Published online Dec 27, 2025. doi: 10.4240/wjgs.v17.i12.111714
Revised: August 23, 2025
Accepted: October 20, 2025
Published online: December 27, 2025
Processing time: 146 Days and 1.1 Hours
Endoscopic submucosal dissection (ESD) is a minimally invasive, safe, and efficient treatment technique for patients diagnosed with early esophageal cancer. However, postoperative disease recurrence remains an important clinical cha
To assess factors that contribute to the risk for disease recurrence after ESD for early esophageal cancer.
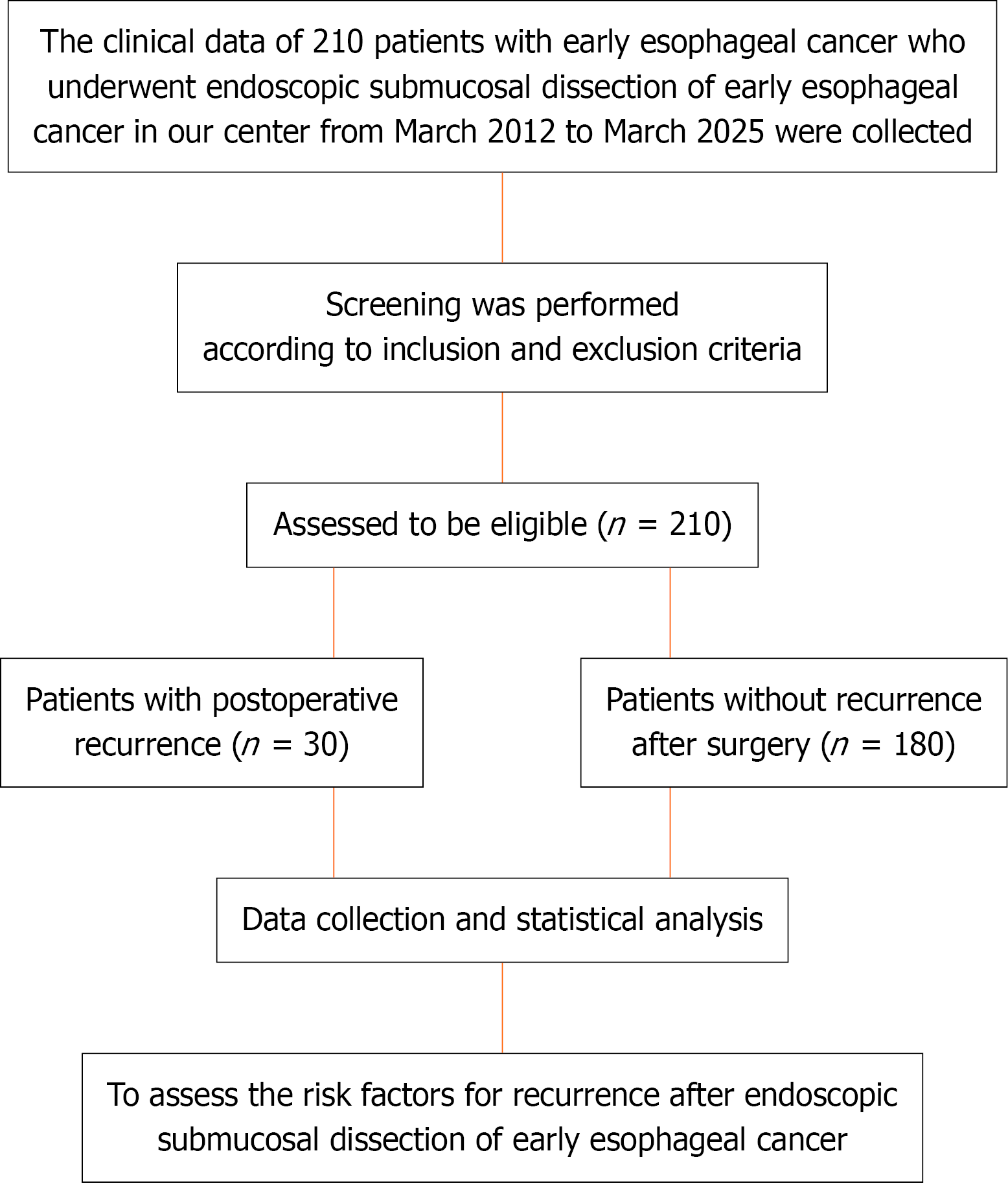
Clinical data from 210 patients diagnosed with early stage esophageal cancer, who underwent ESD at the authors’ center between March 2012 and March 2025, were retrospectively collected and analyzed. Patients were categorized into 2 groups according to postoperative disease recurrence: Recurrence (n = 30), and without recurrence (n = 180). Disease recurrence was defined as the appearance of new tumor lesions or pathologically confirmed tumor recurrence during the post
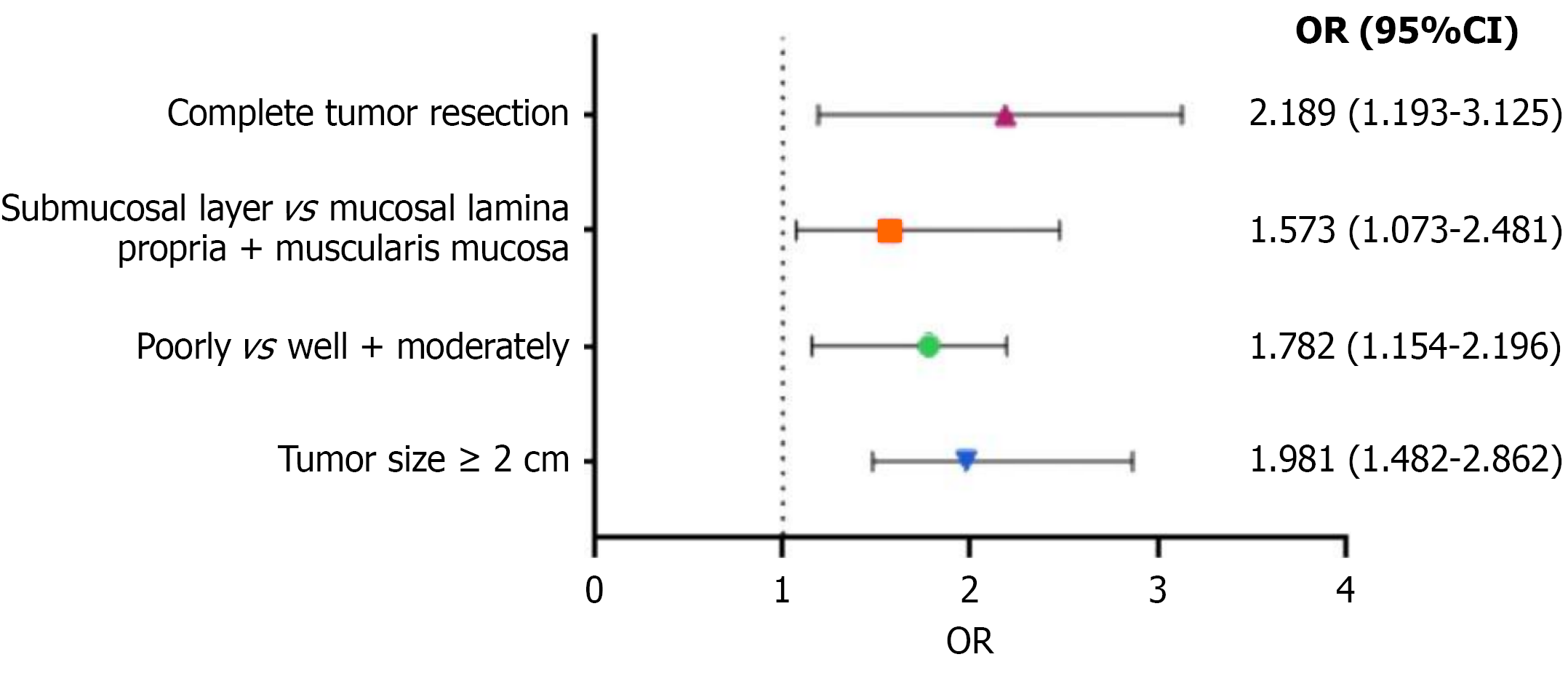
During the follow-up period, 30 patients experienced tumor recurrence, corresponding to a recurrence rate of 14.19%. Multivariate analysis revealed that poor differentiation was a significant potential cause of esophageal cancer recurrence [odds ratio (OR) = 1.782, 95% confidence interval (CI): 1.154-2.196; P < 0.001]. Tumors infiltrating the submucosa were more likely to recur than those penetrating the lamina propria or muscularis mucosa (OR = 1.573, 95%CI: 1.073-2.481; P < 0.001). Furthermore, inability to completely resect the tumor greatly increased the likelihood of recurrence (OR = 2.189, 95%CI: 1.193-3.125; P = 0.001). Tumor diameter ≥ 2 cm was an independent risk factor for postoperative recurrence (OR = 1.981, 95%CI: 1.482-2.862; P = 0.005).
Recurrence of early esophageal cancer after ESD is largely influenced by the degree of differentiation, depth of lesion invasion, complete resection status of the tumor, and tumor diameter.
