Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.110323
Revised: July 19, 2025
Accepted: September 9, 2025
Published online: November 27, 2025
Processing time: 174 Days and 19 Hours
Endoscopic submucosal dissection and endoscopic mucosal resection rely on submucosal injection cushions to effectively separate lesions from the muscularis propria, thereby expanding the operative field, improving resection precision, and minimizing procedure-related complications. Although recent advances have yielded cushions with enhanced lift durabilities and biofunctional properties, their widespread clinical adoption remains limited. Key barriers include poor injectability, uncertain long-term biocompatibility, restricted multifunctionality, manufacturing and supply chain constraints, and a lack of robust clinical and rei
Core Tip: Submucosal injection cushions are essential adjuncts for achieving safe and effective endoscopic resection. Despite advances in material design - aimed at enhancing lift durability and incorporating biofunctional properties - their clinical translation remains constrained. Key limiting factors include suboptimal injectability, insufficient long-term biocompatibility data, restricted functional versatility, and substantial hurdles in manufacturing scalability, regulatory approval, and reimbursement. This article provides a critical comparison of conventional and next-generation submucosal injection materials, delineates major translational bottlenecks, and proposes integrative strategies to support their clinical implementation.
- Citation: Xu PW, Xiao ZH, Yu M, Zhang XY. Advancements in injection materials for submucosal lifting in endoscopic interventions. World J Gastrointest Surg 2025; 17(11): 110323
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/110323.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.110323
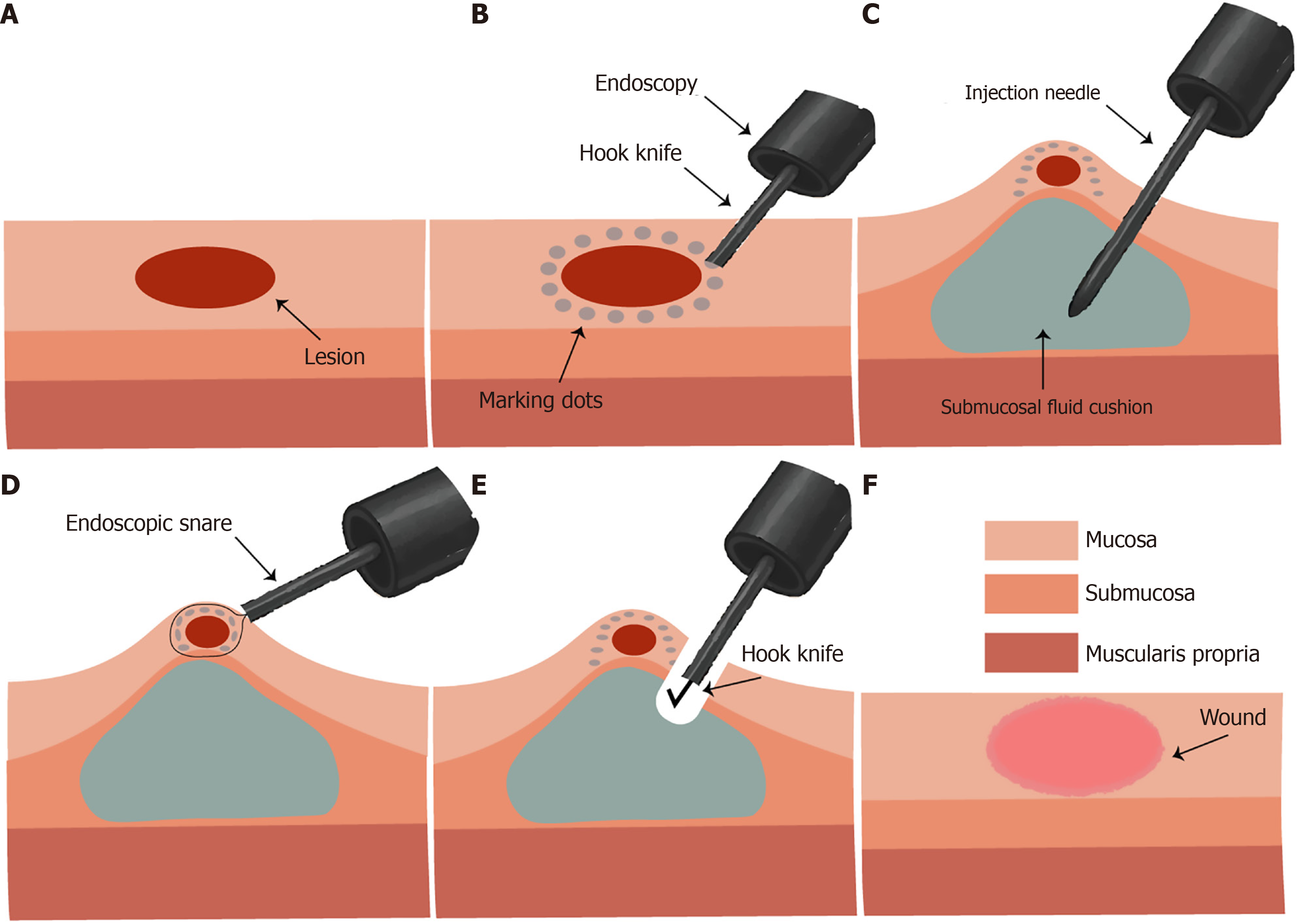
Recent advancements in endoscopic technology, particularly high-definition imaging and artificial intelligence-assisted diagnostics, have significantly enhanced the sensitivity and specificity of early gastrointestinal cancer detection[1,2]. These innovations have facilitated a clinical shift toward earlier diagnosis and minimally invasive management. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are among the cornerstone techniques that enable en bloc resection of precancerous and early stage gastrointestinal neoplasms[3,4]. The stepwise techniques for EMR and ESD are summarized in Figure 1.
A critical technical component of both EMR and ESD involves the creation of a submucosal fluid cushion (SFC) by injecting a fluid into the submucosal layer beneath the lesion. This maneuver elevates the mucosa away from the muscularis propria, establishing a protective barrier that minimizes the risk of perforation and facilitates precise dissection within a stabilized operative field[5,6]. Considering the central role of SFCs in procedural safety and efficacy, various injectates have been investigated.
This article comprehensively reviews the physicochemical properties, biocompatibility, and intraoperative per
In endoscopic procedures, 0.9% normal saline (NS) remains one of the most commonly used submucosal injection solutions for ESD because of its non-toxicity, low cost, and widespread availability. By creating a transient SFC, NS facilitates lesion elevation and reduces the risk of perforation. However, its clinical use is limited by poor durability. As reported by Polymeros et al[7], the median mucosal elevation time with NS was only 12 minutes (interquartile range: 9.25-27.25), which was attributed to its isotonicity and low viscosity. This often necessitates repeated injections, potentially prolonging procedure time and increasing the risk of adverse events.
Hypertonic saline (HTS) has been employed as an alternative submucosal injectate in both EMR and ESD[8]. In an ex vivo porcine model, Fujishiro et al[9] demonstrated that HTS provides a significantly improved initial mucosal elevation and prolonged submucosal cushion persistence compared to NS. These effects are attributed to the higher osmolarity of HTS, which facilitates enhanced fluid retention within the submucosal space, thereby reducing the frequency of reinjection during endoscopic procedures. Although these mechanical advantages may improve procedural efficiency, the hyperosmotic nature of HTS raises concerns regarding tissue injury. Adverse effects, including delayed ulcer healing and potential distortion of histopathological interpretation owing to tissue dehydration, have been reported. Therefore, its clinical application requires careful consideration of the trade-off between mechanical performance and mucosal safety.
As a widely used clinical energy supplement and emergency agent, 50% glucose has attracted attention as a submucosal injection solution for EMR and ESD. In a randomized controlled trial, Varadarajulu et al[10] compared 50% glucose levels with NS in 50 patients who underwent polypectomy via gastroscopy or colonoscopy. Compared to NS, the glucose group required a lower median injection volume (5 mL vs 7 mL, P = 0.02) and fewer injections (1 mL vs 2 mL, P = 0.003). En bloc resection was achieved more frequently, with sustained submucosal elevation observed in 96% of the cases. These results suggest that the high osmolarity of glucose prolongs the cushion duration, reduces the need for reinjection, and enhances procedural efficiency and resection completeness, while maintaining a safety profile comparable to that of NS.
Glycerol-fructose (GF), a hyperosmotic solution comprising 10% glycerol, NS, and 5% fructose, is commonly used as a submucosal injection agent in EMR. In an ex vivo porcine model, Fujishiro et al[9] demonstrated that GF provides superior initial mucosal elevation and sustained cushion height for up to 10 minutes, outperforming NS, 3.75% saline, and 20% glucose.
A clinical retrospective study by Uraoka et al[11] reported that GF improved en bloc and complete resection rates, particularly for colorectal laterally spreading tumors < 20 mm. It reduced the recurrence of small lesions, underscoring its technical advantage in maintaining mucosal elevation. These findings suggest that GF offers strong local retention, enhanced visualization, and procedural efficiency, making it a practical option if moderate duration and stable lifting are required.
Polysaccharides and their derivatives have emerged as promising submucosal injection materials because of their excellent biocompatibility, biodegradability, and capacity to form viscous or gel-like solutions. Derived from natural sources such as marine algae, crustacean shells, and plant gums, they represent tissue-friendly alternatives to conventional agents, such as NS. Extensively studied samples include sodium hyaluronate (SH), hydroxypropyl methylcellulose (HPMC), sodium carboxymethyl cellulose (SCMC), sodium carboxymethyl starch (SCMS), hydroxyethyl starch (HES), and sodium alginate (SA). These high-molecular-weight viscoelastic polymers generate long-lasting submucosal cushions, reduce the frequency of reinjections, and enhance resection accuracy during endoscopic procedures.
