Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.111973
Revised: August 13, 2025
Accepted: September 17, 2025
Published online: November 27, 2025
Processing time: 133 Days and 2.3 Hours
Colorectal cancer is a prevalent malignancy with suboptimal postoperative re
To identify predictors of therapeutic efficacy for probiotics combined with enteral nutrition in postoperative patients with colorectal cancer.
A retrospective study was conducted with 511 patients with colorectal cancer who underwent surgery and received probiotics and enteral nutrition from January 2022 to March 2025. Patients were categorized into the “good efficacy group” (n = 279) and “poor efficacy group” (n = 232) based on outcomes observed 3 months post-surgery. Variables assessed included gut microbiota composition, nutritional intake, immune and inflammatory markers, and demographic characteristics.
Patients with favorable outcomes were typically younger, had higher caloric, protein, and fiber intake, and displayed enhanced intestinal mucosal barrier function with elevated levels of Bifidobacterium and Lactobacillus. Immune markers such as immunoglobulin A, immunoglobulin M, immunoglobulin G, and CD4+/ CD8+ T-cell ratios were significantly higher in the good efficacy group. High numbers of Fusobacterium nucleatum and Bacteroides fragilis and levels of tumor necrosis factor-alpha and interleukin-6 were associated with poor efficacy. Multivariate analysis identified age, tumor node metastasis stage, protein intake, and gut microbiota composition as significant predictors of therapeutic success.
The efficacy of combining probiotics with enteral nutrition in postoperative patients with colorectal cancer was influenced by age, nutritional intake, microbiota balance, immune status, and inflammatory markers.
Core Tip: This retrospective study identifies key predictors influencing the efficacy of probiotics combined with enteral nutrition in 511 postoperative colorectal cancer patients. Younger age, higher protein intake, balanced gut microbiota (elevated Bifidobacterium, reduced Fusobacterium nucleatum), and improved immune markers independently correlated with better outcomes. These findings highlight modifiable factors—nutritional optimization and microbiota modulation—for enhancing recovery. Multivariate analysis confirmed age, tumor node metastasis stage, protein intake, and microbial composition as significant prognostic indicators.
- Citation: Zhang Y, Wu J, Yuan Y, Huang YS, Han H. Factors influencing analysis of the efficacy of probiotics combined with enteral nutrition in postoperative patients with colorectal cancer. World J Gastrointest Surg 2025; 17(11): 111973
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/111973.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.111973
Colorectal cancer, which remains one of the most prevalent malignancies globally, poses significant challenges to healthcare systems and affects a considerable proportion of the adult population. Despite advancements in surgical techniques and adjuvant therapies, postoperative recovery in patients with colorectal cancer remains suboptimal and is often complicated by recurrence, metastasis, and impaired quality of life. The demand for innovative therapeutic stra
In recent years, the integration of probiotics with enteral nutrition has garnered attention as a promising intervention to enhance postoperative outcomes in patients with colorectal cancer. Probiotics, defined as live microorganisms that confer health benefits to the host when administered in adequate amounts, play an integral role in maintaining gut homeostasis[4]. They have been shown to influence immune responses, compete against pathogenic bacteria, and rein
The gut microbiota, a dynamic community of microorganisms residing in the gastrointestinal tract, significantly in
Nutritional intake is a cornerstone of postoperative care and influences recovery through wound healing, muscle maintenance, and overall homeostasis. Enteral nutrition not only ensures continuous supply of vital nutrients but also preserves the functionality of the gastrointestinal tract to provide an environment conducive to probiotic activity and microbial balance[11,12]. High caloric, protein, and fiber intake has been linked with improved gut health and enhanced immune responses, suggesting that targeted nutritional strategies can optimize therapeutic outcomes in cancer patients[13].
Despite the theoretical and preliminary clinical underpinnings supporting the combination of probiotics and enteral nutrition, precise factors influencing their efficacy have not been comprehensively elucidated. This is particularly relevant for understanding why certain patients derive significant benefit while others do not. The interplay between these factors and their collective impact on postoperative recovery remains an area of active investigation and clinical importance[9].
Inflammatory responses play a crucial role in the healing process and are influenced by nutritional and microbial factors[14,15]. Elevated levels of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and in
A retrospective study was performed involving 511 patients with colorectal cancer who underwent surgical procedures and subsequently received a combination of probiotics and enteral nutrition treatment at The First People’s Hospital of Zunyi between January 2022 to March 2025. Patients were divided into two groups based on their therapeutic outcomes observed 3 months post-surgery: The “good efficacy group” (n = 279) and the “poor efficacy group” (n = 232). Patients were classified as belonging to the “poor efficacy group” if they experienced any of the following within 3 months after surgery: Imaging-confirmed tumor recurrence or metastasis; occurrence of a serious post-operative complication re
The study received approval from the institutional review board and ethics committee of The First People’s Hospital of Zunyi. The need for informed consent was waived because of the retrospective nature of the study and the use of anonymized patient data. This exemption was granted in alignment with established regulatory and ethical guidelines for conducting retrospective analyses to ensure that no potential risks or adverse consequences affect the patients.
Inclusion criteria: (1) A confirmed diagnosis of colorectal cancer through pathological examination, without invasion of adjacent tissues or organs and no distant metastasis; (2) First-time diagnosis of colorectal cancer; (3) Aged 18 years or older; (4) Underwent colorectal cancer surgery at The First People's Hospital of Zunyi; (5) Eastern Cooperative Oncology Group performance status (ECOG) score of 0-2 points The ECOG score is a scale used to assess the general health and functional status of cancer patients. This scale classifies patients' activity levels from 0 (fully active, symptomatic but without restricted activity) to 5 (death)[18]; and (6) availability of complete clinical data.
Exclusion criteria: (1) The presence of concurrent malignant tumors; (2) Severe infections or immunological diseases; (3) Significant lesions in vital organs such as the heart, liver, or kidneys; (4) Conditions such as intestinal obstruction or perforation prior to treatment; (5) Use of probiotic preparations or antibiotics within 3 weeks before treatment; (6) Receipt of neoadjuvant therapy, including radiotherapy, chemotherapy, targeted therapy, or immunotherapy, prior to surgery; and (7) Severe malnutrition or obesity.
The enteral nutrition formula used was Youkangli (National Food Registration No. TY20200002), containing 15.8 g protein, 1600 kJ, energy, and 3.5 g dietary fiber per 500 mL. The enteral nutrition protocol was as follows: Postoperative day 1: 100 mL of Youkangli (diluted concentration), with 50 mL per dose at ≥ 2-hour intervals; postoperative day 2: 250 mL of Youkangli at 50 mL per dose at ≥ 2-hour intervals; postoperative day 3: 500 mL of Youkangli at 50 mL per dose at ≥ 2-hour intervals; postoperative day 4: 1000 mL of Youkangli self-administered orally; postoperative day 5: Full enteral nutrition volume (1 g protein/kg/day, 83.7-104.6 kJ energy/kg/day), with any deficit supplemented by paren
Gut microbiota detection: The primary objective was to assess changes in the quantities of four bacterial groups: Bifidobacterium, F. nucleatum, Lactobacillus, and B. fragilis across three time points, namely, before treatment, on day 1, and on day 7 after treatment[19]. The detection method involved collecting fresh fecal samples from patients before starting chemotherapy and on the morning of the fifth day of chemotherapy. A 0.1 g sample of fresh feces was placed in a sterile centrifuge tube and diluted tenfold with 0.9 mL of sterile saline. A 10 μL aliquot of this suspension was serially diluted and inoculated onto selective media. The media used included BBL for Bifidobacterium (Beijing Luqiao Technology Co., Ltd.), CDC anaerobic blood agar for F. nucleatum and B. fragilis (Tianjin Jinzhang Science and Technology Development Co., Ltd.), and MRS agar for Lactobacillus (Hangzhou Baishi Biotechnology Co., Ltd.). Bifidobacterium and Lactobacillus were cultured using an anaerobic jar at 35 °C for 48 hours, whereas F. nucleatum and B. fragilis were cultured in an anaerobic chamber at 35 °C for 72 hours. The VITEK 2 COMPACT automated microbial identification system was used for bacterial identification, and results were expressed as the logarithm of colony-forming units per gram of wet feces (lgCFU/g).