Core Tip: The disease recurrence rate after endoscopic submucosal dissection for early esophageal cancer was 14.19%. A low degree of differentiation, infiltration into the submucosa, incomplete tumor resection, and tumor diameter ≥ 2 cm were independent risk factors for postoperative recurrence. Accurate assessment of these risk factors can help inform personalized follow-up strategies and secondary treatment plans to increase patients’ chances of long-term survival.
- Citation: Yang YM, Dai T, Zou LY, Zhao CJ. Analysis of risk factors for disease recurrence after endoscopic submucosal dissection of early esophageal cancer. World J Gastrointest Surg 2025; 17(12): 111714
- URL: https://www.wjgnet.com/1948-9366/full/v17/i12/111714.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i12.111714
Esophageal cancer is a globally prevalent and aggressive cancer, with significant differences in incidence and mortality rates across different regions[1]. Esophageal cancer, especially squamous cell carcinoma, has high incidence and mortality rates in China[2,3]. According to the 2021 Global Burden of Disease Study[4], the prevalence and mortality rates of early stage esophageal cancer have declined globally, specifically in countries with high sociodemographic indices, and these improvements are primarily driving the downward trend on a global scale. However, despite the overall positive trend, the total number of cases and deaths is increasing due to population expansion and aging[5]. The burden of early stage esophageal cancer remains high, with significant regional differences, which requires international attention and intervention[6,7] because a greater proportion of patients with esophageal cancer are diagnosed at a later stage of disease, with a poor quality of life, and the best time for treatment or even the opportunity for surgery lost, with < 20% of affected individuals surviving 5 additional years[8,9].