SH, a naturally occurring polysaccharide with established use in ophthalmology and orthopedics, has gained prominence as a submucosal injection agent for EMR and ESD. Its high viscosity and prolonged lifting effect contribute to improved procedural safety and efficiency.
In ex vivo porcine and in vivo canine models, Yamamoto et al[12] demonstrated that SH generated a steeper and more sustained mucosal elevation than conventional injectates, particularly enhancing operative stability in anatomically challenging regions, such as the gastric lesser curvature and posterior wall. Importantly, SH was fully absorbed within one week and elicited no local inflammation or adverse tissue responses.
Clinical data further support its use. In a multicenter randomized controlled trial, Kim et al[13] reported that 0.4% SH required significantly less injection volume per unit area than NS (0.03 ± 0.02 mL vs 0.06 ± 0.03 mL, P = 0.0003). Higher procedural satisfaction and usage rates were observed for SH with no major safety concerns, reinforcing its clinical value for gastric ESD.
HPMC is a semi-synthetic, non-ionic cellulose ether derived from natural cellulose characterized by excellent biocompatibility, non-toxicity, and minimal tissue irritation. Although widely used in pharmaceutical formulations, its application as a submucosal injection agent in endoscopy has recently attracted attention[14].
In an ex vivo porcine model, Polymeros et al[7] demonstrated that HPMC provided a significantly longer mucosal elevation than NS (29 minutes vs 12 minutes), with a performance comparable to that of SH. It produced a well-defined submucosal cushion, clearly distinguishing the mucosa and muscularis propria. In vivo studies by Feitoza et al[15] confirmed that HPMC maintained a stable elevation for 45 minutes with minimal histological changes and no significant local adverse effects at one week. Lenz et al[16] evaluated 0.25% HPMC in an in vivo porcine gastric model and reported the most durable submucosal elevation among all tested agents, significantly outperforming NS (P < 0.05). In a phase II clinical trial, Arantes et al[17] demonstrated that 0.4% HPMC provided consistent lifting, excellent tissue compatibility, and no major adverse events in 36 patients who underwent early gastrointestinal neoplasm resection. Collectively, these findings support the potential use of HPMC as a safe and effective long-term submucosal injection material for EMR and ESD.
SCMC is a naturally derived, biocompatible, and widely accessible polymer. It is commonly employed in drug delivery systems, adsorption technologies, and food packaging applications, owing to the presence of hydrophilic carboxyl and hydroxyl functional groups[18]. Its potential as a submucosal injection agent in ESD has been demonstrated in both preclinical and clinical studies.
In ex vivo porcine models, Yamasaki et al[19] showed that SCMC at concentrations ≥ 2.0% provided effective and sustained mucosal elevation. In vivo, 2.5% SCMC enabled successful en bloc resections without bleeding or perforation. Histological analysis confirmed the absence of significant tissue injury, indicating favorable biocompatibility and intraoperative safety.
In a cohort of 98 patients who underwent ESD, Hikichi et al[20] reported en bloc and complete resection rates of 100% and 98%, respectively. The mean procedure time was 75.4 minutes, with an average of 1.7 bleeding episodes. Only one microperforation (1.0%) and four cases of postoperative bleeding (4.0%) were observed, underscoring its clinical safety.
Moreover, in a randomized trial involving 60 patients, Nakamura et al[21] found no significant differences between 1.0% SCMC and 0.4% SH regarding procedure time, en bloc resection rate, hemostasis, injection volume, ulcer healing, or safety outcomes, suggesting that SCMC may serve as a cost-effective alternative to SH.
SCMS is a naturally derived polysaccharide distinguished by its excellent biocompatibility, high water absorbency, cost-effectiveness, and intrinsic hemostatic properties. These characteristics collectively position SCMS as a promising candidate for submucosal injection in endoscopic procedures.
In a study by Dai et al[22], 3.3% SCMS was evaluated in both ex vivo and in vivo models. In porcine stomachs, SCMS achieved significantly higher initial mucosal elevation compared to Eleview™, SH, and NS (P < 0.05), and demonstrated the slowest rate of elevation decline. In vivo, SCMS formed a durable, gel-like cushion that enhanced visualization, minimized bleeding, and outperformed NS, while showing comparable or superior performance to SH and Eleview™. The material was fully biodegradable, non-toxic, non-inflammatory, and did not impair post-procedural healing.
To enhance its mechanical and rheological performance, Wang et al[23] developed SCMS/Laponite (Lap) - a composite hydrogel integrating SCMS with Lap to confer shear-thinning properties. This formulation exhibited low injection resistance, rapid gel recovery, and durable mucosal elevation. In comparative evaluations, SCMS/Lap achieved significantly greater initial lift height (13.16 mm vs 8.98 mm) and improved elevation retention at 2 hours (90% vs 28%) relative to conventional agents. In vitro and in vivo tests confirmed its biocompatibility, biodegradability, and functional superiority, highlighting its potential as a next-generation submucosal injection material for ESD and EMR.
HES, a synthetic plasma expander commonly used for volume resuscitation in hypovolemia, has been investigated as a submucosal injection agent for endoscopic procedures.
In an in vivo porcine gastric model, Sold et al[24] demonstrated that HES produced a more well-defined mucosal elevation than NS and preserved > 90% of its initial height for > 40 minutes, indicating superior submucosal cushion stability. Histological analysis revealed no tissue damage, supporting its biocompatibility.
Clinical evidence from a randomized trial involving 49 patients supports this use. Fasoulas et al[25] showed that 6% HES combined with epinephrine achieved a longer-lasting elevation, required fewer reinjections, and reduced procedure time than NS with epinephrine during EMR for laterally spreading tumors. Postoperative bleeding and perforation rates did not differ significantly between the groups, confirming the safety of HES for clinical use. These findings highlight HES as a promising submucosal injection material with sustained lifting capacity and favorable tissue compatibility.
SA is a naturally occurring anionic polysaccharide extracted from brown algae and other bacteria, widely used in the food, pharmaceutical, and environmental fields owing to its excellent biocompatibility, biodegradability, cost-effectiveness, and functional versatility[26]. In endoscopic therapy, SA has emerged as a promising submucosal injection material, attributed to its favorable rheological properties, mucosal protective effects, and intrinsic hemostatic activity[27,28].
In an ex vivo porcine model, Akagi et al[29] compared 2%-4% SA with 0.4% SH and NS. A 3% SA solution achieved an initial mucosal elevation of 6.1 mm, which was comparable to that of SH and significantly greater than that of NS (4.1 mm), with improved durability. In a pilot clinical study involving 11 patients, 3% SA enabled successful en bloc resection with negative margins, no tumor recurrence at 28-month follow-up, favorable ulcer healing, and no serious adverse events.
To enhance its functionality, Ma et al[30] developed an injectable shear-thinning hydrogel based on SA via dynamic covalent crosslinking. Injectable shear-thinning hydrogel exhibited low injection resistance, self-healing capability, and prolonged lift, producing a 13%-18% higher initial elevation and 87%-92% retention at 2 hours than SH. It showed good biocompatibility and antioxidant activity, although further clinical validation is necessary.
Huang et al[31] engineered a thiolated SA hydrogel incorporating ciprofloxacin to combine mechanical lift and antibacterial efficacy. The hydrogel demonstrated > 95% bacterial inhibition and > 97% ABTS radical scavenging in vitro, offering a dual-functional platform for wound protection. However, further in vivo studies are required to confirm this translational potential.
Furthermore, Moles-Aranda et al[32] formulated SA microparticles using a water-in-oil emulsion that yielded powder and hydrogel forms. These particles exhibited a swelling capacity of > 2000%, strong mucosal adhesion, and sustained elevation for 90-120 minutes. Complete biodegradation was achieved within 24 hours. The formulation demonstrated favorable handling and storage characteristics, supporting its potential as a next-generation submucosal injection platform.
Sodium polyacrylate (SPA), a synthetic superabsorbent polymer, exhibits pronounced pseudoplastic (shear-thinning) rheological behavior, enabling facile injectability under shear stress while maintaining structural integrity and prolonged mucosal elevation upon cessation of flow[33].
In a blinded ex vivo porcine colon model, Hirose et al[34] evaluated the performance of 0.07% SPA in comparison with SH. SPA demonstrated superior elevation retention, smoother injectability, and shorter procedural times relative to SH. It facilitated more efficient resections, with reduced injection volume and fewer reinjections. These advantages were particularly beneficial for operators with limited endoscopic experience, as they helped mitigate procedural challenges. These findings underscore the potential of SPA to improve procedural control, enhance operational efficiency, and support endoscopist training in ESD.
Autologous blood has emerged as a promising SFC material, owing to its high viscosity, excellent biocompatibility, and absence of immunogenicity. In an ex vivo porcine gastric model, Shastri et al[35] compared autologous blood with 0.5% SH, 20% glucose, and NS. Autologous blood achieved mucosal elevation height and width comparable to SH, both significantly outperforming NS and glucose. It also maintained higher elevation at 20 minutes and demonstrated superior durability over glucose throughout the observation period. Histological analysis confirmed preservation of tissue architecture and diagnostic integrity, supporting its safety and effectiveness.
Clinical evidence further supports these findings. In a study involving 28 outpatients with 35 colorectal lesions, Sato et al[36] reported effective mucosal elevation in 34 cases (97.1% en bloc resection rate), with no procedure-related adverse events. These results underscore autologous blood as a safe, accessible, and cost-effective SFC option, particularly suitable for use in outpatient endoscopic practice.
Fibrin glue (FG) is widely used in surgical practice for tissue adhesion, suture reinforcement, and hemostasis, owing to its excellent biocompatibility and robust clot-forming capacity[37]. Its application as a submucosal injectate in endoscopic procedures has recently drawn attention.
