Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.110306
Revised: June 18, 2025
Accepted: September 16, 2025
Published online: November 27, 2025
Processing time: 174 Days and 22.4 Hours
Pancreatic cystic neoplasms (PCNs) are increasingly detected due to advance
To assess the malignancy risk of PCNs using preoperative clinical and routine laboratory parameters.
A retrospective cohort study analyzed 70 patients who underwent surgery for PCNs at Ankara Bilkent City Hospital between February 2019 and March 2023. Patients were categorized into group A (benign or low-grade dysplasia, n = 40) and group B (malignancy or high-grade dysplasia, n = 30) based on postoperative pathology. Preoperative demographic and laboratory parameters, including age, RDW, albumin, and CA 19-9, were com
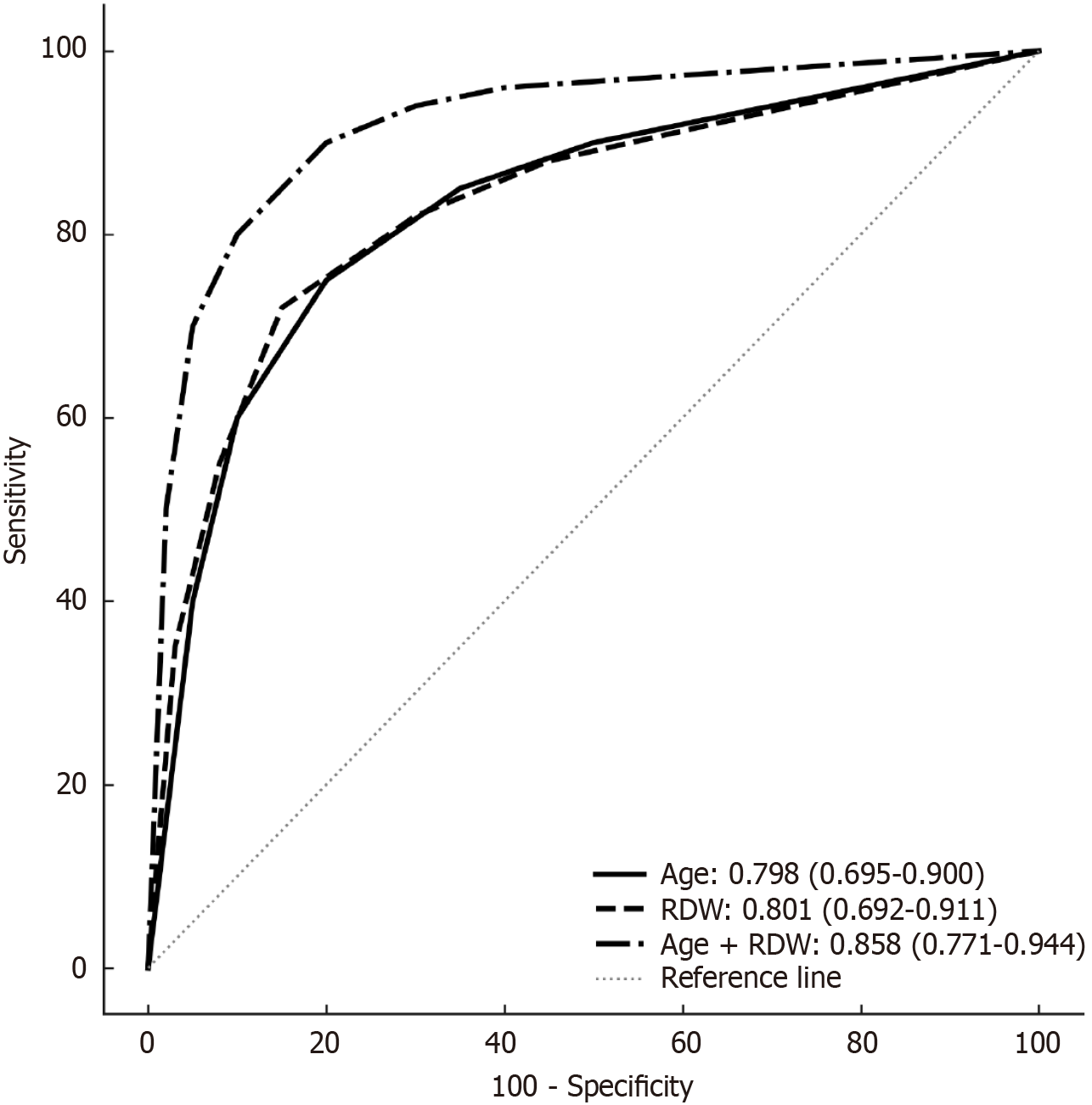
Group B patients were older (69.86 ± 9.58 years vs 52.74 ± 16.85 years, P < 0.001) and had a higher incidence of diabetes mellitus (57.1% vs 21.4%, P = 0.002). RDW (16.2% vs 13.7%, P < 0.001), platelet-lymphocyte ratio (178 vs 126, P = 0.008), and CA 19-9 (21.7 U/mL vs 9.3 U/mL, P = 0.009) were significantly higher in group B, while albumin was lower (41 g/L vs 45 g/L, P = 0.008). Multivariate analysis identified age [odds ratio = 1.067, 95% confidence interval (CI): 1.014-1.122, P = 0.012] and RDW (odds ratio = 1.784, 95%CI: 1.172-2.715, P = 0.007) as independent predictors. The area under the curve for age, RDW, and their combination was 0.798 (95%CI: 0.695-0.900), 0.801 (95%CI: 0.692-0.911), and 0.858 (95%CI: 0.771-0.944), respectively, with bootstrapped validation con
Age and RDW are independent predictors of malignancy in PCNs, aiding in patient selection for advanced dia
Core Tip: This study highlights the utility of simple preoperative parameters - age and red cell distribution width - in predicting malignancy in pancreatic cystic neoplasms. With area under the curve values of 0.798 and 0.801, respectively, and a combined area under the curve of 0.858, these markers can guide patient selection for endoscopic ultrasonography and cytological evaluation, potentially improving surgical decision-making for pancreatic cystic neoplasms.
- Citation: Martli HF, Acehan F, Şimşek A, Şahingöz E, Sürel AA, Er S, Tez M. Preoperative malignancy risk assessment in pancreatic cystic neoplasms using clinical and laboratory parameters. World J Gastrointest Surg 2025; 17(11): 110306
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/110306.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.110306
Pancreatic cystic neoplasms (PCNs) are increasingly detected due to advancements in radiographic techniques, with a prevalence of approximately 15% in the general population[1,2]. These lesions pose a diagnostic challenge due to their heterogeneous nature, ranging from benign to premalignant and malignant[3]. Accurate differentiation is critical, as premalignant and malignant PCNs often require surgical intervention, while benign cysts may only need monitoring unless symptomatic[4]. Current diagnostic approaches rely on cross-sectional imaging (computed tomography, magnetic resonance imaging), endoscopic ultrasonography (EUS), and EUS-guided fine-needle aspiration (FNA)/biopsy[5-7]. However, these methods are specialized, not universally available, and have variable diagnostic accuracy[8,9].
Clinical and laboratory parameters, such as elevated carbohydrate antigen 19-9 (CA 19-9), neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), jaundice, and new-onset diabetes mellitus, have been associated with malignancy risk in PCNs[10]. For instance, PLR has been linked to prognosis in various malignancies, including pancreatic cancer, due to its reflection of systemic inflammation[11,12]. However, only CA 19-9 is supported by guideline-level evidence. Recent studies suggest that simple biochemical markers, such as red cell distribution width (RDW), may aid in preo
This retrospective cohort study was conducted at Ankara Bilkent City Hospital, Department of General Surgery, from February 2019 to March 2023. The study population included 94 patients who underwent surgery for PCNs during this period. After excluding 24 patients who underwent diagnostic EUS ± FNA and were not recommended for surgery, 70 patients were included. Patients were divided into group A (benign or low-grade dysplasia, n = 40) and group B (ma
Preoperative demographic data (age, gender, diabetes mellitus, smoking, comorbidities such as coronary artery disease, hypertension, and others) and laboratory parameters (RDW, NLR, lymphocyte-monocyte ratio, PLR, mean platelet volume, albumin, CA 19-9) were collected from electronic medical records. Imaging characteristics, including cyst size, main pancreatic duct diameter, and presence of mural nodules, were recorded. Pathological findings were obtained from postoperative histopathological reports. Data were retrospectively extracted by trained researchers from the hospital’s electronic database, ensuring consistency through standardized record-keeping protocols.
Statistical analyses were performed using IBM SPSS 26.0 (IBM Corp., Armonk, NY, United States). Normality was assessed with the Shapiro-Wilk test. Categorical variables were analyzed using the Pearson χ2 test (for expected fre
Of the 70 patients, 40 (57.2%) were in group A and 30 (42.8%) in group B. Group B patients were significantly older (69.86 ± 9.58 years vs 52.74 ± 16.85 years, P < 0.001) and had a higher incidence of diabetes mellitus (57.1% vs 21.4%, P = 0.002). Gender distribution showed no significant difference (P = 0.058), with 72.5% females in group A and 45.4% in group B. Group B exhibited higher median RDW (16.2% vs 13.7%, P < 0.001), PLR (178 vs 126, P = 0.008), and CA 19-9 (21.7 U/mL vs 9.3 U/mL, P = 0.009), and lower albumin (41 g/L vs 45 g/L, P = 0.008). Cyst size and lymphadenopathy did not differ between groups, but main pancreatic duct diameter (9.4 mm vs 6.9 mm, P = 0.044) and mural nodule presence (80% vs 5%, P = 0.012) were significantly higher in group B (Table 1).
| Parameters | Overall (n = 70) | Group A (n = 40) | Group B (n = 30) | P value2 |
| Age, years (mean ± SD) | 59.59 ± 16.61 | 52.74 ± 16.85 | 69.86 ± 9.58 | < 0.001 |
| Gender | 0.058 | |||
| Female | 42 (60.0) | 29 (72.5) | 13 (45.4) | |
| Male | 28 (40.0) | 11 (27.5) | 17 (54.6) | |
| Cigarette smoking | 7 (10.0) | 2 (4.8) | 5 (17.9) | 0.107 |
| Presence of chronic pancreatitis | 18 (25.7) | 9 (21.4) | 9 (32.1) | 0.315 |
| Major comorbidity | ||||
| Coronary artery disease | 11 (15.7) | 5 (11.9) | 6 (21.4) | 0.328 |
| Hypertension | 24 (34.3) | 14 (33.3) | 10 (35.7) | 0.837 |
| Diabetes mellitus | 25 (35.7) | 9 (21.4) | 16 (57.1) | 0.002 |
| Other | 9 (12.9) | 3 (7.1) | 6 (21.4) | 0.142 |
| Localization of tumor | ||||
| Head | 39 (55.7) | 17 (42.5) | 22 (73.3) | 0.031 |
| Body | 21 (30.0) | 16 (40) | 5 (16.6) | 0.070 |
| Tail | 9 (12.9) | 6 (15) | 3 (10.1) | |
| Extensive | 1 (1.4) | 1 (2.5) | 0 | |
| Length of tumor (long-axis, mm) | 35 (25-55) | 30 (20-60) | 40 (25-50) | 0.838 |
| Mural nodule (preoperative screening) | 26 (37.1) | 2 (5) | 24 (80) | 0.012 |
| Mural nodule diameter (mm) | 7.8 (5-12) | 5.5 (5-6) | 8.8 (5-12) | 0.035 |
| Pancreatic duct diameter (mm) | 8.8 (< 5-15) | 6.9 (< 5-11) | 9.4 (< 5-15) | 0.044 |
| Pathological diagnosis | ||||
| Serous neoplasms | 9 (12.9) | 9 (22.5) | 0 | |
| Mucinous cystic neoplasm | 11 (15.7) | 9 (22.5) | 2 (6.7) | |
| IPMN | 16 (22.9) | 22 (55) | 26 (86.6) | |
| Other1 | 2 (2.8) | 0 | 2 (6.7) | |
| Laboratory findings, median (IQR) | ||||
| Red cell distribution width (%) | 14.2 (13.5-16) | 13.7 (13.3-14.6) | 16.2 (14.1-17.3) | < 0.001 |
| Neutrophil-lymphocyte ratio | 2.47 (1.95-3.78) | 2.33 (1.90-3.53) | 2.56 (2.14-4.60) | 0.096 |
| Lymphocyte-monocyte ratio | 4.01 (2.73-6.50) | 4.23 (2.81-6.61) | 3.46 (2.50-4.55) | 0.112 |
| Platelet-lymphocyte ratio | 145 (107-194) | 126 (106-157) | 178 (118-238) | 0.008 |
| Mean platelet volume (10-15L) | 8.5 (7.8-9.2) | 8.3 (7.9-8.9) | 8.7 (7.7-9.3) | 0.272 |
| Albumin (g/L) | 43 (40-46) | 45 (41-46) | 41 (34-45) | 0.008 |
| CA 19-9 (U/mL) | 13.6 (5.2-95) | 9.3 (3.2-37.5) | 21.7 (12-211.7) | 0.009 |
In group B, 26 (86.6%) patients had malignancies arising from intraductal papillary mucinous neoplasms (IPMNs), 2 (6.7%) had high-grade dysplasia in mucinous cysts, 1 (3.35%) had high-grade dysplasia in a solid pseudopapillary cyst, and 1 (3.35%) had a neuroendocrine tumor.
Univariate analysis identified age, diabetes mellitus, RDW, PLR, albumin, and CA 19-9 as predictors of malignancy (P < 0.1). Multivariate analysis confirmed age (OR = 1.067, 95%CI: 1.014-1.122, P = 0.012) and RDW (OR = 1.784, 95%CI: 1.172-2.715, P = 0.007) as independent predictors (Table 2). Cut-off values of age ≥ 60 years and RDW ≥ 15.5% were selected to balance sensitivity and specificity, yielding sensitivities of 83.3% and 66.7%, specificities of 70.0% and 82.5%, PPVs of 65.6% and 73.9%, and NPVs of 85.7% and 77.4%, respectively. These thresholds increased malignancy risk 15.3-fold (95%CI: 3.8-61.9) and 22.6-fold (95%CI: 5.2-97.8), respectively (Table 3).
| Parameters | Univariate analysis OR (95%CI) | P value | Multivariate analysis OR (95%CI) | P value |
| Age | 1.095 (1.042-1.150) | < 0.001 | 1.067 (1.014-1.122) | 0.012 |
| Male gender | 2.574 (0.957-6.925) | 0.061 | ||
| Diabetes mellitus | 4.889 (1.710-13.977) | 0.003 | ||
| Red cell distribution width | 2.240 (1.485-3.380) | < 0.001 | 1.784 (1.172-2.715) | 0.007 |
| Neutrophil-lymphocyte ratio | 1.145 (0.927-1.414) | 0.208 | ||
| Platelet-lymphocyte ratio | 1.005 (0.999-1.012) | 0.092 | ||
| Albumin | 0.914 (0.844-0.989) | 0.026 | ||
| CA 19-9 | 1.003 (0.999-1.007) | 0.152 |
The AUC values for age, RDW, and their combination were 0.798 (95%CI: 0.695-0.900), 0.801 (95%CI: 0.692-0.911), and 0.858 (95%CI: 0.771-0.944), respectively (Figure 1). Bootstrapped AUCs confirmed model stability (mean AUC: 0.855, 95%CI: 0.768-0.942).
Accurate preoperative assessment of PCNs remains a significant challenge, despite advances in imaging and endoscopic techniques[8,9]. Established imaging markers such as cyst size, main pancreatic duct dilation, and mural nodules are critical for malignancy risk stratification[5-7]. Clinical parameters like elevated CA 19-9, jaundice, new-onset diabetes mellitus, and PLR have also been linked to malignancy[10]. Our study identifies age and RDW as independent predictors of malignancy in PCNs, with AUC values of 0.798 and 0.801, respectively, and a combined AUC of 0.858, indicating strong discriminatory power for identifying high-risk cases.