Over the past few years, advances have been made in the management of esophageal cancer. Natural polyphenols have attracted attention due to their potential therapeutic effects, and studies have shown that polyphenols in combination with chemotherapy and radiotherapy can overcome current challenges in monotherapy[10]. Furthermore, immune checkpoint inhibitors have demonstrated significant benefits in patients diagnosed with advanced esophageal cancer[11]. However, the early identification of esophageal cancer remains a challenge and, despite significant advances in endoscopic techniques, early detection strategies need to be strengthened to increase the likelihood of patients surviving ≥ 5 years[12].
The etiology of esophageal cancer is complex[13]. Moreover, differences in sex, region, and subtype persist, especially in rural areas where the burden of esophageal cancer remains high[14]. Recent advances in molecular pathology have further revealed the heterogeneity and aggressive potential of esophageal carcinomas, particularly among poorly differentiated subtypes. For instance, TP53 mutations are present in over 80% of poorly differentiated esophageal squamous cell carcinomas, contributing to epithelial-mesenchymal transition and enhanced invasiveness[15,16]. In addition, biomarkers such as programmed cell death ligand 1 expression and microsatellite instability status have been in
Accordingly, this retrospective study analyzed relevant factors to identify patients at high-risk for disease recurrence after undergoing endoscopic submucosal dissection (ESD) for early esophageal cancer.
Clinical data from 210 patients diagnosed with early esophageal cancer, who underwent ESD at the authors’ center between March 2012 and March 2025, were retrospectively collected and analyzed. Participants were divided into 2 groups according to disease recurrence after surgery: Recurrence (n = 30), and without recurrence (n = 180).
Inclusion criteria: (1) Pathology-confirmed diagnosis of early stage esophageal cancer, superficial lesions evident under endoscopy, lymph node metastasis is not visualized on imaging examination, and lesions have not invading the upper one-third of the submucosa (SM1) of the cancer tissue; (2) Underwent successful ESD treatment with good postoperative recovery; (3) Complete clinical data; (4) Underwent regular endoscopic, telephone, or outpatient follow-up after surgery; and (5) Agreed to participate.
Exclusion criteria: (1) ESD failed during surgery and transferred to open surgery; (2) Other acute and chronic serious diseases that affect prognosis; and (3) Lack of complete clinical data.
The hospital ethics review committee approved this study and all participants provided informed written consent.
Information was collected from the hospital’s electronic medical records regarding sex, age, and body weight category (underweight, normal weight, overweight), and alcohol consumption and smoking histories. In addition, data regarding endoscopic morphology of the lesion, entire excision of the lesion, inherent basal injury, pathological classification, degree of mucosal defect around the wound, postoperative complications, lesion location, average diameter of the lesion, and mean operative duration, were also collected.
All ESD procedures were performed by experienced endoscopists. The Dual Knife (Olympus, Japan) was used as the primary electrosurgical knife for circumferential marking and submucosal dissection. In cases with significant fibrosis or difficult locations, the IT Knife (Olympus, Japan) was alternatively employed to complete the dissection. A transparent cap was attached to the endoscope tip to provide constant traction and optimize visualization of the submucosal layer. Submucosal injection was routinely performed using a solution composed of glycerol (10%), fructose (5%), and a small amount of indigo carmine to improve tissue elevation and delineate the dissection plane. For lesions located in areas with expected poor lifting (such as those with fibrosis), sodium hyaluronate solution (0.4%) was selectively applied to achieve sustained submucosal cushioning.
After discharge, patients were followed up by telephone or in the outpatient clinic, and endoscopy was re-examined once in March, June, and December after surgery, and once per year after no recurrence. If a positive or suspicious lesion was found on re-examination, biopsy and pathological diagnosis were required. Routine laboratory investigations and tumor markers were rechecked at each time point, and computed tomography or magnetic resonance imaging, along with other assessments, were performed according to the direction of the suspected metastasis.
Outcome measures included single-variable and logistic regression analyses of postoperative disease recurrence.
Statistical analysis was performed using SPSS version 26.0 (IBM Corp., Armonk, NY, United States). Continuous data were tested for normal distribution using the Kolmogorov-Smirnov method, and are expressed as mean ± SD. The t-test was used to compare groups, while the rate or composition ratio of quantitative data was described, and the χ2 test was used to compare the groups. Independent conditions that contributed to postoperative recurrence were identified using logistic regression analysis for each factor with P ≤ 0.1. Differences with P < 0.05 were considered to be statistically significant.
This study included 210 patients diagnosed with esophageal cancer. A flow-diagram illustrating the patient selection process is presented in Figure 1. The cohort was dominated by males [n = 168 (80%)], with females accounting for 20% (n = 42). The mean (± SD) age of the patients was 62.53 ± 7.82 years. The most common tumors were located in the middle segment [n = 161 (76.67%)], with 49 (23.33%) located in the lower segment. The mean tumor size was 2.49 ± 1.25 cm. Pathological differentiation was distributed as follows: Well differentiated, 50% (n = 105); Moderately differentiated, 36.67% (n = 77); And poorly differentiated, 13.33% (n = 28). Regarding depth of invasion, 63.33% of the tumors invaded the mucosal lamina propria (n = 133), 30% invaded the muscular mucosa (n = 63), and only 6.67% (n = 14) invaded the submucosa. Details are summarized in Table 1.
| Characteristic | Esophageal cancer (n = 210) | Percentage (%) |
| Gender | ||
| Male | 168 | 80 |
| Female | 42 | 20 |
| Age (years) | 62.53 ± 7.82 | |
| Elderly status | ||
| ≥ 65 years | 84 | 40 |
| < 65 years | 126 | 60 |
| Endoscopic ultrasound examination | ||
| Examined | 147 | 70 |
| Not examined | 63 | 30 |
| Tumor location | ||
| Upper third | 0 | 0 |
| Middle third | 161 | 76.67 |
| Lower third | 49 | 23.33 |
| Tumor size (cm) | 2.49 ± 1.25 | |
| Differentiation degree | ||
| Well differentiated | 105 | 50 |
| Moderately differentiated | 77 | 36.67 |
| Poorly differentiated | 28 | 13.33 |
| Invasion depth | ||
| Mucosal lamina propria | 133 | 63.33 |
| Muscularis mucosa | 63 | 30 |
| Submucosal layer | 14 | 6.67 |
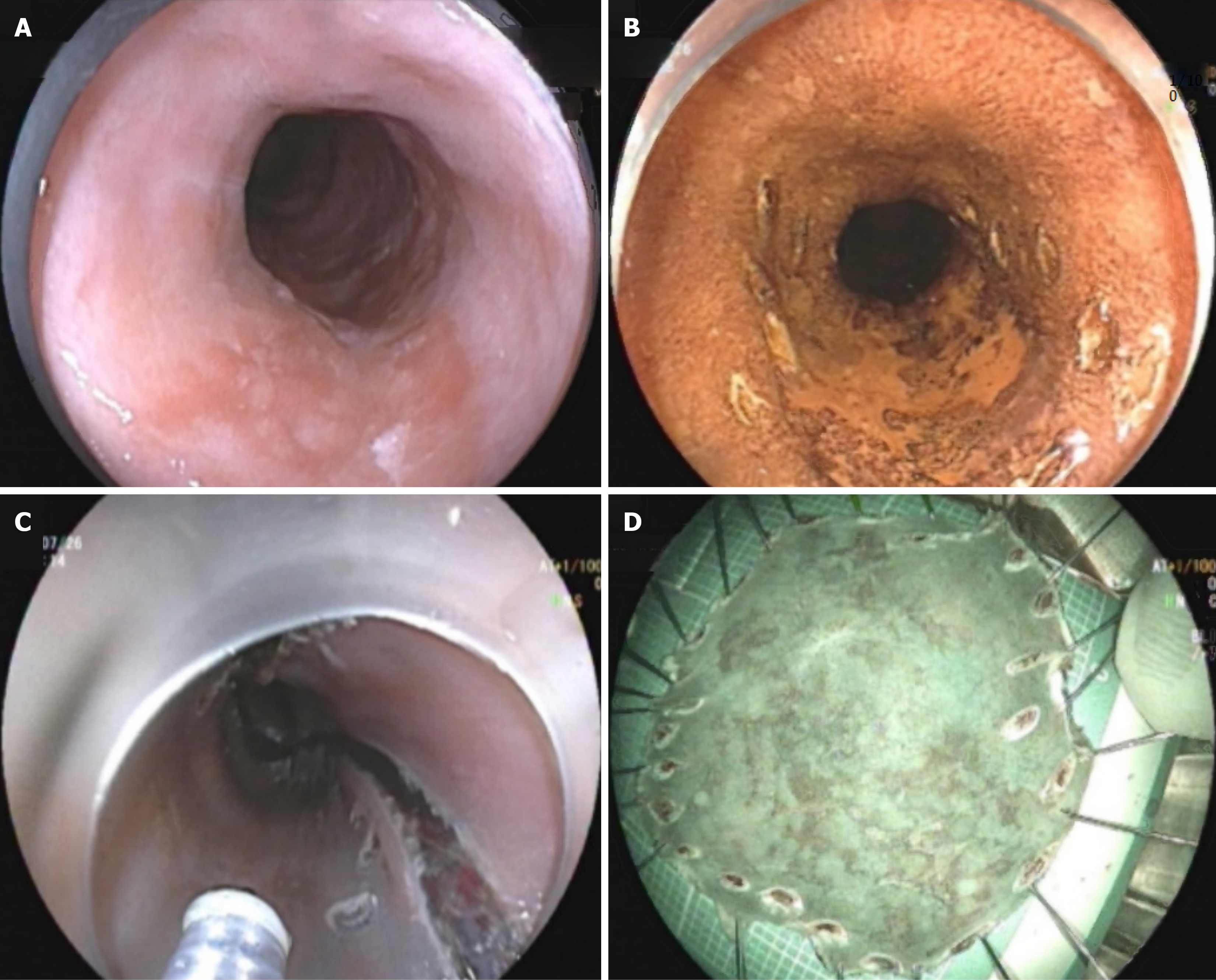
There were 30 patients in the recurrence group, corresponding to a recurrence rate of 14.19% (30/210). There were 180 patients in the non-relapse group. The proportion of males in the recurrence group was 56.67% (17/30), whereas the proportion of males in the non-recurrence group was 83.89% (151/180), with no significant difference (P = 0.068). There were no significant differences in age distribution, endoscopic ultrasound findings, or tumor location between the groups (P > 0.05). The recurrence group had an average tumor diameter of 2.56 ± 1.05 cm, which was notably greater than that in the non-recurrence group (1.87 ± 0.79 cm) (P < 0.001). The proportion of poorly differentiated tumors in the recurrence group was higher [46.67% (14/30)], whereas the proportion of low-grade tumors was significantly lower in the non-recurrence group [7.78% (14/180)] (P < 0.001). Moreover, 36.67% (11/30) of patients who exhibited mucosal infiltration of the lamina propria in the recurrence group was