In a controlled ex vivo porcine gastric model, Takao et al[38] compared FG with SH and NS for use in ESD. FG and SH generated comparable initial mucosal elevation, both significantly outperforming NS. Notably, FG maintained mucosal lift for up to 60 minutes, a result attributed to its cohesive clot formation and limited lateral diffusion, which preserves a stable operative field throughout the procedure. All lesions were resected en bloc without the need for reinjection or technical difficulty. Macroscopic and histological analyses confirm complete resection and dense FG coverage at the resection base. No evidence of thermal injury, residual lesion, or margin compromise was observed, suggesting a potential protective role in mucosal healing. Despite these advantages, the high cost of FG remains a barrier to routine clinical use. For large or fibrotic lesions, optimization of injection technique and the development of tailored delivery systems may be necessary to fully harness its submucosal lifting potential.
The biodegradable elastic hydrogel poly (ethylene glycol) maleate citrate (PEGMC) has attracted considerable attention in tissue engineering and drug delivery due to its favorable physicochemical and biological properties[39-41].
PEGMC-based hydrogels exhibit excellent cytocompatibility and tissue compatibility in both in vitro and in vivo settings. Notably, their degradation kinetics can be precisely tuned, allowing controlled resorption under physiological conditions. These features make PEGMC a promising platform for regenerative applications and localized therapeutic delivery.
Building on this platform, Tran et al[42] developed an injectable, degradable, and elastic hydrogel (IDEEP) derived from PEGMC, utilizing ammonium persulfate as a redox initiator. IDEEP combines favorable injectability, biocompatibility, tunable biodegradation, and sustained drug release capacity. Rebamipide - a mucosal protective agent - was incorporated to assess its therapeutic potential. In vitro studies demonstrated continuous drug release over two weeks. In ex vivo and porcine gastric models, IDEEP was readily injected at pressures comparable to SH and generated higher, more durable submucosal elevations than SH or NS. The hydrogel remained pliable, degraded gradually in situ, and did not interfere with endoscopic resection or visualization. These findings position IDEEP as a multifunctional submucosal injection material, offering both mechanical support and therapeutic delivery - features well suited for advanced endoscopic procedures.
Novel thermosensitive copolymer (LiftUp) is a novel thermosensitive copolymer composed of ethylene oxide and propylene oxide. It remains in a liquid state at ambient temperature and undergoes rapid gelation at physiological temperature, combining excellent injectability with robust in situ gel formation. These properties make it highly suitable for submucosal injection in endoscopic procedures.
In an in vivo porcine model, Wedi et al[43] systematically evaluated LiftUp as a SFC. Upon injection, it rapidly formed a stable gel that maintained mucosal elevation and provided strong mechanical support throughout dissection. Notably, in 90.5% of cases, a single injection was sufficient to complete resection without reinjection, underscoring its procedural efficiency. Follow-up endoscopy at one week demonstrated near-complete mucosal re-epithelialization, while histological analysis at four weeks revealed granulation tissue formation, scar remodeling, and full epithelial coverage. No foreign body reaction or abnormal inflammation was observed, confirming its biocompatibility and in vivo safety. These findings highlight LiftUp as a promising next-generation SFC that offers both mechanical performance and favorable biological responses, supporting its potential for clinical translation in endoscopic interventions.
SIC-8000 (Eleview®) is a low-viscosity, microemulsion-based submucosal injectate containing poloxamer 188. It demonstrates good biocompatibility and has received both Food and Drug Administration 510(k) clearance and Conformité Européenne marking. Upon injection, Eleview forms a stable, long-lasting cushion that provides consistent mucosal elevation, thereby supporting both EMR and ESD procedures.
In a porcine model, Spadaccini et al[44] compared Eleview® with methylene blue–tinted NS during upper and lower gastrointestinal EMR/ESD. Eleview® required significantly lower injection volumes, reflecting more durable mucosal elevation and improved procedural efficiency. While technical success and en bloc resection rates were comparable between groups, Eleview® was associated with a markedly lower adverse event rate (7.7% vs 33.3%, P = 0.03), indicating a superior safety profile. Ulcer healing and histological outcomes were equivalent across groups, with no local or systemic abnormalities and similar inflammatory scores, confirming Eleview®’s biocompatibility. Collectively, these findings support Eleview® as a clinically validated SFC with regulatory approval, offering both procedural advantages and enhanced safety in therapeutic endoscopy.
PS137-25 (LeGoo-endo) is a reverse thermosensitive copolymer composed of 70% polyethylene oxide and 30% polypropylene oxide, structurally derived from the Poloxamer 407 platform. At room temperature, it remains a low-viscosity solution, allowing easy injection. Upon exposure to physiological temperatures, it rapidly undergoes sol-gel transition, forming an in situ hydrogel. These thermoresponsive properties render PS137-25 particularly advantageous as a submucosal injectate for endoscopic procedures.
In a comprehensive evaluation by Fernández-Esparrach et al[45], PS137-25 was tested in both ex vivo and in vivo porcine models of EMR. Ex vivo experiments demonstrated that PS137-25 generated significantly higher and more sustained mucosal elevation compared with NS. In vivo, a single injection sufficed to complete the entire EMR procedure, indicating enhanced procedural efficiency. The material remained well localized within the submucosal layer, forming a stable and clearly defined cushion. This effectively delineated the dissection plane from the muscularis propria, enhancing both operative visibility and procedural safety. No injection-related complications or adverse tissue reactions were observed. Collectively, these findings highlight PS137-25 as a promising next-generation submucosal cushion. It offers temperature-responsive gelation, strong mechanical integrity, and user-friendly handling properties, supporting safer and more efficient therapeutic endoscopy.
Dodecafluoropentane (DDFP) is a biologically inert perfluorocarbon characterized by a low boiling point (29.2 °C), enabling rapid phase transition to gas at physiological temperature. Leveraging this property, Wang et al[46] developed a DDFP-based submucosal injection system designed to generate a gas-liquid interface upon injection, thereby enhancing mucosal lifting through localized cavitation and submucosal expansion. To address the poor injectability of pure DDFP, the authors formulated a composite (DDFP-NS) by emulsifying DDFP with ice-cold NS in a 1:4 ratio.
In an ex vivo porcine gastric model, DDFP-NS achieved a mucosal elevation of approximately 1.5 cm using only 1% of the injection volume required for NS. It retained 75.4% of its initial height at 60 minutes, markedly outperforming both NS and SH in lift durability and efficiency. Moreover, the total procedural duration was reduced by nearly 70% compared to NS. In vivo safety evaluation revealed no evidence of tissue necrosis or organ toxicity up to four weeks post-injection. Notably, the cavitation effect induced by DDFP vaporization may also promote epithelial regeneration. These findings identify DDFP-NS as a novel gas-generating submucosal injectate with superior mechanical lift, enhanced injection efficiency, and potential pro-regenerative properties. It represents a promising alternative for improving the performance and outcomes of endoscopic resection procedures.
Uraoka et al[47] systematically investigated the feasibility of carbon dioxide (CO2) as a submucosal injection agent for ESD, employing both ex vivo and in vivo porcine models. Comparative analyses were conducted against NS and SH. CO2 injection yielded significantly more durable mucosal elevation, with prominent submucosal cushions still visible at 5 minutes post-injection (P < 0.05 vs NS). Histological examination revealed a unique honeycomb-like architecture within the submucosal fibrous network, indicative of sustained structural support. Importantly, no mucosal injury or adverse tissue reactions were detected, affirming the biocompatibility and safety profile of CO2. These findings highlight CO2 as a novel gas-phase submucosal injectate, offering prolonged cushion durability and histologically confirmed mechanical stability. Its favorable safety profile supports further exploration as an alternative or adjunct to conventional liquid-based injectates in therapeutic endoscopy.
Hydrogels are highly hydrated, three-dimensional polymeric networks characterized by exceptional water absorption and retention capabilities. These physicochemical properties have enabled their widespread application across diverse biomedical domains, including tissue engineering, drug delivery, biosensing, and wound healing[48]. More recently, hydrogels have emerged as promising submucosal injection agents in endoscopic interventions. Their ability to maintain a stable mucosal cushion facilitates sustained tissue separation, thereby improving the operative visibility, dissection accuracy, and overall procedural safety.
Chitosan (CS) is a natural polysaccharide derived from crustacean shells, valued for its biocompatibility, biodegradability, inherent antimicrobial activity, and wound-healing capacity, supporting its wide application in biomedicine[49]. Regarding ESD, CS-based hydrogels have shown considerable promise as injectable agents with numerous modifications that improve their performance.
Jeon et al[50] evaluated a thermosensitive CS/β-glycerophosphate (GP) hydrogel for gastric and colonic ESD, in which GP served as a pH- and temperature-responsive crosslinker, inducing sol-gel transition at physiological temperature. The formulation gelled within 28 minutes at 37 °C and achieved effective mucosal elevation with reduced injection volumes, yielding dissection efficiency comparable to SH.
To improve the solubility and gel uniformity, Liu et al[51] incorporated lactobionic acid (LA) into a CS LA/CS/GP hydrogel. LA increased hydrophilicity and reinforced hydrogen bonding within the polymer network, thereby enhancing gel stability. In porcine models, the hydrogel maintained mucosal elevation for > 30 minutes and reduced the operative time by approximately 5 minutes relative to NS or glycerol fructose. Histological analysis revealed near-complete mucosal healing on day 27.
Hattori et al[52] developed a photocrosslinkable CS hydrogel containing azidobenzoic acid moieties that form covalent bonds under ultraviolet light. Photocrosslinkable CS hydrogel provided durable mucosal lifting and degraded within 6 weeks without causing strictures or inflammation. However, concerns regarding phototoxicity may limit its clinical translation[53]. To circumvent external activation, the group later introduced a CS-LA hydrogel that leveraged electrostatic interactions for strong mucosal adhesion and sustained elevation (> 60 minutes) under physiological conditions.