Nutritional intake assessment: Postoperative nutritional intake was assessed by calculating the patient’s meal con
Hematological testing: Peripheral venous blood samples were collected from fasting patients at three time points: Before treatment and on days 1 and 7 post-treatment. Diamine oxidase (DAO) levels and endotoxin concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits [DAO kit: Cusabio Technology LLC, catalog number: CSB-E12759h; endotoxin kit: Danaher (Tianjin) Biotech Co., Ltd.], with a microplate reader [TECAN Sunrise, Danaher (Tianjin) Biotech Co., Ltd.][21]. Intestinal fatty acid binding protein (I-FABP) levels were assessed using an I-FABP ELISA kit (Shanghai Boyan Biotechnology Co., Ltd., catalog number: BY-B0034) and a multifunctional microplate reader [MULTISKAN FC, Thermo Fisher Scientific (China) Co., Ltd.][22]. Immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin G (IgG), and C-reactive protein (CRP) levels were determined using an automatic biochemical analyzer (Hitachi 7180, Hitachi High-Technologies Corporation) through immunonephelometry. TNF-α and IL-6 concentrations were measured using ELISA kits (DTH100 for TNF-α, D6050 for IL-6, R&D Systems, Inc.). Cluster of differentiation 4 positive (CD4+) and cluster of differentiation 8 positive (CD8+) cell ratios were determined using flow cytometry [FACSCanto II, BD Medical Device (Shanghai) Co., Ltd.]. The CD4+/CD8+ ratio was calculated. All procedures were conducted according to the manufacturers’ instructions[23].
The statistical analysis was conducted using SPSS software version 29.0. Continuous variables were presented as means and standard deviations (mean ± SD), and group comparisons were performed using independent samples t-tests. Categorical variables were expressed as percentages, with group differences analyzed via χ² tests. Statistical significance was defined by a P value of less than 0.05. Spearman rank correlation analysis was employed for non-parametric or categorical data. Additionally, factors influencing the efficacy of probiotics combined with enteral nutrition in posto
Patients in the good efficacy group were significantly younger, with a mean age of 49.63 ± 8.54 years compared with
| Parameter | Good efficacy group (n = 279) | Poor efficacy group (n = 232) | t/χ² | P value |
| Age (years) | 49.63 ± 8.54 | 52.66 ± 7.57 | 4.207 | < 0.001 |
| Female/male | 128 (45.88)/151 (54.12) | 111 (47.84)/121 (52.16) | 0.197 | 0.657 |
| Ethnicity (Han/other) | 227 (81.36)/52 (18.64) | 183 (78.88)/49 (21.12) | 0.492 | 0.483 |
| BMI (kg/m²) | 22.64 ± 2.55 | 22.24 ± 2.84 | 1.668 | 0.096 |
| ECOG performance status (0/≥ 1) | 243 (87.1)/36 (12.9) | 185 (79.74)/47 (20.26) | 5.037 | 0.025 |
| Smoking history (yes/no) | 102 (36.56)/177 (63.44) | 83 (35.78)/149 (64.22) | 0.034 | 0.854 |
| Drinking history (yes/no) | 69 (24.73)/210 (75.27) | 67 (28.88)/165 (71.12) | 1.116 | 0.291 |
| Hypertension (yes/no) | 95 (34.05)/184 (65.95) | 85 (36.64)/147 (63.36) | 0.372 | 0.542 |
| Diabetes (yes/no) | 100 (35.84)/179 (64.16) | 78 (33.62)/154 (66.38) | 0.275 | 0.600 |
| Educational level (high school or below/college or above) | 38 (13.62)/241 (86.38) | 39 (16.81)/193 (83.19) | 1.007 | 0.316 |
| Marital status (married/unmarried) | 131 (46.95)/148 (53.05) | 120 (51.72)/112 (48.28) | 1.154 | 0.283 |
| RIP (< 1/1-3/3) | 71 (25.45)/115 (41.22)/93 (33.33) | 64 (27.59)/112 (48.28)/56 (24.14) | 5.313 | 0.070 |
| TNM stage (≤ II/> II) | 137 (49.1)/142 (50.9) | 91 (39.22)/141 (60.78) | 5.004 | 0.025 |
| Tumour size (cm) | 3.38 ± 0.95 | 3.52 ± 1.09 | 1.478 | 0.140 |
The average surgery duration was 189.15 ± 25.11 minutes for the good efficacy group compared with 192.35 ± 27.04 minutes for the poor efficacy group (t = 1.387, P = 0.166; Table 2). Blood loss was similar between groups, with averages of 153.9 ± 30.75 mL and 158.45 ± 50.03 mL for the good and poor efficacy groups, respectively (t = 1.208, P = 0.228). The number of lymph nodes removed also showed no significant difference, with a mean of 17.32 ± 4.54 nodes in the good efficacy group vs 16.87 ± 3.78 nodes in the poor efficacy group (t = 1.212, P = 0.226). The occurrence of immediate postoperative complications was comparable, affecting 8.24% of patients in the good efficacy group and 9.91% in the poor efficacy group (χ² = 0.431, P = 0.511). These findings suggest that the efficacy of probiotics combined with enteral nutrition in postoperative patients with colorectal cancer was not markedly influenced by these surgical-related factors.
