Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.107977
Revised: May 14, 2025
Accepted: July 31, 2025
Published online: September 27, 2025
Processing time: 175 Days and 19.9 Hours
Parastomal hernia (PSH) is a common and challenging complication following preventive ostomy in rectal cancer patients, lacking accurate tools for early risk prediction.
To explore the application of machine learning algorithms in predicting the occurrence of PSH in patients undergoing preventive ostomy after rectal cancer resection, providing valuable support for clinical decision-making.
A retrospective analysis was conducted on the clinical data of 579 patients who underwent rectal cancer resection with preventive ostomy at Tongji Hospital, Huazhong University of Science and Technology, between January 2015 and June 2023. Various machine learning models were constructed and trained using pre
A total of 579 patients were included, with 31 (5.3%) developing PSH. Among the machine learning models, the random forest (RF) model showed the best performance. In the test set, the RF model achieved an area under the curve of 0.900, sensitivity of 0.900, and specificity of 0.725. SHAP analysis revealed that tumor distance from the anal verge, body mass index, and preoperative hypertension were the key factors influencing the occurrence of PSH.
Machine learning, particularly the RF model, demonstrates high accuracy and reliability in predicting PSH after preventive ostomy in rectal cancer patients. This technology supports personalized risk assessment and post
Core Tip: This research proposed and validated a predictive model based on machine learning techniques to assess the risk of parastomal hernia following prophylactic ostomy in individuals with rectal cancer. Among multiple algorithms, the random forest (RF) model achieved the best performance. SHapley Additive exPlanations identified tumor distance from the anal verge, body mass index, and preoperative hypertension as key predictors. An online risk prediction tool based on the RF model has been created to support early screening and individualized postoperative management, offering practical value for clinical decision-making.
- Citation: Yang WS, Su Y, Li YQ, Hu JB, Liu MD, Liu L. Prediction of parastomal hernia in patients undergoing preventive ostomy after rectal cancer resection using machine learning. World J Gastrointest Surg 2025; 17(9): 107977
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/107977.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.107977
Rectal cancer is one of the most common gastrointestinal malignancies, with its incidence rising globally year by year[1]. Particularly in developing countries, changes in lifestyle and diet have led to a trend of increasing incidence, especially among younger populations. With the promotion of total mesorectal excision and advancements in surgical techniques, more and more patients with mid- to low-rectal cancer can undergo sphincter-preserving surgery, significantly improving postoperative quality of life and survival rates[2,3]. However, despite these surgical advancements, post
However, preventive ileostomy is associated with a range of complications, one of the most common and challenging being parastomal hernia (PSH). PSH not only can lead to bowel obstruction, severely affecting the patient's quality of life, but in some cases, it can even be life-threatening[6-8]. Furthermore, PSH can cause changes in the patient's body image, increase psychological burden, and complicate nursing care, ultimately leading to higher healthcare costs. While known risk factors such as body mass index (BMI), age, sex, and surgical duration have been suggested to be associated with PSH development[9,10], the precise mechanisms behind its occurrence remain unclear. Traditional statistical methods have limitations when analyzing the influence of multiple factors, making it difficult to predict individual risk accurately[11].
With the rapid development of artificial intelligence and big data technologies, machine learning has become increasingly applied in the medical field for disease risk prediction and decision-making support[12]. Machine learning can uncover complex patterns within large clinical datasets, providing more accurate risk assessment tools for clinicians, thereby optimizing individualized treatment plans. This study aims to leverage machine learning techniques to develop a predictive model for PSH occurrence in patients undergoing preventive ileostomy following rectal cancer surgery. The goal is to provide new theoretical foundations and practical guidance for postoperative management, individualized treatment, and preventive interventions.
This retrospective study included patients who underwent rectal cancer resection with preventive ostomy at Tongji Hospital, Huazhong University of Science and Technology, from January 2015 to June 2023. The inclusion criteria were as follows: (1) Patients aged ≥ 18 years; (2) Preoperative diagnosis of rectal cancer with planned resection; (3) preventive ostomy performed during surgery; and (4) Availability of postoperative follow-up data for at least 18 months. The exclusion criteria were: (1) Presence of distant metastasis at the time of diagnosis; (2) Incomplete or insufficient follow-up data; (3) Patients with a history of abdominal radiation therapy prior to surgery; and (4) Patients with severe comorbidities that might interfere with postoperative recovery (e.g., advanced heart failure, active infection). Ultimately, 579 patients met the inclusion criteria and were included in the final analysis.