Further optimization by Liu et al[54] led to the formulation of a high-pH CS/polyvinylpyrrolidone/GP hydrogel. polyvinylpyrrolidone, a neutral hydrophilic polymer, formed hydrogen bonds with CS chains, enhancing structural integrity and sol stability at low temperatures. In ex vivo esophageal models, this hydrogel retained > 80% of its initial elevation after 1 hour. In vivo testing in miniature pig ESD procedures demonstrated reduced reinjection frequency and minimized intraoperative complications. Hemolysis rates remained within International Organization for Standardization 10993 standards, and no significant local inflammation was observed.
More recently, Liu et al[55] introduced a succinylated hydroxybutyl CS hydrogel, in which succinic anhydride modification enhanced hydrophilicity and tuned the lower critical solution temperature to approximately 37 °C. This enabled injection at room temperature with rapid gelation upon contact with body temperature. The hydrogel exhibited strong shear-thinning behavior, required lower injection force than SH, and preserved 81% of mucosal elevation at 2 hours. Complete degradation occurred within 16 weeks, and its hemostatic performance in rat models was comparable to gelatin sponges (P < 0.001).
Collectively, these advances highlight the potential of chemically modified CS-based hydrogels as multifunctional submucosal injectates. Structural and rheological modifications have significantly improved the injectability, mechanical strength, in situ gelation, and bioactivity, facilitating their potential clinical application in therapeutic endoscopy.
Cao et al[56] developed an injectable, thermoreversible triblock copolymer hydrogel synthesized via the ring-opening polymerization of D, L-lactide, glycolide, and polyethylene glycol. The resulting formulation exhibited low viscosity at room temperature, enabling facile injection, and underwent a rapid sol-gel transition at physiological temperature to form a stable in situ gel. Engineered for colonic ESD, the hydrogel maintained robust mucosal elevation for at least 30 minutes at concentrations of 15% and 20% in vivo, offering prolonged submucosal support. This facilitated clear dissection plane visualization and efficient lesion removal and minimized the need for repeated electrocautery. Importantly, no muscularis propria injury, significant intraoperative bleeding, or postoperative complications were observed. Complete in vivo degradation occurred without adverse tissue reactions, confirming the biocompatibility and safety of the material. These results highlighted the potential of this hydrogel as a clinically viable submucosal injectate that offers injectability, thermal responsiveness, mechanical robustness, and controlled biodegradation to support safe and effective endoscopic interventions.
Takatori et al[57] developed a temperature-responsive injectable solution comprising pepsin-solubilized collagen (PSC) and genipin (Ge), a natural crosslinker. The formulation retains a fluid state at approximately 30 °C for facile injection and rapidly transitions to a gel at physiological temperature, enabling in situ formation of a durable submucosal cushion.
In preclinical ESD procedures, PSC/Ge demonstrated superior performance to NS and achieved an efficacy comparable to that of SH. The hydrogel provided consistent and durable mucosal elevation without impairing endoscopic visualization or interfering with electrosurgical instruments. No intraoperative complications, such as perforation, bleeding, or thermal injury, were observed. Notably, PSC/Ge remained adherent to post-ESD ulcers, indicating potential secondary benefits for wound protection and the promotion of mucosal healing. These results suggested that PSC/Ge is a multifunctional submucosal injectate with thermal responsiveness, biocompatibility, and wound-protective properties, offering significant potential to improve the safety and therapeutic outcomes of endoscopic procedures.
Nagasaka et al[58] developed an injectable hydrogel [catechol (Cat)-phenylboronic acid (PBA)-Alaska pollock gelatin (ApGltn)] using ApGltn, a marine-sourced biopolymer distinguished by its low hydroxyproline content relative to mammalian gelatin, which confers superior fluidity and injectability. The hydrogel was chemically functionalized with Cat and PBA, enabling rapid gelation via dynamic covalent crosslinking. This design imparted strong tissue adhesion, rapid self-healing, and robust in situ stability.
Upon submucosal injection, the 10% (w/v) Cat-PBA-ApGltn solution underwent rapid gelation within approximately 3 seconds, generating a stable cushion that achieved a mucosal elevation of 7.2 ± 0.4 mm, significantly surpassing that of NS. The hydrogel exhibited excellent localization and retention at the injection site, maintaining structural integrity and wound coverage for over 10 days. Collectively, these findings highlight Cat-PBA-ApGltn as a next-generation submucosal injectate, integrating rapid in situ gelation, durable tissue elevation, and effective wound sealing. Its unique marine-derived composition and dynamic bioadhesiveness suggest substantial potential to enhance both intraoperative performance and post-procedural recovery in endoscopic therapy.
Tang et al[59] developed a shear-thinning hydrogel based on high-acyl gellan gum (GGH) and systematically evaluated its suitability as a submucosal injection agent for endoscopic procedures. The hydrogel remained in a gel-like state at room temperature, exhibited pronounced shear-thinning behavior, and demonstrated rapid self-healing post-injection. Owing to its rheological responsiveness, GGH could be smoothly delivered through a 22G endoscopic needle, requiring significantly lower injection force than SH or SCMC.
In vitro and in vivo biocompatibility assessments revealed only mild and transient inflammatory responses, with no evidence of necrosis, fibrotic encapsulation, or immune rejection. In both ex vivo porcine gastric tissue and in vivo rat models, GGH achieved mucosal elevations comparable to commercial hyaluronic acid formulations and preserved more than 80% of the initial lift height over 120 minutes, significantly outperforming NS and conventional polymeric solutions. Furthermore, GGH was shown to be compatible with epinephrine incorporation, providing potential hemostatic benefits without compromising its injectability or mechanical stability. Collectively, these findings highlight the versatility of GGH as an effective and tunable submucosal cushion material.
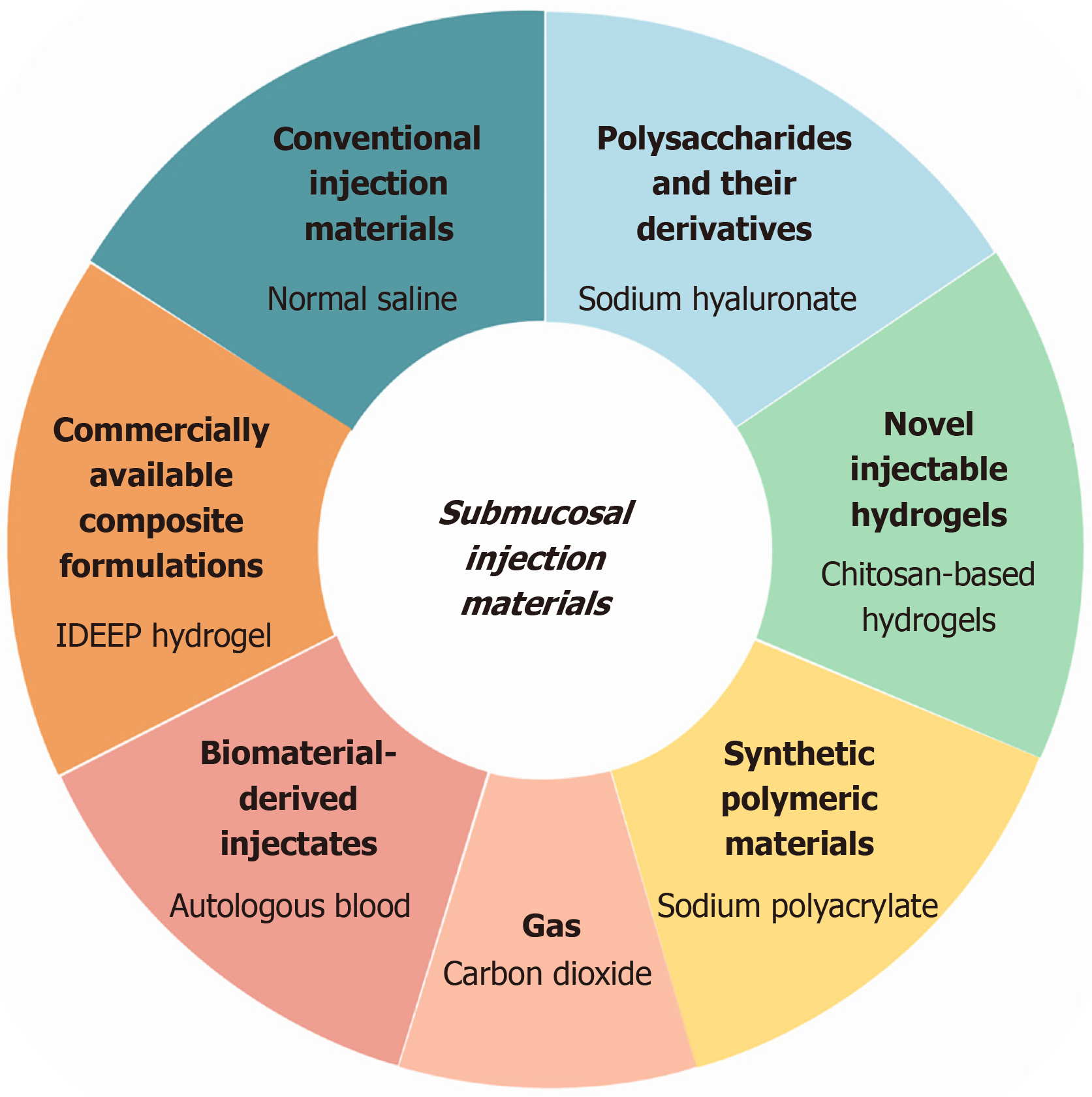
A range of submucosal injectates have now been developed, each offering distinct advantages tailored to specific clinical needs. Table 1 provides a comparative overview of these materials, summarizing their physicochemical pro
| Material | Validation stage | Injection volume (mL) | Initial elevation height/height at 60 minutes (mm) | 60-min retention (%) | Repeat injection | Biocompatibility | Adverse events |
| Normal saline | Clinical use | 1 | NR/0 | 0 | Yes | No observable tissue damage | Increased intraoperative bleeding and perforation risk |
| Hypertonic saline | In vivo | 1 | 4.6/0 | 0 | Yes | No observable tissue damage | None observed |
| 50% glucose injection | Clinical trial | 5 | NR/NR | NA | No | No observable tissue damage | None observed |
| Glycerol fructose | Ex vivo; retrospective clinical study | 1 | 4.6/0 | 0 | Yes | No observable tissue damage | Bleeding: 9% |
| Sodium hyaluronate | Ex vivo; in vivo; multicenter clinical trial | 1 | 6.8/NR | NA | No | Mild inflammation | None observed |
| Hydroxypropyl methylcellulose | Ex vivo; in vivo; phase II clinical trial | 5 | 9/NR | NA | Yes | Minimal tissue reaction | None observed |
| Sodium carboxymethyl cellulose | Ex vivo; in vivo; clinical studies | 2 | 15/14 | 93% | Yes | No observable tissue damage | Intraoperative perforation: 1 case; postoperative bleeding: 4 cases |
| Sodium carboxymethyl starch | Ex vivo; in vivo; preclinical | 23 | NR/NR | NA | Yes | No observable tissue damage | Intraoperative bleeding: 4 cases |
| Hydroxyethyl starch | In vivo; clinical trial | 10.5 | NR/0 | 0 | Yes | No observable tissue damage | Intraoperative bleeding: 1 case; postoperative bleeding: 1 case; macro-perforation: 1 case |
| Sodium alginate | Ex vivo; preclinical; pilot clinical study | 1 | 6.1/4.5 | 74% | No | No observable tissue damage | None observed |
| Sodium polyacrylate | Ex vivo | 2 | 16/15 | 94% | No | NA | None observed |
| Autologous blood | Ex vivo; clinical feasibility study | 7.5 | 6.4/4.3 | 67% | No | Mild local tissue response | None observed |
| Fibrin glue | Ex vivo | 2 | 6.2/5.6 | 90% | No | No observable tissue damage | Gel hardening led to procedural challenge |
| IDEEP hydrogel | Ex vivo | 1 | 5.7/5 | 88% | No | No observable tissue damage | None observed |
| LiftUp | In vivo | 1 | 5.8/4.9 | 84% | No | No observable tissue damage | None observed |
| Eleview® (SIC-8000) | In vivo; clinically validated | 9 | NR/NR | NA | Yes | No observable tissue damage | Postoperative bleeding rate: 2%; postoperative discomfort (abdominal pain/fever): 1% |
| LeGoo-endo (PS137-25) | Ex vivo; in vivo | 5 | 10/NR | NA | No | No observable tissue damage | Mild bleeding: 1 case |
| DDFP-NS | Ex vivo; in vivo | 1 | 5.8/5.0 | 86% | No | No observable tissue damage | Mild bleeding: 1 case |
| Carbon dioxide | Ex vivo; in vivo | 41 | 2/2 | 100% | No | No observable tissue damage | No adverse events reported |
| Chitosan-based hydrogels | Ex vivo; in vivo | 9 | 4.8/3.9 | 81% | No | No observable tissue damage | No adverse events reported |
| PLGA-PEG-PLGA hydrogel | In vivo | 1 | 4.8/3.6 | 75% | No | No observable tissue damage | No adverse events reported |
| PSC/Ge hydrogel | In vivo | 1 | 5.6/4.4 | 79% | No | No observable tissue damage | Mild bleeding (controllable) |
| Cat-PBA-ApGltn hydrogel | Ex vivo; in vivo | 0.5 | 3.8/NR | NA | No | No observable tissue damage | None observed |
| Gellan gum hydrogel | Ex vivo; in vivo | 1 | 5.7/4.7 | 82% | No | No observable tissue damage | Mild bleeding: 1 case |
Submucosal injection cushions are fundamental to the safety and efficacy of ESD and EMR. By providing a transient yet stable separation between the mucosa and the muscularis propria, submucosal injectates expand the operative field, improve endoscopic visualization, and enable precise en bloc resection. Beyond enhancing procedural precision, they also buffer mechanical and thermal stress, thereby reducing the incidence of perforation, bleeding, and other intraoperative complications.
Despite advancements in material design, the clinical adoption of next-generation submucosal injectates remains constrained by multiple factors. Key challenges include poor injectability, limited biocompatibility data, and insufficient functional versatility. In addition, critical translational barriers - such as sterilization compatibility, formulation stability, scalable manufacturing, and regulatory uncertainty - further impede progress. The lack of high-quality clinical evidence and the absence of standardized reimbursement pathways continue to delay their integration into routine endoscopic practice.
Overcoming these multifaceted barriers requires coordinated strategies that integrate rational material design, standardized preclinical evaluation, and early regulatory engagement. Equally important is the generation of robust clinical and economic evidence to support the transition of submucosal injection cushions from experimental innovation to routine endoscopic practice.
Poor injectability remains a key obstacle to the clinical translation of next-generation submucosal cushions. Although many advanced formulations demonstrate favorable gelation behavior and biocompatibility in rheological assays and small-animal models, their high shear viscosity or excessive viscoelasticity often impedes smooth delivery through standard 23-25G endoscopic needles. As a result, administration frequently requires high-pressure syringes, which compromises procedural efficiency and limits practical adoption in routine settings. To enable widespread clinical use, submucosal injectates must combine rapid in situ gelation with pronounced shear-thinning behavior, allowing easy injection through conventional delivery systems and rapid recovery of mechanical integrity post-deployment. This can be achieved through rational polymer design strategies - such as tuning molecular weight, modulating chain entanglement density, or incorporating branched or bottlebrush architectures. These design strategies support the formation of stable networks at rest, which undergo shear-induced disentanglement to lower viscosity and rapidly re-form post-injection, restoring mechanical and functional integrity[60,61].
Current biosafety assessments of submucosal injection materials are predominantly limited to short-term studies in small animal models, with a paucity of long-term follow-up and systematic toxicological data. Existing studies have primarily focused on immediate lift performance and basic histological assessments, whereas key parameters - such as degradation kinetics, chronic toxicity, and immunogenicity - remain insufficiently investigated. This limitation is especially concerning for high-molecular-weight polymers and multifunctional composites containing hemostatic or antimicrobial agents, as they may elicit complex host responses over time.
Moreover, the reliance on small animal models - whose gastrointestinal structure, immune regulation, and metabolic profiles differ markedly from those of humans - limits the predictive value of preclinical findings. To support clinical translation, a standardized biosafety evaluation framework is urgently needed, encompassing both short-term biocompatibility and long-term systemic safety. Future studies should incorporate large animal models with closer physiological resemblance to humans - such as pigs, canines, or non-human primates. Comprehensive evaluation protocols should include histopathological and immunohistochemical analyses, profiling of degradation byproducts, biodistribution studies, and standardized systemic toxicity testing. Such rigorous and integrative approaches are essential to de-risk translational development and validate the clinical potential of next-generation submucosal injection materials.
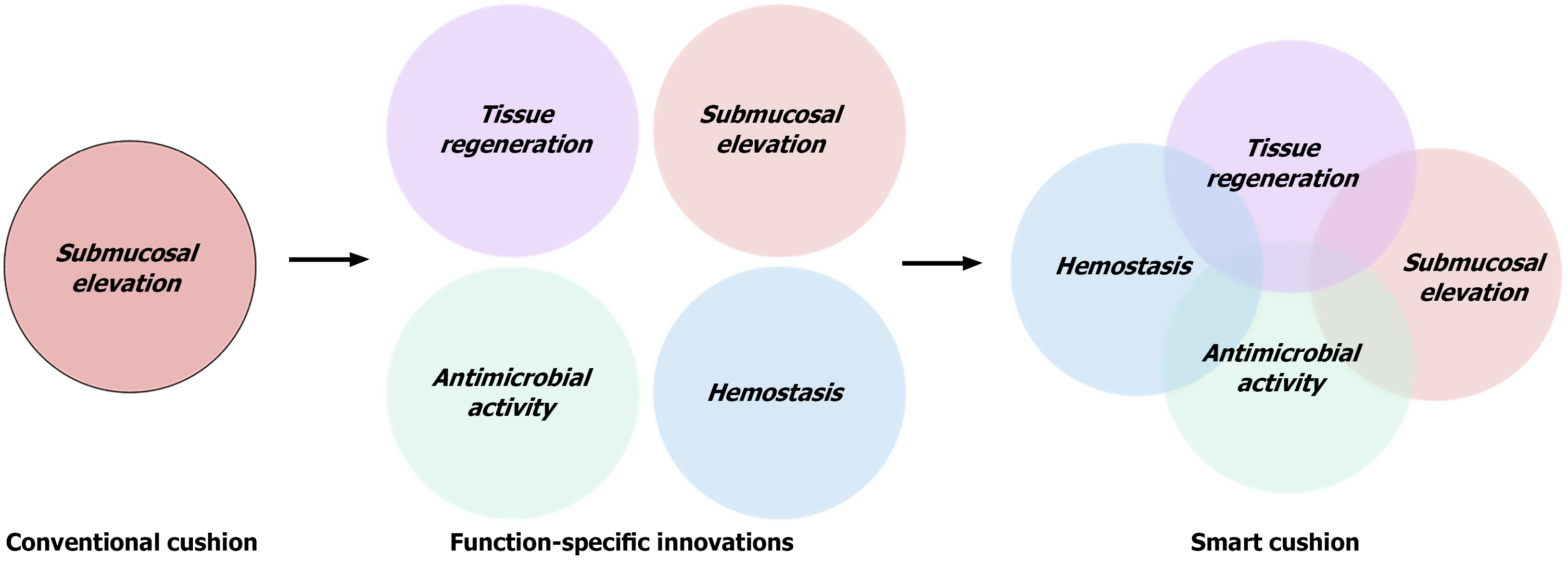
Most submucosal cushions currently used in routine endoscopic practice function solely through passive mechanical elevation. By creating transient separation between tissue layers, these agents enhance visualization and reduce the risk of perforation, adequately meeting the fundamental requirements of conventional mucosal resection. However, they lack any active biological function - offering no control over intraoperative bleeding, microbial contamination, or post-resection healing - thereby limiting their utility in more advanced or high-risk procedures.
As endoscopic therapy advances toward the treatment of larger lesions, deeper dissections, and higher-risk patient populations, there is an increasing clinical need for submucosal injectates that offer more than mechanical support. Materials capable of actively promoting hemostasis, preventing infection, and facilitating tissue regeneration are urgently needed to meet the demands of modern therapeutic endoscopy. The absence of biofunctional attributes in existing formulations constrains clinical utility, compromises cost-effectiveness, and hampers integration into value-based reimbursement models. To catalyze the next generation of submucosal injection materials, a paradigm shift is required - from passive space fillers to multifunctional therapeutic platforms that dynamically engage with the tissue microenvironment. By modulating key biological processes during and after resection, such materials have the potential to enhance intraoperative safety, accelerate postoperative recovery, and ultimately expand the therapeutic scope of endoscopic interventions.
Hemostatic functionalization: Recent advances in submucosal injection design have drawn inspiration from platelet-mediated hemostasis to enable autonomous bleeding control. Hemostasis involves a tightly regulated cascade of platelet adhesion, activation, and aggregation, orchestrated by specific receptor–ligand interactions: GPIbα binds von Willebrand factor, GPIbα-IIa and GPVI recognize exposed collagen, and activated GPIIb-IIIa engages the fibrinogen γ-chain motif - such as the H12 peptide - to stabilize the forming thrombus[62]. Synthetic platforms - such as liposomes or polymeric particles - decorated with von Willebrand factor-binding peptide, collagen-binding peptide, and H12 have successfully recapitulated these steps, demonstrating enhanced hemostatic efficacy in preclinical bleeding models[61,62]. Integration of such platelet-inspired mechanisms into submucosal cushions offers a shift from passive tissue separation to active intraoperative hemostasis, improving both procedural safety and efficiency.
An alternative strategy involves the direct incorporation of coagulation factors. Andrabi et al[63] developed a thrombin-loaded injectable cryogel using a porous polyacrylamide scaffold. Upon submucosal injection, the cryogel rapidly rehydrated and released thrombin in a controlled fashion, achieving complete survival in a porcine model of lethal junctional hemorrhage - markedly outperforming the Food and Drug Administration-approved XStat®. Crucially, thrombin integration did not compromise the material’s injectability or lifting capability, while imparting robust hemostatic functionality suited for intraoperative bleeding control.
Antimicrobial strategies: Infection control and improved wound healing are emerging as critical design objectives for multifunctional cushions. The structural tunability and localized release capacity of hydrogel-based systems render them well-suited for the targeted delivery of antimicrobial agents at the resection site. Various modalities - including silver ions, antibiotics, CS, and antimicrobial peptides - have been explored.
Silver nanoparticles disrupt bacterial membranes, inhibit biofilm formation, and exhibit potent antimicrobial activity in gastrointestinal wound models[64]. The antimicrobial effect of CS originates from its polycationic structure. Under mildly acidic conditions, protonated-NH3+ groups interact with negatively charged cell wall constituents - such as teichoic acids in Gram-positive and lipopolysaccharides in Gram-negative bacteria - compromising membrane integrity and causing bacterial lysis. High-molecular-weight CS forms surface barriers that impede nutrient and ion exchange, while lower molecular weight and oligomeric forms penetrate the cell wall, interact with nucleic acids and proteins, and interfere with gene transcription and translation. In addition, CS can chelate essential metal ions, a mechanism that contributes to membrane destabilization and reinforces its antimicrobial activity[65]. Broad-spectrum antimicrobial peptides offer another promising avenue, providing strong bactericidal effects with minimal resistance development. Their incor
Pro-regenerative functionalization: Recent research has increasingly focused on incorporating pro-regenerative cues into submucosal injection materials to promote mucosal healing beyond resection. Among these, stem cell-derived extracellular vesicles (EVs) have garnered attention due to their rich cargo of regulatory RNAs, proteins, and lipids, which exert immunomodulatory, pro-angiogenic, and tissue-remodeling effects. When encapsulated within hydrogel matrices, EVs benefit from sustained release, enhanced local retention, and responsiveness to the inflammatory or ischemic microenvironment, thereby significantly improving mucosal repair in gastrointestinal injury models[67].
Growth factors, such as epidermal growth factor (EGF) and vascular EGF, have also been embedded in CS - and nanocellulose-based hydrogel systems. These materials accelerate epithelial regeneration and neovascularization. Notably, Maeng et al[68] and Min and Tae[69] developed a sulfated nanocrystalline cellulose hydrogel capable of releasing vascular EGF in a sustained manner over eight weeks, which markedly enhanced vascular infiltration, cell migration, and structural remodeling in intestinal tissues.
The co-delivery of EVs and growth factors offers a synergistic strategy, simultaneously enabling immunomodulation and tissue regeneration. Integrated with stimuli-responsive hydrogels, these systems provide precise spatiotemporal control over therapeutic release, responding dynamically to local changes in pH, enzymatic activity, or temperature, thereby enhancing both intraoperative protection and sustained postoperative healing.
Collectively, these advancements represent a major paradigm shift in submucosal injection design - from passive mechanical injectates toward intelligent, multifunctional platforms integrating hemostatic, antimicrobial, and regenerative properties. Such bioactive systems enhance intraoperative safety and procedural efficiency, actively reduce postoperative complications, and promote durable mucosal healing. Figure 3 illustrates this trajectory, highlighting the evolution from conventional lifting agents to next-generation smart cushions with integrated biological functions.
Material compatibility and industrial scalability are critical determinants for the clinical translation of submucosal injection materials. Compatibility ensures materials maintain structural integrity, bioactivity, and functionality throughout production, sterilization, storage, and clinical use. Industrial scalability encompasses efficient large-scale manufacturing, sterilization, packaging, and distribution, meeting requirements for cost-effectiveness, regulatory compliance, and clinical convenience.
One of the foremost challenges in ensuring material compatibility lies in the inherent sensitivity of hydrogel-based systems to thermal, mechanical, and microbial stressors. Conventional sterilization approaches - including steam autoclaving and gamma irradiation - can compromise polymeric networks, degrade functional moieties, and diminish therapeutic efficacy. High-viscosity formulations are particularly susceptible, as microfiltration techniques often suffer from membrane clogging and inadequate microbial clearance. These limitations underscore the imperative for sterilization-aware material design. For instance, thermally stable zwitterionic hydrogels can tolerate autoclaving at 121 °C for 30 minutes, preserving mechanical integrity. In contrast, heat-sensitive formulations benefit from ethylene oxide sterilization, albeit at the cost of requiring post-sterilization hydration to recover functional architecture. Gamma irradiation is viable for dry-state hydrogels, yet in hydrated states, it may induce free-radical-mediated network damage that impairs material performance[70].
Industrial scalability is further constrained by the reliance on cold-chain logistics, particularly for thermoresponsive or protein-functionalized formulations that necessitate storage below 4 °C to preserve sol-gel behavior and biological activity. These cold-chain requirements not only inflate manufacturing and distribution costs but also limit accessibility in resource-constrained settings. Moreover, repeated freeze-thaw cycles can disrupt supramolecular organization and compromise formulation stability, thereby complicating inventory management and clinical consistency.
Addressing these translational barriers calls for a systems-level, multidisciplinary strategy that synergizes materials engineering, process optimization, and regulatory foresight. Future efforts should focus on developing formulations that are inherently compatible with standard sterilization modalities, exhibit long-term stability under ambient conditions, and can be manufactured at scale in compliance with Good Manufacturing Practice (GMP) standards. Advancing such technologies is essential to realizing the clinical potential of next-generation submucosal injection materials.
The multifunctional evolution of submucosal injection cushions presents substantial regulatory hurdles that constrain clinical translation. These next-generation materials often transcend the conventional classification of medical devices, frequently falling under the category of drug-device combination products or advanced therapeutic technologies. Consequently, they are subject to overlapping regulatory frameworks encompassing medical device, pharmaceutical, and biologic product standards - greatly escalating the complexity of regulatory submissions and prolonging approval timelines.
Ambiguities in product classification remain a central challenge, as the multifunctional nature of these materials blurs the boundaries between established regulatory domains. This uncertainty complicates the selection of appropriate approval pathways and demands exhaustive supporting data packages. Typical data requirements include in vitro functional characterization, in vivo safety and efficacy evaluations, toxicological and immunogenicity assessments, and comprehensive degradation byproduct analyses.
Clinical evaluation introduces additional obstacles. As intraoperative adjuncts, these materials often lack standalone clinical endpoints, making it difficult to design appropriately powered trials, identify meaningful comparators, and obtain ethical clearance. Moreover, the localized and transient nature of their therapeutic action further complicates endpoint definition and clinical outcome attribution.
Overcoming these barriers will require early and sustained dialogue with regulatory agencies to clarify classification criteria and streamline approval routes. Developers should adopt modular and function-specific data frameworks that align with the multifunctional profile of the material. Compliance with GMP or Good Storage Practice standards must be ensured from the outset. In parallel, integrating clinical performance data with health economic assessments - including cost-effectiveness and quality-adjusted life year analyses - is essential to facilitate regulatory approval and reimbursement negotiations. A coordinated regulatory-clinical-economic strategy is thus essential for the successful translation of multifunctional submucosal injection systems into routine clinical practice.
Beyond technical innovation, the clinical integration of submucosal injection cushions also depends on robust evidence and alignment with reimbursement systems - two areas that remain critically underdeveloped. Most formulations have only been evaluated in preclinical models or isolated clinical cases, with few undergoing rigorous multicenter trials to demonstrate superior lifting durability, reduced complication rates, or accelerated mucosal healing. As intraoperative adjuncts, these materials face additional challenges in clinical trial design, including difficulties in defining standalone endpoints, obtaining ethical approval, and selecting appropriate comparators. These barriers collectively hinder the generation of definitive evidence required for regulatory and clinical acceptance.
Moreover, economic and reimbursement constraints further restrict clinical uptake. The high cost of advanced materials, combined with the lack of dedicated insurance codes and unclear reimbursement policies, makes them poorly compatible with Diagnosis-Related Group or Diagnosis-Intervention Packet systems. Under such payment models, costly surgical adjuncts without robust evidence of clinical or economic value are unlikely to meet procurement criteria, particularly in resource-limited healthcare settings.
To bridge the gap between technical feasibility and clinical accessibility, a dual-track evaluation paradigm is urgently needed - one that integrates clinical efficacy with economic value. This includes prospective comparative trials, health economic analyses, and quality-adjusted life year modeling to demonstrate cost-effectiveness and inform payer decision-making. Generating such evidence will be critical to support value-based pricing strategies, facilitate negotiations with reimbursement agencies, and expand institutional adoption.
In summary, submucosal injection cushions are undergoing a paradigm shift - from passive mechanical lifting agents to multifunctional therapeutic platforms that combine hemostatic, antimicrobial, and pro-regenerative properties. However, their clinical translation is impeded by persistent challenges in injectability, biocompatibility validation, scalable manufacturing, regulatory approval, and reimbursement integration. Addressing these multifaceted barriers will require a coordinated, multidisciplinary approach encompassing materials science, translational research, health economics, and regulatory strategy. Future development should prioritize the establishment of standardized evaluation frameworks, GMP-compliant manufacturing pipelines, and robust clinical and economic evidence bases. Only through such integrated efforts can next-generation submucosal injection materials fulfill their translational potential and deliver meaningful improvements in the safety, efficiency, and therapeutic outcomes of endoscopic interventions.
| 1. | Tang Y, Anandasabapathy S, Richards-Kortum R. Advances in optical gastrointestinal endoscopy: a technical review. Mol Oncol. 2021;15:2580-2599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Sinonquel P, Eelbode T, Bossuyt P, Maes F, Bisschops R. Artificial intelligence and its impact on quality improvement in upper and lower gastrointestinal endoscopy. Dig Endosc. 2021;33:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 346] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 4. | Bourke MJ, Neuhaus H, Bergman JJ. Endoscopic Submucosal Dissection: Indications and Application in Western Endoscopy Practice. Gastroenterology. 2018;154:1887-1900.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 5. | ASGE Technology Committee; Bhatt A, Bucobo JC, Abdi M, Akshintala VS, Chen D, Chen YI, Copland AP, Das KK, Desilets DJ, Girotra M, Han S, Kahn A, Krishnan K, Leung G, Lichtenstein DR, Mishra G, Muthusamy VR, Obando JV, Onyimba FU, Pawa S, Rustagi T, Sakaria SS, Saumoy M, Shahnavaz N, Trikudanathan G, Trindade AJ, Vinsard DG, Yang J, Law R; ASGE Technology Committee Chair. Submucosal injection fluid and tattoo agents. Gastrointest Endosc. 2024;100:797-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Castro R, Libânio D, Pita I, Dinis-Ribeiro M. Solutions for submucosal injection: What to choose and how to do it. World J Gastroenterol. 2019;25:777-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 84] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (5)] |
| 7. | Polymeros D, Kotsalidis G, Triantafyllou K, Karamanolis G, Panagiotides JG, Ladas SD. Comparative performance of novel solutions for submucosal injection in porcine stomachs: An ex vivo study. Dig Liver Dis. 2010;42:226-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Hirao M, Masuda K, Asanuma T, Naka H, Noda K, Matsuura K, Yamaguchi O, Ueda N. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 267] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Enomoto S, Kakushima N, Kobayashi K, Hashimoto T, Iguchi M, Shimizu Y, Ichinose M, Omata M. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy. 2004;36:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Varadarajulu S, Tamhane A, Slaughter RL. Evaluation of dextrose 50 % as a medium for injection-assisted polypectomy. Endoscopy. 2006;38:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Uraoka T, Fujii T, Saito Y, Sumiyoshi T, Emura F, Bhandari P, Matsuda T, Fu KI, Saito D. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Yamamoto H, Yube T, Isoda N, Sato Y, Sekine Y, Higashizawa T, Ido K, Kimura K, Kanai N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Kim YD, Lee J, Cho JY, Kim SW, Kim SH, Cho YK, Jang JS, Han JS, Cho JY. Efficacy and safety of 0.4 percent sodium hyaluronate for endoscopic submucosal dissection of gastric neoplasms. World J Gastroenterol. 2013;19:3069-3076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Tundisi LL, Mostaço GB, Carricondo PC, Petri DFS. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur J Pharm Sci. 2021;159:105736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Feitoza AB, Gostout CJ, Burgart LJ, Burkert A, Herman LJ, Rajan E. Hydroxypropyl methylcellulose: A better submucosal fluid cushion for endoscopic mucosal resection. Gastrointest Endosc. 2003;57:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Lenz L, Di Sena V, Nakao FS, Andrade GP, Rohr MR, Ferrari AP Jr. Comparative results of gastric submucosal injection with hydroxypropyl methylcellulose, carboxymethylcellulose and normal saline solution in a porcine model. Arq Gastroenterol. 2010;47:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Arantes V, Albuquerque W, Benfica E, Duarte DL, Lima D, Vilela S, Lima G, Sakai P, Filho FM, Artifon E, Halwan B, Kumar A. Submucosal injection of 0.4% hydroxypropyl methylcellulose facilitates endoscopic mucosal resection of early gastrointestinal tumors. J Clin Gastroenterol. 2010;44:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Zhang Z, Li F, Heo JW, Kim JW, Kim MS, Xia Q, Kim YS. Decoration of sodium carboxymethylcellulose gel microspheres with modified lignin to enhanced methylene blue removal. Int J Biol Macromol. 2023;242:125041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 19. | Yamasaki M, Kume K, Yoshikawa I, Otsuki M. A novel method of endoscopic submucosal dissection with blunt abrasion by submucosal injection of sodium carboxymethylcellulose: an animal preliminary study. Gastrointest Endosc. 2006;64:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Hikichi T, Yamasaki M, Watanabe K, Nakamura J, Sato M, Takagi T, Suzuki R, Sugimoto M, Kikuchi H, Konno N, Waragai Y, Asama H, Takasumi M, Ejiri Y, Watanabe H, Ohira H, Obara K. Gastric endoscopic submucosal dissection using sodium carboxymethylcellulose as a new injection substance. Fukushima J Med Sci. 2016;62:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Nakamura J, Hikichi T, Watanabe K, Hashimoto M, Kato T, Takagi T, Suzuki R, Sugimoto M, Takasumi M, Sato Y, Irie H, Kobashi R, Kikuchi H, Waragai Y, Kobayakawa M, Yamasaki M, Ohira H. Efficacy of Sodium Carboxymethylcellulose Compared to Sodium Hyaluronate as Submucosal Injectant for Gastric Endoscopic Submucosal Dissection: A Randomized Controlled Trial. Digestion. 2021;102:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Dai MS, Hu KW, Wu W, Yin GJ, Hu DM. EndoClot(®)SIS Polysaccharide Injection as a Submucosal Fluid Cushion for Endoscopic Mucosal Therapies: Results of Ex Vivo and In Vivo Studies. Dig Dis Sci. 2019;64:2955-2964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Wang P, Li R, Ma J, Zhang W, Shen H, Ren Y, Zhang X, Li S, Chi B. Facilitating safe and sustained submucosal lift through an endoscopically injectable shear-thinning carboxymethyl starch sodium hydrogel. Carbohydr Polym. 2024;336:122128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 24. | Sold MG, Grobholz R, Post S, Enderle MD, Kaehler GF. Submucosal cushioning with water jet before endoscopic mucosal resection : Which fluids are effective? Surg Endosc. 2008;22:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Fasoulas K, Lazaraki G, Chatzimavroudis G, Paroutoglou G, Katsinelos T, Dimou E, Geros C, Zavos C, Kountouras J, Katsinelos P. Endoscopic mucosal resection of giant laterally spreading tumors with submucosal injection of hydroxyethyl starch: comparative study with normal saline solution. Surg Laparosc Endosc Percutan Tech. 2012;22:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Abka-Khajouei R, Tounsi L, Shahabi N, Patel AK, Abdelkafi S, Michaud P. Structures, Properties and Applications of Alginates. Mar Drugs. 2022;20:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 244] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 27. | Daigo K, Wada Y, Yamada C, Yamaji M, Okuda S, Okada M, Miyazato T. [Pharmacological studies of sodium alginate. I. Protective effect of sodium alginate on mucous membranes of upper-gastrointestinal tract]. Yakugaku Zasshi. 1981;101:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Daigo K, Yamaji M, Yamada C, Wada Y, Okuda S, Okada M, Miyazato T. [Pharmacological studies of sodium alginate. III. Acceleration of fibrin formation by sodium alginate]. Yakugaku Zasshi. 1981;101:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Akagi T, Yasuda K, Tajima M, Suzuki K, Inomata M, Shiraishi N, Sato Y, Kitano S. Sodium alginate as an ideal submucosal injection material for endoscopic submucosal resection: preliminary experimental and clinical study. Gastrointest Endosc. 