The association of RDW with malignancy aligns with previous studies suggesting its role as an inflammatory marker influenced by erythropoietin inhibition and oxidative stress[12]. Hu et al[13] and Yüksel et al[14] reported RDW’s prognostic value in gastric and pancreatic cancers, with elevated levels (cut-off approximately 15%-16%) correlating with poor outcomes. Dang et al[15] further demonstrated RDW’s prognostic significance in patients undergoing pancreatic surgery, linking it to poor immunonutritional status, with a cut-off of 14.8% yielding 68% sensitivity and 72% specificity. Our finding of RDW ≥ 15.5% as a predictor with a 73.9% PPV is consistent with these reports, extending RDW’s utility to preoperative PCN risk assessment. However, unlike studies emphasizing NLR or PLR[10], our analysis found RDW superior, possibly due to its sensitivity to broader inflammatory and oxidative pathways. Celli et al[10] reported NLR’s utility in IPMN malignancy with a sensitivity of 56%-73.1% and specificity of 58%-87% (cut-off approximately 2.5-3.0), but our RDW sensitivity (66.7%) and specificity (82.5%) suggest a more balanced predictive role in PCNs.
Age ≥ 60 years as a predictor is consistent with epidemiological data showing increased pancreatic cancer risk with age, attributed to cumulative exposure to risk factors such as smoking, diabetes, and alcohol, as well as age-related epigenetic changes[11,16]. Our study’s NPV of 85.7% for patients under 60 years supports a conservative approach, aligning with Gonda et al[17], who noted age as a risk modifier in PCN progression (cut-off approximately 55-60 years). This finding contrasts with studies focusing solely on imaging markers[18], highlighting the additive value of clinical parameters. Sun et al[18] emphasized imaging features (e.g., main duct diameter > 10 mm, large mural nodules) without integrating laboratory markers like RDW, achieving high specificity but lacking the non-invasive simplicity of our model.
Compared to existing literature, our study offers a distinct perspective by integrating simple clinical and laboratory markers with imaging. Brugge et al[8] and Yao et al[19] underscored EUS ± FNA’s diagnostic accuracy (25.5%-70%), but noted operator dependency and complication risks, which our age-RDW model could help mitigate by preselecting candidates. Wei et al[20] highlighted the limitations of computed tomography/magnetic resonance imaging alone, supporting our approach of combining age and RDW with imaging to enhance diagnostic precision. Our model’s high NPV and PPV could reduce unnecessary EUS procedures, addressing the accessibility and cost barriers noted by Taya et al[9].
Our findings differ from molecular approaches exploring Kirsten Rat Sarcoma viral oncogene homolog and guanine nucleotide-binding protein alpha-stimulating activity polypeptide mutations in intraductal papillary mucinous neoplasms, which are often multifocal and multiclonal[21]. While molecular analysis can improve cyst classification, its routine clinical application remains limited due to cost and availability, unlike the widely accessible RDW and age parameters we propose. The study, single-center design and small sample size (n = 70) limit generalizability, introducing potential selection bias by including only surgically managed patients. This contrasts with multicenter studies like Ohtsuka et al[5], which provide broader guidelines. Future research should validate our findings across diverse populations, explore RDW’s mechanistic role (e.g., via inflammatory cytokines), and conduct prospective studies with larger cohorts. A nomogram integrating age and RDW could enhance translational impact, though this requires further validation.
Age and RDW are independent predictors of malignancy in PCNs, offering a simple, non-invasive approach to risk stratification. These parameters can guide patient selection for advanced diagnostics and surgical intervention, potentially improving outcomes. Larger, multicenter studies are needed to validate these findings and elucidate RDW’s underlying mechanisms in PCN malignancy.
| 1. | Farrell JJ. Prevalence, Diagnosis and Management of Pancreatic Cystic Neoplasms: Current Status and Future Directions. Gut Liver. 2015;9:571-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2708] [Article Influence: 451.3] [Reference Citation Analysis (3)] |
| 3. | Megibow AJ. Pancreatic Cysts: Radiology. Gastrointest Endosc Clin N Am. 2023;33:519-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Tjaden C, Sandini M, Mihaljevic AL, Kaiser J, Khristenko E, Mayer P, Hinz U, Gaida MM, Berchtold C, Diener MK, Schneider M, Mehrabi A, Müller-Stich BP, Strobel O, Hackert T, Büchler MW. Risk of the Watch-and-Wait Concept in Surgical Treatment of Intraductal Papillary Mucinous Neoplasm. JAMA Surg. 2021;156:818-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Ohtsuka T, Fernandez-Del Castillo C, Furukawa T, Hijioka S, Jang JY, Lennon AM, Miyasaka Y, Ohno E, Salvia R, Wolfgang CL, Wood LD. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2024;24:255-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 236] [Article Influence: 118.0] [Reference Citation Analysis (1)] |
| 6. | Oh D, Pyo JS, Son BK. Prognostic Roles of Inflammatory Markers in Pancreatic Cancer: Comparison between the Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio. Gastroenterol Res Pract. 2018;2018:9745601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1144] [Cited by in RCA: 967] [Article Influence: 120.9] [Reference Citation Analysis (1)] |
| 8. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 917] [Article Influence: 41.7] [Reference Citation Analysis (4)] |
| 9. | Taya M, Hecht EM, Huang C, Lo GC. Pancreatic Cystic Lesions: Imaging Techniques and Diagnostic Features. Gastrointest Endosc Clin N Am. 2023;33:497-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Celli R, Tang LH, Cai G, Freedman-Weiss M, Colunga M, Salem RR, Majumdar S, Jain D. Proinsulin Expressing Neuroendocrine Tumors of the Pancreas: An Underrecognized Entity. Pancreas. 2019;48:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Mishra A, Hunold TM, Peddu DK, Philips GM, Wamsteker EJ, Kwon RS, Schulman AR, Shi J, Carpenter ES, Machicado JD. Histologic Diagnosis of Pancreatic Cystic Lesions with Endoscopic Ultrasound Fine Needle Biopsy and Impact on Management Decisions. Dig Dis Sci. 2025;70:2873-2881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 774] [Article Influence: 64.5] [Reference Citation Analysis (1)] |
| 13. | Hu L, Li M, Ding Y, Pu L, Liu J, Xie J, Cabanero M, Li J, Xiang R, Xiong S. Prognostic value of RDW in cancers: a systematic review and meta-analysis. Oncotarget. 2017;8:16027-16035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 14. | Yüksel C, Erşen O, Culcu S, Bakırarar B, Unal AE, Demirci S. Prognostic Role of Red Distribution Width (RDW) Value in Gastric Cancer. J Coll Physicians Surg Pak. 2021;31:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Dang C, Wang M, Qin T, Qin R. Clinical importance of preoperative red-cell volume distribution width as a prognostic marker in patients undergoing radical surgery for pancreatic cancer. Surg Today. 2022;52:465-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 472] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 17. | Gonda TA, Cahen DL, Farrell JJ. Pancreatic Cysts. N Engl J Med. 2024;391:832-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 18. | Sun L, Wang W, Zhu H, Jiang F, Peng L, Jin G, Jin Z. High-Risk Characteristics Associated with Advanced Pancreatic Cystic Lesions: Results from a Retrospective Surgical Cohort. Dig Dis Sci. 2021;66:2075-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Yao DW, Qin MZ, Jiang HX, Qin SY. Comparison of EUS-FNA and EUS-FNB for diagnosis of solid pancreatic mass lesions: a meta-analysis of prospective studies. Scand J Gastroenterol. 2024;59:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Wei W, Li H, Li N, Sun H, Li Q, Shen X. WNT5A/JNK signaling regulates pancreatic cancer cells migration by Phosphorylating Paxillin. Pancreatology. 2013;13:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Gentiluomo M, Corradi C, Arcidiacono PG, Crippa S, Falconi M, Belfiori G, Farinella R, Apadula L, Lauri G, Bina N, Rizzato C, Canzian F, Morelli L, Capurso G, Campa D. Role of pancreatic ductal adenocarcinoma risk factors in intraductal papillary mucinous neoplasm progression. Front Oncol. 2023;13:1172606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |