of LARC, achieving the greatest prognostic advancement to date[47,48]. The outlook for patients diagnosed with LARC changed radically, with local recurrence rates dropping from over 30% with the previous surgical technique to below 10% (some reports even indicate rates as low as 4%)[49]. The 5-year overall survival (OS) rate, previously around 45%, increased to 75%[50]. While it is bold to attribute all the credit to TME, due to the retrospective and observational nature of most studies, there is sufficient evidence to assign it a central role in these improvements. However, TME has also presented a new challenge for surgeons, who must navigate the technical difficulty of preserving the Holy Plane in typically adverse situations: Locally advanced tumors (pT3-pT4), extensive lymph node involvement, and those requiring abdominoperineal resection[48,51,52]. The increased technical difficulty, combined with the well-known hostility of the pelvis, has contributed to an increase in the incidence of urinary and sexual complications. Nonetheless, the systematic practice of this procedure worldwide as the new gold standard for RC surgery has significantly improved these outcomes[53,54].
The second major advance was the incorporation of CT and RT into the treatment of LARC, strategies that had previously been reserved for patients with non-curable disease. In 1985, a study by the gastrointestinal tumor study group involving 227 patients compared surgery alone with adjuvant treatment using CT, RT, and chemoradiotherapy (CRT), showing promising data in favor of CRT following curative surgery, reducing both local and distant recurrence rates[55]. Although no effect on OS was observed, it paved the way for the future development of these therapies. In 1988, Fisher et al[56] achieved positive results with adjuvant CT, improving both OS and disease-free survival (DFS) in patients, with certain factors (male sex, age, and tumor stage) influencing the outcomes. In contrast, patients treated with adjuvant RT showed fewer local recurrences, but their OS was not significantly altered. In 1991, Krook et al[57] confirmed the usefulness of adjuvant CRT in reducing both local and distant recurrences, improving OS as well as DFS.
The Swedish RC group published the results in 1997 of a comparison between patients who received neoadjuvant RT followed by surgical intervention vs those who underwent surgery alone. In this case, not only was a significant improvement observed in the incidence of local recurrence, but they also confirmed improvements in both OS and DFS[58]. Another study conducted by Kapiteijn et al[59] in 2001 made a similar comparison, but at a time when TME had already become the standard technique for all colorectal surgeons. In this case, the benefit on local recurrence was confirmed, although there was no impact on OS. A subsequent review did observe an improvement in survival at 10 years for patients with locally advanced stages[60].
Although the usefulness of CT and RT in combination with TME in the treatment of LARC became clearer, several aspects still needed to be determined regarding the best way to apply these treatments. A study by Frykholm et al[61] in 1993 supported the use of preoperative RT, which demonstrated greater efficacy in reducing local recurrences and a lower incidence of RT-related complications, without significantly increasing the number of surgical complications[62]. The CAO/ARO/AIO-94 study, which compared preoperative CRT vs postoperative CRT in patients with advanced non-metastatic RC, showed similar overall and disease-specific survival rates for both groups, although better local control was observed with neoadjuvant treatment and improved tolerability[63,64]. Meanwhile, the European Organization for Research and Treatment of Cancer group conducted a study comparing all combinations of CT with preoperative RT in patients with resectable T3 and T4 stage RC. No benefit in overall or disease-specific survival was observed when adding CT in any of its modalities, although a significant reduction in local recurrences was noted[65]. Thanks to all these studies, the role of preoperative RT has become established as the standard treatment alongside TME for LARC (Table 1).
| Ref. | Patient included | Treatment1 | Survival | P value | Survival free of disease | P value | Local recurrence | P value |
| Gastrointestinal Tumor Study Group[55], 1985 | 227 | Surgery | 36 | 0.20 | 45 | 0.85 | 55 | < 0.009 |
| Surgery + RT | 46 | 0.20 | 55 | 0.85 | 48 | < 0.009 | ||
| Surgery + CT | 46 | 0.20 | 60 | 0.85 | 46 | < 0.009 | ||
| Surgery + CTRT | 56 | 0.20 | 70 | 0.85 | 33 | < 0.009 | ||
| Fisher et al[56], 1988 | 555 | Surgery, control | 43 | 30 | 24.5 | 0.06 | ||
| Surgery + RT | 45 | 0.7 | 33 | 0.4 | 16.3 | 0.06 | ||
| Surgery + CT | 53 | 0.05 | 42 | 0.006 | 21.4 | 0.06 | ||
| Krook et al[57], 1991 | 204 | Surgery + RT | 62 | 0.043 | 62 | 0.0025 | ||
| Surgery + CTRT | 49 | 0.043 | 40 | 0.0025 | ||||
| Frykholm et al[61], 1993 | 471 | RT + surgery | 54 | 0.5 | 13 | 0.02 | ||
| Surgery + RT | 52 | 0.5 | 22 | 0.02 | ||||
| Swedish Rectal Cancer Trial[58], 1997 | 1168, < 80 years | RT + surgery | 74 | 0.002 | 11 | < 0.001 | ||
| Surgery | 65 | 0.002 | 27 | < 0.001 | ||||
| Kapiteijn et al[59], 2001 | 1861 | RT + surgery, TME | 82 | 0.84 | 2.4 | < 0.001 | ||
| Surgery, TME | 81.2 | 0.84 | 8.2 | < 0.001 | ||||
| Bosset et al[65], 2006 | 1011, T3-4 | RT + surgery, TME | 64.8 | 0.84 | 54.4 | 0.52 | 17.1 | 0.002 |
| CTRT + surgery, TME | 65.8 | 0.84 | 56.1 | 0.52 | 8.7 | 0.002 | ||
| RT + surgery, TME + CT | 64.8 | 0.84 | 54.4 | 0.52 | 9.6 | 0.002 | ||
| CTRT + surgery, TME + CT | 65.8 | 0.84 | 56.1 | 0.52 | 7.6 | 0.002 | ||
| van Gijn et al[60], 2011 | 1861 | RT + surgery, TME, TNM III, circumferential margin negative | 50 | 0.032 | 5 | < 0.0001 | ||
| Surgery, TME, TNM III, circumferential margin negative | 40 | 0.032 | 11 | < 0.0001 | ||||
| Sauer et al[64], 2012 | 823 | CTRT + surgery, TME + CT | 59.6 | 0.85 | 68.1 | 0.65 | 7.1 | 0.048 |
| Surgery, TME + CTRT | 59.9 | 0.85 | 67.8 | 0.65 | 10.1 | 0.048 |
When analyzing the value of RT, especially when applied preoperatively, two main protocols have been primarily used: Long-course RT (LCRT), consisting of 50.4 Gy administered in 28 sessions, and short-course RT (SCRT), in which 25 Gy is delivered in 5 sessions. The preference for one or the other has mainly depended on the experience of each center, with strong advocates for both approaches. In a meta-analysis published in 2018, it was concluded that OS, complication rates, and incidence of metastases were comparable between LCRT and SCRT[66]. However, more recent studies suggest a superiority of LCRT for low and bulky tumors in achieving greater tumor regression. They also recommend the use of LCRT in cases where an OPT is being considered, as while it does not improve OS, it appears to yield better outcomes in avoiding rectal resection[67-70]. Given the wide discrepancy in results, this aspect of LARC treatment remains to be clarified. However, the current trend favors the use of LCRT for patients with more advanced lesions and for those who are candidates for rectal preservation[71-75].
Although for much of the first two decades of the 21st century, the treatment of LARC has consisted of preoperative CRT followed by surgery with TME and subsequent adjuvant CT, various studies have explored the option of administering 100% of oncologic treatment before surgical intervention, without adjuvant CT. This approach has been termed total neoadjuvant therapy (TNT). Most research groups working with this treatment modality have reported promising results. The 2021 RAPIDO trial observed a greater reduction in the incidence of treatment failure (local or distant relapse or treatment-related mortality) in patients treated with TNT (23.7%) compared to those managed with standard therapy (30.4%), suggesting the utility of TNT as a new treatment standard[76]. However, a five-year follow-up analysis of this same study revealed a higher incidence of local disease recurrence[77]. Another important study, the UNICANCER-PRODIGE 23 trial, incorporated preoperative FOLFIRINOX into the standard treatment regimen, reducing the duration of adjuvant CT. When comparing the new treatment scheme to the classical approach, an improvement in DFS and a decrease in treatment-associated complications were observed[78]. This and other studies[79-81] argue that better patient tolerance, along with potentially superior prognostic outcomes, make TNT the future of oncologic treatment for LARC, although several aspects still need to be determined before it becomes a widespread reality[82-84].
Further surgical advances: Since the introduction of laparoscopy in the mid-1980s, efforts have been made to incorporate these new techniques into colorectal surgery. The first laparoscopically assisted colonic resections were performed in 1991[85] and since then, the development of these techniques has been continuous. Initially, there were critics of the approach, with concerns regarding its safety compared to open surgery for CRC. However, studies such as CLASICC and COLOR II were soon published, demonstrating oncologic equivalence while improving recovery time, reducing hospital stay, and minimizing complications associated with abdominal wall trauma[86-88].
The introduction of laparoscopy led to rapid technical advancements, which in turn facilitated the development of new approaches for managing rectal lesions. Minimally invasive transanal techniques have played a particularly significant role in the treatment of RC. The first of these techniques to be developed was transanal endoscopic microsurgery (TEM), introduced in 1983 as an alternative to purely transanal techniques and in parallel with laparoscopy[89]. TEM employs a 40 mm-diameter proctoscope, through which a combination of optics, ports, and an insufflation-aspiration system allows for the resection of rectal lesions that are not amenable to conventional endoscopic removal. Although initially designed for the excision of adenomas, TEM was soon utilized for the resection of early-stage adenocarcinomas (cT1, cN0). In 2010, combining the principles of TEM and single-port laparoscopy, the first case of transanal minimally invasive surgery (TAMIS) was reported[90]. While sacrificing TEM’s depth perception and its dedicated insufflation-aspiration system, TAMIS provided greater maneuverability along the entire rectal circumference, regardless of patient positioning. Additionally, it allowed the use of standard laparoscopic instruments in combination with any single-port system, significantly reducing costs and improving accessibility[91]. Although TAMIS is technically more complex, many surgeons experienced in advanced laparoscopic surgery have preferred it over TEM due to its advantages. The application of transanal techniques has progressively expanded to include larger and more advanced lesions. Currently, guidelines from several international societies endorse transanal resections using TEM or TAMIS for early-stage RC (cT1) and for selected cT2 tumors in patients who are not candidates for more aggressive surgical treatments[92,93].
The future, towards non-surgical treatment: Every professional dedicated to the treatment of neoplastic diseases envisions an ideal future where tumors can be managed medically, effectively, and with minimal sequelae for the patient. Since the adoption of neoadjuvant CRT as the standard treatment for LARC in the early 21st century, a considerable proportion of patients have been observed to achieve complete tumor response, both clinically complete response (cCR) on restaging assessments and pathologically CR (pCR) upon histopathological examination of the resected specimen after radical surgery. Given these findings and the significant morbidity associated with both CRT and surgical resection, it is inevitable to question whether certain patients, specifically those with favorable prognostic factors and a strong response to neoadjuvant therapy, may be subjected to overtreatment. This concern has fueled growing interest in non-operative management strategies aimed at preserving organ function while maintaining oncologic safety.
In response to this, Habr-Gama et al[94] published the experience in 2004 with 265 patients treated with neoadjuvant CRT, of whom 26.8% achieved a cCR and were closely observed without immediate surgery. These patients were compared to those who underwent surgical treatment and were found to have a pCR on final histological examination. The comparison revealed a slightly higher OS rate for patients who achieved cCR compared to those who underwent surgery, although no significant differences were observed in DFS or recurrence rates. Following these initial findings, the term “watch and wait” was introduced, highlighting the fact that the treatment of LARC offered far more possibilities than had been previously utilized.
However, it is possible that these patients harbored biologically less aggressive tumors with a lower metastatic potential, which may have contributed to the high survival rates observed, regardless of the treatment strategy employed. This possibility should be taken into account when interpreting the results, as not all patients with a cCR share the same biological profile. Although 5-year and 10-year OS was statistically superior in the observation group (P = 0.01), this difference may reflect favorable patient selection rather than a direct effect of the nonoperative management strategy. In contrast, the difference in DFS did not reach statistical significance (P = 0.09), further underscoring the need to interpret these findings with caution. Currently, the WW strategy is beginning to appear in oncology treatment guidelines as a valid alternative for select patients, typically within the context of prospective studies[84,92,95]. Before considering non-surgical treatment or OPT as a new standard of care for RC with a good clinical response to CRT, further investigation is required to address several critical questions for which no definitive answers yet exist.
Which patients can benefit from OPT strategies: Many ongoing studies focus on patients who already have an indication for CRT or TNT, meaning those with locally advanced tumors classified as T3-4, often with nodal involvement (N+)[96-100]. However, the subgroup that may have the greatest potential for response to this treatment consists of patients with early-stage lesions who are typically not offered neoadjuvant therapy and are instead directed toward radical surgical resection[101-103].
Several publications have reported experiences in managing cT2-3 cN0 patients using different preoperative CRT protocols followed by OPT, yielding promising results. For example, in 2022, González et al[104] treated patients who achieved a cCR (25.6%) with TEM. The correlation between cCR and pCR was 65.4%, and 21% of patients required radical surgery due to an insufficient pathological response (ypT3-4). In this series, only one local recurrence and three distant recurrences were observed, with five-year OS and DFS rates of 91.7% and 89.5%, respectively. A French study including cT2-3 cN0-1 patients treated with a combination of preoperative CRT and brachytherapy, followed by either a WW approach or TEM, achieved rectal preservation in 96% of patients, with a three-year DFS rate of 88%. Additionally, the study determined that T2 tumors and those measuring less than 3 cm had a significantly lower risk of local recurrence[105]. Another study also identified lymph node involvement as a negative predictor for cCR[106]. In light of this evidence, CRT for patients with stage T2 tumors without nodal involvement or distant metastasis, with subsequent rectal preservation, is now being incorporated into selected clinical practice guidelines[95].
What is the role of local surgery in OPT: One of the major challenges that have emerged with the introduction of the WW strategy is establishing a clear definition of cCR and correlating it with pCR. In this context, minimally invasive surgery techniques such as TEM and TAMIS provide a reasonable alternative. These approaches not only allow for the removal of potential residual tumor cells but also serve as a salvage option for lesions exhibiting near-complete responses. However, several studies raise doubts about this intuitive assertion. In a study by Jones et al[107], where they presents their experience and reviews previous studies in 2021, it is suggested that the value of local resection in patients with cCR may be insufficient to justify the potential complications, as long as proper follow-up is ensured. Moreover, the relative safety of TEM/TAMIS, assumed from the experience gained with the resection of adenomatous lesions, may not be accurate. In 2014, Gornicki et al[108] published an analysis of quality of life and functional capacity in patients with locally advanced RC (cT1-3, cN0) who underwent local full rectal wall surgery after receiving CRT or SCRT. These patients were compared with a retrospective cohort of similar patients who underwent direct anterior resection. The results showed a significant loss of quality of life and severe rectal symptoms in up to 20% of patients treated with OPT, as well as a 20% decrease in sexual quality of life, similar to the worsening seen in the control group. Similar results were observed in the CARTS study[109], where up to 50% of patients experienced major symptoms of low anterior resection syndrome. These data suggest that the damage caused to the rectum after radiation therapy, when undergoing local resection, is greater than expected and must be considered when including such surgeries in treatment protocols.
What is the optimal interval between the completion of RT and the assessment of clinical response: Numerous studies and extensive experience in the administration of neoadjuvant RT in LARC have established an approximate minimum period of 8 weeks between the completion of RT and definitive surgery to allow sufficient benefit from the treatment[110]. However, there is now growing evidence questioning the adequacy of this timeframe in organ-preserving strategies for LARC[106,111-114]. In the study published by Asoglu et al[115] in 2022, only 44% of patients achieving a cCR did so within the 8 to 10 weeks interval, particularly in cases of larger or more advanced tumors. Additionally, a large study conducted by the International Watch and Wait Database found no significant differences in organ preservation rates at two years, OS or metastasis-free survival between patients who achieved a cCR at first evaluation and those who attained it later at a second assessment[116]. The therapeutic effects of CRT in LARC appear to require sufficient time to fully develop. To maximize its benefits, prolonged intervals before making treatment decisions may be necessary. However, the optimal waiting period remains uncertain, with some studies suggesting delays of up to 30 weeks[113].
What is the role of TNT in OPT: TNT applies the entire oncological treatment before surgery. The evidence regarding the feasibility, safety, and effectiveness of TNT, particularly in terms of improved local control and survival outcomes in LARC, comes from multiple retrospective studies and phase II clinical trials, such as the CAO/ARO/AIO-12[117]. More recently, four phase III clinical trials have provided additional evidence (Table 2)[78,118-120]. Combined CT is the core of TNT, being the primary factor responsible for the superior outcomes of TNT compared to standard neoadjuvant therapy. It is recommended to administer CT for 3 to 4 months, with 6 cycles of CAPOX or between 6 and 9 cycles of FOLFOX/FOLFIRINOX according to different regimens. CT can be administered at the beginning of treatment, followed by RT in an induction strategy. It may also be given after RT, in a consolidation strategy. Alternatives are being explored where RT is completely omitted if the response to induction CT is sufficient to achieve a cCR[121,122].
| Ref. | Clinical trials | Patients | Stage | Treatments1 | pCR rates | Locorregional control | MFS | DFS |
| Bujko et al[118], 2016 | POLISH II | 261 | T3-4 | SCRT + CT | 16 | 78 | 70 | 53 |
| RT + CT | 12 | 79 | 73 | 52 | ||||
| Bahadoer et al[76], 2021 | RAPIDO | 462 | T4, N2 | SCRT + CT | 28 | 92 | 80 | 76 |
| RT + CT | 14 | 94 | 73 | 70 | ||||
| Conroy et al[78], 2021 | UNICANCER-PRODIGE 23 | 231 | T3-4, N1-2 | CT + RT + CT | 28 | 96 | 79 | 76 |
| RT + CT | 13 | 94 | 72 | 69 | ||||
| Jin et al[120], 2022 | STELLAR | 298 | T3-4, N1-2 | SCRT + CT + CT | 17 | 92 | 77 | 65 |
| RT + CT | 12 | 89 | 75 | 62 |
In the context of TNT, both SCRT and CRT (LCRT with capecitabine) have been employed in different experimental designs, without evidence of one being superior to the other at this time. However, certain aspects of each regimen deserve consideration, as they may make one preferable over the other depending on individual cases. Currently, clinical trials are underway that directly compare both regimens as initial treatment within TNT, such as the ACO/ARO/AIO-18.1 study.
The potential benefit of TNT in RC results in two main clinical achievements: Improved survival and increased local control, allowing for the implementation of OPT. TNT has been shown to increase DFS and metastasis-free survival. The observed improvement ranges from 5% to 10% at 3 years, with a 3-year DFS of approximately 75% and a distant metastasis rate of less than 20%. OS only showed a significant increase in the STELLAR study, seemingly due to the low DFS in the control group[120]. It is important to note that none of the studies had sufficient statistical power to detect differences in OS due to the short duration of follow-up (median less than 5 years). A recent update of the PRODIGE 23 trial, with a follow-up of over 6 years, showed an improvement in estimated 7-year DFS and metastasis-free survival, as well as a significant difference in estimated 7-year OS (82% vs 76%) in the TNT group compared to the standard treatment group[123]. TNT has also doubled the rate of pCR, reaching values above 25% (Table 2).
Although TNT has demonstrated a reduction in distant metastasis rates, no significant improvement in OS has been observed. This phenomenon, known as adjuvant therapy-related shortening of survival, suggests that treatment intensification may not necessarily translate into prolonged survival for all patients. To elucidate the role of adjuvant therapy-related shortening of survival as a potential explanation for the lack of OS benefit despite gains in DFS, post-relapse survival should be compared between arms in randomized trials investigating neoadjuvant treatment strategies[124].
The potential of TNT in OPT makes it an attractive option for early-stage RC (stage I)[125]. Currently, multiple phase I and II studies with different designs are underway, including patients with T1-3 N0-2 RC, aiming to evaluate its impact. Until the results of these studies, and preferably also those from larger randomized trials, are available, it is crucial to consider two key aspects: (1) In early-stage RC (stage I), it has not yet been determined whether TNT provides any additional benefit over standard neoadjuvant RT in terms of local disease control, while exposing patients to the adverse effects of combined CT[126]; and (2) In patients with middle and high RC in stage I, who could be cured with surgery alone without significant long-term impairment of their quality of life, especially without the need for a permanent colostomy, the benefits of non-surgical management are highly questionable. This is due to the burden of CT and RT, the need for continuous active surveillance, and the risks of local or distant recurrence.
As a novel alternative, OPT can be offered to patients with a good response to TNT, based on findings from the OPRA study and other published studies. Non-surgical management should only be considered within the context of a highly competent and experienced multidisciplinary team, in patients with excellent treatment adherence, well-informed, and willing to undergo intensive follow-up. A locoregional recurrence rate of between 20% and 40% has been documented in non-surgical management. In the OPRA study 2022, no differences were described between groups in terms of DFS, metastasis-free survival, or global survival. Most of these cases can be rescued through radical surgery without affecting DFS[125,127]. However, the main concern with non-surgical management is the risk of distant metastatic spread in patients under surveillance. According to available, albeit limited, evidence, this risk can occur in 5% to 15% of patients and is primarily associated with locoregional recurrence. While the combination of radical surgery and systemic therapy can offer a curative option, metastatic spread remains a potentially fatal event and should be discussed in detail with patients[128].
While TNT represents a significant advancement in the treatment of LARC, as has been extensively discussed, its implementation should be carefully considered by weighing the potential benefits against the associated risks and side effects. Appropriate patient selection, based on individual factors and tumor characteristics, is essential to maximize the advantages of TNT and minimize its drawbacks. The RAPIDO and PRODIGE-23 trials reported higher rates of severe (grade 4) adverse events in the TNT arms compared to standard treatment arms. In RAPIDO, 6.5% of patients experienced grade 4 adverse events in the experimental arm, vs 2.3% in the standard arm. In PRODIGE-23, 7% of patients developed grade 4 adverse events during the neoadjuvant CT phase, including one treatment-related death[129].
RT, a key component of TNT, can lead to significant long-term morbidities, including bowel dysfunction, fecal incontinence, urgency, sexual dysfunction, and bowel obstructions. Studies have shown that patients treated with neoadjuvant SCRT experience higher rates of fecal incontinence and sexual dysfunction compared to those who underwent surgery alone[130]. TNT may lead to overtreatment in patients with low-risk RC, unnecessarily exposing them to toxicities without a clear survival benefit. The indiscriminate adoption of TNT could result in treatment intensification beyond what is necessary for certain patient subgroups[129].
Limitations and controversies of organ-preserving therapies: Despite the favorable outcomes achieved with the WW strategy and TEM/TAMIS, several concerns remain regarding the implementation of these approaches and the associated risks, which must be thoroughly evaluated. Key issues include the correlation between cCR and pCR, as well as the added complexity of salvage surgery after prolonged intervals following RT or local excision. Evidence on the correlation between cCR and pCR remains inconsistent. As previously mentioned, González et al[104] reported a 65% concordance rate. In contrast, a meta-analysis by Dossa et al[96], which reviewed 23 studies, found no significant differences in DFS, OS, local recurrence, or cancer-specific mortality when comparing patients with cCR treated with WW and those with pCR following standard therapy. These discrepancies may stem from various factors, including differences in diagnostic methods and criteria for defining cCR, the timing of reassessment, and the interval between completion of neoadjuvant therapy and surgery.
Currently, most groups assess treatment response using rectal magnetic resonance imaging[131], rectoscopy, and digital rectal examination, typically excluding biopsies due to their low sensitivity[132]. Emerging techniques such as liquid biopsy[133], may soon play a valuable role in enhancing the clinical prediction of pCR. Until the correlation between cCR and pCR approaches near-perfect concordance, close surveillance remains essential. Follow-up should be particularly rigorous during the first two years post-treatment, when the risk of local recurrence is highest. Nonetheless, as Dijkstra et al[77] noted in his five-year analysis of the RAPIDO trial, late local recurrences can still occur, even in patients with pCR after radical surgery. Residual tumor does not necessarily compromise oncological outcomes[134], although non-response to treatment appears to be associated with more aggressive tumor biology. Furthermore, as the interval between treatment completion and potential salvage surgery continues to lengthen, driven by attempts to optimize RT response, increased fibrosis and greater surgical complexity are becoming more frequent. Dijkstra et al[77] reported a higher incidence of incomplete mesorectal excision in patients who underwent prolonged treatment courses. While the relationship between surgical timing and specimen quality has not yet been definitively established[135], nor its association with local recurrence risk, these are reasonable concerns that should be taken into account when designing treatment protocols.
Novel targeted therapies: Just as RC and CC were treated as a single disease throughout the late 20th century, LARC has been managed uniformly until now, regardless of its prognostic factors and biological characteristics. A notable example is the one of LARC patients with mismatch repair deficiency (deficient mismatch repair/MSI). Although they account for only 3%-10% of CRC cases and have a strong association with Lynch syndrome, they present actionable therapeutic targets responsive to immunotherapy. In 2023 and 2024, Cercek et al[136,137] published the results of a study on patients treated with dostarlimab (a monoclonal anti-programmed cell death-1 antibody) as monotherapy. The initial cCR rate was 100% and none of the patients experienced local or distant recurrence after a median follow-up of 26.3 months. Other similar studies, testing different immunotherapeutic agents, have also reported high cCR rates[138-141]. Although these findings are still in early stages and long-term efficacy and safety need further evaluation, immunotherapy is emerging as a key tool for a specific subset of patients.
Beyond its safety profile, it is also essential to investigate the potential benefits for patients with proficient mismatch repair and microsatellite stable, as evidence suggests that combining immunotherapy with anti-programmed cell death-1 agents may enhance the effectiveness of CT and RT[142]. Several well-established tumor-specific risk and prognostic factors could be utilized in clinical practice to tailor treatment strategies according to current evidence levels. Gandini et al[121] has proposed a therapeutic algorithm that incorporates these factors along with patient and physician preferences. Another approach that could further personalize treatment is tumor epigenetic profiling. As explored by George et al[143] in a 2019 publication, specific tumor mutations may determine the optimal radiosensitizing agent, thereby enhancing the effects of RT, promoting tumor regression, and increasing cCR and pCR rates. Achieving truly personalized treatment tailored to each patient’s tumor characteristics requires further research to refine molecular profiling and identify the most effective therapeutic agents for each clinical scenario[144].
Over the past century, RC treatment has advanced significantly, driven by a deeper understanding of both the disease and the anatomy and biology of the rectum, now recognized as more than just the fixed, distal segment of the large intestine. Surgical approaches have evolved from tumor-focused resections to interventions aimed at eradicating all local disease, and more recently, to strategies that seek to eliminate cancer without organ resection. These advancements prioritize reducing treatment-related side effects and preserving patients’ quality of life without compromising survival.
This article has highlighted many key advances in the treatment of LARC. However, certain developments, such as robotic surgery and transanal approaches for TME, were not discussed. While these techniques represent significant surgical innovations transforming modern practice, they have not revolutionized RC treatment to the same extent as other “breakthroughs.” Recent innovations, particularly in the fields of TNT and immunotherapy, are reshaping treatment paradigms and deserve further emphasis. For instance, the PRODIGE 23 update (2023) reinforces the benefit of intensified neoadjuvant CT in improving DFS. Similarly, Cercek et al[136,137] demonstrated the remarkable potential of immunotherapy in mismatch repair-deficient tumors, with unprecedented rates of clinical complete response and organ preservation. Additionally, the STELLAR trial (2022) has provided important data regarding personalized treatment approaches based on tumor biology, highlighting the importance of stratifying patients beyond traditional staging.
Maintaining the current pace of progress requires a coordinated, multidisciplinary effort from all professionals involved in RC management. In the near future, LARC treatment will likely adopt an approach similar to that of breast cancer, where tumor location, well-defined histological and biological risk factors, and molecular profiling will guide personalized treatment strategies. This evolution could not only improve overall and DFS rates but also increase the number of patients who achieve optimal outcomes without the need for surgery or RT.
I would like to express my deepest gratitude to my colleagues in the department and the dedicated multidisciplinary team involved in the management of locally advanced rectal cancer at Hospital Universitario de Cuenca. Your unwavering commitment, expertise, and collaborative spirit have been instrumental in advancing patient care and pushing the boundaries of treatment. This work would not have been possible without your continuous support, insightful discussions, and shared dedication to improving outcomes for our patients.
| 1. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1515] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 2. | Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, Ghidini M, Turati L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann Surg. 2020;271:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 3. | Bruckstein AH. Update on colorectal cancer. Risk factors, diagnosis, and treatment. Postgrad Med. 1989;86:83-85, 88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Silverberg E, Boring CC, Squires TS. Cancer statistics, 1990. CA Cancer J Clin. 1990;40:9-26. [PubMed] |
| 5. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68690] [Article Influence: 13738.0] [Reference Citation Analysis (201)] |
| 6. | Paschke S, Jafarov S, Staib L, Kreuser ED, Maulbecker-Armstrong C, Roitman M, Holm T, Harris CC, Link KH, Kornmann M. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Int J Mol Sci. 2018;19:2577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 7. | Cheng L, Eng C, Nieman LZ, Kapadia AS, Du XL. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol. 2011;34:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 8. | Cress RD, Morris C, Ellison GL, Goodman MT. Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992-2001. Cancer. 2006;107:1142-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Rhodes JB, Holmes FF, Clark GM. Changing distribution of primary cancers in the large bowel. JAMA. 1977;238:1641-1643. [PubMed] |
| 10. | Ghahremani GG, Dowlatshahi K. Colorectal carcinomas: diagnostic implications of their changing frequency and anatomic distribution. World J Surg. 1989;13:321-324; discussion 324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. J Clin Gastroenterol. 2007;41:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Safizadeh F, Mandic M, Hoffmeister M, Brenner H. Colorectal Cancer and Central Obesity. JAMA Netw Open. 2025;8:e2454753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Stein MJ, Baurecht H, Bohmann P, Fervers B, Fontvieille E, Freisling H, Friedenreich CM, Konzok J, Peruchet-Noray L, Sedlmeier AM, Leitzmann MF, Weber A. Diurnal timing of physical activity and risk of colorectal cancer in the UK Biobank. BMC Med. 2024;22:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 14. | Ionescu VA, Gheorghe G, Bacalbasa N, Chiotoroiu AL, Diaconu C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina (Kaunas). 2023;59:1646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 15. | Toyoda Y, Nakayama T, Ito Y, Ioka A, Tsukuma H. Trends in colorectal cancer incidence by subsite in Osaka, Japan. Jpn J Clin Oncol. 2009;39:189-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Seydaoğlu G, Özer B, Arpacı N, Parsak CK, Eray IC. Trends in colorectal cancer by subsite, age, and gender over a 15-year period in Adana, Turkey: 1993-2008. Turk J Gastroenterol. 2013;24:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Zou D, Xin X, Xu Y, Xu H, Xu T. A cross-sectional study on the association between physical activity and the risk of colon cancer based on NHANES 2007-2018. Sci Rep. 2025;15:3297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Halle M, Schoenberg MH. Physical activity in the prevention and treatment of colorectal carcinoma. Dtsch Arztebl Int. 2009;106:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Sun M, Wang Y, Sundquist J, Sundquist K, Ji J. Temporal Trends of Sex Disparity in Incidence and Survival of Colorectal Cancer: Variations by Anatomical Site and Age at Diagnosis. Clin Epidemiol. 2020;12:73-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Ungvari Z, Fekete M, Varga P, Lehoczki A, Fekete JT, Ungvari A, Győrffy B. Overweight and obesity significantly increase colorectal cancer risk: a meta-analysis of 66 studies revealing a 25-57% elevation in risk. Geroscience. 2025;47:3343-3364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 22. | Slattery ML, Friedman GD, Potter JD, Edwards S, Caan BJ, Samowitz W. A description of age, sex, and site distributions of colon carcinoma in three geographic areas. Cancer. 1996;78:1666-1670. [PubMed] [DOI] [Full Text] |
| 23. | Safizadeh F, Mandic M, Schöttker B, Hoffmeister M, Brenner H. Central obesity may account for most of the colorectal cancer risk linked to obesity: evidence from the UK Biobank prospective cohort. Int J Obes (Lond). 2025;49:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Abdominal Key. Anatomy and Embryology of the Anus, Rectum, and Colon. [cited 28 August 2024]. Available from: https://abdominalkey.com/anatomy-and-embryology-of-the-anus-rectum-and-colon/. |
| 25. | Hurtado CW. Embryology and Anatomy of the Gastrointestinal Tract. [cited 28 August 2024]. Available from: https://naspghan.org/files/documents/pdfs/training/curriculum-resources/physiology-series/Embryology_Final_NASPGHAN.pdf. |
| 26. | Carmichael JC, Mills S. Anatomy and Embryology of the Colon, Rectum, and Anus. In: Steele SR, Hull TL, Hyman N, Maykel JA, Read TE, Whitlow CB. The ASCRS Textbook of Colon and Rectal Surgery. 4th ed. Switzerland: Springer Nature, 2022. |
| 27. | Roa I, Meruane M. Desarrollo del Aparato Digestivo. Int J Morphol. 2012;30:1285-1294. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | DʼSouza N, de Neree Tot Babberich MPM, d'Hoore A, Tiret E, Xynos E, Beets-Tan RGH, Nagtegaal ID, Blomqvist L, Holm T, Glimelius B, Lacy A, Cervantes A, Glynne-Jones R, West NP, Perez RO, Quadros C, Lee KY, Madiba TE, Wexner SD, Garcia-Aguilar J, Sahani D, Moran B, Tekkis P, Rutten HJ, Tanis PJ, Wiggers T, Brown G. Definition of the Rectum: An International, Expert-based Delphi Consensus. Ann Surg. 2019;270:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 29. | Culligan K, Coffey JC, Kiran RP, Kalady M, Lavery IC, Remzi FH. The mesocolon: a prospective observational study. Colorectal Dis. 2012;14:421-428; discussion 428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (2)] |
| 30. | Poirer P, Charpy A, Nicolas A, Prenant A, Poirier P, Jonnesco T. Appareil de la digestion. In: Traité d’anatomie humaine. 2nd ed. Paris: Masson et Cie., 1901: 372-373. |
| 31. | Takahashi T, Ueno M, Azekura K, Ohta H. Lateral node dissection and total mesorectal excision for rectal cancer. Dis Colon Rectum. 2000;43:S59-S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 32. | van Ophoven A, Roth S. The anatomy and embryological origins of the fascia of Denonvilliers: a medico-historical debate. J Urol. 1997;157:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Kulaylat MN. Mesorectal excision: Surgical anatomy of the rectum, mesorectum, and pelvic fascia and nerves and clinical relevance. World J Surg Proced. 2015;5:27. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (8)] |
| 34. | Lunniss PJ, Standring S. Vascular supply and lymphatic drainage of the hindgut. In: Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 41st ed. London: Elsevier, 2016: 1150-1152. |
| 35. | Hugen N, van de Velde CJH, de Wilt JHW, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 370] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 36. | Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, Liao X, Waldron L, Hoshida Y, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 501] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 37. | Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan P, Roth AD, Klingbiel D, Bosman FT, Delorenzi M, Tejpar S. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 521] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 38. | Kornmann M, Staib L, Wiegel T, Kron M, Henne-Bruns D, Link KH, Formentini A; Study Group Oncology of Gastrointestinal Tumors (FOGT). Long-term results of 2 adjuvant trials reveal differences in chemosensitivity and the pattern of metastases between colon cancer and rectal cancer. Clin Colorectal Cancer. 2013;12:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Lee YC, Lee YL, Chuang JP, Lee JC. Differences in survival between colon and rectal cancer from SEER data. PLoS One. 2013;8:e78709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 40. | Tamas K, Walenkamp AM, de Vries EG, van Vugt MA, Beets-Tan RG, van Etten B, de Groot DJ, Hospers GA. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat Rev. 2015;41:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 41. | Gaetani RS, Ladin K, Abelson JS. Journey through the Decades: The Evolution in Treatment and Shared Decision Making for Locally Advanced Rectal Cancer. Cancers (Basel). 2024;16:2807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 42. | Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908). CA Cancer J Clin. 1971;21:361-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 198] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Hartmann MH. Nouveau procédé d’ablation des cancers de la partie terminale du colon pelvien. Bull Soc Chir Paris. 1921;30:441. |
| 44. | Dixon CF. Anterior Resection for Malignant Lesions of the Upper Part of the Rectum and Lower Part of the Sigmoid. Ann Surg. 1948;128:425-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1973] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 46. | Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med. 1988;81:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 508] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 47. | Ridgway PF, Darzi AW. The role of total mesorectal excision in the management of rectal cancer. Cancer Control. 2003;10:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Kitz J, Fokas E, Beissbarth T, Ströbel P, Wittekind C, Hartmann A, Rüschoff J, Papadopoulos T, Rösler E, Ortloff-Kittredge P, Kania U, Schlitt H, Link KH, Bechstein W, Raab HR, Staib L, Germer CT, Liersch T, Sauer R, Rödel C, Ghadimi M, Hohenberger W; German Rectal Cancer Study Group. Association of Plane of Total Mesorectal Excision With Prognosis of Rectal Cancer: Secondary Analysis of the CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trial. JAMA Surg. 2018;153:e181607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 49. | McAnena OJ, Heald RJ, Lockhart-Mummery HE. Operative and functional results of total mesorectal excision with ultra-low anterior resection in the management of carcinoma of the lower one-third of the rectum. Surg Gynecol Obstet. 1990;170:517-521. [PubMed] |
| 50. | Enker WE. Total mesorectal excision--the new golden standard of surgery for rectal cancer. Ann Med. 1997;29:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 51. | Garlipp B, Ptok H, Schmidt U, Stübs P, Scheidbach H, Meyer F, Gastinger I, Lippert H. Factors influencing the quality of total mesorectal excision. Br J Surg. 2012;99:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Leonard D, Penninckx F, Fieuws S, Jouret-Mourin A, Sempoux C, Jehaes C, Van Eycken E; PROCARE, a multidisciplinary Belgian Project on Cancer of the Rectum. Factors predicting the quality of total mesorectal excision for rectal cancer. Ann Surg. 2010;252:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Ivorra García-Moncó P. Estudio prospectivo de calidad de vida tras la cirugía del cáncer de recto. 2009. [cited 28 January 2025]. Available from: http://hdl.handle.net/10550/15906. |
| 54. | Eveno C, Lamblin A, Mariette C, Pocard M. Sexual and urinary dysfunction after proctectomy for rectal cancer. J Visc Surg. 2010;147:e21-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 918] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 56. | Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, Wickerham DL, Fisher ER, Caplan R, Jones J, Lerner H. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 728] [Article Influence: 19.2] [Reference Citation Analysis (48)] |
| 57. | Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, Kubista TP, Poon MA, Meyers WC, Mailliard JA. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1419] [Cited by in RCA: 1307] [Article Influence: 37.3] [Reference Citation Analysis (14)] |
| 58. | Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1838] [Article Influence: 63.4] [Reference Citation Analysis (11)] |
| 59. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3160] [Article Influence: 126.4] [Reference Citation Analysis (9)] |
| 60. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1387] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 61. | Frykholm GJ, Glimelius B, Påhlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum. 1993;36:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 355] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 62. | Korkolis DP, Plataniotis GD, Gondikakis E, Xinopoulos D, Koulaxouzidis GV, Katsilieris J, Vassilopoulos PP. Short-term preoperative radiotherapy is a safe approach for treatment of locally advanced rectal cancer. Int J Colorectal Dis. 2006;21:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Sauer R, Fietkau R, Wittekind C, Rödel C, Martus P, Hohenberger W, Tschmelitsch J, Sabitzer H, Karstens JH, Becker H, Hess C, Raab R; German Rectal Cancer Group. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis. 2003;5:406-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 64. | Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1540] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 65. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC; EORTC Radiotherapy Group Trial 22921. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2073] [Article Influence: 103.7] [Reference Citation Analysis (5)] |
| 66. | Chen K, Xie G, Zhang Q, Shen Y, Zhou T. Comparison of short-course with long-course preoperative neoadjuvant therapy for rectal cancer: A meta-analysis. J Cancer Res Ther. 2018;14:S224-S231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Ngan SY. Preoperative Treatment of Locally Advanced Rectal Cancer: Assets and Drawbacks of Short Course and Long Course in Clinical Practice. Semin Radiat Oncol. 2016;26:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Kammar PS, Garach NR, Masillamany S, de'Souza A, Ostwal V, Saklani AP. Downstaging in Advanced Rectal Cancers: A Propensity-Matched Comparison Between Short-Course Radiotherapy Followed by Chemotherapy and Long-Course Chemoradiotherapy. Dis Colon Rectum. 2022;65:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Bercz A, Park BK, Pappou E, Nemirovsky D, Sarkar R, Yamner M, Omer D, Verheij FS, Alvarez J, Atri P, Reyngold M, Yaeger R, Wei IH, Wu A, Raj N, Widmar M, Hajj C, Kim MJ, Rao D, Nash GM, Williams V, Shia J, Segal NH, Diaz L, Ganesh K, Weiser MR, Gollub MJ, Paty PB, Horvat N, Zinovoy M, Roth O'Brien D, Sanchez-Vega F, Saltz LB, Crane CH, Cercek A, Gonen M, Garcia-Aguilar J, Smith JJ, Romesser PB. Organ preservation after neoadjuvant long-course chemoradiotherapy versus short-course radiotherapy. Ann Oncol. 2024;35:1003-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 70. | Kairevičė L, Latkauskas T, Tamelis A, Petrauskas A, Paužas H, Žvirblis T, Jaruševičius L, Saladžinskas Ž, Pavalkis D, Jančiauskienė R. Preoperative long-course chemoradiotherapy plus adjuvant chemotherapy versus short-course radiotherapy without adjuvant chemotherapy both with delayed surgery for stage II-III resectable rectal cancer: 5-Year survival data of a randomized controlled trial. Medicina (Kaunas). 2017;53:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | Kim JS, Chung MJ, Lee DY, Lee SH, Jeong SK, Yoo BE, Chung CS, Chung WK. Clinicopathological Outcomes in Patients With Locally Advanced Rectal Cancer Undergoing Preoperative Short-Versus Long-course Chemoradiotherapy With Delayed Surgery. In Vivo. 2023;37:2768-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | Qiaoli W, Yongping H, Wei X, Guoqiang X, Yunhe J, Qiuyan L, Cheng L, Mengling G, Jiayi L, Wei X, Yi Y. Preoperative short-course radiotherapy (5 × 5 Gy) with delayed surgery versus preoperative long-course radiotherapy for locally resectable rectal cancer: a meta-analysis. Int J Colorectal Dis. 2019;34:2171-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Amariyil A, Pathy S, Sharma A, Kumar S, Pramanik R, Bhoriwal S, Pandey RM. Randomized Controlled Trial of Neoadjuvant Short-Course Radiotherapy Followed by Consolidation Chemotherapy Versus Long-Course Chemoradiotherapy in Locally Advanced Rectal Cancer: Comparison of Overall Response Rates. J Gastrointest Cancer. 2024;55:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Chakrabarti D, Rajan S, Akhtar N, Qayoom S, Gupta S, Verma M, Srivastava K, Kumar V, Bhatt MLB, Gupta R. Short-course radiotherapy with consolidation chemotherapy versus conventionally fractionated long-course chemoradiotherapy for locally advanced rectal cancer: randomized clinical trial. Br J Surg. 2021;108:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 75. | Pu W, Chen W, Jing H, Li J, Jiang Y, Li S, Wen W, Xu Z, Jin J. Total neoadjuvant therapy based on short-course radiotherapy versus standard long-course chemoradiotherapy for locally advanced rectal cancer: a systematic review and meta-analysis of randomized controlled trials. Front Oncol. 2024;14:1515756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 1118] [Article Influence: 223.6] [Reference Citation Analysis (1)] |
| 77. | Dijkstra EA, Nilsson PJ, Hospers GAP, Bahadoer RR, Meershoek-Klein Kranenbarg E, Roodvoets AGH, Putter H, Berglund Å, Cervantes A, Crolla RMPH, Hendriks MP, Capdevila J, Edhemovic I, Marijnen CAM, van de Velde CJH, Glimelius B, van Etten B; Collaborative Investigators. Locoregional Failure During and After Short-course Radiotherapy Followed by Chemotherapy and Surgery Compared With Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial. Ann Surg. 2023;278:e766-e772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 241] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 78. | Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardière C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C; Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 878] [Article Influence: 175.6] [Reference Citation Analysis (0)] |
| 79. | Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, Safont MJ, Salud A, Vera R, Massuti B, Escudero P, Alonso V, Bosch C, Martin M, Minsky BD. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial†. Ann Oncol. 2015;26:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 80. | Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K; Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 542] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 81. | Marco MR, Zhou L, Patil S, Marcet JE, Varma MG, Oommen S, Cataldo PA, Hunt SR, Kumar A, Herzig DO, Fichera A, Polite BN, Hyman NH, Ternent CA, Stamos MJ, Pigazzi A, Dietz D, Yakunina Y, Pelossof R, Garcia-Aguilar J; Timing of Rectal Cancer Response to Chemoradiation Consortium. Consolidation mFOLFOX6 Chemotherapy After Chemoradiotherapy Improves Survival in Patients With Locally Advanced Rectal Cancer: Final Results of a Multicenter Phase II Trial. Dis Colon Rectum. 2018;61:1146-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 82. | Riesco-Martinez MC, Fernandez-Martos C, Gravalos-Castro C, Espinosa-Olarte P, La Salvia A, Robles-Diaz L, Modrego-Sanchez A, Garcia-Carbonero R. Impact of Total Neoadjuvant Therapy vs. Standard Chemoradiotherapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis of Randomized Trials. Cancers (Basel). 2020;12:3655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 83. | Kasi A, Abbasi S, Handa S, Al-Rajabi R, Saeed A, Baranda J, Sun W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e2030097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 290] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 84. | Langenfeld SJ, Davis BR, Vogel JD, Davids JS, Temple LKF, Cologne KG, Hendren S, Hunt S, Garcia Aguilar J, Feingold DL, Lightner AL, Paquette IM; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer 2023 Supplement. Dis Colon Rectum. 2024;67:18-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 85. | Blackmore AE, Wong MT, Tang CL. Evolution of laparoscopy in colorectal surgery: an evidence-based review. World J Gastroenterol. 2014;20:4926-4933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 86. | Colon Cancer Laparoscopic or Open Resection Study Group; Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1076] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 87. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM; MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2325] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 88. | van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1249] [Article Influence: 96.1] [Reference Citation Analysis (1)] |
| 89. | Buess G, Theiss R, Günther M, Hutterer F, Pichlmaier H. Endoscopic surgery in the rectum. Endoscopy. 1985;17:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 137] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc. 2010;24:2200-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 403] [Article Influence: 25.2] [Reference Citation Analysis (2)] |
| 91. | Thompson EV, Bleier JI. Transanal Minimally Invasive Surgery. Clin Colon Rectal Surg. 2017;30:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1310] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
| 93. | Benson AB, Venook AP, Adam M, Chang G, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Haste P, Hecht JR, Hoffe S, Hunt S, Hussan H, Johung KL, Joseph N, Kirilcuk N, Krishnamurthi S, Malla M, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Shogan B, Skibber JM, Sofocleous CT, Tavakkoli A, Willett CG, Wu C, Jones F, Gurski L. NCCN Guidelines® Insights: Rectal Cancer, Version 3.2024. J Natl Compr Canc Netw. 2024;22:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 128] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 94. | Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711-717; discussion 717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1120] [Cited by in RCA: 1403] [Article Influence: 63.8] [Reference Citation Analysis (9)] |
| 95. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 511] [Article Influence: 127.8] [Reference Citation Analysis (1)] |
| 96. | Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 437] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 97. | Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A, Widmar M, Ganesh K, Yaeger R, Cercek A, Weiser MR, Nash GM, Guillem JG, Temple LKF, Chalasani SB, Fuqua JL, Petkovska I, Wu AJ, Reyngold M, Vakiani E, Shia J, Segal NH, Smith JD, Crane C, Gollub MJ, Gonen M, Saltz LB, Garcia-Aguilar J, Paty PB. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019;5:e185896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 434] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 98. | Lai CL, Lai MJ, Wu CC, Jao SW, Hsiao CW. Rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy, surgery, or "watch and wait". Int J Colorectal Dis. 2016;31:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 99. | Habr-Gama A, Perez RO, Nadalin W, Nahas SC, Ribeiro U Jr, Silva E Sousa AH Jr, Campos FG, Kiss DR, Gama-Rodrigues J. Long-term results of preoperative chemoradiation for distal rectal cancer correlation between final stage and survival. J Gastrointest Surg. 2005;9:90-99; discussion 99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 100. | Habr-Gama A, Gama-Rodrigues J, São Julião GP, Proscurshim I, Sabbagh C, Lynn PB, Perez RO. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 434] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 101. | Fernandez LM, São Julião GP, Vailati BB, Habr-Gama A, Perez RO. Nonoperative Management for T2 Low Rectal Cancer: A Western Approach. Clin Colon Rectal Surg. 2020;33:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 102. | Olson CH. Current Status of the Management of Stage I Rectal Cancer. Curr Oncol Rep. 2020;22:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 103. | Rouleau-Fournier F, Brown CJ. Can less be more? Organ preservation strategies in the management of rectal cancer. Curr Oncol. 2019;26:S16-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 104. | González JEB, Lavernia HC, Fraga JGP, Lemus SQ. Long-term outcomes of transanal endoscopic microsurgery for clinical complete response after neoadjuvant treatment in T2-3 rectal cancer. Surg Endosc. 2022;36:2906-2913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 105. | Gérard JP, Barbet N, Gal J, Dejean C, Evesque L, Doyen J, Coquard R, Gugenheim J, Benizri E, Schiappa R, Baudin G, Benezery K, François E. Planned organ preservation for early T2-3 rectal adenocarcinoma: A French, multicentre study. Eur J Cancer. 2019;108:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 106. | Bitterman DS, Resende Salgado L, Moore HG, Sanfilippo NJ, Gu P, Hatzaras I, Du KL. Predictors of Complete Response and Disease Recurrence Following Chemoradiation for Rectal Cancer. Front Oncol. 2015;5:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 107. | Jones HJS, Al-Najami I, Baatrup G, Cunningham C. Local excision after (near) complete response of rectal cancer to neoadjuvant radiation: does it add value? Int J Colorectal Dis. 2021;36:1017-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Gornicki A, Richter P, Polkowski W, Szczepkowski M, Pietrzak L, Kepka L, Rutkowski A, Bujko K. Anorectal and sexual functions after preoperative radiotherapy and full-thickness local excision of rectal cancer. Eur J Surg Oncol. 2014;40:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Stijns RCH, de Graaf EJR, Punt CJA, Nagtegaal ID, Nuyttens JJME, van Meerten E, Tanis PJ, de Hingh IHJT, van der Schelling GP, Acherman Y, Leijtens JWA, Bremers AJA, Beets GL, Hoff C, Verhoef C, Marijnen CAM, de Wilt JHW; CARTS Study Group. Long-term Oncological and Functional Outcomes of Chemoradiotherapy Followed by Organ-Sparing Transanal Endoscopic Microsurgery for Distal Rectal Cancer: The CARTS Study. JAMA Surg. 2019;154:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 110. | Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15:2661-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 111. | Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, Fazio VW. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 290] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 112. | Karimi M, Osterlund P, Hammarström K, Imam I, Frodin JE, Glimelius B. Associations between Response to Commonly Used Neo-Adjuvant Schedules in Rectal Cancer and Routinely Collected Clinical and Imaging Parameters. Cancers (Basel). 2022;14:6238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 113. | Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA, Tanis PJ; Dutch Surgical Colorectal Audit. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg. 2013;100:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 114. | Moore HG, Gittleman AE, Minsky BD, Wong D, Paty PB, Weiser M, Temple L, Saltz L, Shia J, Guillem JG. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 206] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 115. | Asoglu O, Goksoy B, Aliyev V, Mustafayev TZ, Atalar B, Bakir B, Guven K, Demir G, Goksel S. Watch and Wait Strategy for Rectal Cancer: How Long Should We Wait for a Clinical Complete Response? Surg Technol Int. 2022;40:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | Temmink SJD, Peeters KCMJ, Bahadoer RR, Kranenbarg EM, Roodvoets AGH, Melenhorst J, Burger JWA, Wolthuis A, Renehan AG, Figueiredo NL, Pares O, Martling A, Perez RO, Beets GL, van de Velde CJH, Nilsson PJ; International Watch & Wait Database (IWWD) Consortium. Watch and wait after neoadjuvant treatment in rectal cancer: comparison of outcomes in patients with and without a complete response at first reassessment in the International Watch & Wait Database (IWWD). Br J Surg. 2023;110:676-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 117. | Fokas E, Schlenska-Lange A, Polat B, Klautke G, Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL, Kirste S, Jacobasch L, Allgäuer M, Flentje M, Germer CT, Grützmann R, Hildebrandt G, Schwarzbach M, Bechstein WO, Sülberg H, Friede T, Gaedcke J, Ghadimi M, Hofheinz RD, Rödel C; German Rectal Cancer Study Group. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022;8:e215445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (1)] |
| 118. | Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, Michalski W, Olędzki J, Kuśnierz J, Zając L, Bednarczyk M, Szczepkowski M, Tarnowski W, Kosakowska E, Zwoliński J, Winiarek M, Wiśniowska K, Partycki M, Bęczkowska K, Polkowski W, Styliński R, Wierzbicki R, Bury P, Jankiewicz M, Paprota K, Lewicka M, Ciseł B, Skórzewska M, Mielko J, Bębenek M, Maciejczyk A, Kapturkiewicz B, Dybko A, Hajac Ł, Wojnar A, Leśniak T, Zygulska J, Jantner D, Chudyba E, Zegarski W, Las-Jankowska M, Jankowski M, Kołodziejski L, Radkowski A, Żelazowska-Omiotek U, Czeremszyńska B, Kępka L, Kolb-Sielecki J, Toczko Z, Fedorowicz Z, Dziki A, Danek A, Nawrocki G, Sopyło R, Markiewicz W, Kędzierawski P, Wydmański J; Polish Colorectal Study Group. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 331] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 119. | Bahadoer RR, Hospers GAP, Marijnen CAM, Peeters KCMJ, Putter H, Dijkstra EA, Kranenbarg EM, Roodvoets AGH, van Etten B, Nilsson PJ, Glimelius B, van de Velde CJH; collaborative investigators. Risk and location of distant metastases in patients with locally advanced rectal cancer after total neoadjuvant treatment or chemoradiotherapy in the RAPIDO trial. Eur J Cancer. 2023;185:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 120. | Jin J, Tang Y, Hu C, Jiang LM, Jiang J, Li N, Liu WY, Chen SL, Li S, Lu NN, Cai Y, Li YH, Zhu Y, Cheng GH, Zhang HY, Wang X, Zhu SY, Wang J, Li GF, Yang JL, Zhang K, Chi Y, Yang L, Zhou HT, Zhou AP, Zou SM, Fang H, Wang SL, Zhang HZ, Wang XS, Wei LC, Wang WL, Liu SX, Gao YH, Li YX. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J Clin Oncol. 2022;40:1681-1692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 317] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 121. | Gandini A, Sciallero S, Martelli V, Pirrone C, Puglisi S, Cremante M, Grassi M, Andretta V, Fornarini G, Caprioni F, Comandini D, Pessino A, Mammoliti S, Sobrero A, Pastorino A. A Comprehensive Approach to Neoadjuvant Treatment of Locally Advanced Rectal Cancer. Cancers (Basel). 2025;17:330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 122. | Boublikova L, Novakova A, Simsa J, Lohynska R. Total neoadjuvant therapy in rectal cancer: the evidence and expectations. Crit Rev Oncol Hematol. 2023;192:104196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 123. | Conroy T, Etienne P, Rio E, Evesque L, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouche O, Boileve A, Delaye M, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, De La Fouchardiere C, Morand C, Pezzella V, Rullier E, Castan F, Borg C. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: 7-year results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol. 2023;41:LBA3504-LBA3504. [DOI] [Full Text] |
| 124. | Socha J, Bujko K. Does the Gain of Total Neoadjuvant Therapy Outweigh the Harm in Rectal Cancer? Importance of the ATRESS (neoAdjuvant Therapy-RElated Shortening of Survival) Phenomenon: A Systematic Review. Cancers (Basel). 2023;15:1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 125. | Chin RI, Roy A, Pedersen KS, Huang Y, Hunt SR, Glasgow SC, Tan BR, Wise PE, Silviera ML, Smith RK, Suresh R, Badiyan SN, Shetty AS, Henke LE, Mutch MG, Kim H. Clinical Complete Response in Patients With Rectal Adenocarcinoma Treated With Short-Course Radiation Therapy and Nonoperative Management. Int J Radiat Oncol Biol Phys. 2022;112:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 126. | Wo JY, Ashman JB, Bhadkamkar NA, Bradfield L, Chang DT, Hanna N, Hawkins M, Holtz M, Kim E, Kelly P, Ling DC, Olsen JR, Palta M, Raldow AC, Ruiz-Garcia E, Sheybani A, Stitzenberg KB, Das P. Radiation Therapy for Rectal Cancer: An ASTRO Clinical Practice Guideline Focused Update. Pract Radiat Oncol. 2025;15:124-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 127. | Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, Verheij FS, Omer DM, Lee M, Dunne RF, Marcet J, Cataldo P, Polite B, Herzig DO, Liska D, Oommen S, Friel CM, Ternent C, Coveler AL, Hunt S, Gregory A, Varma MG, Bello BL, Carmichael JC, Krauss J, Gleisner A, Paty PB, Weiser MR, Nash GM, Pappou E, Guillem JG, Temple L, Wei IH, Widmar M, Lin S, Segal NH, Cercek A, Yaeger R, Smith JJ, Goodman KA, Wu AJ, Saltz LB. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J Clin Oncol. 2022;40:2546-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 636] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 128. | Goffredo P, Quezada-Diaz FF, Garcia-Aguilar J, Smith JJ. Non-Operative Management of Patients with Rectal Cancer: Lessons Learnt from the OPRA Trial. Cancers (Basel). 2022;14:3204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 129. | Sclafani F, Corrò C, Koessler T. Debating Pros and Cons of Total Neoadjuvant Therapy in Rectal Cancer. Cancers (Basel). 2021;13:6361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Conces ML, Mahipal A. Adoption of Total Neoadjuvant Therapy in the Treatment of Locally Advanced Rectal Cancer. Curr Oncol. 2024;31:366-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 131. | López-Campos F, Martín-Martín M, Fornell-Pérez R, García-Pérez JC, Die-Trill J, Fuentes-Mateos R, López-Durán S, Domínguez-Rullán J, Ferreiro R, Riquelme-Oliveira A, Hervás-Morón A, Couñago F. Watch and wait approach in rectal cancer: Current controversies and future directions. World J Gastroenterol. 2020;26:4218-4239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (3)] |
| 132. | Alexandrescu ST, Dumitru AV, Babiuc RD, Costea RV. Assessment of clinical and pathological complete response after neoadjuvant chemoradiotherapy in rectal adenocarcinoma and its therapeutic implications. Rom J Morphol Embryol. 2021;62:411-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 133. | Wang Y, Yang L, Bao H, Fan X, Xia F, Wan J, Shen L, Guan Y, Bao H, Wu X, Xu Y, Shao Y, Sun Y, Tong T, Li X, Xu Y, Cai S, Zhu J, Zhang Z. Utility of ctDNA in predicting response to neoadjuvant chemoradiotherapy and prognosis assessment in locally advanced rectal cancer: A prospective cohort study. PLoS Med. 2021;18:e1003741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 134. | On J, Shim J, Aly EH. Systematic review and meta-analysis on outcomes of salvage therapy in patients with tumour recurrence during 'watch and wait' in rectal cancer. Ann R Coll Surg Engl. 2019;101:441-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 135. | Figueiredo N, Panteleimonitis S, Popeskou S, Cunha JF, Qureshi T, Beets GL, Heald RJ, Parvaiz A. Delaying surgery after neoadjuvant chemoradiotherapy in rectal cancer has no influence in surgical approach or short-term clinical outcomes. Eur J Surg Oncol. 2018;44:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 136. | Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386:2363-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1087] [Article Influence: 271.8] [Reference Citation Analysis (0)] |
| 137. | Cercek A, Sinopoli JC, Shia J, Weiss JA, Temple L, Smith JJ, Saltz LB, Widmar M, Fumo G, Aparo S, Romesser PB, Walch HS, Patel M, Jayaprakasam VS, Kim T, Paty P, Gonen M, Garcia-Aguilar J, Weiser MR, Diaz LA. Durable complete responses to PD-1 blockade alone in mismatch repair deficient locally advanced rectal cancer. J Clin Oncol. 2024;42:LBA3512-LBA3512. [DOI] [Full Text] |
| 138. | Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, Liu M, Lin JZ, Wang FL, Li C, Quan TT, Xi SY, Zhang HZ, Pan ZZ, Wang F, Xu RH. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol. 2023;8:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 97] [Reference Citation Analysis (0)] |
| 139. | Ludford K, Ho WJ, Thomas JV, Raghav KPS, Murphy MB, Fleming ND, Lee MS, Smaglo BG, You YN, Tillman MM, Kamiya-Matsuoka C, Thirumurthi S, Messick C, Johnson B, Vilar E, Dasari A, Shin S, Hernandez A, Yuan X, Yang H, Foo WC, Qiao W, Maru D, Kopetz S, Overman MJ. Neoadjuvant Pembrolizumab in Localized Microsatellite Instability High/Deficient Mismatch Repair Solid Tumors. J Clin Oncol. 2023;41:2181-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 132] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 140. | Tosi F, Salvatore L, Tamburini E, Artale S, Lonardi S, Marchetti S, Pastorino A, Pietrantonio F, Puccini A, Rojas-Llimpe FL, Vincenzi B, Mariano S, Negri F, Bencardino K, Pinto C, Aschele C, Siena S. Curative immune checkpoint inhibitors therapy in patients with mismatch repair-deficient locally advanced rectal cancer: a real-world observational study. ESMO Open. 2024;9:103929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 141. | Wang QX, Xiao BY, Cheng Y, Wu AW, Zhang T, Wang H, Zhang X, Huang WX, Tang JH, Jiang W, Steele SR, Krishnamurthi S, Li Y, Cai J, Kong LH, Li DD, Pan ZZ, Zhang XS, Ding PR. Anti-PD-1-based immunotherapy as curative-intent treatment in dMMR/MSI-H rectal cancer: A multicentre cohort study. Eur J Cancer. 2022;174:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 142. | Zhang H, Huang J, Xu H, Yin N, Zhou L, Xue J, Ren M. Neoadjuvant immunotherapy for DNA mismatch repair proficient/microsatellite stable non-metastatic rectal cancer: a systematic review and meta-analysis. Front Immunol. 2025;16:1523455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 143. | George TJ, Franke AJ, Chakravarthy AB, Das P, Dasari A, El-Rayes BF, Hong TS, Kinsella TJ, Landry JC, Lee JJ, Monjazeb AM, Jacobs SA, Raben D, Rahma OE, Williams TM, Wu C, Coleman CN, Vikram B, Ahmed MM. National Cancer Institute (NCI) state of the science: Targeted radiosensitizers in colorectal cancer. Cancer. 2019;125:2732-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 144. | Dienstmann R, Guinney J, Delorenzi M, De Reynies A, Roepman P, Sadanandam A, Vermeulen L, Schlicker A, Missiaglia E, Soneson C, Marisa L, Homicsko K, Wang X, Simon I, Laurent-Puig P, Wessels LFA, Medema JP, Kopetz S, Friend SH, Tejpar S; Colorectal Cancer Subtyping Consortium. Colorectal Cancer Subtyping Consortium (CRCSC) identification of a consensus of molecular subtypes. J Clin Oncol. 2014;32:3511-3511. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/