notably greater than those in the non-recurrence group (67.78% (122/180) (P < 0.001), while the proportion of patients with submucosal invasion was also higher in the recurrence group [33.33% (10/30)]. The percentage of patients with submucosal invasion was greater in the recurrence group [33.33% (10/30)], in contrast to the non-recurrence group [2.22% (4/180)]. Furthermore, the difference in total resection between the groups was statistically significant (P < 0.001) (Table 2). Data from representative cases are presented in Figure 2.
| Characteristic | Recurrence group (n = 30) | Non-recurrence group (n = 180) | P value |
| Gender | 0.068 | ||
| Male | 17 (56.67) | 151 (83.89) | |
| Female | 13 (43.33) | 29 (16.11) | |
| Age (years) | 0.473 | ||
| ≥ 65 years | 11 (36.67) | 73 (40.56) | |
| < 65 years | 19 (63.33) | 107 (59.44) | |
| Endoscopic ultrasound examination | 0.262 | ||
| Examined | 16 (53.33) | 131 (72.78) | 0.713 |
| Not examined | 14 (46.67) | 49 (27.22) | |
| Tumor location | |||
| Upper third | 0.173 | ||
| Middle third | 18 (60) | 143 (79.44) | |
| Lower third | 12 (40) | 37 (20.56) | |
| Tumor size (cm) | 2.56 ± 1.05 | 1.87 ± 0.79 | < 0.001 |
| Differentiation degree | < 0.001 | ||
| Well differentiated | 9 (30) | 96 (53.33) | |
| Moderately differentiated | 7 (23.33) | 70 (38.89) | |
| Poorly differentiated | 14 (46.67) | 14 (7.78) | |
| Invasion depth | < 0.001 | ||
| Mucosal lamina propria | 11 (36.67) | 122 (67.78) | |
| Muscularis mucosa | 9 (30) | 54 (30) | |
| Submucosal layer | 10 (33.33) | 4 (2.22) | |
| Complete tumor resection | < 0.001 | ||
| Yes | 19 (63.33) | 169 (93.89) | |
| No | 11 (36.67) | 11 (6.11) |
Univariate analysis revealed significant differences in tumor size, degree of differentiation, invasion depth, and complete tumor resection (P < 0.05). Multivariate logistic regression analysis was performed on the 4 significantly different factors, with postoperative recurrence as the dependent variable (Table 3). Multivariate analysis indicated that poor differentiation greatly increased the likelihood of esophageal cancer recurrence [odds ratio (OR) = 1.782, 95% confidence interval (CI): 1.154-2.196; P < 0.001]. Tumors infiltrating the submucosa were more likely to recur than those infiltrating the lamina propria or muscularis mucosa (OR = 1.573, 95%CI: 1.073-2.481; P < 0.001). Moreover, inability to completely resect the tumor greatly heightened the likelihood of recurrence (OR = 2.189, 95%CI: 1.193-3.125; P = 0.001). Tumor diameter ≥ 2 cm was also a major risk determinant (OR = 1.981, 95%CI: 1.482-2.862; P = 0.005). The results are summarized in Table 4 and Figure 3.
| Item | Assignment |
| Tumor size (cm) | Tumor size ≥ 2 cm = 1; tumor size < 2 cm = 0 |
| Differentiation degree | Poorly differentiated = 1; well + moderately differentiated = 0 |
| Invasion depth | Submucosal layer = 1; mucosal lamina propria + muscularis mucosa = 0 |
| Complete tumor resection | No = 1; yes = 0 |
| Risk factor | P value | OR | 95%CI |
| Poorly vs well + moderately | < 0.001 | 1.782 | 1.154-2.196 |
| Submucosal layer vs mucosal lamina propria + muscularis mucosa | < 0.001 | 1.573 | 1.073-2.481 |
| Complete tumor resection | 0.001 | 2.189 | 1.193-3.125 |
| Tumor size ≥ 2 cm | 0.005 | 1.981 | 1.482-2.862 |
Disease recurrence rate is an important indicator for assessing the effect of treatment. ESD is a minimally invasive procedure for esophageal cancer. However, the disease recurrence rate of esophageal cancer after ESD surgery remains a concern. The results of this study revealed that the recurrence rate of esophageal cancer after ESD surgery was 14.19% (30/210), compared with only 0%-2.6% reported in previous studies[19,20]. This discrepancy can be attributed to several factors. Positive surgical margins are important risk factors for recurrence. Both positive vertical and horizontal margins have been shown to significantly increased the risk for recurrence[21]. In addition, lymphovascular invasion is also an important pathological factor, increasing the likelihood of postoperative recurrence[21]. However, for tumors with submucosal invasion > 200 microns, due to the elevated risk for lymph node metastasis, there is a relative increase in the recurrence rate[20,22]. Nevertheless, the recurrence rate remains high after ESD.
The present study also found that a low degree of differentiation, infiltration of the submucosa, incomplete tumor resection, and tumor diameter ≥ 2 cm independently contributed to the risk for recurrence after surgery, consistent with findings from several previous studies. In terms of the degree of tumor differentiation, this study confirmed that poorly differentiated tumors greatly increased the likelihood of postoperative recurrence (OR = 1.782, P < 0.001). Previous studies have reported that patients with poorly differentiated esophageal cancer are more likely to develop lymph node metastasis and disease recurrence after surgery[23,24], which may be due to the more aggressive biological behavior of poorly differentiated tumors, which aligns with the findings of this study.
The results of this study also revealed that the risk for tumor recurrence infiltrating the submucosa was 1.573 for entry into the mucosal lamina propria or muscularis mucosa (P < 0.001). A possible explanation for this is that the submucosal layer is rich in blood and lymphatic vessels and, once the tumor has infiltrated this point, it has obtained a more convenient metastatic pathway, making the tumor cells more susceptible to distant metastasis or local retention, thereby increasing the probability of recurrence. This is consistent with previous studies[25,26].
Additionally, incomplete tumor resection and R0 resection did not significantly increase the risk for recurrence. Studies have shown that tumors that are not completely resected are more likely to develop local recurrence after surgery; therefore, it is critical to achieve complete resection during surgery, and the inability to completely eliminate the tumor greatly increases the likelihood of recurrence (OR = 2.189, P = 0.001). Residual tumor cells are the source of postoperative recurrence, and it is difficult to completely remove these residual lesions, even with adjuvant therapy. The results of this investigation are confirmed by those of previous studies[27,28].
The findings of this research indicated that tumor diameter ≥ 2 cm was an independent risk factor for recurrence after surgery (OR = 1.981, P = 0.005). The findings of this study are consistent with those of previous studies because a larger tumor volume indicates an increased number of tumor cells, tumor margins are difficult to define, and small residual lesions are more likely to occur during surgery. At the same time, large tumors may have multicentric origin or early invasion, which together contribute to an increased risk for postoperative recurrence[29,30]. Thus, it is essential to enhance clinical efforts for patients with early stage esophageal cancer, focusing on screening and identifying high-risk factors for recurrence after ESD, and strengthen postoperative management for those at high risk. Targeted interventions should be provided promptly to reduce the probability of postoperative recurrence, prolong survival, and improve survival rates.
For patients with poorly differentiated tumors and submucosal infiltration, adjuvant radiotherapy or chemotherapy may be required. For patients with lymphatic invasion, studies have shown that adjuvant radiotherapy can significantly improve survival rates, whereas for those without lymphatic involvement, the effectiveness of adjuvant radiotherapy is not significant[31]. Additionally, research indicates that immediate adjuvant therapies such as chemoradiotherapy or surgery following esophageal cancer surgery (ESD) can substantially enhance survival rates in patients at risk of metastasis[32].
Nevertheless, the present study had some limitations, the first of which were its retrospective, single-center design and relatively small sample size, which may have introduced selection bias. As such, the findings require further validation for general applicability. Second, there were individual differences in the follow-up time; some patients had a short follow-up, which may have underestimated the risk for long-term recurrence. In addition, the study only analyzed a few risk factors that are common in clinical practice and did not deeply explore potential molecular biological factors, such as certain gene mutations and protein expression levels, which may also be associated with postoperative recurrence. Finally, the recurrence rate in the present investigation was somewhat greater than that reported in earlier studies, which is likely due to confounding factors that were not strictly controlled for in this study. It should be noted that the recurrence rate observed in this study was higher than that typically reported in high-volume medical centers in East Asia. As recurrence rates are influenced by multiple factors rather than a single cause, this discrepancy may be attributable to several confounding variables, such as the duration of data collection, variations in surgical experience among operators, and differences in annual procedure volumes. Future research also needs to propose a practical scoring system to stratify patients based on recurrence risk. This approach may support individualized postoperative management, including enhanced follow-up of high-risk patients and consideration of adjuvant therapy, ultimately aiming to improve survival outcomes. Multicenter, large-sample prospective studies are required to validate the results of the present study.
The recurrence of esophageal cancer after ESD for initial disease was largely influenced by the degree of tumor differentiation, depth of lesion invasion, complete resection status of the tumor, and tumor diameter. Clinicians should accurately assess these risk factors using endoscopic ultrasonography, pathological biopsy, and other methods before surgery to provide more effective treatment strategies to improve long-term patient survival.
| 1. | Aadam AA, Abe S. Endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2018;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | An W, Liu MY, Zhang J, Cui YP, Gao J, Wang LP, Chen Y, Yang LX, Chen HZ, Jin H, Liu F, Chen J, Li ZS, Wang LW, Shi XG, Sun C. Endoscopic submucosal dissection versus esophagectomy for early esophageal squamous cell carcinoma with tumor invasion to different depths. Am J Cancer Res. 2020;10:2977-2992. [PubMed] |
| 3. | Chen R, Zheng R, Zhang S, Wang S, Sun K, Zeng H, Li L, Wei W, He J. Patterns and trends in esophageal cancer incidence and mortality in China: An analysis based on cancer registry data. J Natl Cancer Cent. 2023;3:21-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 4. | Cho YA, Lee H, Kim DG, Kim H, Ha SY, Choi YL, Jang KT, Kim KM. PD-L1 Expression Is Significantly Associated with Tumor Mutation Burden and Microsatellite Instability Score. Cancers (Basel). 2021;13:4659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Doumbe-Mandengue P, Pellat A, Belle A, Ali EA, Hallit R, Beuvon F, Terris B, Chaussade S, Coriat R, Barret M. Endoscopic submucosal dissection versus endoscopic mucosal resection for early esophageal adenocarcinoma. Clin Res Hepatol Gastroenterol. 2023;47:102138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 6. | Funakawa K, Uto H, Sasaki F, Nasu Y, Mawatari S, Arima S, Nakazawa J, Taguchi H, Hashimoto S, Kanmura S, Setoyama H, Numata M, Tsubouchi H, Ido A. Effect of endoscopic submucosal dissection for superficial esophageal neoplasms and risk factors for postoperative stricture. Medicine (Baltimore). 2015;94:e373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Furukawa H, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M, Doki Y. PRIMA-1 induces p53-mediated apoptosis by upregulating Noxa in esophageal squamous cell carcinoma with TP53 missense mutation. Cancer Sci. 2018;109:412-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Gu L, Khadaroo PA, Chen L, Li X, Zhu H, Zhong X, Pan J, Chen M. Comparison of Long-Term Outcomes of Endoscopic Submucosal Dissection and Surgery for Early Gastric Cancer: a Systematic Review and Meta-analysis. J Gastrointest Surg. 2019;23:1493-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Hatta W, Koike T, Takahashi S, Shimada T, Hikichi T, Toya Y, Tanaka I, Onozato Y, Hamada K, Fukushi D, Watanabe K, Kayaba S, Ito H, Mikami T, Oikawa T, Takahashi Y, Kondo Y, Yoshimura T, Shiroki T, Nagino K, Hanabata N, Funakubo A, Hirasawa D, Ohira T, Nakamura J, Matsumoto T, Nakamura T, Nakaya N, Iijima K, Fukuda S, Masamune A; Tohoku GI Endoscopy Group. Risk of metastatic recurrence after endoscopic resection for esophageal squamous cell carcinoma invading into the muscularis mucosa or submucosa: a multicenter retrospective study. J Gastroenterol. 2021;56:620-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Jiang H, Tian B, Gao Y, Bian Y, Yu C, Xu J, Wang W, Lin H, Xin L, Wang L. Risk and pathologic factors of recurrence after endoscopic resection for superficial esophageal squamous cell carcinoma: a systematic review and meta-analysis. Gastrointest Endosc. 2024;100:1006-1019.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Jin W, Huang K, Ding Z, Zhang M, Li C, Yuan Z, Ma K, Ye X. Global, regional, and national burden of esophageal cancer: a systematic analysis of the Global Burden of Disease Study 2021. Biomark Res. 2025;13:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 12. | Kadota T, Yano T, Fujita T, Daiko H, Fujii S. Submucosal Invasive Depth Predicts Lymph Node Metastasis and Poor Prognosis in Submucosal Invasive Esophageal Squamous Cell Carcinoma. Am J Clin Pathol. 2017;148:416-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Katada C, Yokoyama T, Hirasawa D, Iizuka T, Kikuchi D, Yano T, Hombu T, Yoshio T, Yoshimizu S, Ono H, Yabuuchi Y, Terai S, Hashimoto S, Takahashi K, Tanaka S, Urabe Y, Arima M, Tanabe S, Wada T, Furue Y, Oyama T, Takahashi A, Sakamoto Y, Muto M. Curative Management After Endoscopic Resection for Esophageal Squamous Cell Carcinoma Invading Muscularis Mucosa or Shallow Submucosal Layer-Multicenter Real-World Survey in Japan. Am J Gastroenterol. 2023;118:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Kume K. Endoscopic therapy for early gastric cancer: standard techniques and recent advances in ESD. World J Gastroenterol. 2014;20:6425-6432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Liu GM, Ji X, Lu TC, Duan LW, Jia WY, Liu Y, Sun ML, Luo YG. Comprehensive multi-omics analysis identified core molecular processes in esophageal cancer and revealed GNGT2 as a potential prognostic marker. World J Gastroenterol. 2019;25:6890-6901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Lorenzo D, Barret M, Leblanc S, Terris B, Beuvon F, Coriat R, Chaussade S, Prat F. Outcomes of endoscopic submucosal dissection for early oesophageal squamous cell neoplasia at a Western centre. United European Gastroenterol J. 2019;7:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Lu Z, Geng M, Han Y, Cao J, Wang J, Liu T, Yuan X, Meng X, Zhang Y, Zhao R, Wan L, Li E, Wang W, Li Z, Shi D, Qian J, Shi S, Dong F, Shen L. Retrospective analysis of disease characteristics and treatment patterns among patients with esophageal cancer across 14 surgically represented centers. Cancer Biol Med. 2025;21:1171-1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Merkow RP, Bilimoria KY, Keswani RN, Chung J, Sherman KL, Knab LM, Posner MC, Bentrem DJ. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst. 2014;106:dju133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Niu C, Zhang J, Okolo PI 3rd. Unlocking the Therapeutic Potential of Natural Polyphenols in Esophageal Cancer. Curr Treat Options Oncol. 2025;26:278-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Pei G, Huang Y, Yang Y, Wang S, Meng S, Liu J, Jiang H. Global Burden of Early-Onset Esophageal Cancer From 1990 to 2021: A Systematic Analysis of the Global Burden of Disease Study 2021. Thorac Cancer. 2025;16:e70082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Probst A, Kappler F, Ebigbo A, Albers D, Faiss S, Steinbrück I, Wannhoff A, Allgaier HP, Denzer U, Rempel V, Reinehr R, Dakkak D, Mende M, Pohl J, Schaller T, Märkl B, Muzalyova A, Fleischmann C, Messmann H. Endoscopic submucosal dissection for early esophageal adenocarcinoma: low rates of metastases in mucosal cancers with poor differentiation. Gastrointest Endosc. 2024;100:626-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Pubu S, Zhang JW, Yang J. Early diagnosis of esophageal cancer: How to put "early detection" into effect? World J Gastrointest Oncol. 2024;16:3386-3392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Qiu S, Xie B, Liao J, Luo J, Liu X, He L, Huang Y, Peng L. Blood trace elements in association with esophageal squamous cell carcinoma risk, aggressiveness and prognosis in a high incidence region of China. Sci Rep. 2025;15:5208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Ran X, Zeng H, Zheng R, Sun K, Han B, Wang S, Chen R, Li L, Wei W, He J. Geographic, sex and socioeconomic disparities in esophageal cancer incidence in China: A population-based study. Int J Cancer. 2024;154:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 25. | Sun L, Zhao K, Liu X, Meng X. Global, regional, and national burden of esophageal cancer using the 2019 global burden of disease study. Sci Rep. 2025;15:3284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Takahashi H, Arimura Y, Masao H, Okahara S, Tanuma T, Kodaira J, Kagaya H, Shimizu Y, Hokari K, Tsukagoshi H, Shinomura Y, Fujita M. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc. 2010;72:255-264, 264.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (5)] |
| 27. | Talagala IA, Arambepola C. Changes in quality of life following initial treatment of oesophageal carcinoma: a cohort study from Sri Lanka. BMC Cancer. 2018;18:1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Teng Y, Xia C, Cao M, Yang F, Yan X, He S, Cao M, Zhang S, Li Q, Tan N, Wang J, Chen W. Esophageal cancer global burden profiles, trends, and contributors. Cancer Biol Med. 2024;21:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 29. | Tong X, Jin M, Wang L, Zhang D, Yin Y, Shen Q. Prognostic biomarkers for immunotherapy in esophageal cancer. Front Immunol. 2024;15:1420399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 30. | Wang ZX, Li LS, Su S, Li JP, Zhang B, Wang NJ, Liu SZ, Wang SS, Zhang S, Bi YW, Gao F, Shao Q, Xu N, Shao BZ, Yao Y, Liu F, Linghu EQ, Chai NL. Linked color imaging vs Lugol chromoendoscopy for esophageal squamous cell cancer and precancerous lesion screening: A noninferiority study. World J Gastroenterol. 2023;29:1899-1910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 31. | Yang B, Chen Q, Wan C, Sun S, Zhu L, Zhao Z, Zhong W, Wang B. Transgelin Inhibits the Malignant Progression of Esophageal Squamous Cell Carcinomas by Regulating Epithelial-Mesenchymal Transition. Front Oncol. 2021;11:709486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Yang X, Zhao L, Shi A, Chen C, Cao J, Zhang Y, Zhu H, Wang J, Zhou W, Li X, Hu S, Men Y, Wang J, Xue L, Liu Y, Dou L, Zhang Y, Sun S, Yuan M, Bao Y, Ma Z, Liu Y, Zhang W, Bi N, Wang G, Hui Z. Radiotherapy Improves Survival of Patients With Lymphovascular Invasion in pT1b Esophageal Squamous Cell Cancer After Endoscopic Submucosal Dissection. Am J Gastroenterol. 2023;118:1344-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/