Published online Sep 27, 2025. doi: 10.4240/wjgs.v17.i9.108127
Revised: May 27, 2025
Accepted: July 31, 2025
Published online: September 27, 2025
Processing time: 171 Days and 7.9 Hours
Current guidelines recommend providing malnourished individuals immunonutrition before major gastrointestinal surgery. Nonetheless, the advantages of pre
To analyses the effects of preoperative immunonutrition and standard oral nutrition supplements on colorectal surgery outcomes.
This study employed a prospective single-center randomized double-blinded comparative approach and was conducted at Hospital Universiti Sains Malaysia between September 2023 and September 2024. In this study, the participants in the experimental group were supplied with a specialized oral supplement enriched with immune-modulating nutrients. Meanwhile, a conventional oral nutrition supplement was provided to the control group. The time to first flatus and the time to first bowel evacuation were the primary outcomes recorded. Incidence of nosocomial infections, surgical site infections, and the total length of hospital stay were considered secondary data.
This study involved 58 patients who were allocated into two groups. No dropouts were documented. The mean age of the participants was 61.20 ± 12.96, and most were males (63.38%). All participants’ baseline and surgical characteristics in both arms were also generally comparable. The participants in this study underwent colorectal surgery, where most had laparoscopic surgery (58%). Based on the results, no significant statistical differences were observed regarding the duration from the first flatus to the first bowel evacuation, the onset of a normal diet, and hospital stay between the experimental and control groups. Both groups also recorded 10 (17.24%) infectious complications.
The findings indicated no notable variations in the primary and secondary endpoints despite the theoretical benefits of immune-modulating nutrients. Conclusively, routine preoperative immunonutrition may not provide additional advantages over standard nutrition in this demographic.
Core Tip: This randomized controlled trial evaluated the effects of preoperative immunonutrition vs standard oral nutrition supplements in patients undergoing colorectal surgery. Despite theoretical benefits, immunonutrition did not significantly improve postoperative gastrointestinal recovery, reduce infectious complications, or shorten hospital stay. All baseline and surgical variables were comparable between groups. The findings suggest that routine use of immunonutrition may not confer additional clinical advantages over standard supplementation in this patient population, challenging current recommendations and supporting a more individualized nutritional approach.
- Citation: Pannirselvam M, Zakaria Z, Wong MPK, Abdul Satar MHS, Jusoh NS, Zakaria AD, Othman MF. Effects of preoperative immunonutrition vs standard oral nutrition in patients undergoing colorectal surgery: A randomized controlled trial. World J Gastrointest Surg 2025; 17(9): 108127
- URL: https://www.wjgnet.com/1948-9366/full/v17/i9/108127.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i9.108127
Stress hormones, inflammatory mediators, and metabolic alterations can be triggered by major surgeries, leading to considerable catabolic effects. The effects will then induce substantial general host homeostasis, defence mechanisms, and inflammatory response impairments[1]. These outcomes influence patients physiologically and psychologically. Consequently, the delayed recovery of normal bowel function, postoperative complications, and extended hospital stays are among the primary challenges associated with colorectal surgery. Promptly commencing oral intake, integrating nutritional support, metabolic regulation, and early mobilization are essential in considerable postoperative patient care improvements[2]. Accordingly, perioperative nutritional management in surgical patients includes immunomodulating enteral nutrition as a therapeutic strategy[3]. Immunonutrition is the modulation of either the activities of the immune system or the consequences of its activation through nutrients or specific food items supplemented above normal diet amounts[4].
Typically, an immunonutrition formula comprises arginine, omega-3 fatty acids, glutamine, and nucleotides. Each component has a substantial role in immunomodulating via separate mechanisms. Arginine is a precursor for proline, glutamate, and polyamines, which are vital in the urea cycle[5]. Arginine also promotes growth hormones and insulin-like growth factor 1 secretions. Both hormones enhance wound healing extensively[6,7]. Furthermore, arginine generates highly reactive nitric oxide, which facilitates vascular tone regulation and improves blood flow.
Omega-6 fatty acids are present in vegetable oils and are a conventional lipid energy source. The fatty acids are precursors for the 2- and 4-series prostanoids, which have vasoconstrictive attributes and enhance platelet aggregation[8]. The prostanoids also exhibit anti-inflammatory properties, including lowering blood leukocyte counts and serum C-reactive protein (CRP) levels, inhibiting inflammatory cytokines production by endotoxin-stimulated mononuclear cells, and enhancing leukotriene B5 generation by activated neutrophils. Furthermore, omega-3 fatty acids reduce arachidonic acid levels while promoting resolvins and protectins synthesis, contributing to inflammation resolution and wound healing[9,10].
Glutamine is a vital amino acid essential in B-cell differentiation, neutrophil superoxide generation, T-cell proliferation, and phagocytosis. The amino acid is also critical in modulating inflammatory responses[11]. Although glutamine treatment in surgical patients is linked to reduced infection sequelae and hospital stays, it does not affect mortality[12]. Nucleotides, another component of immunonutrition formula, are fundamental RNA and DNA units. Consequently, nucleotides are essential for almost all biochemical processes, such as rapid cell replication, including T cells, maturation, proliferation, and function[13].
Administering immunonutrition to patients at high risk of malnutrition preoperatively for major oncologic surgeries is the current guideline globally. Braga et al[14] documented improved gut microperfusion and intraoperative oxygenation in patients supplemented with immunonutrition. The observations suggested that earlier bowel return was enhanced by preoperative immunonutrition consumption. In this study, the clinical outcomes of patients undergoing colorectal cancer surgery were evaluated regarding the effects of preoperative immune-modulating enteral nutrition vs standard oral nutrition supplements.
This study applied a randomized controlled method and was conducted at Hospital Universiti Sains Malaysia from September 2023 to September 2024. The protocol was approved by the Jawatankuasa Etika Penyelidikan Manusia Universiti Sains Malaysia. Written informed consents were also obtained from all participants. Finally, the trial was registered at www.clinicaltrials.gov with the ID NCT06128798.
Only patients scheduled for colorectal surgery at Hospital Universiti Sains Malaysia were considered for participation in this study. The patients were also 18 years or older and were undergoing elective colorectal surgery. Meanwhile, patients with a history of allergy to milk, fish, and soy, and fluid restrictions, including end-stage renal failure, were not included. Patients exhibiting decompensated heart failure and scheduled for emergency surgery were also excluded from this study.
This study utilized was a double-blinded approach. The participants were not informed of the type of nutritional supplement they received. Both beverages employed in this study, immunonutrition, Oral Impact®, and standard nutrition, Nutren Optimum®, were repackaged by a dietitian not involved in any part of patient recruitment, clinical care, or outcome assessment in unlabelled containers. Furthermore, the supplements had similar appearance and texture, and the participants were not permitted to cross-taste the products. Clinical care providers, surgeons, ward staff, and outcome assessors in this study were also blinded to the group allocations. The selected participants were divided into experimental (Oral Impact) and control (Nutren Optimum) groups using permutated block randomization with multiple random block size methods. An independent statistician generated and stored the randomization sequence. The statistician had no contact with the clinical team, ensuring allocation concealment and minimizing performance and detection bias. A total of 58 random sequences were generated prior to the recruitment of the participants.
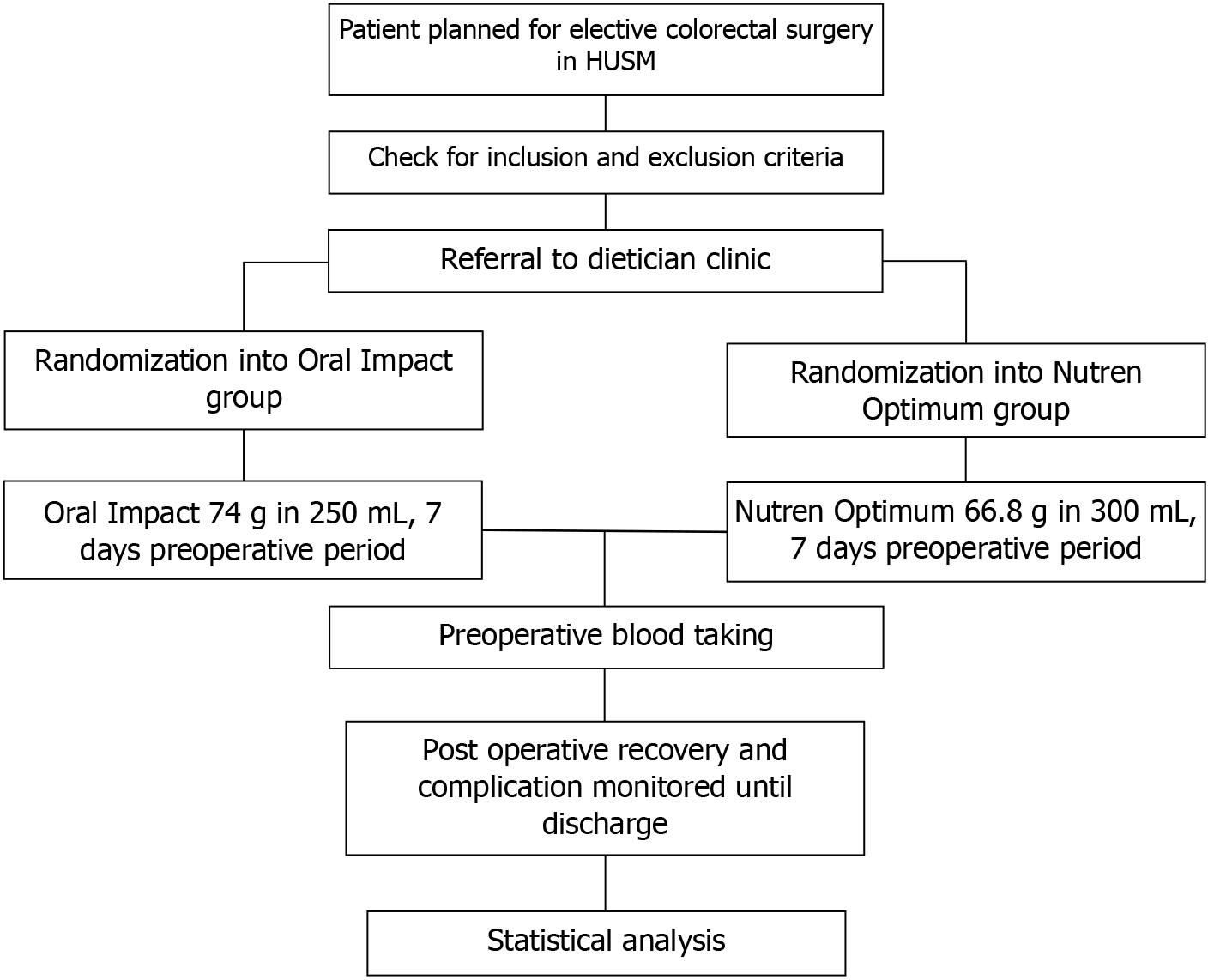
Patients requiring colorectal surgery were review at surgical outpatient clinic. Eligible participants meeting the inclusion criteria in this study were recruited in the surgical outpatient clinic. Subsequently, the participants were referred to the dietitian clinic for preoperative beverages according to the randomized sequence previously generated. Each patient received their study material, which was wrapped to hide the content, separately at the dietitian clinic. The participants in the experimental group consumed Oral Impact thrice daily. A serving of Oral Impact consisted of 74 g of the beverage mixed with 250 mL of water. Meanwhile, the patients in the control group consumed 3 servings of Nutren Optimum daily. A serving of Nutren Optimum had 66.8 g of the beverage and 300 mL of water. The participants consumed the beverages for 7 days before their scheduled operation dates. A dietitian provided constant reminders to each participant regarding consuming their respective beverage via phone calls and text messages every other day.
Standard hospital preoperative protocol was applied to all participants in this study, including routine preoperative blood procedures in the surgical ward a day prior to admission. All participants were also provided with prophylactic antibiotics, cefoperazone and metronidazole. In this study, the colorectal surgeries were performed by colorectal surgeons, colorectal trainees, or general surgeons. Surgical access, intraoperative difficulty, the duration of surgery, blood loss, and transfusion were recorded. Figure 1 illustrates the procedure flow chart employed in this study.
On the 3rd postoperative day, the venous blood of all participants was sampled, and their CRP levels were documented. Furthermore, clinical observations on the return of bowel function (time of first flatus and first bowel evacuation), surgical site and nosocomial infections, and hospital stay duration were recorded in the proforma. Based on the standards of care, the attending team decided the antibiotic prescription, empirical or therapeutic, for each participant. The patients were also scheduled for a review in the surgical outpatient clinic 2 and 4 weeks after being discharged.
This study primarily analyzed participants’ clinical responses, the time of the first flatus and the first bowel evacuation, following the colorectal surgery. Meanwhile, nosocomial and surgical site infection (SSI) incidences and the length of hospital stay were recorded as secondary outcomes. Nosocomial infections are infections, excluding SSIs, that develop in a patient during hospital treatment, are absent during admission, and manifest 48 hours post-hospitalization. SSI is a surgical wound infection manifesting within 30 days post-surgery without implants. Meanwhile, this study defined hospital stay duration from postoperative day 1.
This study required 58 samples to achieve a study power of 80%, a 0.05 significance level, and a 10% dropout. The sample size for this study was calculated based on formula two mean with the PS size calculation version 3.1.2 software. Following evaluation of each outcome, a 58 sample size was required to allow null hypothesis rejection. The protocol analysis was implemented during the results assessment. Firstly, all data were entered and analyzed utilizing Microsoft Excel and R version 4.4.0. Descriptive statistics were also employed to summarize patient profiles and other relevant clinical information. In this study, numerical variables were denoted as mean ± SD or median (interquartile range) when involving non-normally distributed data, whereas categorical variables were presented as n (%). For normally and non-normally distributed variables, independent t-tests and Mann-Whitney tests were performed, enabling comparisons between Oral Impact and Nutren Optimum outcomes. Finally, χ2 distribution and Fisher’s exact tests were conducted on categorical variables.
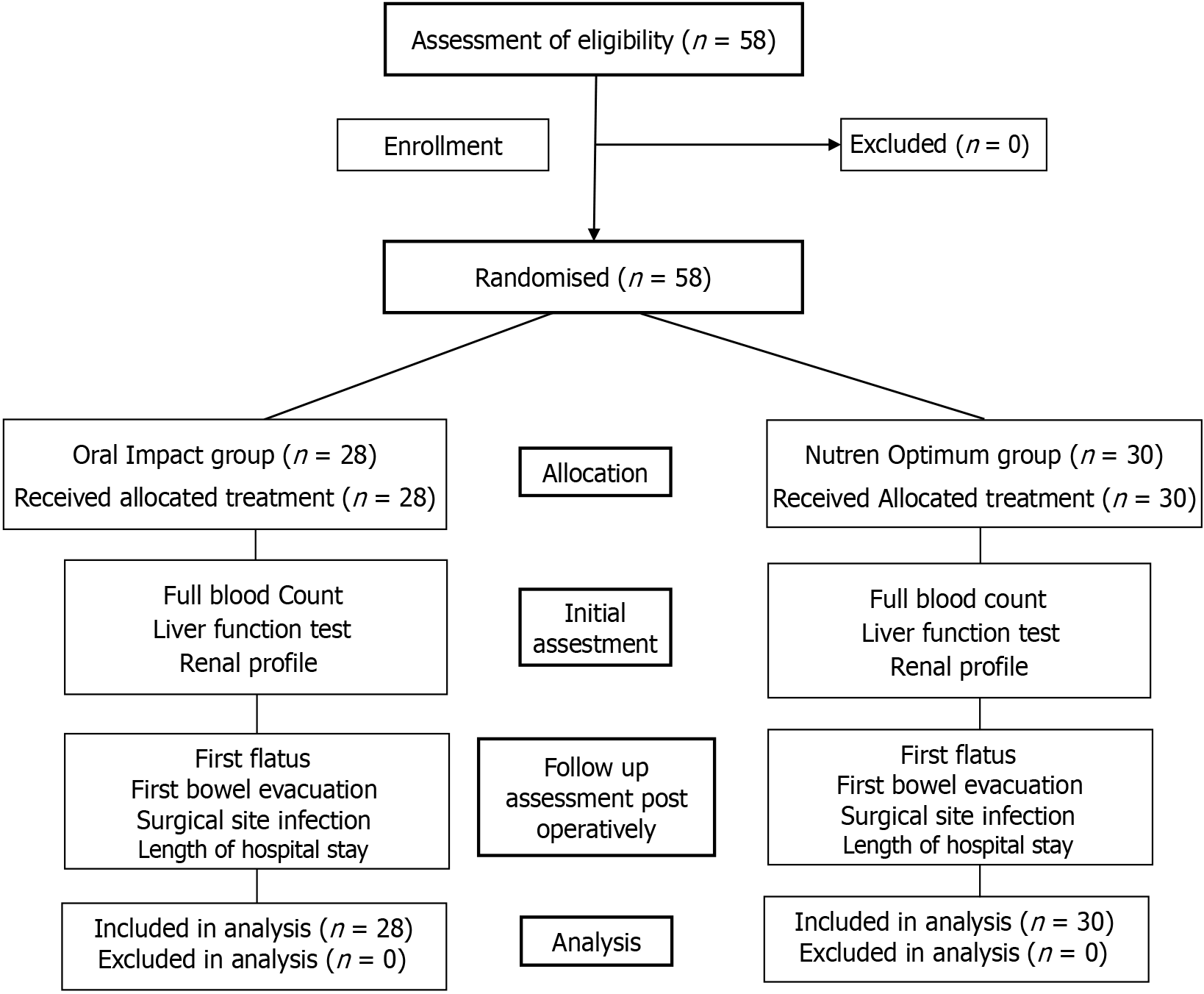
Figure 2 demonstrates CONSORT flowchart implemented in this study. No dropouts were recorded from the 58 participants recruited in this study. The participants were randomly allocated into Oral Impact, n = 28 (experimental group), and Nutren Optimum, n = 30 (control group). The data collection period was 12 months. The mean age of the participants in this study was 60, with no statistically significant differences (P = 0.787). Most of the participants, 62.1%, were also males. Furthermore, similar body mass index distributions were observed in the experimental and control groups, with no notable variations (P = 0.767). Both groups in this study also documented comparable baseline data, including age, sex, body mass index, and comorbidities. The sociodemographic and preoperative information are summarized in Table 1.
| Variable | Oral Impact (n = 28) | Nutren Optimum (n = 30) | P value |
| Age (years) | 59.6 ± 13.5 | 60.6 ± 13.4 | 0.787 |
| Sex | 0.455 | ||
| Female | 12 (42.9) | 10 (33.3) | |
| Male | 16 (57.1) | 20 (66.7) | |
| Body mass index (kg/m2) | 0.767 | ||
| < 18.5 | 0 (0.0) | 1 (3.3) | |
| > 30 | 1 (3.6) | 1 (3.3) | |
| 18.5-24.9 | 19 (67.9) | 22 (73.3) | |
| 25-29.9 | 8 (28.6) | 6 (20.0) | |
| NRS score | 0.584 | ||
| 0-3 | 23 (85.2) | 24 (80.0) | |
| 4 | 3 (11.1) | 2 (6.7) | |
| 5-7 | 1 (3.7) | 4 (13.3) | |
| Unknown | 1 | 0 | |
| ASA class | 0.050 | ||
| 1 | 13 (46.4) | 15 (50.0) | |
| 2 | 15 (53.6) | 10 (33.3) | |
| 3 | 0 (0.0) | 5 (16.7) | |
| Hemoglobin (g/dL) | 12.3 ± 1.4 | 11.8 ± 1.6 | 0.266 |
| Total white cell count | 8.4 ± 2.2 | 8.7 ± 2.8 | 0.657 |
| Serum albumin (g/dL) | 37.3 ± 5.0 | 37.5 ± 5.9 | 0.882 |
| Urea (mmol/L) | 4.9 ± 2.0 | 4.8 ± 1.8 | 0.772 |
| Creatinine (mmol/L) | 75.9 ± 25.1 | 81.6 ± 20.9 | 0.359 |
| Unknown | 0 | 1 | |
| Diabetes mellitus | 15 (53.6) | 9 (30.0) | 0.069 |
| Hypertension | 14 (50.0) | 21 (70.0) | 0.120 |
| Chronic kidney disease | 2 (7.1) | 1 (3.3) | 0.605 |
| Ischemic heart disease | 5 (17.9) | 4 (13.3) | 0.726 |
| Previous abdominal surgery | 9 (32.1) | 10 (33.3) | 0.923 |
According to the results, 48.2% (28), 43.1% (25), and 8.6% (5) of the participants were in the I, II, and III American Society of Anesthesiology (ASA) classifications, respectively. Nonetheless, substantial statistical differences, P = 0.050, were observed in the experimental group, which recorded a higher proportion of ASA class 1 participants than their control counterparts. The participants in the experimental group also documented a considerably higher diabetes prevalence (P = 0.069) than the control group.
Based on the baseline preoperative results in Table 1, no significant differences were observed between the control and experimental groups regarding their hemoglobulin, leukocytes, albumin, serum urea, and creatinine levels at the time of recruitment. Meanwhile, the participants’ nutrition risk screening 2002 scores indicated that 81% (n = 47) were at low risk, 10.3% (n = 6) were at risk, and 8.6% (n = 8.6) were at substantial risk of malnutrition.
A total of 87.9% (n = 51) of participants in the control and experimental groups were diagnosed with malignant disease. Most of the participants, 63.79% (n = 37), also underwent laparoscopic surgery, while 8.6% (n = 5) had open surgeries. Nevertheless, 27.86% (n = 16) of the laparoscopically intervened patients required conversion to open surgery. Among the various colorectal surgeries performed in this study, anterior resection was conducted on 50% (n = 29) of the participants. No statistically significant differences were observed regarding the frequencies of specific procedures between the participants in the control and experimental groups (all P > 0.05). A similar average surgery time was also documented between groups, approximately 4.9 hours. Comparable intraoperative data were recorded by the participants in the control and experimental groups in this study. The disease, surgery type performed, surgical team composition, and procedure duration exhibited no statistically significant differences. Nonetheless, the slight variation in blood loss and transfusion requirements was not statistically notable. Table 2 summarizes the intraoperative procedure information.
| Variable | Oral Impact (n = 28) | Nutren Optimum (n = 30) | P value |
| Nature of disease | > 0.999 | ||
| Benign | 3 (10.7) | 4 (13.3) | |
| Malignant | 25 (89.3) | 26 (86.7) | |
| Mode of surgery | 0.396 | ||
| Laparoscopic | 14 (50.0) | 13 (43.3) | |
| Laparoscopic assisted | 4 (14.3) | 6 (20.0) | |
| Laparoscopic convert open | 6 (21.4) | 10 (33.3) | |
| Open surgery | 4 (14.3) | 1 (3.3) | |
| Surgery performed | 0.794 | ||
| Abdominoperineal resection | 1 (3.6) | 2 (6.7) | |
| Anterior resection with stoma | 2 (7.1) | 5 (16.7) | |
| Anterior resection without stoma | 9 (32.1) | 13 (43.3) | |
| Closure of stoma | 3 (10.7) | 1 (3.3) | |
| Extended right hemicolectomy | 1 (3.6) | 1 (3.3) | |
| Hartmann’s procedure | 1 (3.6) | 1 (3.3) | |
| Left hemicolectomy | 3 (10.7) | 1 (3.3) | |
| Reversal of Hartmann’s procedure | 3 (10.7) | 3 (10.0) | |
| Right hemicolectomy | 4 (14.3) | 2 (6.7) | |
| Sigmoid colectomy | 0 (0.0) | 1 (3.3) | |
| Subtotal colectomy | 1 (3.6) | 0 (0.0) | |
| Operating surgeon | 0.973 | ||
| Colorectal surgeon | 1 (3.6) | 1 (3.3) | |
| Colorectal surgeon, colorectal trainee | 13 (46.4) | 16 (53.3) | |
| Colorectal surgeon, colorectal trainee, general surgeon | 2 (7.1) | 1 (3.3) | |
| Colorectal surgeon, general surgeon | 0 (0.0) | 1 (3.3) | |
| Colorectal trainee | 6 (21.4) | 6 (20.0) | |
| Colorectal trainee, general surgeon | 6 (21.4) | 5 (16.7) | |
| Duration of surgery, mean ± SD | 4.9 ± 1.8 | 4.8 ± 1.9 | 0.895 |
| Total blood loss | 0.380 | ||
| < 500 mL | 24 (85.7) | 23 (76.7) | |
| > 500 mL | 4 (14.3) | 7 (23.3) | |
| Blood transfusion | 4 (14.3) | 7 (23.3) | 0.380 |
According to the per-protocol analysis results, the time of the first flatus between the experimental and control groups was 2 days, while the time of bowel output was 3 days (P = 0.2). The duration the control and experimental participants recorded progressing through different stages of their postoperative diet (clear fluids, nourishing fluids, soft diet, and normal diet) demonstrated not statistically significant variation (all P > 0.05). Similarly, the total length of hospital stays of the experimental and control groups was 6 and 7 days, respectively, which was not statistically notable (P = 0.353; Table 3). Postoperative complications were also statistically insignificant in both arms, recording P > 0.05 (Table 4).
| Variable | Oral Impact (n = 28) | Nutren Optimum (n = 30) | P value |
| Initiation of clear fluid (days) | 1.0 (0.3) | 1.0 (0.8) | 0.783 |
| Initiation of nourishing fluid (days) | 2.0 (1.0) | 2.0 (1.0) | 0.877 |
| Initiation of soft diet (days) | 4.0 (1.0) | 4.0 (2.0) | 0.274 |
| Return to normal diet (days) | 4.0 (1.0) | 5.0 (1.8) | 0.189 |
| Flatus (days) | 2.0 (1.0) | 2.0 (1.0) | 0.640 |
| Bowel output (days) | 3.0 (0.0) | 3.0 (1.0) | 0.200 |
| Unknown | 1 | 1 | |
| C-reactive protein (day 3 post surgery), mean ± SD | 78.2 ± 57.7 | 102.1 ± 56.1 | 0.116 |
| Duration of antibiotic (days) | 4.0 (3.3) | 5.0 (2.8) | 0.100 |
| Indication of antibiotic | 0.192 | ||
| Prophylactic | 16 (57.1) | 12 (40.0) | |
| Treatment | 12 (42.9) | 18 (60.0) | |
| ICU stay (days) | 11 (39.3) | 15 (50.0) | 0.412 |
| Total length of hospital stays (days) | 6.0 (2.3) | 7.0 (3.0) | 0.353 |
| Variable | Oral Impact (n = 28) | Nutren Optimum (n = 30) | P value |
| Prolonged post operative ileus | 1 (3.6) | 1 (3.4) | > 0.999 |
| Unknown | 0 | 1 | |
| Surgical site infection | 3 (10.7) | 4 (13.3) | > 0.999 |
| Nosocomial infection | 1 (3.6) | 3 (10.0) | 0.612 |
Malignancies and surgical stress can detrimentally affect the immune system and various defence mechanisms of patients. Furthermore, infections pose a significant challenge following major abdominal surgeries, leading to increased morbidity and mortality rates and healthcare costs. The association between malnutrition and unfavourable clinical outcomes also resulted in the widely recognized nutritional support concept. Numerous clinical and experimental studies in the past 10 years revealed that enteral immunonutrition effectively enhanced immune responses, regulated inflammatory reactions, and improved intestinal microperfusion[15-19]. Meta-analyses between 1999 and 2003 also demonstrated a consistent association between immunonutrition and diminished infectious complications and hospital stay durations in elective surgical patients, without affecting mortality rates[20-22].
Most reports administered immunonutrition postoperatively. For instance, Daly et al[15] documented reduced infectious complications in patients administered postoperative immunonutrition following major upper gastrointestinal malignancy surgery. Nevertheless, Heslin et al[23] recorded no benefit from early postoperative immunonutrition in a similar patient population. Conversely, Braga et al[24] recorded superior outcomes in malnourished (≥ 10% weight loss over 6 months) gastrointestinal cancer patients subjected to perioperative immunonutrition. Nonetheless, the study reported similar efficacy of preoperative and perioperative immunonutrition supplementations in well-nourished patients. A recent study by Sacks et al[25] highlighted the benefits of preoperative immunonutrition over postoperative administration.
McCowen and Bistrian[26] suggested that effective immunonutrition formulae should contain at least 12 g/L of arginine and be administered for over 3 days (ideally 5-10 days). An efficient supplementation should also aim for a caloric daily intake approaching 25 kcal/kg, with a fluid volume exceeding 800 mL/day. For patients undergoing major surgery, the French guidelines recommend preoperative nutritional intervention. The guideline suggested the Oral Impact® nutritional drink enriched with electrolytes, vitamins, and immune nutrients, including omega-3, arginine, and nucleotides. The recommended consumption is thrice daily for 7 days before surgery.
The findings in this study did not support the Oral Impact® superiority hypothesis. Overall statistical analysis also revealed no variations. Nonetheless, several parameters demonstrated clinically relevant findings. Participants in the experimental group documented a shorter average hospital stay (6 days) than the control group (7 days). A 2017 British Journal of Surgery meta-analysis reported similar observations, a significant 1.79-day reduction in hospital stays after major abdominal surgery in patients subjected to a nutritional intervention. Although mean and standard deviation values were not used for non-parametric data, this trend remains notable. The data supported previous studies evaluating immunonutrition effects on surgical outcomes across diverse patient populations with different gas
Regarding intensive care unit stays, the participants in the intervention group documented a shorter duration, 11 days, than the control group, which recorded 15 days. The mean CRP level on postoperative day 3 was lower in the im
Although this study had adequate power for the primary endpoint, the relatively small sample size might be underpowered to detect smaller but clinically meaningful differences in secondary outcomes, including the 1-day reduction in hospital stay and decreased postoperative inflammatory markers. The non-significant trends might also reflect a type II error arising from the limited sample size. Furthermore, potential immunonutrition benefits in select subgroups could be underestimated. Larger, sufficiently powered multicenter trials are required to validate the findings recorded in this study. Nutritional risk (e.g., nutrition risk screening > 3)-based subgroup assessments could also contribute to identifying patients who might derive greater advantages from immunonutrition. The small number of at-risk patients in this cohort restricted the feasibility of such an analysis, requiring future studies with larger and stratified samples. Notable baseline imbalances between groups, including elevated ASA class I and diabetes prevalences in the experimental group, were observed. Although not statistically significant, the variations might have contributed to residual confounding, particularly regarding infection rates and recovery metrics. The matter requires consideration when interpreting outcomes. In this study, the participants’ adherence to the prescribed seven-day nutritional regimen was not objectively assessed through direct measures, such as logs or returned supplement counts. This issue might have limited the internal validity of the intervention despite regular reminders being provided to the participants. Ne
Although the results in this study did not demonstrate significant statistical differences between the control and experimental groups in the outcomes measured, considerable clinical variations were documented in several parameters. Consequently, null findings do not dispute the potential benefits of immunonutrition. Future studies should consider evaluating larger sample sizes and extending the duration of the study with subgroup analyses. The complexity of the immune response and its interaction with surgical stress highlighted the necessity for a more nuanced approach to determining the benefits of immunonutrition in colorectal surgery patients.
The authors would like to express gratitude to Associate Professor Dr Maya Mazuwin Yahya, Head of the Department of Surgery, and all faculty members for their assistance and contributions to this study.
| 1. | Chang HR, Bistrian B. The role of cytokines in the catabolic consequences of infection and injury. JPEN J Parenter Enteral Nutr. 1998;22:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, Nygren J, Hausel J, Soop M, Andersen J, Kehlet H. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1001] [Cited by in RCA: 1058] [Article Influence: 50.4] [Reference Citation Analysis (1)] |
| 3. | Klek S, Szybinski P, Szczepanek K. Perioperative immunonutrition in surgical cancer patients: a summary of a decade of research. World J Surg. 2014;38:803-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Grimble RF. Basics in clinical nutrition: Immunonutrition – Nutrients which influence immunity: Effect and mechanism of action. E Spen Eur E J Clin Nutr Metab. 2009;4:e10-e13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Böger RH, Bode-Böger SM. The clinical pharmacology of L-arginine. Annu Rev Pharmacol Toxicol. 2001;41:79-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 211] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Arnold M, Barbul A. Nutrition and wound healing. Plast Reconstr Surg. 2006;117:42S-58S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Stechmiller JK, Childress B, Cowan L. Arginine supplementation and wound healing. Nutr Clin Pract. 2005;20:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Gurr MI. The role of lipids in the regulation of the immune system. Prog Lipid Res. 1983;22:257-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Shaikh SR, Jolly CA, Chapkin RS. n-3 Polyunsaturated fatty acids exert immunomodulatory effects on lymphocytes by targeting plasma membrane molecular organization. Mol Aspects Med. 2012;33:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Bharadwaj S, Trivax B, Tandon P, Alkam B, Hanouneh I, Steiger E. Should perioperative immunonutrition for elective surgery be the current standard of care? Gastroenterol Rep (Oxf). 2016;4:87-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients. 2018;10:1564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 775] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 12. | Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med. 2002;30:2022-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 425] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 13. | Hess JR, Greenberg NA. The role of nucleotides in the immune and gastrointestinal systems: potential clinical applications. Nutr Clin Pract. 2012;27:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 275] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 15. | Daly JM, Lieberman MD, Goldfine J, Shou J, Weintraub F, Rosato EF, Lavin P. Enteral nutrition with supplemental arginine, RNA, and omega-3 fatty acids in patients after operation: immunologic, metabolic, and clinical outcome. Surgery. 1992;112:56-67. [PubMed] |
| 16. | Senkal M, Mumme A, Eickhoff U, Geier B, Späth G, Wulfert D, Joosten U, Frei A, Kemen M. Early postoperative enteral immunonutrition: clinical outcome and cost-comparison analysis in surgical patients. Crit Care Med. 1997;25:1489-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 162] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Senkal M, Zumtobel V, Bauer KH, Marpe B, Wolfram G, Frei A, Eickhoff U, Kemen M. Outcome and cost-effectiveness of perioperative enteral immunonutrition in patients undergoing elective upper gastrointestinal tract surgery: a prospective randomized study. Arch Surg. 1999;134:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 212] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Braga M, Gianotti L, Vignali A, Cestari A, Bisagni P, Di Carlo V. Artificial nutrition after major abdominal surgery: impact of route of administration and composition of the diet. Crit Care Med. 1998;26:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 127] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Braga M, Gianotti L, Radaelli G, Vignali A, Mari G, Gentilini O, Di Carlo V. Perioperative immunonutrition in patients undergoing cancer surgery: results of a randomized double-blind phase 3 trial. Arch Surg. 1999;134:428-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 248] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Heys SD, Walker LG, Smith I, Eremin O. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: a meta-analysis of randomized controlled clinical trials. Ann Surg. 1999;229:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 298] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Beale RJ, Bryg DJ, Bihari DJ. Immunonutrition in the critically ill: a systematic review of clinical outcome. Crit Care Med. 1999;27:2799-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 300] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 22. | Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 581] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 23. | Heslin MJ, Latkany L, Leung D, Brooks AD, Hochwald SN, Pisters PW, Shike M, Brennan MF. A prospective, randomized trial of early enteral feeding after resection of upper gastrointestinal malignancy. Ann Surg. 1997;226:567-577; discussion 577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 243] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Braga M, Gianotti L, Nespoli L, Radaelli G, Di Carlo V. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg. 2002;137:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 288] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Sacks GS, Genton L, Kudsk KA. Controversy of immunonutrition for surgical critical-illness patients. Curr Opin Crit Care. 2003;9:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | McCowen KC, Bistrian BR. Immunonutrition: problematic or problem solving? Am J Clin Nutr. 2003;77:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Jakobson T, Karjagin J, Vipp L, Padar M, Parik AH, Starkopf L, Kern H, Tammik O, Starkopf J. Postoperative complications and mortality after major gastrointestinal surgery. Medicina (Kaunas). 2014;50:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, Macfie J, Liberman AS, Soop M, Hill A, Kennedy RH, Lobo DN, Fearon K, Ljungqvist O; Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg. 2013;37:259-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 868] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 29. | Sica GS, Iaculli E, Biancone L, Di Carlo S, Scaramuzzo R, Fiorani C, Gentileschi P, Gaspari AL. Comparative study of laparoscopic vs open gastrectomy in gastric cancer management. World J Gastroenterol. 2011;17:4602-4606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Bailón-Cuadrado M, Pérez-Saborido B, Sánchez-González J, Rodríguez-López M, Velasco-López R, C Sarmentero-Prieto J, I Blanco-Álvarez J, Pacheco-Sánchez D. Prognostic Nutritional Index predicts morbidity after curative surgery for colorectal cancer. Cir Esp (Engl Ed). 2019;97:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Moya P, Miranda E, Soriano-Irigaray L, Arroyo A, Aguilar MD, Bellón M, Muñoz JL, Candela F, Calpena R. Perioperative immunonutrition in normo-nourished patients undergoing laparoscopic colorectal resection. Surg Endosc. 2016;30:4946-4953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/