| Parameter | Good efficacy group (n = 279) | Poor efficacy group (n = 232) | t/χ² | P value |
| Surgery duration (minute) | 189.15 ± 25.11 | 192.35 ± 27.04 | 1.387 | 0.166 |
| Blood loss (mL) | 153.9 ± 30.75 | 158.45 ± 50.03 | 1.208 | 0.228 |
| Lymph nodes removed | 17.32 ± 4.54 | 16.87 ± 3.78 | 1.212 | 0.226 |
| Immediate post-operative complications, n (%) | 23 (8.24)/256 (91.76) | 23 (9.91)/209 (90.09) | 0.431 | 0.511 |
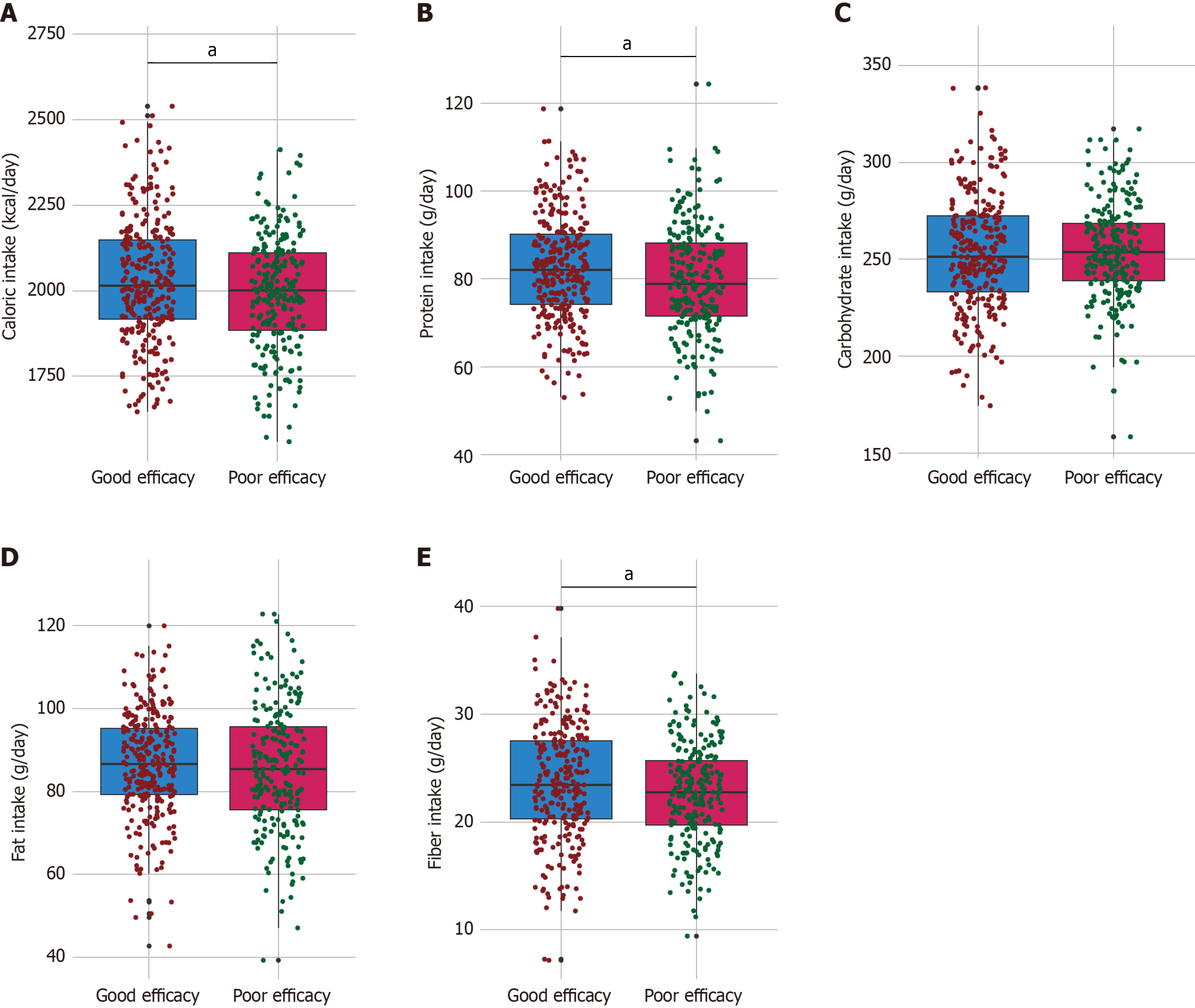
The good efficacy group had a higher mean caloric intake, consuming 2029.48 ± 180.64 kcal/day compared with 1991.33 ± 165.72 kcal/day in the poor efficacy group (t = 2.468, P = 0.014; Figure 1). Protein intake was also significantly higher in the good efficacy group, with an average of 82.56 ± 11.92 g/day vs 79.87 ± 12.61 g/day in the poor efficacy group (t = 2.48, P = 0.013). Additionally, fiber intake was higher in the good efficacy group, measuring 23.69 ± 5.38 g/day compared with 22.68 ± 4.64 g/day in the poor efficacy group (t = 2.291, P = 0.022). Conversely, no significant differences were observed in carbohydrate intake, with means of 253.24 ± 29.28 g/day and 254.31 ± 25.05 g/day (t = 0.444, P = 0.658), and fat intake, with means of 86.63 ± 12.51 g/day and 85.67 ± 15.22 g/day (t = 0.77, P = 0.442) between the two groups. These findings suggest that higher caloric, protein, and fiber intakes were associated with improved efficacy of probiotics combined with enteral nutrition in postoperative patients with colorectal cancer.
Preoperative values for DAO, I-FABP, lipopolysaccharide (LPS), and D-lactate (D-Lac) levels showed no significant differences, with t-values ranging from 0.066 to 0.876 and P values > 0.382, indicating comparable baseline levels (Table 3). By postoperative day 7, the good efficacy group exhibited significantly lower averages for DAO (3.98 ± 0.62
| Index | Good efficacy group (n = 279) | Poor efficacy group (n = 232) | t value | P value |
| DAO (U/L) | ||||
| Preoperative | 4.27 ± 0.48 | 4.27 ± 0.39 | 0.066 | 0.947 |
| Postoperative day 1 | 5.73 ± 1.07 | 5.69 ± 1.06 | 0.41 | 0.682 |
| Postoperative day 7 | 3.98 ± 0.62 | 4.11 ± 0.58 | 2.493 | 0.013 |
| I-FABP (μg/L) | ||||
| Preoperative | 50.53 ± 12.95 | 51.42 ± 11.07 | 0.832 | 0.406 |
| Postoperative day 1 | 70.65 ± 11.54 | 69.88 ± 11.92 | 0.746 | 0.456 |
| Postoperative day 7 | 47.54 ± 10.27 | 50.21 ± 12.83 | 2.564 | 0.011 |
| LPS (U/L) | ||||
| Preoperative | 4.53 ± 0.84 | 4.47 ± 0.62 | 0.876 | 0.382 |
| Postoperative day 1 | 7.64 ± 1.52 | 7.59 ± 1.97 | 0.298 | 0.766 |
| Postoperative day 7 | 4.59 ± 1.32 | 4.87 ± 1.55 | 2.218 | 0.027 |
| D-Lac (mg/L) | ||||
| Preoperative | 6.89 ± 1.24 | 6.92 ± 1.48 | 0.247 | 0.805 |
| Postoperative day 1 | 13.27 ± 2.31 | 13.18 ± 2.24 | 0.457 | 0.648 |
| Postoperative day 7 | 6.63 ± 1.22 | 7.02 ± 2.37 | 2.243 | 0.026 |
Preoperative values did not differ significantly between the groups, with t values ranging from 0.445 to 1.642 and P values > 0.101, indicating comparable baseline microbiota profiles (Table 4). However, by postoperative day 7, the good efficacy group showed significantly higher levels of Bifidobacterium (9.01 ± 0.53 lgCFU/g vs 8.87 ± 0.68 lgCFU/g; t = 2.492, P = 0.013) and Lactobacillus (8.75 ± 0.75 lgCFU/g vs 8.62 ± 0.53 lgCFU/g; t = 2.145, P = 0.032) and lower levels of F. nucleatum (5.94 ± 0.45 lgCFU/g vs 6.03 ± 0.20 lgCFU/g; t = 3.014, P = 0.003) and B. fragilis (5.68 ± 0.29 lgCFU/g vs 5.74 ± 0.27 lgCFU/g; t = 2.75, P = 0.006). These findings suggest that the efficacy of probiotics combined with enteral nutrition in postoperative patients with colorectal cancer was associated with the modulation of gut microbiota, potentially contributing to the improved postoperative outcomes observed in the good efficacy group.
| Index | Good efficacy group (n = 279) | Poor efficacy group (n = 232) | t value | P value |
| Bifidobacterium | ||||
| Preoperative | 9.27 ± 0.47 | 9.25 ± 0.49 | 0.445 | 0.656 |
| Postoperative day 1 | 6.33 ± 0.56 | 6.29 ± 0.58 | 0.683 | 0.495 |
| Postoperative day 7 | 9.01 ± 0.53 | 8.87 ± 0.68 | 2.492 | 0.013 |
| Fusobacterium nucleatum | ||||
| Preoperative | 11.39 ± 0.64 | 11.46 ± 0.39 | 1.642 | 0.101 |
| Postoperative day 1 | 7.38 ± 0.46 | 7.41 ± 0.32 | 0.932 | 0.352 |
| Postoperative day 7 | 5.94 ± 0.45 | 6.03 ± 0.2 | 3.014 | 0.003 |
| Lactobacillus | ||||
| Preoperative | 9.07 ± 0.55 | 9.02 ± 0.59 | 0.863 | 0.389 |
| Postoperative day 1 | 6.27 ± 0.49 | 6.26 ± 0.5 | 0.375 | 0.708 |
| Postoperative day 7 | 8.75 ± 0.75 | 8.62 ± 0.53 | 2.145 | 0.032 |
| Bacteroides fragilis | ||||
| Preoperative | 12.07 ± 0.68 | 11.98 ± 0.75 | 1.381 | 0.168 |
| Postoperative day 1 | 8.27 ± 0.59 | 8.17 ± 0.72 | 1.721 | 0.086 |
| Postoperative day 7 | 5.68 ± 0.29 | 5.74 ± 0.27 | 2.75 | 0.006 |
Preoperative levels of immunoglobulins (IgA, IgM, IgG) and CD4+/CD8+ ratios were similar between the two groups, and the differences were not significant (t values ranging from 0.073 to 1.312; P values > 0.19; Table 5). However, by postoperative day 7, the good efficacy group exhibited significantly higher levels of IgA (2.46 ± 0.48 g/L vs 2.34 ± 0.63
| Index | Good efficacy group (n = 279) | Poor efficacy group (n = 232) | t value | P value |
| IgA (g/L) | ||||
| Preoperative | 2.38 ± 0.42 | 2.37 ± 0.47 | 0.241 | 0.809 |
| Postoperative day 1 | 2.21 ± 0.57 | 2.2 ± 0.39 | 0.008 | 0.993 |
| Postoperative day 7 | 2.46 ± 0.48 | 2.34 ± 0.63 | 2.472 | 0.014 |
| IgM (g/L) | ||||
| Preoperative | 1.28 ± 0.24 | 1.28 ± 0.18 | 0.073 | 0.941 |
| Postoperative day 1 | 1.05 ± 0.12 | 1.04 ± 0.16 | 0.551 | 0.582 |
| Postoperative day 7 | 1.31 ± 0.22 | 1.25 ± 0.31 | 2.553 | 0.011 |
| IgG (g/L) | ||||
| Preoperative | 9.57 ± 1.62 | 9.39 ± 1.43 | 1.312 | 0.19 |
| Postoperative day 1 | 8.67 ± 1.36 | 8.61 ± 1.47 | 0.449 | 0.653 |
| Postoperative day 7 | 11.23 ± 1.89 | 10.71 ± 1.73 | 3.216 | 0.001 |
| CD4+/CD8+ | ||||
| Preoperative | 1.56 ± 0.37 | 1.55 ± 0.39 | 0.431 | 0.667 |
| Postoperative day 1 | 1.43 ± 0.32 | 1.45 ± 0.3 | 0.686 | 0.493 |
| Postoperative day 7 | 1.65 ± 0.35 | 1.57 ± 0.33 | 2.521 | 0.012 |
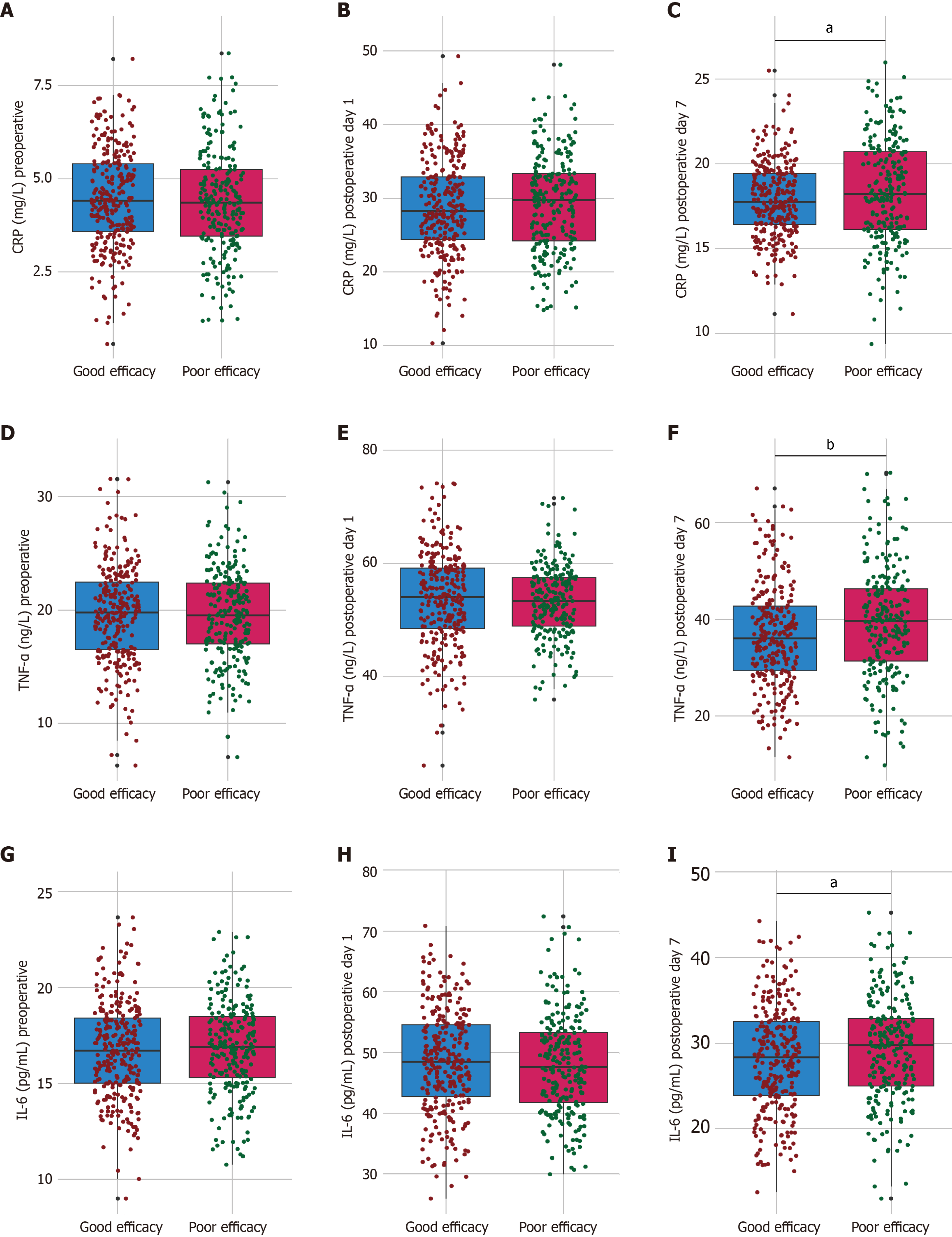
Preoperative levels of CRP, TNF-α, and IL-6 were comparable between the groups, and the differences were not significant (t-values ranging from 0.193 to 0.604; P values > 0.546; Figure 2). By postoperative day 7, the good efficacy group showed significantly lower levels of CRP (17.86 ± 2.19 mg/L vs 18.37 ± 3.04 mg/L; t = 2.136, P = 0.033), TNF-α (36.58 ± 10.46 ng/L vs 39.27 ± 11.53 ng/L; t = 2.761, P = 0.006), and IL-6 (28.14 ± 6.23 pg/mL vs 29.27 ± 6.07 pg/mL; t = 2.062, P = 0.04) than the poor efficacy group. These findings suggest that the observed clinical benefits in the good efficacy group may be mediated by reduced systemic inflammation, potentially contributing to improved postoperative recovery in patients with colorectal cancer receiving probiotics and enteral nutrition.
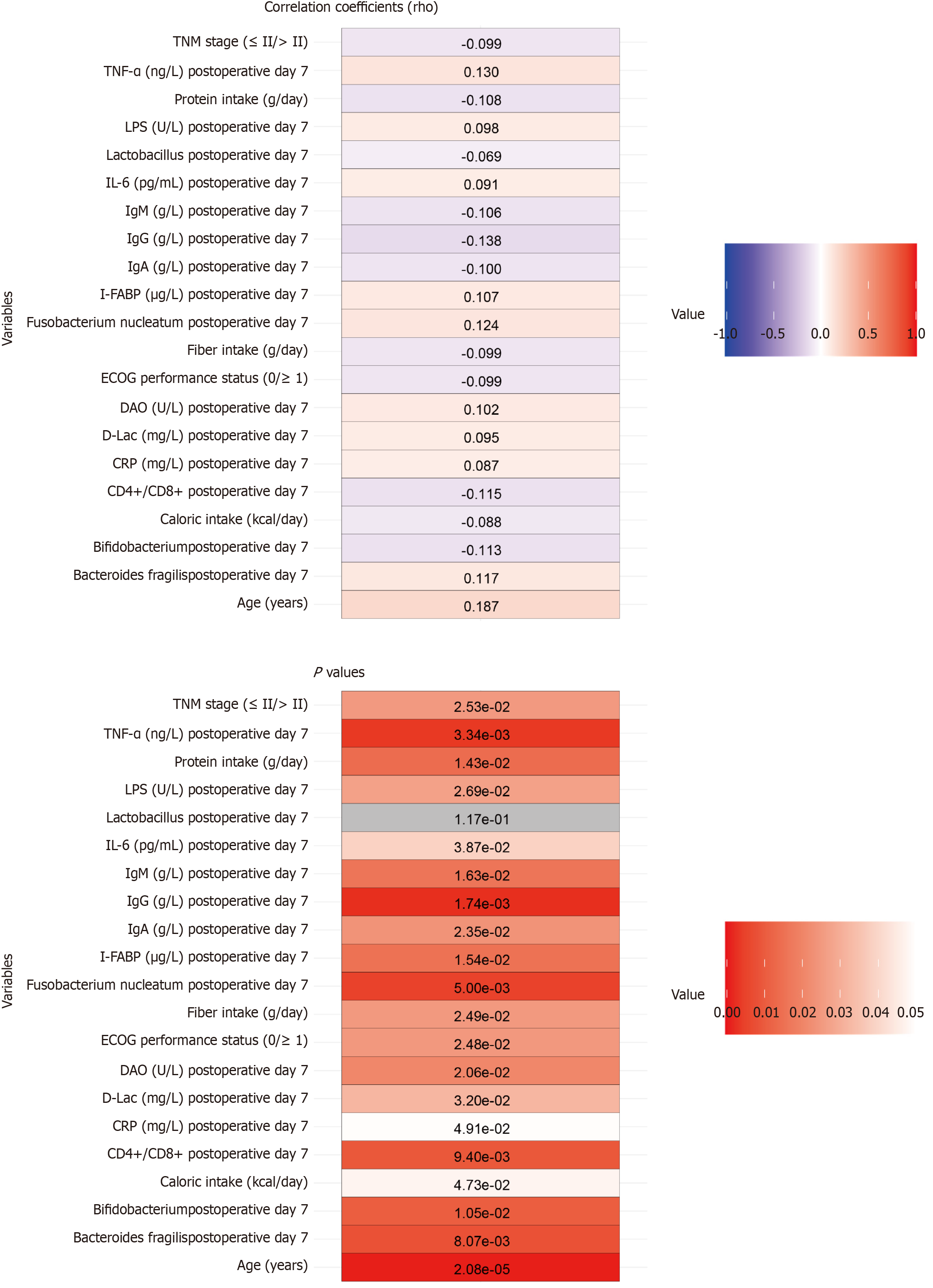
Age showed a modest positive correlation with therapeutic efficacy (rho = 0.187, P < 0.001), suggesting potential age-related differences in treatment response (Figure 3). Negative correlations were observed in ECOG performance status (rho = -0.099, P = 0.025) and TNM stage (rho = -0.099, P = 0.025), indicating that worse baseline functional status and more advanced disease stage may slightly undermine therapeutic outcomes. Nutritional factors such as caloric intake (rho =
Increasing age was a positive predictor of efficacy (coefficient = 0.046, SE = 0.011, P < 0.001, OR = 1.047, 95%CI: 1.025-1.071; Table 6). Conversely, a poor ECOG performance status and advanced TNM stage were associated with decreased efficacy, with coefficients of -0.539 (P = 0.026, OR = 0.583, 95%CI: 0.361-0.935) and -0.402 (P = 0.026, OR = 0.669, 95%CI: 0.469-0.951), respectively. Nutritional factors including lower caloric (coefficient = -0.001, P = 0.015, OR = 0.999) and protein intake (coefficient = -0.018, P = 0.014, OR = 0.982) and reduced fiber intake (coefficient = -0.040, P = 0.025, OR = 0.961) were inversely related to efficacy. Inflammatory and microbial markers, such as higher postoperative day 7 levels of DAO (coefficient = 0.371, P = 0.014, OR = 1.448), I-FABP (coefficient = 0.020, P = 0.010, OR = 1.020), LPS (coefficient = 0.141, P = 0.026, OR = 1.151), and D-Lac (coefficient = 0.115, P = 0.019, OR = 1.122), were positively associated with therapeutic efficacy. Bifidobacterium and Lactobacillus levels on postoperative day 7 exhibited protective effects (coefficients = -0.376 and -0.282, P = 0.012 and P = 0.039, ORs = 0.687 and 0.754, respectively). Higher numbers of F. nucleatum and B. fragilis were associated with reduced efficacy (coefficients = 0.705 and 0.876, P = 0.005 and 0.007, ORs = 2.023 and 2.401, respectively). Immune markers such as IgA, IgM, IgG, and CD4+/CD8+ ratios were negatively correlated with efficacy, indicating a complex relationship between immune modulation and therapeutic success. Elevated CRP, TNF-α, and IL-6 levels were also significantly associated with efficacy, suggesting that inflammation plays a critical role in patient outcomes. These insights highlight the multifaceted nature of factors influencing treatment efficacy in this patient cohort.
| Factor | Coefficient | SE | Wald | P value | OR | CI lower | CI upper |
| Age (years) | 0.046 | 0.011 | 4.074 | < 0.001 | 1.047 | 1.025 | 1.071 |
| ECOG performance status (0/≥ 1) | -0.539 | 0.242 | 2.228 | 0.026 | 0.583 | 0.361 | 0.935 |
| TNM stage (≤ II/> II) | -0.402 | 0.180 | 2.233 | 0.026 | 0.669 | 0.469 | 0.951 |
| Caloric intake (kcal/day) | -0.001 | 0.001 | 2.444 | 0.015 | 0.999 | 0.998 | 1.000 |
| Protein intake (g/day) | -0.018 | 0.007 | 2.455 | 0.014 | 0.982 | 0.968 | 0.996 |
| Fiber intake (g/day) | -0.040 | 0.018 | 2.243 | 0.025 | 0.961 | 0.928 | 0.995 |
| DAO (U/L) postoperative day 7 | 0.371 | 0.150 | 2.468 | 0.014 | 1.448 | 1.082 | 1.951 |
| I-FABP (μg/L) postoperative day 7 | 0.020 | 0.008 | 2.586 | 0.010 | 1.020 | 1.005 | 1.036 |
| LPS (U/L) postoperative day 7 | 0.141 | 0.063 | 2.233 | 0.026 | 1.151 | 1.018 | 1.304 |
| D-Lac (mg/L) postoperative day 7 | 0.115 | 0.049 | 2.343 | 0.019 | 1.122 | 1.02 | 1.237 |
| Bifidobacterium postoperative day 7 | -0.376 | 0.149 | 2.522 | 0.012 | 0.687 | 0.511 | 0.917 |
| Fusobacterium nucleatum postoperative day 7 | 0.705 | 0.252 | 2.792 | 0.005 | 2.023 | 1.240 | 3.341 |
| Lactobacillus postoperative day 7 | -0.282 | 0.137 | 2.065 | 0.039 | 0.754 | 0.575 | 0.984 |
| Bacteroides fragilis postoperative day 7 | 0.876 | 0.323 | 2.714 | 0.007 | 2.401 | 1.283 | 4.555 |
| IgA (g/L) postoperative day 7 | -0.407 | 0.163 | 2.503 | 0.012 | 0.666 | 0.482 | 0.913 |
| IgM (g/L) postoperative day 7 | -0.884 | 0.340 | 2.601 | 0.009 | 0.413 | 0.211 | 0.800 |
| IgG (g/L) postoperative day 7 | -0.158 | 0.050 | 3.157 | 0.002 | 0.854 | 0.773 | 0.941 |
| CD4+/CD8+ postoperative day 7 | -0.663 | 0.266 | 2.494 | 0.013 | 0.515 | 0.304 | 0.864 |
| CRP (mg/L) postoperative day 7 | 0.075 | 0.034 | 2.183 | 0.029 | 1.078 | 1.008 | 1.154 |
| TNF-α (ng/L) postoperative day 7 | 0.022 | 0.008 | 2.725 | 0.006 | 1.023 | 1.006 | 1.039 |
| IL-6 (pg/mL) postoperative day 7 | 0.030 | 0.015 | 2.050 | 0.040 | 1.030 | 1.001 | 1.060 |
The multivariate logistic regression analysis identified several independent factors that were significantly associated with therapeutic efficacy in postoperative patients with colorectal cancer receiving probiotics combined with enteral nutrition (Table 7). Age was a strong positive predictor of efficacy, with each additional year increasing the odds of a positive therapeutic outcome (coefficient = 0.049, P < 0.001, OR = 1.050, 95%CI: 1.023-1.078). Conversely, more advanced TNM stage was associated with reduced efficacy (coefficient = -0.427, P = 0.041, OR = 0.653, 95%CI: 0.434-0.982). Nutritional components such as protein intake also showed an inverse relationship with efficacy (coefficient = -0.018, P = 0.028, OR = 0.982, 95%CI: 0.966-0.998), whereas caloric and fiber intake did not reach conventional levels of statistical significance. Among inflammatory and mucosal markers, higher DAO levels on postoperative day 7 were associated with increased efficacy (coefficient = 0.408, P = 0.020, OR = 1.504, 95%CI: 1.065-2.125), while LPS levels (coefficient = 0.204, P = 0.005,
| Factor | Coefficient | SE | Wald Stat | P value | OR | OR CI lower | OR CI upper |
| Age (years) | 0.049 | 0.013 | 3.670 | < 0.001 | 1.050 | 1.023 | 1.078 |
| ECOG performance status (0/≥ 1) | -0.498 | 0.281 | -1.774 | 0.076 | 0.607 | 0.350 | 1.054 |
| TNM stage (≤ II/> II) | -0.427 | 0.209 | -2.047 | 0.041 | 0.653 | 0.434 | 0.982 |
| Caloric intake (kcal/day) | -0.001 | 0.001 | -1.954 | 0.051 | 0.999 | 0.998 | 1.000 |
| Protein intake (g/day) | -0.018 | 0.008 | -2.193 | 0.028 | 0.982 | 0.966 | 0.998 |
| Fiber intake (g/day) | -0.038 | 0.020 | -1.863 | 0.062 | 0.963 | 0.925 | 1.002 |
| DAO (U/L) postoperative day 7 | 0.408 | 0.176 | 2.317 | 0.020 | 1.504 | 1.065 | 2.125 |
| I-FABP (μg/L) postoperative day 7 | 0.017 | 0.009 | 1.886 | 0.059 | 1.017 | 0.999 | 1.036 |
| LPS (U/L) postoperative day 7 | 0.204 | 0.073 | 2.802 | 0.005 | 1.226 | 1.063 | 1.414 |
| D-Lac (mg/L) postoperative day 7 | 0.099 | 0.056 | 1.768 | 0.077 | 1.104 | 0.989 | 1.231 |
| Bifidobacterium postoperative day 7 | -0.451 | 0.174 | -2.592 | 0.010 | 0.637 | 0.453 | 0.896 |
| Fusobacterium nucleatum postoperative day 7 | 0.808 | 0.287 | 2.814 | 0.005 | 2.244 | 1.278 | 3.941 |
| Lactobacillus postoperative day 7 | -0.256 | 0.157 | -1.633 | 0.102 | 0.774 | 0.569 | 1.053 |
| Bacteroides fragilis postoperative day 7 | 0.842 | 0.373 | 2.257 | 0.024 | 2.322 | 1.117 | 4.824 |
| IgA (g/L) postoperative day 7 | -0.330 | 0.187 | -1.765 | 0.078 | 0.719 | 0.498 | 1.037 |
| IgM (g/L) postoperative day 7 | -0.926 | 0.388 | -2.387 | 0.017 | 0.396 | 0.185 | 0.847 |
| IgG (g/L) postoperative day 7 | -0.183 | 0.058 | -3.152 | 0.002 | 0.832 | 0.743 | 0.933 |
| CD4+/CD8+ postoperative day 7 | -0.619 | 0.301 | -2.056 | 0.040 | 0.539 | 0.299 | 0.971 |
| CRP (mg/L) postoperative day 7 | 0.094 | 0.040 | 2.324 | 0.020 | 1.098 | 1.015 | 1.188 |
| TNF-α (ng/L) postoperative day 7 | 0.017 | 0.010 | 1.805 | 0.071 | 1.018 | 0.999 | 1.037 |
| IL-6 (pg/mL) postoperative day 7 | 0.036 | 0.017 | 2.124 | 0.034 | 1.037 | 1.003 | 1.072 |
Microbiota composition postoperatively had significant effects. High numbers of F. nucleatum predicted reduced efficacy (coefficient = 0.808, P = 0.005, OR = 2.244, 95%CI: 1.278-3.941), and high numbers of Bifidobacterium indicated protective effects (coefficient = -0.451, P = 0.010, OR = 0.637, 95%CI: 0.453-0.896). In terms of immune markers, decreased IgM and IgG levels were associated with improved efficacy (IgM coefficient = -0.926, P = 0.017, OR = 0.396; IgG
In this study, we aimed to investigate factors influencing the efficacy of probiotics combined with enteral nutrition in postoperative patients with colorectal cancer.
An interesting observation from our study is the correlation between younger age and better therapeutic outcomes. The finding that younger age correlated with better outcomes aligns with emerging evidence on immunosenescence. A 2022 trial by Essink et al[24] demonstrated that age influences immune function, with younger patients typically having a more robust immune response. The aging immune system, known as immunosenescence, may impair the ability to benefit from treatments intended to modulate gut microbiota and systemic immunity. Additionally, Li et al[25] de
The role of nutritional intake is another significant finding, particularly the higher caloric, protein, and fiber intake associated with improved outcomes. These nutrients are fundamental components that influence gut health and modulate immune responses. Postoperative nutrition is crucial for recovery; it supports not only wound healing and muscle maintenance but also the overall functional integrity of the gut[26,27]. The study by Seifi et al[28] in 2022 corroborates our findings. Their research indicates that enteral nutrition is superior to parenteral nutrition in maintaining the integrity of the intestinal mucosa, thereby supporting an effective intestinal barrier. This enhanced barrier function can boost the efficacy of probiotics. Conversely, inadequate nutrient intake, especially deficiencies in protein and fiber, can hinder mucosal recovery and diminish the efficiency of probiotic colonization in the gut, ultimately resulting in suboptimal outcomes. Fibers are known to ferment into short-chain fatty acids, which are pivotal in maintaining colonic health and providing an energy source for colonocytes to support recovery[29,30].
Our study also highlights the significant impact of gut microbiota modulation on treatment outcomes. An intriguing observation is the elevation of intestinal mucosal barrier function indicators such as DAO and I-FABP on postoperative day 1 compared with preoperative levels and those observed on postoperative day 7. This initial increase likely reflects acute surgical stress and the resultant disruption of the intestinal barrier, leading to increased permeability and systemic absorption of bacterial products and endotoxins[29,31]. By postoperative day 7, the gradual recovery of the intestinal barrier function is indicated by the reduction in these markers, suggesting the beneficial effects of probiotics combined with enteral nutrition in restoring gut integrity. Conversely, low numbers of F. nucleatum and B. fragilis were associated with poor outcomes, suggesting that these bacteria might impede recovery possibly through inflammatory processes. F. nucleatum, in particular, has been associated with increased inflammation and cancer progression, which may undermine therapeutic efficacy[32]. Future research should focus on comparing these temporal changes statistically among groups and elucidating the underlying mechanisms, including potential immunomodulatory and anti-inflammatory roles played by probiotics during the early postoperative period.
Immune function was enhanced in patients with improved therapeutic outcomes, as evidenced by high levels of immunoglobulins (IgA, IgM, IgG) and an increased CD4+/CD8+ ratio. Probiotics may enhance mucosal immunity, a crucial aspect of the body’s defense against tumor recurrence and infections, by upregulating these immunoglobulins. Probiotics and enteral nutrition may synergistically protect against postoperative immunosuppression to maintain a balanced immune environment that favors recovery. Furthermore, the observed correlation between high levels of inflammatory markers and poor outcomes underscores the notion that probiotics might mitigate this response, con
The multivariate analysis illuminated the independent factors significant to therapeutic efficacy, thereby reinforcing the interplay of bacterial colonization, inflammation, and immune modulation. The association between high levels of DAO and LPS on postoperative day 7 with good efficacy suggest that these parameters are markers of improved gut barrier function, possibly reflecting successful probiotic and nutritional therapy in strengthening intestinal health. These results indicate that the integrity of the intestinal mucosal barrier plays a crucial role in therapeutic outcomes for posto
This study provides valuable insights, but it also has limitations. The retrospective design limits causative conclusions and may involve inherent biases. Future prospective investigations are warranted to confirm these findings and further explore the mechanistic pathways involved. Incorporating multi-omics technologies could also elucidate the interaction between the microbiome and the immune system clearly, potentially tailoring personalized therapeutic strategies for postoperative patients with colorectal cancer based on their microbiome signatures and immune profiles. Specifically, the exploration of microbiome genomics to identify strains with optimal probiotic potential could further refine and improve treatment efficacy.
Probiotics combined with enteral nutrition can significantly promote gastrointestinal function recovery in postoperative patients with colorectal cancer. The therapeutic effect is influenced by multiple complex factors, including age, nutritional intake, gut microbiota balance, immune status, and inflammation. These findings highlight the importance of comprehensive patient management strategies that integrate nutritional optimization and microbiota modulation to improve postoperative recovery and outcomes. Advancement of such strategies may benefit not only patients with colorectal cancer but also broader surgical and oncological populations, underscoring the value of personalized care in cancer treatment.
| 1. | Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SI, Leal TA, Bekaii-Saab TS, Paweletz CP, Heavey GA, Christensen JG, Velastegui K, Kheoh T, Der-Torossian H, Klempner SJ. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N Engl J Med. 2023;388:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 350] [Article Influence: 116.7] [Reference Citation Analysis (0)] |
| 2. | Barnell EK, Wurtzler EM, La Rocca J, Fitzgerald T, Petrone J, Hao Y, Kang Y, Holmes FL, Lieberman DA. Multitarget Stool RNA Test for Colorectal Cancer Screening. JAMA. 2023;330:1760-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 3. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 2030] [Article Influence: 338.3] [Reference Citation Analysis (0)] |
| 4. | Huang F, Li S, Chen W, Han Y, Yao Y, Yang L, Li Q, Xiao Q, Wei J, Liu Z, Chen T, Deng X. Postoperative Probiotics Administration Attenuates Gastrointestinal Complications and Gut Microbiota Dysbiosis Caused by Chemotherapy in Colorectal Cancer Patients. Nutrients. 2023;15:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 100] [Reference Citation Analysis (0)] |
| 5. | Kaźmierczak-Siedlecka K, Folwarski M, Ruszkowski J, Skonieczna-Żydecka K, Szafrański W, Makarewicz W. Effects of 4 weeks of Lactobacillus plantarum 299v supplementation on nutritional status, enteral nutrition tolerance, and quality of life in cancer patients receiving home enteral nutrition - a double-blind, randomized, and placebo-controlled trial. Eur Rev Med Pharmacol Sci. 2020;24:9684-9694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 6. | Mahboobipour AA, Bitaraf A, Mohammadi P, Khosravifar M, Babaei H, Shahidolahi A. Effects of synbiotics on necrotizing enterocolitis and full enteral feeding in very low birth weight infants: A double-blind, randomized controlled trial. Medicine (Baltimore). 2024;103:e39647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | D'Onofrio V, Del Chierico F, Belci P, Vernocchi P, Quagliariello A, Reddel S, Conta G, Mancino MV, Fadda M, Scigliano MC, Morelli R, De Francesco A, Guagnini F, Fassio F, Galletti R, Putignani L. Effects of a Synbiotic Formula on Functional Bowel Disorders and Gut Microbiota Profile during Long-Term Home Enteral Nutrition (LTHEN): A Pilot Study. Nutrients. 2020;13:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Seifi N, Rezvani R, Sedaghat A, Nematy M, Khadem-Rezaiyan M, Safarian M. The effects of synbiotic supplementation on enteral feeding tolerance, protein homeostasis, and muscle wasting of critically ill adult patients: a randomized controlled trial. Trials. 2022;23:846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Orlandoni P, Jukic Peladic N, Amoruso A, Pane M, Di Rosa M, Vedruccio J, Santini F. Safety and Efficacy of Probiotic Supplementation in Reducing the Incidence of Infections and Modulating Inflammation in the Elderly with Feeding Tubes: A Pilot, Double-Blind, Placebo-Controlled Study, "IntegPRO". Nutrients. 2021;13:391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Morton D, Seymour M, Magill L, Handley K, Glasbey J, Glimelius B, Palmer A, Seligmann J, Laurberg S, Murakami K, West N, Quirke P, Gray R; FOxTROT Collaborative Group. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J Clin Oncol. 2023;41:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 268] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 11. | Wang YS, Shen W, Yang Q, Lin R, Tang LX, Bai RM, Yang D, Zhang J, Zhang YJ, Yu WT, Song SR, Kong J, Song SY, Mao J, Tong XM, Li ZK, Wu F, Lin XZ. Analysis of risk factors for parenteral nutrition-associated cholestasis in preterm infants: a multicenter observational study. BMC Pediatr. 2023;23:250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 12. | Landais M, Nay MA, Auchabie J, Hubert N, Frerou A, Yehia A, Mercat A, Jonas M, Martino F, Moriconi M, Courte A, Robert-Edan V, Conia A, Bavozet F, Egreteau PY, Bruel C, Renault A, Huet O, Feller M, Chudeau N, Ferrandiere M, Rebion A, Robert A, Giraudeau B, Reignier J, Thille AW, Tavernier E, Ehrmann S; REVA network and CRICS-TriggerSEP F-CRIN research network. Continued enteral nutrition until extubation compared with fasting before extubation in patients in the intensive care unit: an open-label, cluster-randomised, parallel-group, non-inferiority trial. Lancet Respir Med. 2023;11:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Kagan I, Hellerman-Itzhaki M, Bendavid I, Statlender L, Fishman G, Wischmeyer PE, de Waele E, Singer P. Controlled enteral nutrition in critical care patients - A randomized clinical trial of a novel management system. Clin Nutr. 2023;42:1602-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 14. | Su X, He J, Cui J, Li H, Men J. The effects of aerobic exercise combined with resistance training on inflammatory factors and heart rate variability in middle-aged and elderly women with type 2 diabetes mellitus. Ann Noninvasive Electrocardiol. 2022;27:e12996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 15. | Liu P, Liu Z, Wang J, Wang J, Gao M, Zhang Y, Yang C, Zhang A, Li G, Li X, Liu S, Liu L, Sun N, Zhang K. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat Commun. 2024;15:3003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 110] [Reference Citation Analysis (0)] |
| 16. | Vadell AKE, Bärebring L, Hulander E, Gjertsson I, Lindqvist HM, Winkvist A. Anti-inflammatory Diet In Rheumatoid Arthritis (ADIRA)-a randomized, controlled crossover trial indicating effects on disease activity. Am J Clin Nutr. 2020;111:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | Jackson MK, Bilek LD, Waltman NL, Ma J, Hébert JR, Price S, Graeff-Armas L, Poole JA, Mack LR, Hans D, Lyden ER, Hanson C. Dietary Inflammatory Potential and Bone Outcomes in Midwestern Post-Menopausal Women. Nutrients. 2023;15:4277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Azam F, Latif MF, Farooq A, Tirmazy SH, AlShahrani S, Bashir S, Bukhari N. Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) Score for Cancer Patients by Oncology Healthcare Professionals. Case Rep Oncol. 2019;12:728-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 19. | Yao Q, Tang M, Zeng L, Chu Z, Sheng H, Zhang Y, Zhou Y, Zhang H, Jiang H, Ye M. Potential of fecal microbiota for detection and postoperative surveillance of colorectal cancer. BMC Microbiol. 2021;21:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Khalil M, Munir MM, Woldesenbet S, Endo Y, Tsilimigras DI, Kalady MF, Huang E, Husain S, Harzman A, Pawlik TM. Association of county-level food deserts and food swamps on postoperative outcomes among patients undergoing colorectal surgery. J Gastrointest Surg. 2024;28:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 21. | Sun W, Li F, Wang X, Liu H, Mo H, Pan D, Wen S, Zhou A. Effects of Dexmedetomidine on Patients Undergoing Laparoscopic Surgery for Colorectal Cancer. J Surg Res. 2021;267:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Yi M, Wu Y, Li M, Zhang T, Chen Y. Effect of remote ischemic preconditioning on postoperative gastrointestinal function in patients undergoing laparoscopic colorectal cancer resection. Int J Colorectal Dis. 2023;38:68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 23. | Zaharuddin L, Mokhtar NM, Muhammad Nawawi KN, Raja Ali RA. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019;19:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 194] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 24. | Essink B, Sabharwal C, Cannon K, Frenck R, Lal H, Xu X, Sundaraiyer V, Peng Y, Moyer L, Pride MW, Scully IL, Jansen KU, Gruber WC, Scott DA, Watson W. Pivotal Phase 3 Randomized Clinical Trial of the Safety, Tolerability, and Immunogenicity of 20-Valent Pneumococcal Conjugate Vaccine in Adults Aged ≥18 Years. Clin Infect Dis. 2022;75:390-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 25. | Li A, Wang Y, Li Z, Qamar H, Mehmood K, Zhang L, Liu J, Zhang H, Li J. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb Cell Fact. 2019;18:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Koponen S, Nykänen I, Savela RM, Välimäki T, Suominen AL, Schwab U. Individually tailored nutritional guidance improved dietary intake of older family caregivers: a randomized controlled trial. Eur J Nutr. 2022;61:3585-3596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 27. | Funk Debleds P, Chambrier C, Slim K. Postoperative nutrition in the setting of enhanced recovery programmes. Eur J Surg Oncol. 2024;50:106866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Seifi N, Amin Mohammadi M, Dabagh AE, Sedaghat A, Rezvani R, Khadem-Rezaiyan M, Nematy M, Safarian M. The effect of early enteral nutrition supplemented with synbiotics on lipid and glucose homeostasis in critically ill patients: A randomized controlled trial. Diabetes Metab Syndr. 2022;16:102352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Yang J, Yang X, Wu G, Huang F, Shi X, Wei W, Zhang Y, Zhang H, Cheng L, Yu L, Shang J, Lv Y, Wang X, Zhai R, Li P, Cui B, Fang Y, Deng X, Tang S, Wang L, Yuan Q, Zhao L, Zhang F, Zhang C, Yuan H. Gut microbiota modulate distal symmetric polyneuropathy in patients with diabetes. Cell Metab. 2023;35:1548-1562.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 30. | Ortiz-Reyes L, Patel JJ, Jiang X, Coz Yataco A, Day AG, Shah F, Zelten J, Tamae-Kakazu M, Rice T, Heyland DK. Early versus delayed enteral nutrition in mechanically ventilated patients with circulatory shock: a nested cohort analysis of an international multicenter, pragmatic clinical trial. Crit Care. 2022;26:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Zhu R, Fang Y, Li H, Liu Y, Wei J, Zhang S, Wang L, Fan R, Wang L, Li S, Chen T. Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front Immunol. 2023;14:1158137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 32. | Li H, Zhang L, Li J, Wu Q, Qian L, He J, Ni Y, Kovatcheva-Datchary P, Yuan R, Liu S, Shen L, Zhang M, Sheng B, Li P, Kang K, Wu L, Fang Q, Long X, Wang X, Li Y, Ye Y, Ye J, Bao Y, Zhao Y, Xu G, Liu X, Panagiotou G, Xu A, Jia W. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat Metab. 2024;6:578-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 103] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 33. | Lai H, Li Y, He Y, Chen F, Mi B, Li J, Xie J, Ma G, Yang J, Xu K, Liao X, Yin Y, Liang J, Kong L, Wang X, Li Z, Shen Y, Dang S, Zhang L, Wu Q, Zeng L, Shi L, Zhang X, Tian T, Liu X. Effects of dietary fibers or probiotics on functional constipation symptoms and roles of gut microbiota: a double-blinded randomized placebo trial. Gut Microbes. 2023;15:2197837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 118] [Reference Citation Analysis (1)] |