PSH after rectal cancer resection was diagnosed according to the guidelines recommended by the European Hernia Society. PSH is defined as an abnormal protrusion of abdominal contents through a defect in the abdominal wall caused by an ostomy[13]. Diagnosis was based on clinical presentation (e.g., abdominal bulge), physical examination (palpation of the abdominal defect), and imaging studies (e.g., computed tomography, ultrasound).
Preoperative and intraoperative variables were collected, including gender, age, American Society of Anesthesiologists score, BMI, smoking history, alcohol use, abdominal surgery history, comorbidities (e.g., diabetes, hypertension), preoperative tumor markers (carcinoembryonic antigen), hemoglobin, albumin, electrolytes, intraoperative blood loss, surgical approach, surgical duration, tumor stage, tumor size, tumor distance from the anal verge, and neoadjuvant treatment. All data were retrieved from the hospital's electronic medical record system and verified by three independent researchers for accuracy and completeness. To identify key predictors of PSH risk, the Boruta algorithm was employed. This algorithm evaluates the relative importance of each feature by comparing the original features with random shadow features, allowing for the identification of variables significantly associated with the risk of PSH[14].
Based on the selected features, several machine learning models were built to predict the risk of PSH in patients undergoing preventive ostomy after rectal cancer resection. Due to the complexity and variability in predicting this outcome, models including eXtreme gradient boosting (XGBoost), logistic regression (LR), random forest (RF), support vector machine (SVM), and k-nearest neighbors (KNN) were evaluated. The dataset was randomly split into training and testing sets in an 80:20 ratio, with 10-fold cross-validation used for model parameter optimization, and grid search applied to find the best parameters. Evaluation metrics included the area under the curve (AUC) from receiver operating characteristic analysis, sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV). Decision curve analysis (DCA) and calibration curves were used to assess the clinical utility of the models. The contribution of each feature to the prediction results was analyzed using SHapley Additive exPlanation (SHAP).
Data analysis and model construction were performed using R software (version 4.3.0) and Python software (version 3.11.6). Categorical data were presented as frequencies and percentages, and intergroup comparisons were performed using the χ2 test or Fisher's exact test. Continuous variables were expressed as mean ± SD, with comparisons between groups made using t-tests or Mann-Whitney U tests. A P-value < 0.05 was considered statistically significant.
A total of 579 patients were included in this study, with a 5.3% incidence of PSH. The patients were randomly assigned to either the training group (n = 405) or the testing group (n = 174) at a 7:3 ratio. Comparative analysis revealed no significant differences in baseline clinical characteristics between the two groups (P > 0.05), indicating that the datasets were representative and balanced (Table 1).
| Characteristic | All (n = 579) | Training cohort (n = 405) | Testing cohort (n = 174) | P value |
| Female | 207 (35.7) | 139 (34.3) | 68 (39.1) | 0.27 |
| Age (≥ 65 years) | 190 (32.8) | 132 (32.6) | 58 (333.3) | 0.86 |
| BMI, kg/m2 | 23.1 (3.0) | 23.0 (3.0) | 23.1 (3.0) | 0.80 |
| Smoking | 141 (24.4) | 102 (25.2) | 39 (22.4) | 0.48 |
| Alcohol use | 86 (14.9) | 58 (14.3) | 28 (16.1) | 0.49 |
| Hypertension | 154 (26.6) | 105 (25.9) | 49 (28.1) | 0.58 |
| Diabetes | 72 (12.4) | 49 (12.1) | 23 (13.2) | 0.71 |
| Previous abdominal surgery | 89 (15.3) | 62 (15.3) | 27 (15.5) | 0.95 |
| Tumorous obstruction | 27 (4.7) | 19 (4.7) | 8 (4.6) | 0.96 |
| Neoadjuvant therapy | 89 (15.3) | 65 (16.1) | 24 (13.8) | 0.49 |
| Electrolyte disorders | 89 (15.4) | 62 (15.3) | 27 (15.5) | 0.95 |
| Albumin, g/L | 40.1 (4.0) | 40.1 (3.9) | 40.1 (4.1) | 0.97 |
| CEA (> 5 ng/mL) | 143 (24.7) | 95 (23.5) | 48 (27.6) | 0.29 |
| Tumor size, cm | 3.4 (1.3) | 3.4 (1.2) | 3.5 (1.3) | 0.76 |
| Tumor distance, cm | 6.4 (2.4) | 6.4 (2.3) | 6.5 (2.5) | 0.57 |
| Laparoscopic surgery | 563 (97.2) | 394 (97.2) | 169 (97.1) | 0.96 |
| Operative time, minutes | 219.1 (57.1) | 220.4 (57.4) | 215.9 (56.4) | 0.39 |
| Intraoperative bleeding, ml | 75.8 (122.6) | 79.1 (122.5) | 68.3 (123.1) | 0.34 |
| ASA | 0.97 | |||
| I, II | 507 (91.0) | 354 (91.8) | 153 (91.5) | |
| III, IV | 72 (9.0) | 51 (8.1) | 21 (8.5) | |
| Clinical stages | 0.38 | |||
| 1 | 158 (27.2) | 113 (27.9) | 45 (25.9) | |
| 2 | 189 (32.6) | 1255 (30.8) | 64 (36.8) | |
| 3 | 232 (40.1) | 167 (41.2) | 65 (37.3) | |
| PSF | 31 (5.3) | 21 (5.1) | 10 (5.7) | 0.42 |
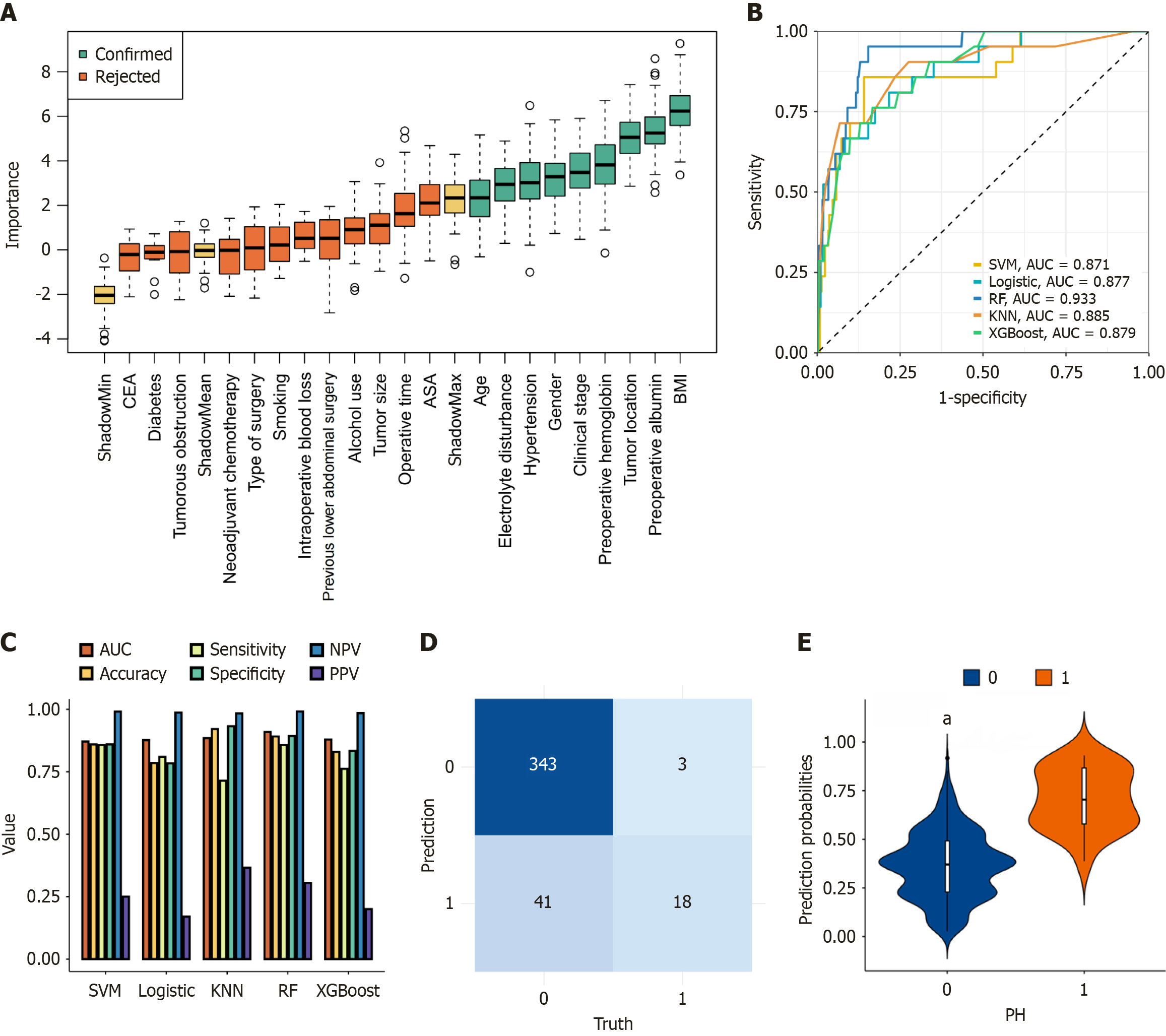
In the model-building phase, we utilized the Boruta method to perform feature selection and assess the importance of individual variables. Figure 1A illustrates the ranking and statistical distribution of variable importance. A total of nine key predictors were identified, including BMI, preoperative albumin levels, tumor distance from the anal verge, preoperative hemoglobin levels, clinical staging, gender, hypertension, electrolyte disturbances, and age. These factors were determined to be crucial in predicting surgical-related risks in the machine learning model.
Using the selected nine predictors, we constructed five different machine learning models: RF, LR, KNN, XGBoost, and SVM. Table 2 summarizes the performance of each model in the training cohort.
| Model | AUC (95%CI) | Accuracy | Sensitivity | Specificity | PPV | NPV |
| RF | 0.909 (0.844-0.974) | 0.891 | 0.857 | 0.893 | 0.305 | 0.991 |
| LR | 0.876 (0.800-0.953) | 0.785 | 0.809 | 0.783 | 0.170 | 0.986 |
| KNN | 0.884 (0.825-0.954) | 0.920 | 0.714 | 0.932 | 0.365 | 0.983 |
| XGBoost | 0.879 (0.812-0.945) | 0.829 | 0.761 | 0.833 | 0.200 | 0.984 |
| SVM | 0.870 (0.786-0.955) | 0.859 | 0.857 | 0.859 | 0.250 | 0.990 |
Among all models, the RF model achieved the best performance with an AUC of 0.909 (95%CI: 0.844-0.974), accuracy of 0.891, specificity of 0.893, and NPV of 0.991. KNN exhibited the highest overall accuracy (0.920) but had lower sensitivity (0.714). LR and XGBoost showed relatively balanced sensitivity and specificity, while SVM delivered consistent classification with slightly lower AUC (0.870) (Figure 1B and C; Table 2). The confusion matrix of the RF model in the training set is shown in Figure 1D. Figure 1E demonstrates strong calibration between predicted and observed PSH probabilities.
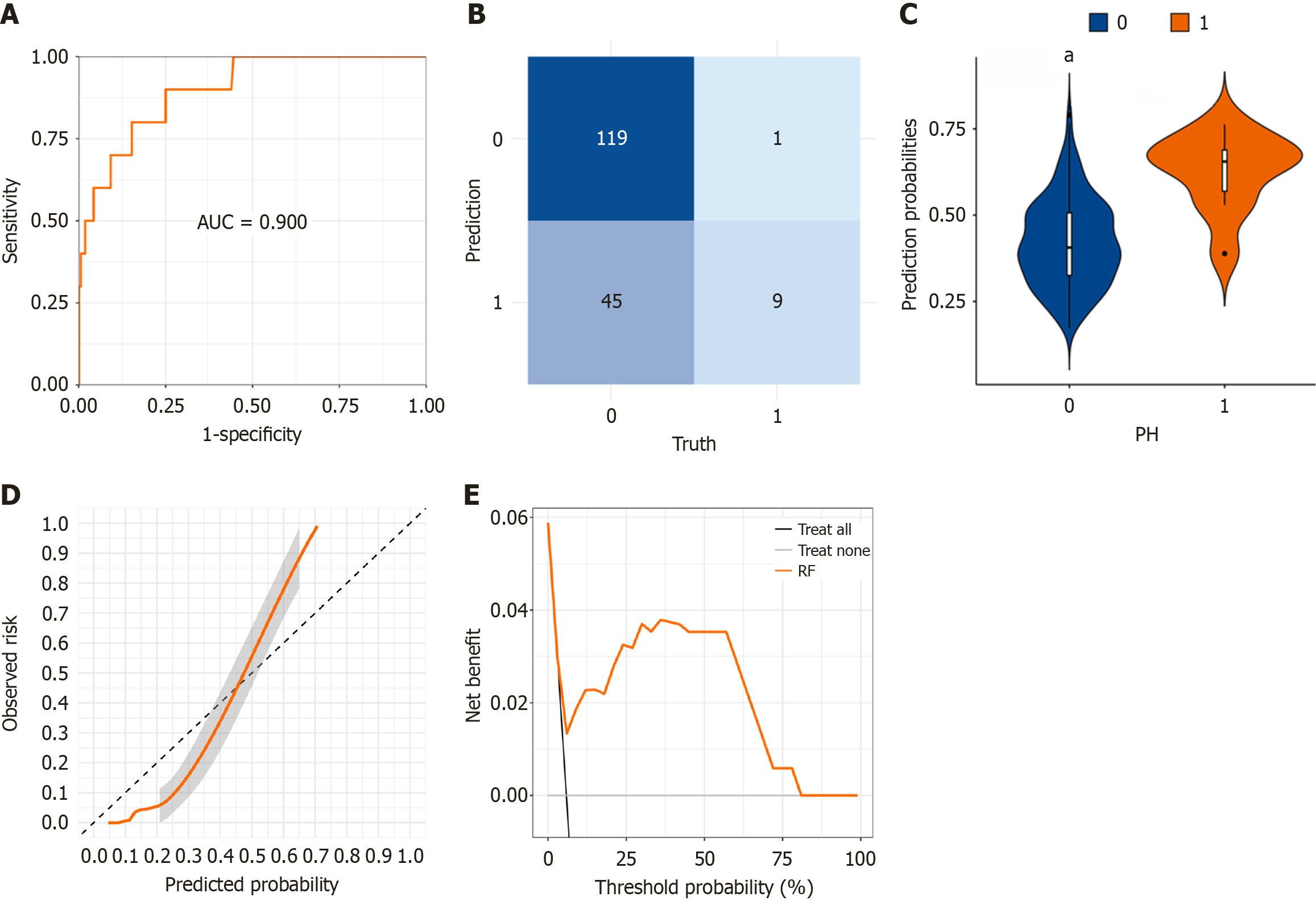
To assess the effectiveness of the RF model in predicting the risk of PSH, we employed an independent testing cohort. The RF model demonstrated excellent discriminatory ability in identifying PSH. Key performance indicators derived from the confusion matrix revealed an AUC of 0.900, a specificity of 0.725, a sensitivity of 0.900, a PPV of 0.166, and a NPV of 0.991 (Figure 2A and B). Furthermore, the distribution of predicted probabilities differed significantly between patients with and without PSH (P < 0.001) (Figure 2C). Calibration curve analysis further confirmed that the model's predictions closely aligned with the actual incidence of PSH (Figure 2D). DCA revealed substantial net benefit across a broad range of threshold probabilities, indicating that the RF model can serve as a valuable tool for clinicians in assessing PSH risk, thereby facilitating more informed decision-making across diverse clinical thresholds (Figure 2E). Given the excellent performance of the RF model, we developed a user-friendly online prediction platform to assist clinicians with an accessible and intuitive interface (https://yangsu2023.shinyapps.io/parastomal_hernia/). By entering patient-specific predictive factors, physicians can quickly assess the likelihood of PSH, aiding in clinical decision-making.
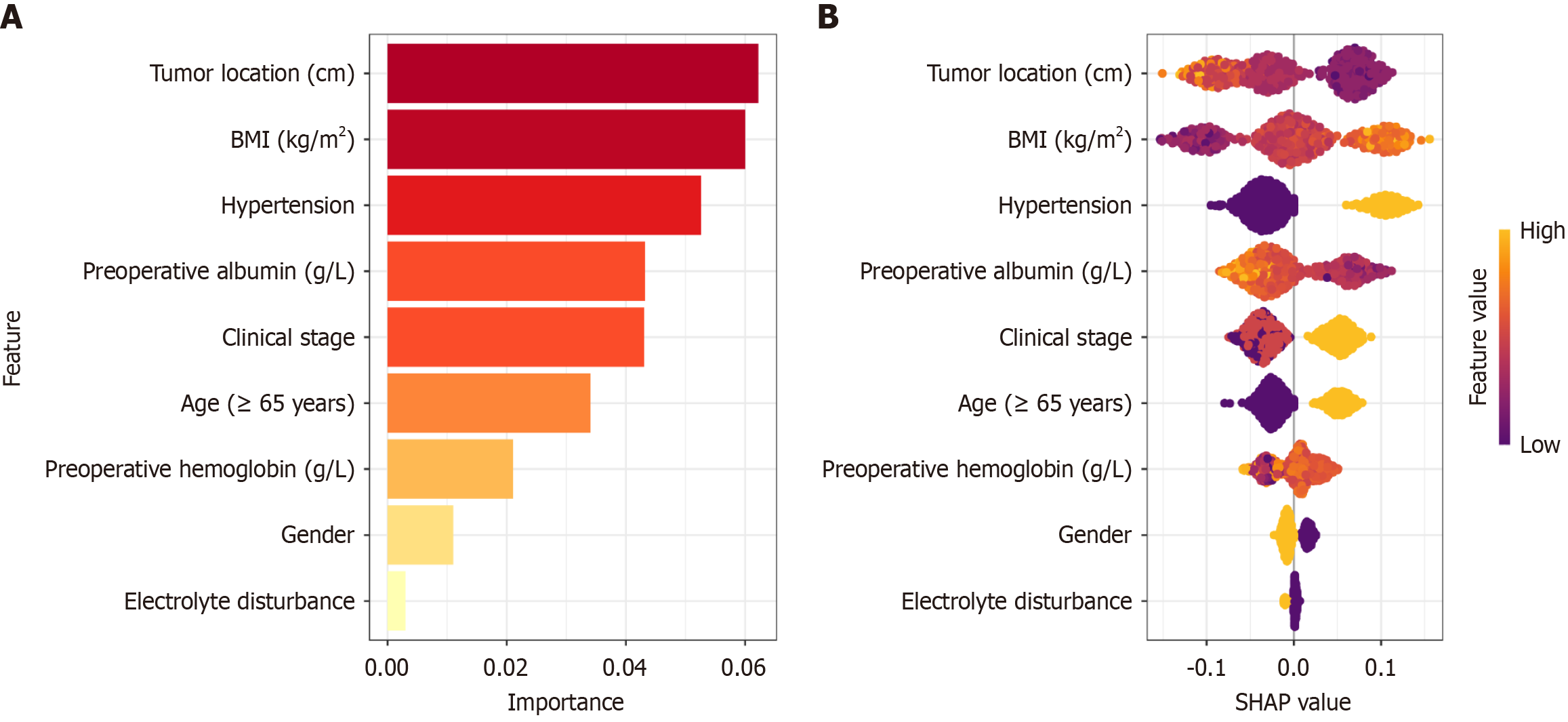
To quantify the contribution of each feature to the RF model's predictions, we performed an explanation analysis using the SHAP algorithm. The analysis identified three key factors influencing the prediction of PSH risk: Tumor distance from the anal verge, BMI and hypertension (Figure 3A). Additionally, BMI, hypertension, and longer surgery duration were associated with an increased likelihood of PSH. In contrast, tumor height from the anus and preoperative albumin levels appeared to have a protective effect, reducing the risk of PSH occurrence (Figure 3B).
PSH is one of the most common and severe complications in patients undergoing preventive ostomy after rectal cancer resection. It can cause symptoms such as abdominal deformity, swelling, and, in severe cases, lead to ostomy dysfunction, bowel obstruction, or hernia content incarceration[15,16]. These complications not only increase the patient's life risk and economic burden but also significantly prolong recovery time and impair quality of life. Therefore, early prediction of PSH occurrence is of great clinical significance. However, effective predictive methods remain scarce in current clinical practice. Traditional risk assessment methods mainly rely on statistical models, which often consider only a few variables and fail to capture the complexity of individual patient differences and multifactorial interactions, limiting their predictive accuracy and ability to support personalized medical decisions[17]. Thus, developing a model that integrates multiple clinical factors and demonstrates high predictive accuracy is of considerable clinical value for early identification of high-risk PSH patients.
With the development of big data and artificial intelligence, machine learning offers a new approach to solving this issue. The RF model, as an ensemble learning method, has shown excellent performance in various medical prediction tasks due to its ability to handle nonlinear relationships and its robustness and interpretability[18].
In our study, the incidence of PSH was 5.3%, which is within the range of 3.1% to 16.5% reported in the literature[17,19,20]. This difference may be attributed to variations in diagnostic criteria, follow-up duration, and study populations. By utilizing preoperative and intraoperative clinical data, our study constructed a machine learning model that effectively predicted the risk of PSH following preventive ostomy in rectal cancer patients. The results indicate that the RF model performs well with an AUC of 0.900 on the test set, demonstrating its high accuracy and robustness. Additionally, SHAP analysis identified tumor distance from the anal verge, BMI, preoperative hypertension, and hypoalbuminemia as key factors influencing the occurrence of PSH. These findings are consistent with previous studies[17,21], suggesting that patients with a closer tumor to the anal verge, higher BMI, preoperative hypertension, and hypoalbuminemia are at significantly increased risk for developing PSH postoperatively. For example, Zhu et al[22] found that overweight (BMI ≥ 25 kg/m²) is significantly associated with PSH formation after abdominoperineal resection for rectal cancer. A shorter tumor distance from the anal verge may increase the risk of permanent ostomy due to postoperative complications, such as anastomotic leaks and stenosis, leading to a higher likelihood of PSH[4,12,22]. Hypertension could increase the risk of postoperative thrombosis, reduce tissue oxygenation, and impair wound healing, thereby promoting the development of PSH. Hypoalbuminemia, through its impact on collagen synthesis and granulation tissue formation, may also increase the risk of hernia formation[23]. Additionally, factors such as longer surgical time, older age, clinical staging, and female sex were found to contribute positively to PSH occurrence, which aligns with previous research findings[22,24-26].
Despite the valuable findings obtained in this study, several limitations should be acknowledged. First, this is a single-center retrospective study, with patient data derived from a single institution. This may introduce selection bias due to institutional-specific surgical techniques, perioperative management protocols, and regional demographic characteristics. Such homogeneity could limit the generalizability of our findings to more diverse clinical settings. Second, although the sample size of 579 patients is sufficient for initial model development and internal validation, it may not fully capture the heterogeneity present in broader populations. Subgroups with rare complications or unique demographic traits may be underrepresented, potentially affecting the robustness and external applicability of the predictive model. In particular, performance metrics such as sensitivity and PPV may vary in real-world multicenter use. Third, while we employed well-established machine learning techniques including feature selection with the Boruta algorithm and cross-validation, emerging methods such as deep learning, ensemble hybrid frameworks, and temporal modeling could be explored in future studies to further enhance predictive performance and adaptability. To address these limitations, we have already initiated a prospective, multicenter validation study involving three independent institutions. This ongoing effort aims to assess the reproducibility and clinical utility of our model in varied healthcare environments. Furthermore, we plan to incorporate patient-reported outcome measures, such as ostomy-related discomfort and quality of life scores, into the predictive framework to better capture patient-centered dimensions of postoperative recovery. These future directions will not only refine the predictive capacity of the model but also support its integration into clinical workflows, enabling personalized postoperative risk stratification and more informed decision-making in rectal cancer management.
This study developed an online predictive tool (https://yangsu2023.shinyapps.io/parastomal_hernia/) based on the RF model to assess the risk of PSH after preventive ostomy in rectal cancer patients. By integrating preoperative and intraoperative clinical data, the RF model effectively identifies high-risk patients with excellent predictive performance. Key factors such as tumor distance from the anal verge, BMI, preoperative hypertension, and hypoalbuminemia significantly influence the development of PSH. This online platform provides clinicians with a convenient, personalized tool for early risk assessment, helping to optimize postoperative management, reduce complication rates, and improve patients' quality of life. As technology continues to evolve, machine learning models are expected to further improve and become more widely applicable in clinical practice.
We sincerely thank the Experimental Medicine Center of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, for their generous provision of essential resources and facilities that supported this research.
| 1. | Jokhadze N, Das A, Dizon DS. Global cancer statistics: A healthy population relies on population health. CA Cancer J Clin. 2024;74:224-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 2. | Stitzenberg KB, Barnes E. Advances in Rectal Cancer Surgery. Clin Colorectal Cancer. 2022;21:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Nocera F, Angehrn F, von Flüe M, Steinemann DC. Optimising functional outcomes in rectal cancer surgery. Langenbecks Arch Surg. 2021;406:233-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Shao S, Zhao Y, Lu Q, Liu L, Mu L, Qin J. Artificial intelligence assists surgeons' decision-making of temporary ileostomy in patients with rectal cancer who have received anterior resection. Eur J Surg Oncol. 2023;49:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 5. | Ahmad NZ, Abbas MH, Khan SU, Parvaiz A. A meta-analysis of the role of diverting ileostomy after rectal cancer surgery. Int J Colorectal Dis. 2021;36:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Carne PW, Robertson GM, Frizelle FA. Parastomal hernia. Br J Surg. 2003;90:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 426] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 7. | Aquina CT, Iannuzzi JC, Probst CP, Kelly KN, Noyes K, Fleming FJ, Monson JR. Parastomal hernia: a growing problem with new solutions. Dig Surg. 2014;31:366-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Fleming AM, Wood EH. Repair of Parastomal Hernias. Adv Surg. 2024;58:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Andersen RM, Klausen TW, Danielsen AK, Vinther A, Gögenur I, Thomsen T. Incidence and risk factors for parastomal bulging in patients with ileostomy or colostomy: a register-based study using data from the Danish Stoma Database Capital Region. Colorectal Dis. 2018;20:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019;20:e262-e273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 743] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 11. | Su Y, Li Y, Chen W, Yang W, Qin J, Liu L. Automated machine learning-based model for predicting benign anastomotic strictures in patients with rectal cancer who have received anterior resection. Eur J Surg Oncol. 2023;49:107113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Antoniou SA, Agresta F, Garcia Alamino JM, Berger D, Berrevoet F, Brandsma HT, Bury K, Conze J, Cuccurullo D, Dietz UA, Fortelny RH, Frei-Lanter C, Hansson B, Helgstrand F, Hotouras A, Jänes A, Kroese LF, Lambrecht JR, Kyle-Leinhase I, López-Cano M, Maggiori L, Mandalà V, Miserez M, Montgomery A, Morales-Conde S, Prudhomme M, Rautio T, Smart N, Śmietański M, Szczepkowski M, Stabilini C, Muysoms FE. European Hernia Society guidelines on prevention and treatment of parastomal hernias. Hernia. 2018;22:183-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 273] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 13. | Hamidi F, Gilani N, Arabi Belaghi R, Yaghoobi H, Babaei E, Sarbakhsh P, Malakouti J. Identifying potential circulating miRNA biomarkers for the diagnosis and prediction of ovarian cancer using machine-learning approach: application of Boruta. Front Digit Health. 2023;5:1187578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 14. | Maskal SM, Ellis RC, Fafaj A, Costanzo A, Thomas JD, Prabhu AS, Krpata DM, Beffa LRA, Tu C, Zheng X, Miller BT, Rosen MJ, Petro CC. Open Retromuscular Sugarbaker vs Keyhole Mesh Placement for Parastomal Hernia Repair: A Randomized Clinical Trial. JAMA Surg. 2024;159:982-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Ramli R, Ng ZQ, Diab J, Gilmore A. Acute Parastomal Hernia Presentations: A 10-Year Review of Management and Outcomes. J Abdom Wall Surg. 2024;3:13364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Dai T, Bao M, Zhang M, Wang Z, Tang J, Liu Z. A risk prediction model based on machine learning algorithm for parastomal hernia after permanent colostomy. BMC Med Inform Decis Mak. 2024;24:224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | Hu J, Szymczak S. A review on longitudinal data analysis with random forest. Brief Bioinform. 2023;24:bbad002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 280] [Reference Citation Analysis (0)] |
| 18. | Hardt J, Seyfried S, Weiß C, Post S, Kienle P, Herrle F. A pilot single-centre randomized trial assessing the safety and efficacy of lateral pararectus abdominis compared with transrectus abdominis muscle stoma placement in patients with temporary loop ileostomies: the PATRASTOM trial. Colorectal Dis. 2016;18:O81-O90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Edwards DP, Leppington-Clarke A, Sexton R, Heald RJ, Moran BJ. Stoma-related complications are more frequent after transverse colostomy than loop ileostomy: a prospective randomized clinical trial. Br J Surg. 2001;88:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Robertson I, Leung E, Hughes D, Spiers M, Donnelly L, Mackenzie I, Macdonald A. Prospective analysis of stoma-related complications. Colorectal Dis. 2005;7:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Su Y, Li Y, Zhang H, Yang W, Liu M, Luo X, Liu L. Machine learning model for prediction of permanent stoma after anterior resection of rectal cancer: A multicenter study. Eur J Surg Oncol. 2024;50:108386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Zhu L, Li S, Wang F. Risk factors for parastomal hernia after abdominoperineal resection of rectal cancer. Front Oncol. 2024;14:1470113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Manole TE, Daniel I, Alexandra B, Dan PN, Andronic O. Risk Factors for the Development of Parastomal Hernia: A Narrative Review. Saudi J Med Med Sci. 2023;11:187-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | Zhang HY, Wang ZJ, Han JG. [Risk factors and prevent strategy of parastomal hernia]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Niu N, Du S, Yang D, Zhang L, Wu B, Zhi X, Li J, Xu D, Zhang Y, Meng A. Risk factors for the development of a parastomal hernia in patients with enterostomy: a systematic review and meta-analysis. Int J Colorectal Dis. 2022;37:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Fox SS, Johnson R, Fischer JP, Eckhauser F, Hope WW. Prophylactic Mesh for Hernia Prevention: Has the Time Arrived? Plast Reconstr Surg. 2018;142:180S-186S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/