2011;74:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Ma J, Wang P, Tang C, Liao H, Zhang W, Yang R, Shi T, Tan X, Chi B. Injectable shear-thinning sodium alginate hydrogels with sustained submucosal lift for endoscopic submucosal dissection. Int J Biol Macromol. 2022;223:939-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 31. | Huang L, Jiang Y, Zhang P, Li M, Liu B, Tang K. Injectable Modified Sodium Alginate Microspheres for Enhanced Operative Efficiency and Safety in Endoscopic Submucosal Dissection. Biomacromolecules. 2024;25:2953-2964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Moles-Aranda C, Pérez-González N, Calpena-Campmany AC, Martín-Villena MJ, Otero-Espinar FJ, Severino P, Souto EB, Morales-Molina JA, Clares-Naveros B. Preparation and ex vivo investigation of an injectable microparticulate formulation for gastrointestinal mucosa polyp resection. Eur J Pharm Biopharm. 2022;178:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Bigi A, Boanini E, Botter R, Panzavolta S, Rubini K. Alpha-tricalcium phosphate hydrolysis to octacalcium phosphate: effect of sodium polyacrylate. Biomaterials. 2002;23:1849-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Hirose R, Yoshida N, Naito Y, Yoshida T, Bandou R, Daidoji T, Inoue K, Dohi O, Konishi H, Nakaya T, Itoh Y. Development of Sodium Polyacrylate-Based High-Performance Submucosal Injection Material with Pseudoplastic Fluid Characteristics. ACS Biomater Sci Eng. 2019;5:6794-6800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Shastri YM, Kriener S, Caspary WF, Schneider A. Autologous blood as a submucosal fluid cushion for endoscopic mucosal therapies: results of an ex vivo study. Scand J Gastroenterol. 2007;42:1369-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Sato T. A novel method of endoscopic mucosal resection assisted by submucosal injection of autologous blood (blood patch EMR). Dis Colon Rectum. 2006;49:1636-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Spotnitz WD. Fibrin Sealant: The Only Approved Hemostat, Sealant, and Adhesive-a Laboratory and Clinical Perspective. ISRN Surg. 2014;2014:203943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 38. | Takao M, Takegawa Y, Takao T, Sakaguchi H, Nakano Y, Tanaka S, Morita Y, Toyonaga T, Umegaki E, Kutsumi H, Kodama Y. Fibrin glue: Novel submucosal injection agent for endoscopic submucosal dissection. Endosc Int Open. 2021;9:E319-E323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Gyawali D, Nair P, Zhang Y, Tran RT, Zhang C, Samchukov M, Makarov M, Kim HK, Yang J. Citric acid-derived in situ crosslinkable biodegradable polymers for cell delivery. Biomaterials. 2010;31:9092-9105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Tran RT, Thevenot P, Gyawali D, Chiao JC, Tang L, Yang J. Synthesis and characterization of a biodegradable elastomer featuring a dual crosslinking mechanism. Soft Matter. 2010;6:2449-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | Gyawali D, Tran RT, Guleserian KJ, Tang L, Yang J. Citric-acid-derived photo-cross-linked biodegradable elastomers. J Biomater Sci Polym Ed. 2010;21:1761-1782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Tran RT, Palmer M, Tang SJ, Abell TL, Yang J. Injectable drug-eluting elastomeric polymer: a novel submucosal injection material. Gastrointest Endosc. 2012;75:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Wedi E, Ho CN, Conrad G, Weiland T, Freidinger S, Wehrmann M, Meining A, Ellenrieder V, Gottwald T, Schurr MO, Hochberger J. Preclinical evaluation of a novel thermally sensitive co-polymer (LiftUp) for endoscopic resection. Minim Invasive Ther Allied Technol. 2019;28:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Spadaccini M, Hassan C, Maselli R, D'Amico F, Lamonaca L, Craviotto V, Repici A. Efficacy and safety of SIC-8000 (Eleview®) for submucosal injection for endoscopic mucosal resection and endoscopic submucosal dissection in an in vivo porcine model. Dig Liver Dis. 2018;50:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Fernández-Esparrach G, Shaikh SN, Cohen A, Ryan MB, Thompson CC. Efficacy of a reverse-phase polymer as a submucosal injection solution for EMR: a comparative study (with video). Gastrointest Endosc. 2009;69:1135-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Wang M, Wang K, Chen Y, Liu W, Liu L, Wang X, Zhao L, Fan Z. Thermoresponsive aerification and tissue vacuolization for facilitating endoscopic submucosal resection. Dig Endosc. 2018;30:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Uraoka T, Kawahara Y, Ohara N, Kato J, Hori K, Okada H, Yamamoto K. Carbon dioxide submucosal injection cushion: an innovative technique in endoscopic submucosal dissection. Dig Endosc. 2011;23:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Mellati A, Hasanzadeh E, Gholipourmalekabadi M, Enderami SE. Injectable nanocomposite hydrogels as an emerging platform for biomedical applications: A review. Mater Sci Eng C Mater Biol Appl. 2021;131:112489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 49. | Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 889] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 50. | Jeon HJ, Choi HS, Bang EJ, Lee KW, Kim SH, Lee JM, Kim ES, Keum B, Tae Jeen Y, Lee HS, Chun HJ, Jeong S, Kim JH. Efficacy and safety of a thermosensitive hydrogel for endoscopic submucosal dissection: An in vivo swine study. PLoS One. 2021;16:e0260458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 51. | Liu J, Ni P, Wang Y, Zhou Z, Li J, Chen T, Yuan T, Liang J, Fan Y, Shan J, Sun X, Zhang X. Design and validation of performance-oriented injectable chitosan thermosensitive hydrogels for endoscopic submucosal dissection. Biomater Adv. 2023;146:213286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 52. | Hattori H, Ishihara M. Development of Mucoadhesive Chitosan Derivatives for Use as Submucosal Injections. Polymers (Basel). 2018;10:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Ishihara M, Kumano I, Hattori H, Nakamura S. Application of hydrogels as submucosal fluid cushions for endoscopic mucosal resection and submucosal dissection. J Artif Organs. 2015;18:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Liu Y, Lang C, Zhang K, Feng L, Li J, Wang T, Sun S, Sun G. Injectable chitosan-polyvinylpyrrolidone composite thermosensitive hydrogels with sustained submucosal lifting for endoscopic submucosal dissection. Int J Biol Macromol. 2024;276:133165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 55. | Liu S, Ju R, Zhang Z, Jiang Z, Cui J, Liu W, Han B, Wang S. Temperature-sensitive injectable chitosan-based hydrogel for endoscopic submucosal dissection. Int J Biol Macromol. 2024;282:136566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 56. | Cao L, Li Q, Zhang C, Wu H, Yao L, Xu M, Yu L, Ding J. Safe and Efficient Colonic Endoscopic Submucosal Dissection Using an Injectable Hydrogel. ACS Biomater Sci Eng. 2016;2:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Takatori Y, Uraoka T, Sasaki M, Narita T, Yunoki S, Shiraishi J, Yahagi N. Potential of temperature-response collagen-genipin sol as a novel submucosal injection agent for endoscopic resection: Acute and chronic phase study using living animals. Dig Endosc. 2023;35:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Nagasaka K, Komatsu H, Ito S, Palai D, Nishiguchi A, Taguchi T. Elevation and adhesion properties of injectable hydrogels based on catechol/boronic acid-modified Alaska pollock gelatin for endoscopic submucosal dissection. Colloids Surf B Biointerfaces. 2025;245:114307. [PubMed] [DOI] [Full Text] |
| 59. | Tang Y, Hu M, Tang F, Huang R, Wang H, Wu D, Lan P. Easily-injectable shear-thinning hydrogel provides long-lasting submucosal barrier for gastrointestinal endoscopic surgery. Bioact Mater. 2022;15:44-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Ha TY, Jeong SH, Baig C. Interfacial Polymer Rheology of Entangled Short-Chain Branched Ring Melts in Shear Flow. Macromolecules. 2024;57:6177-6189. [DOI] [Full Text] |
| 61. | Yan D, Wang W, Zhu S. Effect of long chain branching on rheological properties of metallocene polyethylene. Polymer. 1999;40:1737-1744. [RCA] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 130] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Modery-Pawlowski CL, Kuo HH, Baldwin WM, Sen Gupta A. A platelet-inspired paradigm for nanomedicine targeted to multiple diseases. Nanomedicine (Lond). 2013;8:1709-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Andrabi SM, Shahriar SMS, Al-Gahmi AM, Wilczewski BL, Carlson MA, Xie J. Injectable and rapidly expandable thrombin-decorated cryogels achieve rapid hemostasis and high survival rates in a swine model of lethal junctional hemorrhage. Bioact Mater. 2024;38:154-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Aldakheel FM, Sayed MME, Mohsen D, Fagir MH, El Dein DK. Green Synthesis of Silver Nanoparticles Loaded Hydrogel for Wound Healing; Systematic Review. Gels. 2023;9:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 65. | Yan D, Li Y, Liu Y, Li N, Zhang X, Yan C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules. 2021;26:7136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 66. | Mhlongo JT, Waddad AY, Albericio F, de la Torre BG. Antimicrobial Peptide Synergies for Fighting Infectious Diseases. Adv Sci (Weinh). 2023;10:e2300472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 67. | Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2023;18:100522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 247] [Article Influence: 82.3] [Reference Citation Analysis (37)] |
| 68. | Maeng JH, Bang BW, Lee E, Kim J, Kim HG, Lee DH, Yang SG. Endoscopic application of EGF-chitosan hydrogel for precipitated healing of GI peptic ulcers and mucosectomy-induced ulcers. J Mater Sci Mater Med. 2014;25:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Min K, Tae G. Cellular infiltration in an injectable sulfated cellulose nanocrystal hydrogel and efficient angiogenesis by VEGF loading. Biomater Res. 2023;27:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 70. | Galante R, Pinto TJA, Colaço R, Serro AP. Sterilization of hydrogels for biomedical applications: A review. J Biomed Mater Res B Appl Biomater. 2018;106:2472-2492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |