Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.107796
Revised: April 22, 2025
Accepted: July 14, 2025
Published online: September 27, 2025
Processing time: 179 Days and 20.2 Hours
Chronic postsurgical pain (CPSP) following gastrointestinal (GI) surgery is a common issue that poses significant challenges to patients’ recovery and quality of life. Given the importance of vitamin D in inflammation reduction, nerve repair, bone health, and immune regulation, its potential role in pain management has gained increasing attention. Preliminary evidence suggests that many patients who undergo GI surgery have lower perioperative vitamin D levels. Patients with vitamin D deficiency have increased opioid use and heightened pain sensitivity after colorectal cancer surgery. Patients with lower vitamin D levels experience greater pain three months after arthroscopic rotator cuff repair or video-assisted thoracoscopic surgery. However, research on the relationship between vitamin D and CPSP after GI surgery is limited. Larger, well-designed clinical trials are needed to determine the causal relationship between low vitamin D levels and CPSP, determine the optimal perioperative vitamin D levels, and provide more reliable evidence for clinical application. Moreover, vitamin D has positive effects on various systemic diseases and postoperative recovery, including immune regulation, infection prevention, wound healing, tissue regeneration, nutritional status, and metabolic health. These findings indicate that vitamin D has broad clinical application potential. We hope to provide a new insight into postoperative recovery and pain management strategies for GI surgeries.
Core Tip: Vitamin D may play a beneficial role in managing chronic pain following gastrointestinal surgery and postoperative recovery by modulating immune responses, aiding neural repair, and regulating gastrointestinal function. While preliminary evidence is promising, further large-scale clinical trials are essential to confirm its effects in these patients.
- Citation: Cheng CC, Yu L, Zheng N, Zhang F, Liao Q. Role of vitamin D in the management of chronic pain after gastrointestinal surgery. World J Gastrointest Surg 2025; 17(9): 107796
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/107796.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.107796
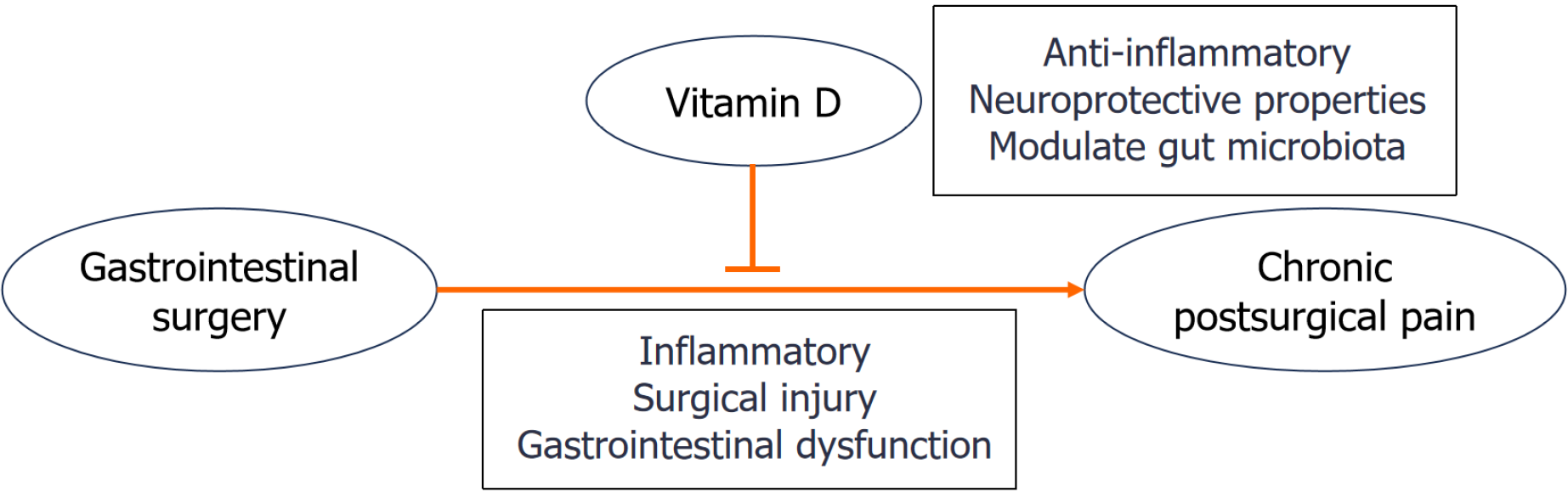
Gastrointestinal (GI) surgeries, including gastrectomy, colectomy and bariatric procedures, are commonly performed to treat conditions such as cancer, inflammatory bowel disease, and obesity[1]. While these surgeries can be life-saving, up to 30% of patients experience chronic postsurgical pain (CPSP) lasting more than 3-6 months[2]. This pain is often caused by multiple factors, including surgical injury, inflammation and GI dysfunction[3,4]. Vitamin D has neuroprotective, anti-inflammatory, and gut microbiota modulatory properties[5]. Recent studies have highlighted vitamin D deficiency as a modifiable risk factor for CPSP[6,7]. Low levels of vitamin D have been associated with increased pain sensitivity in various conditions, such as fibromyalgia, neuropathy, and chronic musculoskeletal pain[8-11]. It is thought to be linked to the inflammatory and neurogenic effects of low vitamin D[8]. In the context of postoperative pain, vitamin D deficiency may exacerbate pain and hinder recovery[12].
To date, there has been limited research specifically establishing a link between vitamin D and chronic pain after GI surgery. This review examines the mechanisms of chronic pain after GI surgeries and the role of vitamin D in pain modulation. Given the similar mechanisms underlying CPSP across different surgeries such as nerve injury and inflammation, we extrapolate findings from other surgical fields to GI surgery.
Vitamin D is a fat-soluble steroid that plays a critical role in numerous physiological processes. It is synthesized in the skin upon sunlight exposure and can also be obtained from dietary sources such as fatty fish, fortified foods, and supplements[13]. Once synthesized or ingested, vitamin D is converted into its active form, calcitriol (1,25-dihydroxyvitamin D), in the liver and kidneys. Calcitriol binds to vitamin D receptors (VDRs) in various cells, including immune cells, neurons, and GI cells. Upon activation, the VDR, a nuclear transcription factor, forms a heterodimer with the retinoid X receptor. This complex then binds to vitamin D response elements in target genes, modulating gene expression to regulate bone metabolism, immune responses, inflammatory pathways, neuronal health, and gut function[5,14].
Vitamin D may improve chronic pain after GI surgery, mainly by regulating the immune system, nervous system, and digestive system.
Immune system: Vitamin D plays a key role in regulating the immune system by balancing proinflammatory and anti-inflammatory responses[15,16]. It inhibits proinflammatory cytokines, including tumor necrosis factor-α and interleukin-6, which play crucial roles in pain pathways[17]. Moreover, vitamin D promotes the production of anti-inflammatory cytokines such as interleukin-10, helping to counteract excessive inflammation[18], a common trigger of chronic pain[17,18]. This balance is crucial in conditions where inflammation contributes to pain, such as autoimmune diseases and chronic pain conditions. By regulating the immune system to ensure a balance between proinflammatory and anti-inflammatory signals[15,16], vitamin D can help alleviate inflammation-related pain.
Nervous system: Vitamin D influences neuronal function by modulating the expression of nerve growth factor, which is essential for neuron growth, maintenance, and survival[19]. Additionally, VDRs are activated by vitamin D to regulate the calcium balance within neurons, which is essential for proper nerve function and pain perception. It also helps reduce neuroinflammation, which can contribute to neuropathic pain[18]. By suppressing neuroinflammation, vitamin D may alleviate pain associated with nerve damage or dysfunction[20], making it particularly beneficial for postoperative pain management. Finally, as a novel endogenous regulator, vitamin D also modulates the expression of pain-related genes, such as transient receptor potential vanilloid 1 channel (TRPV1) and glial cell line-derived neurotrophic factor[21,22]. By regulating the expression of these pain-related genes, vitamin D can effectively diminish pain sensitivity, thereby alleviating chronic pain[18].
Digestive system: Vitamin D plays a crucial role in regulating the gut-brain axis by modulating microbiome composition, influencing immune responses and supporting neuronal health[23]. This regulation is vital for GI function, especially in conditions such as visceral hypersensitivity, where abdominal organs become overly sensitive to stimuli[24]. Specifically, vitamin D may help alleviate the visceral pain commonly associated with irritable bowel syndrome (IBS) by enhancing intestinal integrity, promoting ion exchange, restoring epithelial function, and protecting against inflammatory damage[25].
Vitamin D supplementation can relieve chronic pain[26]. Vitamin D plays a crucial role in chronic pain management because of its anti-inflammatory, neuroprotective, and microbiome-regulating effects[27,28].
Anti-inflammatory role in chronic pain: In the context of chronic pain, the nuclear factor kappa-B (NF-κB) signaling pathway is crucial for triggering the release of proinflammatory cytokines and sensitizing pain receptors[29]. By reducing NF-κB activity, vitamin D helps alleviate inflammation and decreases pain perception[30,31]. Preclinical animal studies have demonstrated that vitamin D can reduce visceral pain in patients with inflammatory bowel diseases[32-35]. These findings suggest that vitamin D may offer potential therapeutic benefits for individuals with chronic pain associated with inflammation.
Neuroprotection and pain alleviation: In a rat model of peroneal nerve injury, vitamin D promoted axonal sparing/regeneration and improved physiological maturation. Vitamin D can enhance myelination and nerve function[36]. By interacting with the VDR in dorsal root ganglia and spinal cord neurons[5], vitamin D helps maintain calcium balance in neurons and regulates pain-related genes such as TRPV1, thereby maintaining normal neuronal function, reducing pain sensitivity, and supporting the recovery of neurological function after injury or surgery[21,37].
Gut microbiota and pain: The gut microbiome plays crucial roles in systemic inflammation and pain signaling[38-40]. In humans, the gut microbiome has been implicated in the onset of postoperative pain. Concurrently, animal research has highlighted the pivotal role of the gut microbiome in neuropathic pain, which is mediated by immunomodulatory processes[41]. However, the composition of the gut microbiome changes after general anesthesia and surgery[41]. In individuals with vitamin D deficiency, the impact of microbial imbalance may be more pronounced[42,43]. Individuals with sufficient vitamin D levels are better equipped to maintain a balanced microbiome, potentially reducing the severity of pain after surgery or in conditions such as IBS[44]. A study showed that regulating the intestinal flora can help alleviate chronic pain in rats after spared nerve injury[45]. Vitamin D may prevent dysbiosis (an imbalance in gut bacteria) after GI surgery[46,47], thereby reducing inflammation and subsequent pain[48].
During GI surgery, various forms of surgical trauma, such as tissue manipulation, nerve damage, and inflammation, can lead to chronic pain[49]. This pain may persist long after healing, often due to scar tissue, adhesions, or nerve entrapment[50].
Preoperative factors may increase the risk of CPSP. Patients with chronic pain conditions, such as fibromyalgia or a history of abdominal pain, are more prone to experience ongoing pain[51]. High levels of psychological stress, anxiety, or depression can exacerbate pain perception and make postoperative pain management more challenging. Preoperative malnutrition can also impede wound healing[52]. Certain nutrient deficiencies, such as vitamin D deficiency, are particularly common in patients undergoing GI surgery, especially in those with Crohn’s disease or obesity[53-57]. Vitamin D plays a key role in immune function, tissue repair, and pain regulation[5]. Low levels of vitamin D (below 20 ng/mL) can increase inflammation, increase pain sensitivity and slow tissue repair, leading to increased pain and delayed recovery.
Surgical procedures, especially laparoscopic surgeries, may inadvertently damage surrounding nerves, leading to persistent neuropathic pain or burning sensation. After surgery, scar tissue (adhesions) may form internally, causing organs or tissues that should remain separate to become attached. These adhesions can lead to lifelong complications, including adhesive small bowel obstruction (ASBO), chronic pain, infertility, and difficulties during reoperations[58]. Additionally, abdominal organs may become abnormally sensitive after surgery. This heightened sensitivity may manifest as increased pain from normal abdominal sensations, a condition known as visceral hypersensitivity[59]. This hypersensitivity is closely linked to GI dysfunction and clinical pain syndromes, such as postoperative ileus, ASBO-related pain, or visceral pain after colectomy[59-61].
Inflammation plays a key role in the development of CPSP. Even after surgery, inflammation may persist due to surgical trauma[62]. This ongoing inflammation can be triggered by the changes in the gut microbiota or immune system dysregulation, both of which can prolong pain[63]. Additionally, a prolonged immune response can cause tissue damage, exacerbate pain, and delay recovery[64].
Several studies have shown that chronic pain after GI surgery is associated with surgical injury, inflammation, and GI dysfunction (Table 1)[2,4,65-70]. Although there is no strong evidence linking vitamin D to this pain, many patients who undergo GI surgery have low vitamin D levels during the perioperative period[71-73]. In a prospective observational study involving 112 patients who underwent colorectal cancer surgery, patients with vitamin D levels less than 20 ng/mL had increased perioperative opioid use and heightened pain sensitivity compared with those with vitamin D levels ≥ 20 ng/mL[74]. In addition, a cohort study involving 89 patients who underwent arthroscopic rotator cuff repair surgery revealed that patients with lower vitamin D levels experienced greater pain three months after surgery[6]. In video-assisted thoracoscopic surgery, low vitamin D levels were associated with higher pain scores at three months after surgery[12]. These studies highlight that vitamin D deficiency may lead to higher rates of acute and chronic pain after surgery.
| GI surgery | Finding | Related factors | Ref. |
| Hernia repair surgery | Laparo-endoscopic hernia surgery leads to a lower incidence of chronic inguinal pain compared to Lichtenstein repair | Surgical injury (tissue injury, nerve damage) | Lillo-Albert et al[65] |
| Roux-en-Y gastric bypass | A substantial proportion of patients experienced chronic abdominal pain and symptoms 5 years after Roux-en-Y gastric bypass | Gastrointestinal dysfunction | Høgestøl et al[4]; Blom-Høgestøl et al[66] |
| Lower gastrointestinal tract surgery | In one study following 198 patients after for adhesive small bowel obstruction, 40% of patients developed chronic abdominal pain | Surgical injury; Inflammation | ten Broek et al[67] |
| Colorectal surgery | CPSP was reported by 32.1% of patients at 3 months and 21.8% at 6 months after colorectal surgery | Surgical injury | Jin et al[2] |
| Total pancreactectomy | Symptoms of chronic gastrointestinal dysmotility and chronic abdominal pain are common post-surgery | Gastrointestinal dysfunction | John et al[68] |
| Gastrointestinal laparotomy | The prevalence of chronic pain four years after surgery was 18%, with pain predominantly of a neuropathic nature | Surgical injury (nerve damage) | Bruce and Krukowski[69] |
| Bariatric surgery | Chronic abdominal pain was the most prevalent complication to bariatric surgery | Surgical injury; Gastrointestinal dysfunction | Simoni et al[70] |
A meta-analysis of studies on bariatric bypass surgery revealed that vitamin D supplementation effectively reduced chronic axial back pain in patients after surgery[75]. However, a randomized clinical trial examining brain tumor surgery reported inconsistent results, suggesting that the effects of vitamin D supplements vary across different types of surgeries[76].
In summary, observational studies have shown that vitamin D deficiency may increase the risk of pain and opioid dependency after surgery[77], and vitamin D supplementation can help reduce pain and opioid consumption[78]. However, more research is needed to understand its effects on pain after GI surgery.
Postoperative recovery represents a critical phase following surgery, during which the body initiates a complex series of physiological processes to repair and regenerate tissues. Notably, vitamin D has emerged as a promising candidate for optimizing these recovery processes.
Vitamin D plays a pivotal role in modulating the immune system, particularly during the postoperative recovery phase. Studies have shown that vitamin D can enhance the function of immune cells, including macrophages and T cells, thereby reducing the risk of postoperative infections[79,80]. By upregulating the expression of antimicrobial peptides, vitamin D helps strengthen the body’s innate immune defenses, providing a robust shield against potential pathogens[81].
Surgical procedures inherently carry a risk of infection, which can prolong the recovery period, complicate the rehabilitation process, and prolong the hospital stay. The anti-inflammatory and antimicrobial properties of vitamin D make it a valuable asset for preventing postoperative infections. Clinical studies have shown that supplementation with vitamin D may lower the incidence of surgical site infections and other postoperative complications[82].
Vitamin D is also involved in the process of wound healing and tissue regeneration[83]. It facilitates the differentiation of mesenchymal stem cells into osteoblasts and a variety of other cell types and is essential for the repair of bone and soft tissues[83,84]. Moreover, vitamin D helps increase collagen synthesis and reduce scar formation[85], thereby enhancing both aesthetic outcomes and functional performance.
Patients often face nutritional stress, which can severely affect their recovery after surgery. Vitamin D helps improve nutritional status by enhancing calcium absorption and promoting bone mineralization[86,87]. Additionally, vitamin D may also affect metabolic processes[88], thus profoundly affecting the overall recovery process after GI surgery.
Accumulating evidence indicates that vitamin D has expansive roles in postoperative recovery, extending beyond its well-known benefits for bone health. It has the potential to modulate the immune system, prevent infections, facilitate wound healing, and support nutritional and metabolic health, making vitamin D a promising therapeutic agent for perioperative care.
The use of vitamin D supplementation in surgery presents several challenges due to methodological heterogeneity across studies, which complicates the interpretation of findings. The main sources of variability include the type of surgery, pain assessment methods, timing of vitamin D measurement, and supplementation protocols.
For example, the type of surgery (e.g., orthopedic vs abdominal surgeries) can influence pain outcomes and the body’s response to vitamin D, and different methods of pain assessment, such as self-report scales and objective measurements, may lead to inconsistent results. The timing of vitamin D measurement, from pre-surgery to post-surgery, is also a critical factor, as surgical stress can cause fluctuations in vitamin D levels. Furthermore, supplementation protocols (dosage and duration) vary widely, hindering the ability to determine the optimal supplementation regimen.
Moreover, the pain mechanisms differ across surgeries, such as laparoscopic and open procedures, making it challenging to generalize the findings. Other factors, including obesity, malnutrition, and comorbidities, can also affect vitamin D levels and pain outcomes. To address these challenges, future research should focus on large-scale trials of patients with GI surgery who are vitamin D-deficient, explore the role of vitamin D in gut-nerve interactions and the microbiome’s impact on pain, and develop standardized clinical guidelines for vitamin D screening and supplementation during surgical care. These efforts will help provide more reliable, generalizable conclusions and improve clinical practices for surgical patients.
The limitations of this study include the lack of clinical trials specifically examining the impact of vitamin D on postoperative pain in patients undergoing GI surgery. Most of the available studies are observational in nature and involve diverse patient populations, which may introduce potential biases and limit the generalizability of the findings. Furthermore, there is a significant gap in research that directly investigates the role of vitamin D in pain management within the context of GI surgery. These limitations underscore the necessity for more focused and targeted research in GI surgery settings to better understand the value of vitamin D in this specific patient group.
Chronic pain after GI surgery is influenced by various factors, including surgical trauma, inflammation, pre-existing health conditions, and nutritional status. Vitamin D may improve CPSP and promote postoperative recovery after GI surgery through immune regulation, neuroprotection and regulation of GI function (Figure 1). However, further research is needed to determine the optimal methods for integrating vitamin D into postoperative recovery and pain management strategies.
| 1. | Utrilla Fornals A, Costas-Batlle C, Medlin S, Menjón-Lajusticia E, Cisneros-González J, Saura-Carmona P, Montoro-Huguet MA. Metabolic and Nutritional Issues after Lower Digestive Tract Surgery: The Important Role of the Dietitian in a Multidisciplinary Setting. Nutrients. 2024;16:246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Jin J, Chen Q, Min S, Du X, Zhang D, Qin P. Prevalence and predictors of chronic postsurgical pain after colorectal surgery: A prospective study. Colorectal Dis. 2021;23:1878-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Gu D, Xia Y, Ding Z, Qian J, Gu X, Bai H, Jiang M, Yao D. Inflammation in the Peripheral Nervous System after Injury. Biomedicines. 2024;12:1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 4. | Høgestøl IK, Chahal-Kummen M, Eribe I, Brunborg C, Stubhaug A, Hewitt S, Kristinsson J, Mala T. Chronic Abdominal Pain and Symptoms 5 Years After Gastric Bypass for Morbid Obesity. Obes Surg. 2017;27:1438-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Habib AM, Nagi K, Thillaiappan NB, Sukumaran V, Akhtar S. Vitamin D and Its Potential Interplay With Pain Signaling Pathways. Front Immunol. 2020;11:820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 6. | Chen J, Lou J, Wang W, Xu G. Association of Preoperative Vitamin D Deficiency With Retear Rate and Early Pain After Arthroscopic Rotator Cuff Repair: A Retrospective Cohort Study. Orthop J Sports Med. 2022;10:23259671221130315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 7. | Lee A, Chan SKC, Samy W, Chiu CH, Gin T. Effect of Hypovitaminosis D on Postoperative Pain Outcomes and Short-Term Health-Related Quality of Life After Knee Arthroplasty: A Cohort Study. Medicine (Baltimore). 2015;94:e1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Alonso-Pérez JL, Martínez-Pérez I, Romero-Morales C, Abuín-Porras V, López-Bueno R, Rossettini G, Leigheb M, Villafañe JH. Relationship Between Serum Vitamin D Levels and Chronic Musculoskeletal Pain in Adults: A Systematic Review. Nutrients. 2024;16:4061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Ali OME. Prevalence of Vitamin D Deficiency and Its Relationship with Clinical Outcomes in Patients with Fibromyalgia: a Systematic Review of the Literature. SN Compr Clin Med. 2022;4:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Lakkireddy M, Karra ML, Patnala C, Iyengar R, Cherukuri N, Hussain KSA, Chodavarapu LM, Kiran Kumar KK, Aluka SK, Bodla AK, Badavath RR, Peddamadyam SK. Efficiency of vitamin D supplementation in patients with mechanical low back ache. J Clin Orthop Trauma. 2019;10:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Yong WC, Sanguankeo A, Upala S. Effect of vitamin D supplementation in chronic widespread pain: a systematic review and meta-analysis. Clin Rheumatol. 2017;36:2825-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Zeng X, Chen X, Li C, Shi H. Preoperative Vitamin D Level is Associated with Acute Pain After Video-Assisted Thoracoscopic Surgery: A Retrospective Cohort Study. J Pain Res. 2022;15:3189-3196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Janoušek J, Pilařová V, Macáková K, Nomura A, Veiga-Matos J, Silva DDD, Remião F, Saso L, Malá-Ládová K, Malý J, Nováková L, Mladěnka P. Vitamin D: sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit Rev Clin Lab Sci. 2022;59:517-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 14. | Fenercioglu AK. The Anti-Inflammatory Roles of Vitamin D for Improving Human Health. Curr Issues Mol Biol. 2024;46:13514-13525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (1)] |
| 15. | Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 851] [Cited by in RCA: 794] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 16. | Gominak SC. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a "pro-inflammatory" state associated with atherosclerosis and autoimmunity. Med Hypotheses. 2016;94:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Athanassiou L, Mavragani CP, Koutsilieris M. The Immunomodulatory Properties of Vitamin D. Mediterr J Rheumatol. 2022;33:7-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Chauss D, Freiwald T, McGregor R, Yan B, Wang L, Nova-Lamperti E, Kumar D, Zhang Z, Teague H, West EE, Vannella KM, Ramos-Benitez MJ, Bibby J, Kelly A, Malik A, Freeman AF, Schwartz DM, Portilla D, Chertow DS, John S, Lavender P, Kemper C, Lombardi G, Mehta NN, Cooper N, Lionakis MS, Laurence A, Kazemian M, Afzali B. Autocrine vitamin D signaling switches off pro-inflammatory programs of T(H)1 cells. Nat Immunol. 2022;23:62-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 19. | Dewi MM, Imron A, Risan NA, Mediana G, Judistiani RTD, Setiabudiawan B. The Association of Vitamin D, Nerve Growth Factor (NGF), Brain-Derived Neurotrophic Factor (BDNF), and Glial Cell-Derived Neurotrophic Factor (GDNF) with Development in Children. Children (Basel). 2025;12:60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Plantone D, Primiano G, Manco C, Locci S, Servidei S, De Stefano N. Vitamin D in Neurological Diseases. Int J Mol Sci. 2022;24:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Long W, Fatehi M, Soni S, Panigrahi R, Philippaert K, Yu Y, Kelly R, Boonen B, Barr A, Golec D, Campbell SA, Ondrusova K, Hubert M, Baldwin T, Lemieux MJ, Light PE. Vitamin D is an endogenous partial agonist of the transient receptor potential vanilloid 1 channel. J Physiol. 2020;598:4321-4338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Pertile RAN, Cui X, Hammond L, Eyles DW. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J. 2018;32:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Ogbu D, Xia E, Sun J. Gut instincts: vitamin D/vitamin D receptor and microbiome in neurodevelopment disorders. Open Biol. 2020;10:200063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Teng M, Li Y, Zhao X, White JC, Zhao L, Sun J, Zhu W, Wu F. Vitamin D modulation of brain-gut-virome disorder caused by polystyrene nanoplastics exposure in zebrafish (Danio rerio). Microbiome. 2023;11:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Uberti F, Trotta F, Cavalli R, Galla R, Caldera F, Ferrari S, Mulè S, Brovero A, Molinari C, Pagliaro P, Penna C. Enhancing Vitamin D3 Efficacy: Insights from Complexation with Cyclodextrin Nanosponges and Its Impact on Gut-Brain Axes in Physiology and IBS Syndrome. Int J Mol Sci. 2024;25:2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 26. | Putz Z, Tordai D, Hajdú N, Vági OE, Kempler M, Békeffy M, Körei AE, Istenes I, Horváth V, Stoian AP, Rizzo M, Papanas N, Kempler P. Vitamin D in the Prevention and Treatment of Diabetic Neuropathy. Clin Ther. 2022;44:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Vernia F, Valvano M, Longo S, Cesaro N, Viscido A, Latella G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients. 2022;14:269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 28. | Saija C, Bertuccio MP, Scoglio A, Macaione V, Cacciola F, Micalizzi G, Caccamo D, Muscoli C, Currò M. Role of Vitamin D Status and Alterations in Gut Microbiota Metabolism in Fibromyalgia-Associated Chronic Inflammatory Pain. Biomedicines. 2025;13:139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Kim DH, Meza CA, Clarke H, Kim JS, Hickner RC. Vitamin D and Endothelial Function. Nutrients. 2020;12:575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 30. | Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J Biol Chem. 2013;288:19450-19458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 31. | Huang D, Guo Y, Li X, Pan M, Liu J, Zhang W, Mai K. Vitamin D(3)/VDR inhibits inflammation through NF-κB pathway accompanied by resisting apoptosis and inducing autophagy in abalone Haliotis discus hannai. Cell Biol Toxicol. 2023;39:885-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Meeker S, Seamons A, Maggio-Price L, Paik J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J Gastroenterol. 2016;22:933-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 33. | Meeker S, Seamons A, Paik J, Treuting PM, Brabb T, Grady WM, Maggio-Price L. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res. 2014;74:4398-4408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 34. | Mariani M, Viganò P, Gentilini D, Camisa B, Caporizzo E, Di Lucia P, Monno A, Candiani M, Somigliana E, Panina-Bordignon P. The selective vitamin D receptor agonist, elocalcitol, reduces endometriosis development in a mouse model by inhibiting peritoneal inflammation. Hum Reprod. 2012;27:2010-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Xiong X, Cheng Z, Zhou Y, Wu F, Xie L, Lawless L, Dong R, Zhao Y, Yu L, Chen G. HuanglianGanjiang Tang alleviates DSS-induced colitis in mice by inhibiting necroptosis through vitamin D receptor. J Ethnopharmacol. 2022;298:115655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 36. | Chabas JF, Stephan D, Marqueste T, Garcia S, Lavaut MN, Nguyen C, Legre R, Khrestchatisky M, Decherchi P, Feron F. Cholecalciferol (vitamin D₃) improves myelination and recovery after nerve injury. PLoS One. 2013;8:e65034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Shi Y, Shi Y, Jie R, He J, Luo Z, Li J. Vitamin D: The crucial neuroprotective factor for nerve cells. Neuroscience. 2024;560:272-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Dimitrov V, White JH. Vitamin D signaling in intestinal innate immunity and homeostasis. Mol Cell Endocrinol. 2017;453:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Pagnini C, Di Paolo MC, Graziani MG, Delle Fave G. Probiotics and Vitamin D/Vitamin D Receptor Pathway Interaction: Potential Therapeutic Implications in Inflammatory Bowel Disease. Front Pharmacol. 2021;12:747856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Del Pinto R, Ferri C, Cominelli F. Vitamin D Axis in Inflammatory Bowel Diseases: Role, Current Uses and Future Perspectives. Int J Mol Sci. 2017;18:2360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Minerbi A, Shen S. Gut Microbiome in Anesthesiology and Pain Medicine. Anesthesiology. 2022;137:93-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 42. | Xia Y, Zhou J, Zhao HM, You JY. [Mechanism of action and exogenous supplementation of vitamin D in Crohn's disease]. Zhongguo Dang Dai Er Ke Za Zhi. 2023;25:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Ramasamy B, Magne F, Tripathy SK, Venugopal G, Mukherjee D, Balamurugan R. Association of Gut Microbiome and Vitamin D Deficiency in Knee Osteoarthritis Patients: A Pilot Study. Nutrients. 2021;13:1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Matthews SW, Heitkemper MM, Kamp K. Early Evidence Indicates Vitamin D Improves Symptoms of Irritable Bowel Syndrome: Nursing Implications and Future Research Opportunities. Gastroenterol Nurs. 2021;44:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Li S, Huang J, Luo D, Fu W, Liu J. Electro-acupuncture inhibits HDAC2 via modulating gut microbiota to ameliorate SNI-induced pain and depression-like behavior in rats. J Affect Disord. 2024;360:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Yang Y, Zhou HY, Zhou GM, Chen J, Ming R, Zhang D, Jiang HW. The impact of different gastrointestinal reconstruction techniques on gut microbiota after gastric cancer surgery. Front Microbiol. 2024;15:1494049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 47. | Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. 2017;14:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 48. | Masaud K, Collins JM, Rubio RC, Corrigan M, Cotter PD, O'Brien N, Bluett R, Jimenez CK, O'Mahony SM, Shorten GD. The gut microbiota in persistent post-operative pain following breast cancer surgery. Sci Rep. 2024;14:12401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Strik C, van den Beukel B, van Rijckevorsel D, Stommel MWJ, Ten Broek RPG, van Goor H. Risk of Pain and Gastrointestinal Complaints at 6Months After Elective Abdominal Surgery. J Pain. 2019;20:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Milkias M, Mekonnen S, Ahmed S, Getachew H, Adamu Y, Mola S, Gugsa T. Evidence-based guideline on chronic postsurgical pain management in adult patients in resource-restricted setting, 2023: systematic review and guideline. Ann Med Surg (Lond). 2023;85:5593-5603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 51. | Fregoso G, Wang A, Tseng K, Wang J. Transition from Acute to Chronic Pain: Evaluating Risk for Chronic Postsurgical Pain. Pain Physician. 2019;22:479-488. [PubMed] |
| 52. | Zhang Q, Sun H, Xin Y, Li X, Shao X. Studies on Pain Associated with Anxiety or Depression in the Last 10 Years: A Bibliometric Analysis. J Pain Res. 2024;17:133-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 53. | Karampela I, Sakelliou A, Vallianou N, Christodoulatos GS, Magkos F, Dalamaga M. Vitamin D and Obesity: Current Evidence and Controversies. Curr Obes Rep. 2021;10:162-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 54. | Lespessailles E, Toumi H. Vitamin D alteration associated with obesity and bariatric surgery. Exp Biol Med (Maywood). 2017;242:1086-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Soheilipour F, Hamidabad NM. Vitamin D and Calcium Status Among Adolescents with Morbid Obesity Undergoing Bariatric Surgery. Obes Surg. 2022;32:738-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 56. | Ananthakrishnan AN, Cagan A, Gainer VS, Cai T, Cheng SC, Savova G, Chen P, Szolovits P, Xia Z, De Jager PL, Shaw SY, Churchill S, Karlson EW, Kohane I, Plenge RM, Murphy SN, Liao KP. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn's disease. Inflamm Bowel Dis. 2013;19:1921-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 57. | Grant WB. Lower Vitamin D Status May Explain why African Americans Have Poorer Outcomes than Non-African Americans After Surgery for Crohn's Disease. J Crohns Colitis. 2017;11:761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Ten Broek RPG, Toneman MK, van Goor H. [Adhesions after abdominal surgery: developments in diagnosis and treatment]. Ned Tijdschr Geneeskd. 2023;167:D7320. [PubMed] |
| 59. | Xie GS, Ma L, Zhong JH. Recovery of gastrointestinal functional after surgery for abdominal tumors: A narrative review. Medicine (Baltimore). 2024;103:e40418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 60. | Bayrer JR, Castro J, Venkataraman A, Touhara KK, Rossen ND, Morrie RD, Maddern J, Hendry A, Braverman KN, Garcia-Caraballo S, Schober G, Brizuela M, Castro Navarro FM, Bueno-Silva C, Ingraham HA, Brierley SM, Julius D. Gut enterochromaffin cells drive visceral pain and anxiety. Nature. 2023;616:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 61. | Xie Z, Feng J, Hibberd TJ, Chen BN, Zhao Y, Zang K, Hu X, Yang X, Chen L, Brookes SJ, Spencer NJ, Hu H. Piezo2 channels expressed by colon-innervating TRPV1-lineage neurons mediate visceral mechanical hypersensitivity. Neuron. 2023;111:526-538.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 62. | Bhutta NK, Xu X, Jian C, Wang Y, Liu Y, Sun J, Han B, Wu S, Javeed A. Gut microbiota mediated T cells regulation and autoimmune diseases. Front Microbiol. 2024;15:1477187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (1)] |
| 63. | Kohl BA, Deutschman CS. The inflammatory response to surgery and trauma. Curr Opin Crit Care. 2006;12:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 64. | Matsuo K, Iwasa Y. Modeling Innate Immunity Causing Chronic Inflammation and Tissue Damage. Bull Math Biol. 2025;87:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 65. | Lillo-Albert G, Villa EB, Boscà-Robledo A, Carreño-Sáenz O, Bueno-Lledó J, Martínez-Hoed J, Pous-Serrano S. Chronic inguinal pain post-hernioplasty. Laparo-endoscopic surgery vs lichtenstein repair: systematic review and meta-analysis. Hernia. 2024;28:1427-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Blom-Høgestøl IK, Stubhaug A, Kristinsson JA, Mala T. Diagnosis and treatment of chronic abdominal pain 5 years after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2018;14:1544-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | ten Broek RP, Issa Y, van Santbrink EJ, Bouvy ND, Kruitwagen RF, Jeekel J, Bakkum EA, Rovers MM, van Goor H. Burden of adhesions in abdominal and pelvic surgery: systematic review and met-analysis. BMJ. 2013;347:f5588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 421] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 68. | John GK, Singh VK, Moran RA, Warren D, Sun Z, Desai N, Walsh C, Kalyani RR, Hall E, Hirose K, Makary MA, Stein EM. Chronic Gastrointestinal Dysmotility and Pain Following Total Pancreatectomy with Islet Autotransplantation for Chronic Pancreatitis. J Gastrointest Surg. 2017;21:622-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Bruce J, Krukowski ZH. Quality of life and chronic pain four years after gastrointestinal surgery. Dis Colon Rectum. 2006;49:1362-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Simoni AH, Ladebo L, Christrup LL, Drewes AM, Johnsen SP, Olesen AE. Chronic abdominal pain and persistent opioid use after bariatric surgery. Scand J Pain. 2020;20:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Giustina A, di Filippo L, Facciorusso A, Adler RA, Binkley N, Bollerslev J, Bouillon R, Casanueva FF, Cavestro GM, Chakhtoura M, Conte C, Donini LM, Ebeling PR, Fassio A, Frara S, Gagnon C, Latella G, Marcocci C, Mechanick JI, Minisola S, Rizzoli R, Santini F, Shaker JL, Sempos C, Ulivieri FM, Virtanen JK, Napoli N, Schafer AL, Bilezikian JP. Vitamin D status and supplementation before and after Bariatric Surgery: Recommendations based on a systematic review and meta-analysis. Rev Endocr Metab Disord. 2023;24:1011-1029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 72. | Mahamid A, Kazlow E, David AM, Abu-Zaydeh O, Abu Shtaya A, Froylich D, Khoury W, Sadot E, Haddad R. The Association between Preoperative Vitamin D Levels and Postoperative Complications in Patients Undergoing Colorectal Liver Metastasis Surgery. J Clin Med. 2023;13:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Musella M, Berardi G, Vitiello A, Dayan D, Schiavone V, Franzese A, Abu-Abeid A. Vitamin D Deficiency in Patients with Morbid Obesity before and after Metabolic Bariatric Surgery. Nutrients. 2022;14:3319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Xia J, Li D, Yu G, Xu B, Gao X, Wang H, Ma Y, Li X, Xiong Y. Effects of Hypovitaminosis D on Preoperative Pain Threshold and Perioperative Opioid Use in Colorectal Cancer Surgery: A Cohort Study. Pain Physician. 2022;25:E1009-E1019. [PubMed] |
| 75. | Epstein NE. Bariatric bypasses contribute to loss of bone mineral density but reduce axial back pain in morbidly obese patients considering spine surgery. Surg Neurol Int. 2017;8:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Hajimohammadebrahim-Ketabforoush M, Shahmohammadi M, Khoundabi B, Shariatpanahi ZV. Effect of Vitamin D Supplementation on Postcraniotomy Pain After Brain Tumor Surgery: A Randomized Clinical Trial. World Neurosurg. 2019;130:e105-e111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Bergman P, Sperneder S, Höijer J, Bergqvist J, Björkhem-Bergman L. Low vitamin D levels are associated with higher opioid dose in palliative cancer patients--results from an observational study in Sweden. PLoS One. 2015;10:e0128223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Laubner Sakalauskienė G, Stražnickaitė I, Miškinytė S, Zdanavičius L, Šipylaitė J, Badaras R. Baseline Vitamin D Levels on Quality of Life and Pain Perception Among Patients with Chronic Pain with Long-Term Prescription Opioid Use: A Prospective Study. J Clin Med. 2025;14:645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 79. | Martucci G, Volpes R, Panarello G, Tuzzolino F, Di Carlo D, Ricotta C, Gruttadauria S, Conaldi PG, Luca A, Amrein K, Arcadipane A. Vitamin D levels in liver transplantation recipients and early postoperative outcomes: Prospective observational DLiverX study. Clin Nutr. 2021;40:2355-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 80. | Meng J, Li X, Liu W, Xiao Y, Tang H, Wu Y, Xiong Y, Gao S. The role of vitamin D in the prevention and treatment of SARS-CoV-2 infection: A meta-analysis of randomized controlled trials. Clin Nutr. 2023;42:2198-2206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 81. | Meng J, Li X, Xiong Y, Wu Y, Liu P, Gao S. The role of vitamin D in the prevention and treatment of tuberculosis: a meta-analysis of randomized controlled trials. Infection. 2025;53:1129-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 82. | Abdehgah AG, Monshizadeh A, Tehrani MM, Afhami S, Molavi B, Jafari M, Nasiri S, Soroush A. Relationship Between Preoperative 25-Hydroxy Vitamin D and Surgical Site Infection. J Surg Res. 2020;245:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Bikle DD. Role of vitamin D and calcium signaling in epidermal wound healing. J Endocrinol Invest. 2023;46:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 84. | Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol. 2016;164:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Kotze J, Nortje E, Phulukdaree A, Fear MW, Wood F, Bester J. Unveiling the Link: The Potential Roles of Vitamin D in Keloid Pathophysiology. Exp Dermatol. 2025;34:e70043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 86. | Fleet JC. Vitamin D-Mediated Regulation of Intestinal Calcium Absorption. Nutrients. 2022;14:3351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 87. | Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 802] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 88. | Delrue C, Speeckaert MM. Vitamin D and Vitamin D-Binding Protein in Health and Disease. Int J Mol Sci. 2023;24:4642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 69] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/