Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.110036
Revised: July 21, 2025
Accepted: September 9, 2025
Published online: November 27, 2025
Processing time: 181 Days and 4.4 Hours
Biliary stent placement and removal are common medical procedures, but they carry risks of chyme regurgitation and residual common bile duct stones (CBDS), highlighting the necessity of intra-biliary cleansing during secondary endoscopic stent removal.
To compare the incidence of chyme reflux into the common bile duct and residual or recurrent CBDS, and the safety of intra-biliary cleansing during secondary duodenoscopic removal of duodenal bend vs single pigtail biliary stents.
We included 554 patients undergoing secondary duodenoscopy for biliary stent removal and intra-biliary cleansing from March 2019 to September 2024. Patients were divided into a single pigtail biliary stent group and a duodenal bend biliary stent group (DBBSG). Chyme reflux and CBDS occurrences were compared using the Cox proportional hazards model.
The median age of the patients included was 62 years (interquartile range: 51-70), with 53.11% being female. During stent removal, DBBSG showed higher rates of chyme reflux (23.27% vs 9.65%, P < 0.001) and CBDS (42.77% vs 21.05%, P < 0.001) compared to the single pigtail biliary stent group. No significant differences were found in the incidence of adverse reactions between the two groups (P > 0.05), and no serious events or deaths occurred. DBBSG patients had increased risks of chyme reflux (hazard ratio = 2.793; 95% confidence interval: 1.695-4.603; P < 0.001) and CBDS (hazard ratio: 2.475; 95% confidence interval: 1.732-3.536; P < 0.001).
Duodenal bend biliary stents increase the risk of chyme reflux into the common bile duct and CBDS. The safety of intra-biliary cleaning during stent removal has been validated, and as a result, it is recommended that endoscopists perform intra-biliary cleaning during duodenoscopic removal of duodenal bend biliary stents.
Core Tip: In clinical practice, compared to single-pigtail biliary stents, patients with duodenal bend biliary stent have a significantly increased risk of chyme reflux into the bile duct and common bile duct stones (residual or recurrent). Performing intra-biliary cleansing after stent removal can improve patient prognosis. The finding of this study will increase endoscopists’ awareness of the importance of intra-biliary cleansing during biliary stent placement and replacement, thereby optimising clinical practice and improving patient prognosis.
- Citation: Zhang HL, Zhang C, Qiu C, Zhang BS, Huang AH, Yang JS, Jiang ZY, Zheng L, Hu H, Yang YL. Intra-biliary cleansing during secondary duodenoscopic removal of duodenal bend biliary stents: A retrospective cohort study. World J Gastrointest Surg 2025; 17(11): 110036
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/110036.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.110036
Endoscopic biliary stenting is the primary minimally invasive treatment for failed choledochal stone retrieval[1-3], benign biliary strictures[1,4,5], palliative drainage of malignant biliary obstruction[1,6,7], and bile leakage[1,8,9]. In clinical practice, bile duct stenting is commonly used to manage duodenal papillary thermal injury caused by endoscopic sphincterotomy (EST) and mechanical injury to the duodenal papilla caused by stone retrieval baskets or balloons[10]. To prevent temporary bile duct drainage obstruction due to duodenal papillary oedema after endoscopic retrograde cholangiopancreatography (ERCP), bile duct stents are usually placed after achieving treatment goals [such as complete removal of common bile duct stones (CBDS)] to maintain bile duct patency[11], until the stent either dislodges naturally or is removed through reoperation in a short period. However, the type of biliary stent and the timing of its replacement not only affect biliary patency but may also contribute to the formation of medically induced choledochal stones[2,10,11].
Recent studies recommend that biliary stents should be replaced at regular intervals, with the usual recommended interval being every three months[2]. However, studies have found that some patients have their stents replaced when they become symptomatic; by this time, the stent is usually obstructed, which can lead to serious biliary infections such as acute cholangitis, with a high mortality rate, particularly in older adults[12]. When replacing a stent during an ERCP operation, it is common to find intestinal chyme reflux in the bile duct or stones forming between the end of the stent and the biliary wall[13]. Some of these were residual stones that were incompletely removed during the last extraction; how
Most endoscopists clean bile ducts when replacing biliary stents in patients who require long-term stent placement and periodic replacement. However, in patients who have completed ERCP therapy and are scheduled for short-term stent removal, many endoscopists only remove the stent through duodenoscopy and observe the duodenal papilla for active bleeding, often ignoring the possible presence of chyme reflux and residual or new bile duct stones in the biliary tract. This increases the risk of distant, medically induced CBDS[2,10,11]. In the European Society of Gastrointestinal Endos
The types of biliary stents mainly include plastic stents and metal stents. According to different stent designs and clinical conditions, they also include duodenal bend, centre bend, straight, pigtail, winged stents, and so on. The metal stent is a type of mesh stent with self-expanding characteristics and large-diameter openings at both ends, which is mainly used for long-term drainage of malignant biliary obstruction. It is inherently prone to intestinal biliary reflux and CBDS and is therefore insufficient to serve the main population of this study. The single pigtail biliary stent is an improved type of stent that is now widely used in clinical practice. In addition to this, another widely used biliary stent in clinical practice is the duodenal bend biliary stent. However, the acute angulation inherent to duodenal bend biliary stent can disrupt the natural axis of bile flow, including regions of recirculation and vortex formation at the bend site. These flow disruptions create zones of stagnant bile flow and localized negative pressure, which can facilitate retrograde movement of duodenal contents into the bile duct. This effect is amplified when the stent tip is close to or within the duodenal lumen, as fluctuations in intraduodenal pressure during peristalsis further promote enterobiliary reflux. The mechanical pressure exerted by the stent’s bend on the sphincter of Oddi may further diminish its competence. The irregular inner surface and localized stagnation zones at the stent bend favor the deposition of bile sludge, bacterial colonization, and subsequent biofilm development[14-16], setting the stage for stone nucleation and growth.
Therefore, this study was based on a single pigtail biliary stent as a standard, and the main objective was to compare the incidence of chyme reflux into the bile duct and residual or recurrent CBDS and the safety of intra-biliary cleansing during secondary duodenoscopic removal of the duodenal bend biliary stent. We hope that this study will increase endoscopists’ awareness of the importance of intra-biliary cleansing during biliary stent removal and replacement, thereby optimizing clinical practice and improving patient prognosis.
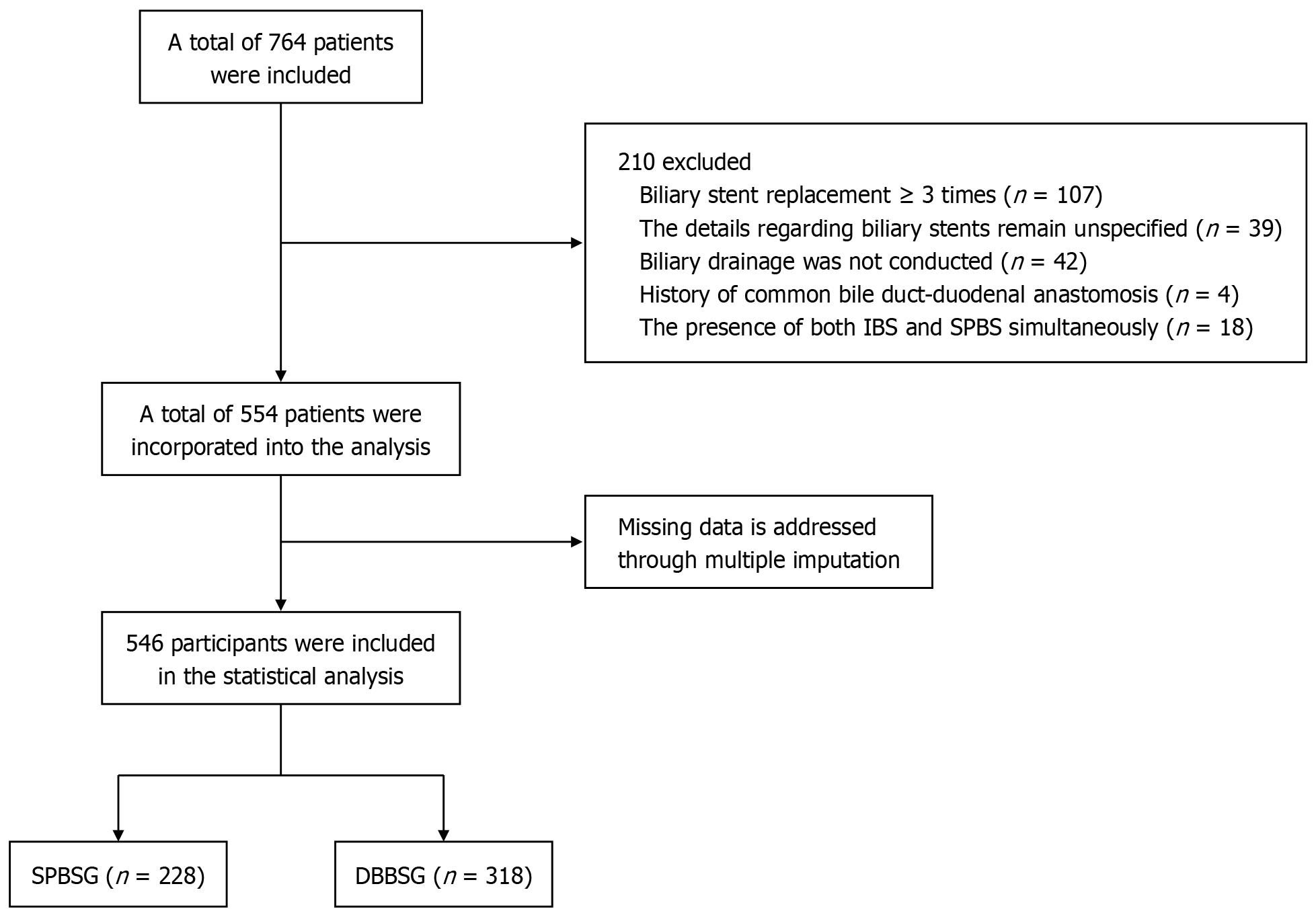
This was a retrospective cohort study including 554 patients who underwent secondary duodenoscopy for biliary stent removal with intra-biliary cleansing between March 2019 and September 2024 at our institution. The inclusion criteria for selecting participants were: (1) Age ≥ 14 years old, regardless of sex; (2) Patients with a biliary stent placed during ERCP treatment in our hospital and had no stent dislodge as confirmed by abdominal computerized tomography review; (3) Detailed surgical records or ERCP imaging data retained; (4) Admitted to the hospital for duodenoscopic removal of biliary stent and had signed the informed consent form; and (5) Stenting of bile ducts or (and) pancreatic ducts met the indications for stenting. The exclusion criteria were: (1) Duodenoscopic removal of biliary stent without intra-biliary cleansing; (2) Due to previous choledocho-jejunal anastomosis, food easily flowed into the common bile duct; (3) Patient could not tolerate the surgery when removing the biliary stent and intra-biliary cleansing for various reasons, resulting in failure to assess the bile duct situation in detail; (4) For various reasons, it was impossible to determine the time of the last placement of the biliary stent; (5) Biliary stent type was unknown; (6) Repeated replacement of biliary stents (≥ 3 times); and (7) The presence of both duodenal bend biliary stent and single pigtail biliary stent in the body. A flowchart of the study is shown in Figure 1.
The study was approved by the local Institutional Review Board, approval No.[2022] Research Review (107). Each patient provided written informed consent before the endoscopic interventions. All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Stent type was chosen at the discretion of the attending endoscopist, based on anatomical considerations, anticipated risk of migration, and operator experience. While single pigtail biliary stents are the standard of care at our institution, duodenal bend stents are preferentially used in cases with a challenging anatomy or risk of migration.
Basic information of all patients at the time of initial ERCP biliary stent placement was collected from the electronic medical record system, including sex, age, body mass index, hypertension, hyperlipidaemia, diabetes mellitus, coronary heart disease, and biliary system diseases (fatty liver, gallstones, congenital choledochal cysts, and extrahepatic bile duct stenosis). Additionally, history of pancreatitis and biliary surgeries (ERCP, cholecystectomy, and common bile duct exploration) and hospitalization duration (for the second ERCP procedure to remove the biliary stent, with durations categorized as ≤ 1 day, 2 days, 3 days, and ≥ 4 days) were collected.
Tests included lipid markers (serum total cholesterol and triglyceride levels at the time of initial ERCP choledochal stent placement) and liver function (within 24 hours of the second duodenoscopy to remove the biliary stent and perform intra-biliary cleansing).
ERCP surgical records included classifications of duodenal papillopathy (DP)[17], the presence of periampullary diverticula and their relative position to the papilla, sphincter of Oddi dysfunction (SOD)[18], pancreaticobiliary maljunction (PBM)[17,19], length of EST incision (mm), final dilated diameter achieved during endoscopic papillary balloon dilation (mm), pancreatic duct stent (PDS), and types of biliary stents (single pigtail biliary stents, duodenal bend biliary stents, and metal-covered stents).
DP can be categorised into different types based on its relative position to the diverticulum[17]: (1) Papilla adjacent to the duodenal diverticulum. The root of the papillae was within 3 cm of the diverticulum; and (2) Papilla within the duodenal diverticulum. The root of the papillae was located inside the diverticulum, including the lateral wall and base of the diverticulum.
Intra-biliary cleansing: Following intubation of the descending part of the duodenum with the duodenoscope, the duodenal papilla was identified. The existing biliary stent was then captured and removed using a snare. A sphincterotomy was performed, and a guidewire was advanced into the bile duct. Cholangiography was conducted to observe the morphology of the bile ducts and the possible presence of lesions. Subsequently, the bile ducts were cleared using a stone extraction basket and balloon catheter, followed by repeated irrigation. The procedure was concluded after confirming the absence of contrast extravasation on final cholangiography. Prior to the procedure, all patients and their families were thoroughly informed of the purpose and potential adverse effects of the operation. The biliary cleansing intervention was only performed after obtaining written informed consent.
The patients were divided into either a control group or a study group according to the type of biliary stent selected during the initial ERCP procedure. The control group consisted of patients who received a single pigtail biliary stent during initial ERCP, which represents the routine standard of care. This group was also designated as the single pigtail biliary stent group (SPBSG). The study group, also designated as the duodenal bend biliary stent group (DBBSG), consisted of patients who underwent single pigtail biliary stent placement during the initial ERCP procedure. Biliary stent indwelling time was defined as the time from the date of successful placement of the biliary stent during the initial ERCP procedure to the date of removal of the biliary stent and intra-biliary cleansing during the second ERCP procedure. This time (in weeks) was used as the follow-up period in this study. Indwelling duration was analyzed as a time-dependent variable using Cox proportional risk models.
The primary endpoints were the occurrence of chyme reflux into the bile duct (defined as the presence of visible, macroscopic food particles or turbid, non-biliary fluid aspirated from the bile duct at the time of ERCP) and CBDS (the presence of stones in the common bile duct during the second ERCP for stent removal and intra-biliary cleansing, regardless of whether they were residual or recurrent stones, source surgical records, or ERCP images retained). The above results were assessed by both the endoscopist and the assistant. For reliability, a blinded second reviewer inde
The secondary endpoints included the occurrence of acute cholangitis (symptoms that met the diagnostic criteria for acute cholangitis during the second ERCP for stent removal) and stent displacement rates[20].
Evaluation of safety of the second ERCP for stent removal and intra-biliary cleansing was based on the following parameters: Changes in liver function, inflammatory markers, and ERCP-related adverse events within 24 hours postoperatively (ERCP-related adverse events[21] occurring during hospitalization after the second ERCP for stent removal and intra-biliary cleansing, based on electronic medical records and nursing records), primarily including post-ERCP acute cholangitis, post-ERCP pancreatitis, hyperamylasemia[22], postoperative nausea and vomiting, bleeding, and duodenal perforation during hospitalization.
Based on preliminary data from our institution, the incidence of chyme reflux was estimated at 21% in the DBBSG and 8% in the SPBSG. To ensure adequate statistical power, we performed a sample size calculation using PASS software, specifying a two-sided alpha of 0.05 and 90% power (1-β = 0.9). PASS determined that a minimum of 142 subjects per group would be required to detect this difference. Our final sample comprised 318 DBBSG and 228 SPBSG patients.
Continuous data are summarized as the mean and standard deviation or median and interquartile range, and comparisons between groups were performed using independent samples t-tests or Mann-Whitney U tests. Count data are summarized as numbers and percentages, and comparisons between groups were performed using the χ2 test or Fisher’s exact test. In this study, missing data were addressed using the “mice” package in R for multiple imputation, under the assumption of missing at random. Predictive mean matching was applied for continuous variables and logistic regression for categorical variables, generating five complete datasets. Results were combined using Rubin’s rules. Sensitivity analyses with complete-case data were performed to confirm the robustness of our findings.
The Kaplan-Meier method was used to estimate the cumulative risks, and the results were compared using the log-rank test. Cox proportional hazard regression models were used to determine the effect of covariates on outcomes and to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). Model 1 was an unadjusted Cox proportional hazards model. Model 2 was based on model 1 and additionally adjusted for age, sex, PBM, and extrahepatic bile duct strictures. Model 3 was based on model 2 and further adjusted for duodenal papilla length, EST incision length, DP, SOD, metal-covered biliary stents, and PDS. Restricted cubic splines were used to explore potential nonlinear relationships between the covariates of age, EST incision length, and CBDS.
To observe the effects of different biliary stents on primary outcomes in various subgroups, we set up six subgroups based on age (< 60 and ≥ 60), sex, hyperlipidaemia, extrahepatic biliary strictures, SOD, and PDS and assessed interactions in the Cox model. Regarding sensitivity analyses, we excluded two patient groups to avoid confounding the primary outcomes: (1) Patients with SOD, due to their potentially compromised biliary integrity; and (2) Those with metal-covered biliary stents, because the larger openings of these stents may facilitate reflux. Both the loss of normal regulation of the duodenal papilla due to SOD and the large-calibre opening of the metal stent itself, by design, predispose patients to enterobiliary reflux. We, therefore, excluded the above groups from the sensitivity analysis.
All statistical analyses were performed using R language (version 4.3.2) and SPSS software (version 27.0, IBM Corp, Armonk, NY, United States). Statistical significance was defined as P < 0.05.
The median age of the overall population was 62 (interquartile range: 51-70) years, and 290 individuals (53.11%) were females (Table 1). Regarding the previous EST incision length and history of gallstones, the DBBSG was significantly longer than the SPBSG; however, the SPBSG had a higher proportion of patients with a history of cholecystectomy and pancreatic duct stenting (all P < 0.05). There were no significant differences in other demographic or disease characteristics between the two groups (all P > 0.05) (Table 1).
| Variable | Total (n = 546) | SPBSG (n = 228) | DBBSG (n = 318) | P value |
| Age, median (Q1, Q3) | 62.00 (51.00, 70.00) | 61.00 (51.00, 69.00) | 62.00 (51.00, 71.00) | 0.320 |
| Sex | 0.875 | |||
| Female | 290 (53.11) | 122 (53.51) | 168 (52.83) | |
| Male | 256 (46.89) | 106 (46.49) | 150 (47.17) | |
| BMI (kg/m²), median (Q1, Q3) | 21.56 (20.07, 23.23) | 21.83 (20.07, 23.23) | 21.30 (19.84, 23.23) | 0.501 |
| TC, median (Q1, Q3) | 4.34 (3.65, 5.25) | 4.27 (3.58, 5.14) | 4.43 (3.71, 5.26) | 0.116 |
| TG, median (Q1, Q3) | 1.19 (0.87, 1.72) | 1.16 (0.86, 1.63) | 1.20 (0.88, 1.87) | 0.222 |
| Duodenal papilla length (cm), median (Q1, Q3) | 1.00 (0.78, 1.30) | 1.02 (0.80, 1.39) | 1.00 (0.76, 1.30) | 0.111 |
| Length of EST incision (mm), median (Q1, Q3) | 3.00 (2.00, 3.58) | 2.40 (1.00, 3.00) | 3.00 (2.00, 4.00) | < 0.001 |
| Diameter of EPBD (mm), median (Q1, Q3) | 6.00 (6.00, 7.80) | 6.00 (6.00, 7.12) | 6.00 (6.00, 8.00) | 0.888 |
| Time (week), median (Q1, Q3) | 11.00 (7.00, 14.00) | 12.00 (7.25, 14.00) | 11.00 (7.00, 14.00) | 0.010 |
| EST | < 0.001 | |||
| No | 139 (25.46) | 78 (34.21) | 61 (19.18) | |
| Yes | 407 (74.54) | 150 (65.79) | 257 (80.82) | |
| EPBD | 0.162 | |||
| No | 82 (15.02) | 40 (17.54) | 42 (13.21) | |
| Yes | 464 (84.98) | 188 (82.46) | 276 (86.79) | |
| Diameter of the stents | < 0.001 | |||
| 5 Fr | 35 (6.41) | 35 (15.35) | 0 (0) | |
| 7 Fr | 193 (35.35) | 172 (75.44) | 21 (6.6) | |
| 8.5 Fr | 105 (19.23) | 19 (8.33) | 86 (27.04) | |
| 10 Fr | 213 (39.01) | 2 (0.88) | 211 (66.35) | |
| Hyperlipidaemia | 0.070 | |||
| No | 474 (86.81) | 205 (89.91) | 269 (84.59) | |
| Yes | 72 (13.19) | 23 (10.09) | 49 (15.41) | |
| DM | 0.100 | |||
| No | 481 (88.10) | 207 (90.79) | 274 (86.16) | |
| Yes | 65 (11.90) | 21 (9.21) | 44 (13.84) | |
| CHD | 0.408 | |||
| No | 504 (92.31) | 213 (93.42) | 291 (91.51) | |
| Yes | 42 (7.69) | 15 (6.58) | 27 (8.49) | |
| Fatty liver | 0.414 | |||
| No | 486 (89.01) | 200 (87.72) | 286 (89.94) | |
| Yes | 60 (10.99) | 28 (12.28) | 32 (10.06) | |
| Gallstones | < 0.001 | |||
| No | 264 (48.35) | 139 (60.96) | 125 (39.31) | |
| Yes | 282 (51.65) | 89 (39.04) | 193 (60.69) | |
| Congenital choledochal cysts | 1.000 | |||
| No | 544 (99.63) | 227 (99.56) | 317 (99.69) | |
| Yes | 2 (0.37) | 1 (0.44) | 1 (0.31) | |
| Extrahepatic bile duct stenosis | 0.059 | |||
| No | 484 (88.64) | 209 (91.67) | 275 (86.48) | |
| Yes | 62 (11.36) | 19 (8.33) | 43 (13.52) | |
| History of pancreatitis | 0.327 | |||
| No | 503 (92.12) | 207 (90.79) | 296 (93.08) | |
| Yes | 43 (7.88) | 21 (9.21) | 22 (6.92) | |
| History of ERCP | 0.983 | |||
| No | 460 (84.25) | 192 (84.21) | 268 (84.28) | |
| Yes | 86 (15.75) | 36 (15.79) | 50 (15.72) | |
| History of cholecystectomy | 0.002 | |||
| No | 359 (65.75) | 133 (58.33) | 226 (71.07) | |
| Yes | 187 (34.25) | 95 (41.67) | 92 (28.93) | |
| History of common bile duct exploration | 0.165 | |||
| No | 527 (96.52) | 223 (97.81) | 304 (95.60) | |
| Yes | 19 (3.48) | 5 (2.19) | 14 (4.40) | |
| DP | 0.138 | |||
| Simple or acute type | 301 (55.13) | 126 (55.26) | 175 (55.03) | |
| Hyperplastic or sclerotic type | 118 (21.61) | 58 (25.44) | 60 (18.87) | |
| Constrictive type | 41 (7.51) | 13 (5.70) | 28 (8.81) | |
| Atrophic type | 86 (15.75) | 31 (13.60) | 55 (17.30) | |
| PAD | 0.377 | |||
| No | 384 (70.33) | 165 (72.37) | 219 (68.87) | |
| Yes | 162 (29.67) | 63 (27.63) | 99 (31.13) | |
| Location of DP to PAD | 0.406 | |||
| Outside | 392 (71.79) | 168 (73.68) | 224 (70.44) | |
| Inside | 154 (28.21) | 60 (26.32) | 94 (29.56) | |
| SOD | 0.209 | |||
| No | 477 (87.36) | 204 (89.47) | 273 (85.85) | |
| Yes | 69 (12.64) | 24 (10.53) | 45 (14.15) | |
| PBM | 0.152 | |||
| No | 506 (92.67) | 207 (90.79) | 299 (94.03) | |
| Yes | 40 (7.33) | 21 (9.21) | 19 (5.97) | |
| PDS | < 0.001 | |||
| No | 256 (46.89) | 74 (32.46) | 182 (57.23) | |
| Yes | 290 (53.11) | 154 (67.54) | 136 (42.77) | |
| Metal-covered stents | 0.239 | |||
| No | 534 (97.80) | 221 (96.93) | 313 (98.43) | |
| Yes | 12 (2.20) | 7 (3.07) | 5 (1.57) | |
In secondary duodenoscopy to remove the biliary stent and for intra-biliary cleansing, the incidences of chyme reflux into the bile duct [74/318 (23.27%) vs 22/228 (9.65%), P < 0.001] and CBDS [136/318 (42.77%) vs 48/228 (21.05%), P < 0.001] were significantly higher in the DBBSG than in the SPBSG. There were no significant differences in the incidence of acute cholangitis or stent displacement rates between the two groups (all P > 0.05) (Table 2).
| Variable | Total (n = 546) | SPBSG (n = 228) | DBBSG (n = 318) | P value |
| Chyme | < 0.001 | |||
| No | 450 (82.42) | 206 (90.35) | 244 (76.73) | |
| Yes | 96 (17.58) | 22 (9.65) | 74 (23.27) | |
| CBDS | < 0.001 | |||
| No | 362 (66.30) | 180 (78.95) | 182 (57.23) | |
| Yes | 184 (33.70) | 48 (21.05) | 136 (42.77) | |
| Acute cholangitis | 0.816 | |||
| No | 521 (95.42) | 217 (95.18) | 304 (95.60) | |
| Yes | 25 (4.58) | 11 (4.82) | 14 (4.40) | |
| Stent displacement | 0.335 | |||
| No | 524 (95.97) | 221 (96.93) | 303 (95.28) | |
| Yes | 22 (4.03) | 7 (3.07) | 15 (4.72) | |
In secondary duodenoscopy to remove the biliary stent and for intra-biliary cleansing, the DBBSG had a longer hospital stay than the SPBSG (P = 0.039); however, overall, most patients (369 individuals, 67.58%) had a hospital stay of no more than 2 days (Supplementary Table 1). The postoperative 24-hour gamma-glutamyl transpeptidase levels were higher in the DBBSG than in the SPBSG (P = 0.009), but the median levels remained within the normal range (Supplementary Table 1). There were no significant differences between the two groups in postoperative adverse reactions (Table 3), liver function at 24 hours postoperatively, inflammatory markers, or amylase levels (all P > 0.05) (Supplementary Table 1).
| Variable | Total (n = 546) | SPBSG (n = 228) | DBBSG (n = 318) | P value |
| Post-ERCP acute cholangitis | 0.311 | |||
| No | 532 (97.44) | 224 (98.25) | 308 (96.86) | |
| Yes | 14 (2.56) | 4 (1.75) | 10 (3.14) | |
| PEP | 0.418 | |||
| No | 545 (99.82) | 227 (99.56) | 318 (100.00) | |
| Yes | 1 (0.18) | 1 (0.44) | 0 (0.00) | |
| Bleeding | 1.000 | |||
| No | 544 (99.63) | 227 (99.56) | 317 (99.69) | |
| Yes | 2 (0.37) | 1 (0.44) | 1 (0.31) | |
| Duodenal perforation | ||||
| No | 546 (100) | 228 (100) | 318 (100) | NA |
| Yes | 0 (0) | 0 (0) | 0 (0) | |
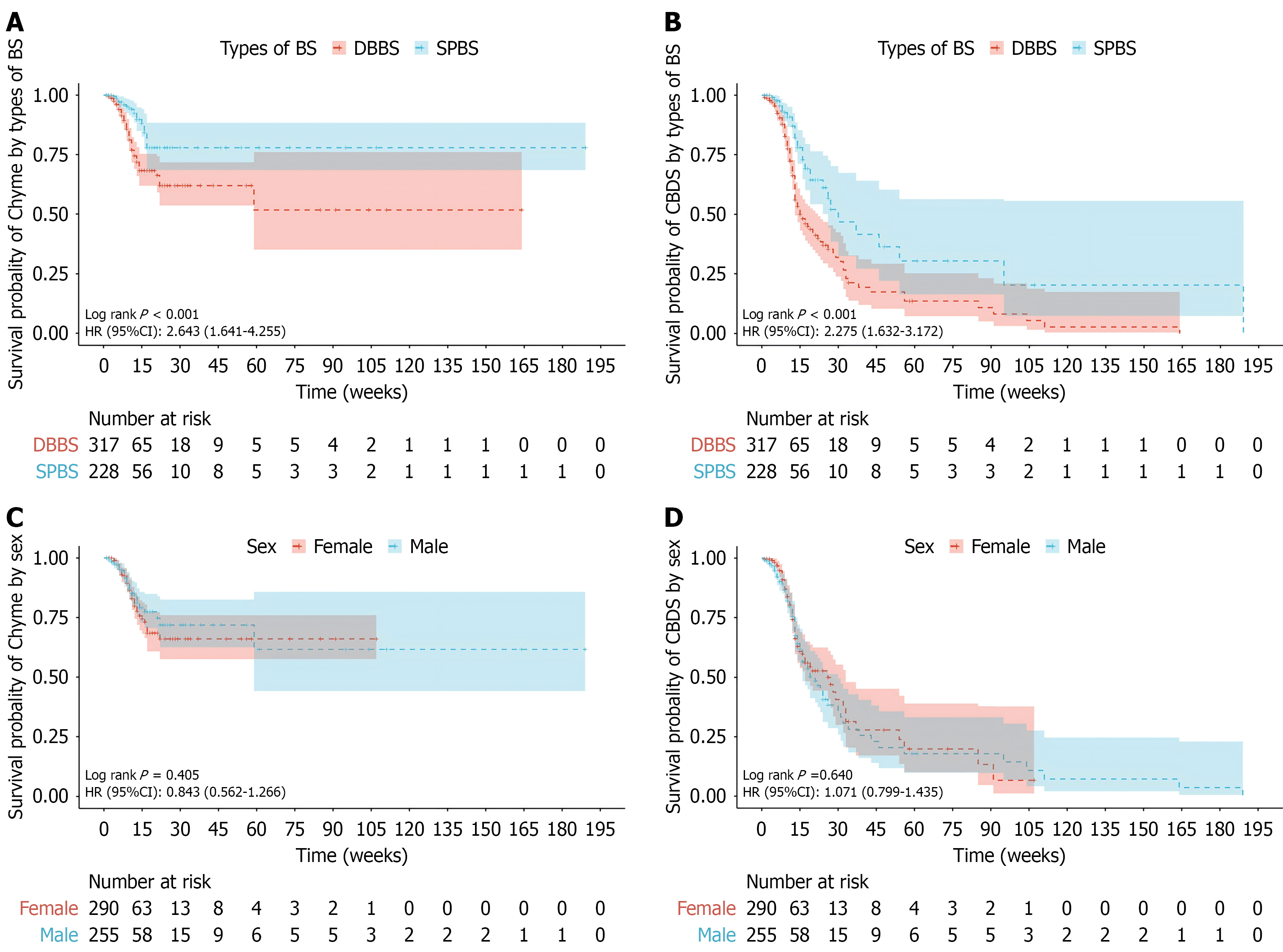
The Kaplan-Meier survival curves for chyme reflux into the bile duct and CBDS in the DBBSG and SPBSG groups are shown in Figure 2. The overall incidence of chyme reflux into the bile duct in the DBBSG was significantly higher than that in the SPBSG, with a HR of 2.643 (95%CI: 1.641-4.255; P < 0.001; Figure 2A). The overall incidence of CBDS in the DBBSG group was significantly higher than that in the SPBSG group, with a HR of 2.275 (95%CI: 1.632-3.172; P < 0.001; Figure 2B). There were no significant differences in the incidence of chyme reflux into the bile duct or CBDS between sexes (Figure 2C and D).
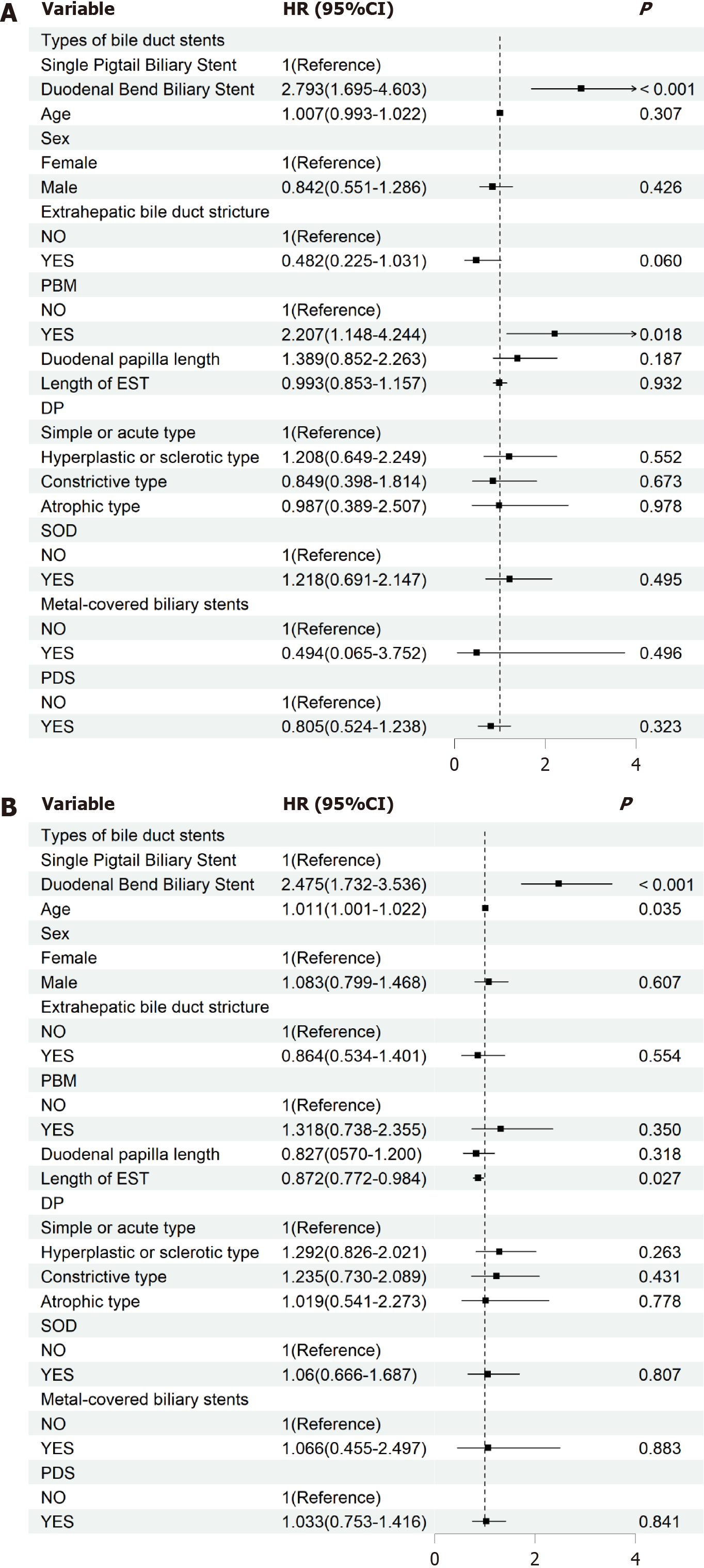
Multivariate Cox proportional hazards regression models revealed that the type of biliary stent was associated with the risk of chyme reflux into the bile duct and CBDS (Tables 4 and 5, Figure 3). When adjusted for factors such as age, sex, PBM, extrahepatic bile duct stricture, duodenal papilla length, length of EST incision, DP, SOD, metal-covered biliary stents, and diameter of the stents and PDS, the DBBSG had a higher risk of developing chyme reflux into the bile duct (HR = 2.793; 95%CI: 1.695-4.603; P < 0.001) and CBDS (HR = 2.475; 95%CI: 1.732-3.536; P < 0.001) than the SPBSG (Tables 4 and 5, Figure 3). Model 3 revealed statistical differences in the continuous variables of age and EST incision length concerning CBDS (both P < 0.05). Further exploration of the nonlinear relationships through RCS revealed no significant nonlinear relationship between age, EST incision length, and CBDS (P-non-linear > 0.05; Supplementary Figure 1).
| Group | Model 1 | Model 2 | Model 3 | |||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| 0 | 1.00 (reference) | - | 1.00 (reference) | - | 1.00 (reference) | - |
| 1 | 2.643 (1.641-4.255) | < 0.001 | 2.868 (1.774-4.637) | < 0.001 | 2.793 (1.695-4.603) | < 0.001 |
| Group | Model 1 | Model 2 | Model 3 | |||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| 0 | 1.00 (reference) | - | 1.00 (reference) | - | 1.00 (reference) | - |
| 1 | 2.275 (1.632-3.172) | < 0.001 | 2.319 (1.659-3.242) | < 0.001 | 2.475 (1.732-3.536) | < 0.001 |
In the main patient subgroups categorized by age (< 60 years and ≥ 60 years), sex, history of hyperlipidaemia, extrahepatic bile duct stricture, SOD, and PDS, there were no significant differences in the relationship between DBBSG and SPBSG with chyme reflux into the bile duct and CBDS (all P-interactions > 0.05, Supplementary Figures 2 and 3). In the sensitivity analysis, the relationship between duodenal bend biliary stent and chyme reflux into the bile duct was similar to that in the primary analysis. When the analysis was limited to patients without SOD, the connection between the two was slightly weakened (Supplementary Table 2). However, when the analysis was limited to patients without metal-covered bile duct stents, the connection between the two was slightly strengthened (Supplementary Table 3). The relationship between duodenal bend biliary stent and CBDS was similar to the results of the primary analysis. When the analysis was limited to patients without SOD or metal-covered bile duct stents, the connection between the two was slightly weakened (Supplementary Tables 4 and 5).
In the current management strategies for biliary stents, the type of stent and the timing of replacement are critical factors; however, there is a lack of clear guidelines regarding the performance of biliary cleaning after stent removal. This study, based on a retrospective cohort analysis of 556 patients, investigated the impact of different types of biliary stents and their dwelling time on the incidence of chyme reflux into the bile duct and CBDS (residual or recurrent) during secondary duodenoscopic stent retrieval and assessed the safety of intra-biliary cleansing. Our study results showed that compared to single pigtail biliary stents, patients with duodenal bend biliary stents had a significantly higher risk of chyme reflux into the bile duct (HR = 2.67; 95%CI: 1.59-4.50; P < 0.001) and CBDS (HR = 2.58; 95%CI: 1.78-3.75; P < 0.001). We performed adjusted Cox regression incorporating length of EST incision, stent diameter, and PDS as potential covariates that may affect the results. The relationship between stent type and primary outcomes was unchanged after adjustment, supporting the robustness of the findings. This result may be related to the design characteristics of the duodenal bend biliary stents, such as their larger diameter and relatively rigid structure, which may increase the pressure in the bile duct and impede the normal drainage of bile, in turn leading to the reflux of intestinal contents into the bile duct[23-25]. In addition, some patients are co-positioned with metal-coated stents because of the complexity of their condition, and the material properties of metal-coated stents may promote chronic inflammatory responses and fibrous tissue proliferation in the biliary lining, further exacerbating the risk of biliary obstruction and reflux[26,27]. This is supported by current literature, which states that differences in the biocompatibility and mechanical properties of different types of biliary stents can significantly affect clinical outcomes[28,29]. The surface properties of duodenal bend biliary stents may be more prone to induce biofilm formation, and the bacteria in these biofilms are able to attach to and colonise the bile ducts, increasing the risk of infection and stone formation[30,31]. These mechanisms explain why endoscopists should be more careful when selecting duodenal bend biliary stents in clinical practice[25], especially in high-risk patients, and should take appropriate precautions during placement and retrieval. The choice of the stent type should be assessed individually according to the patient’s anatomy and disease characteristics.
Studies have shown that in patients undergoing stent placement, the status of the stent should be regularly assessed and replaced in a timely manner[2,12]. The longer the stent was left in place, the higher the incidence of chyme reflux into the bile duct and stone formation. This finding is related to the chronic inflammatory and fibrotic processes in the biliary tract caused by prolonged stent retention, which may lead to cholestasis and bile duct stenosis[24,32]. This study further found that these risks were more prominent when duodenal bend biliary stents were used. It has been shown that regular stent replacement reduces the risk of bile duct infection and stone formation and improves the disease-free survival of patients[27,29]. In addition, the present study further supports the practice of intra-biliary cleansing during biliary stent removal. The study analyzed the safety of patients after secondary duodenoscopic removal of the biliary stent with intra-biliary cleansing and showed no significant difference between the two groups in terms of adverse effects, such as po
Although this study provides new insights into the optimization of biliary stent use, some limitations remain. This study did not investigate the long-term effects of different types of stents on the biliary micro-ecosystem, which may play an important role in the development of biliary complications[29,34]. In addition, future studies should focus on ex
In summary, our study identified DBBS as a risk factor for enterobiliary reflux. Intra-biliary cleansing during secondary stent removal was associated with improved short-term outcomes in our cohort; however, additional long-term studies are required to determine sustained benefits and impacts on patient prognosis. Based on the results of this study, it is re
| 1. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 528] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 2. | Di Giorgio P, Manes G, Grimaldi E, Schettino M, D'Alessandro A, Di Giorgio A, Giannattasio F. Endoscopic plastic stenting for bile duct stones: stent changing on demand or every 3 months. A prospective comparison study. Endoscopy. 2013;45:1014-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Hartery K, Lee CS, Doherty GA, Murray FE, Cullen G, Patchett SE, Mulcahy HE. Covered self-expanding metal stents for the management of common bile duct stones. Gastrointest Endosc. 2017;85:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Coté GA. Metallic vs Plastic Stents for Benign Biliary Strictures--Reply. JAMA. 2016;316:540-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Coté GA, Slivka A, Tarnasky P, Mullady DK, Elmunzer BJ, Elta G, Fogel E, Lehman G, McHenry L, Romagnuolo J, Menon S, Siddiqui UD, Watkins J, Lynch S, Denski C, Xu H, Sherman S. Effect of Covered Metallic Stents Compared With Plastic Stents on Benign Biliary Stricture Resolution: A Randomized Clinical Trial. JAMA. 2016;315:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 6. | Arvanitakis M. Optimizing biliary stenting in patients with distal malignant biliary obstruction. Endoscopy. 2023;55:569-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Irisawa A, Katanuma A, Itoi T. Otaru consensus on biliary stenting for unresectable distal malignant biliary obstruction. Dig Endosc. 2013;25 Suppl 2:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Adler DG, Papachristou GI, Taylor LJ, McVay T, Birch M, Francis G, Zabolotsky A, Laique SN, Hayat U, Zhan T, Das R, Slivka A, Rabinovitz M, Munigala S, Siddiqui AA. Clinical outcomes in patients with bile leaks treated via ERCP with regard to the timing of ERCP: a large multicenter study. Gastrointest Endosc. 2017;85:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Canena J, Liberato M, Meireles L, Marques I, Romão C, Coutinho AP, Neves BC, Veiga PM. A non-randomized study in consecutive patients with postcholecystectomy refractory biliary leaks who were managed endoscopically with the use of multiple plastic stents or fully covered self-expandable metal stents (with videos). Gastrointest Endosc. 2015;82:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Testoni PA, Mariani A, Aabakken L, Arvanitakis M, Bories E, Costamagna G, Devière J, Dinis-Ribeiro M, Dumonceau JM, Giovannini M, Gyokeres T, Hafner M, Halttunen J, Hassan C, Lopes L, Papanikolaou IS, Tham TC, Tringali A, van Hooft J, Williams EJ. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:657-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 429] [Article Influence: 42.9] [Reference Citation Analysis (1)] |
| 11. | Ginsberg GG, Attalla S. Management of Complications of Biliary Stenting. In book: Testoni PA, Inoue H, Wallace MB, editors. Gastrointestinal and Pancreatico-Biliary Diseases: Advanced Diagnostic and Therapeutic Endoscopy. New York: Springer, 2022: 1467-1480. |
| 12. | Hoshi K, Irisawa A, Tominaga K, Goda K, Iijima M. Association of long-term endoscopic biliary stent placement with choledocholithiasis: a literature review. Clin J Gastroenterol. 2021;14:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Tang SJ, Armstrong L, Lara LF, Kortan P. De novo stent-stone complex after long-term biliary stent placement: pathogenesis, diagnosis, and endotherapy. Gastrointest Endosc. 2007;66:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Lee H, Won DS, Park S, Park Y, Kim JW, Han G, Na Y, Kang MH, Kim SB, Kang H, Park JK, Jang TS, Lee SJ, Park SA, Lee SS, Park JH, Jung HD. 3D-printed versatile biliary stents with nanoengineered surface for anti-hyperplasia and antibiofilm formation. Bioact Mater. 2024;37:172-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Tian L, Lu Z, Lei L, Yang N, Chen Z, Lu B, Jin Z, Shen Y, Guo S. Preparation, characterization and primary evaluation of trilayered biliary stent films for anti-cholangiocarcinoma and anti-biofilm formation. Int J Pharm. 2021;606:120869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Flumignan VK, Sircili MP, Franzolin MR, Tavassi AMC, Germano LG, Souza AVDS, Silva NF, Fukumasu NK, Anjos RMD, Otoch JP, Artifon ELA. Comparison between biliary plastic stents with and without application of silver nanoparticles: an in-vitro study of the biofilm formation. Acta Cir Bras. 2025;40:e402825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Gallbladder-Preserving Surgery Committee; Endoscopy Specialist Branch of Chinese Medical Doctor Association; Expert Consensus Collaborative Group on Endoscopic Diagnosis and Treatment of the Benign Pancreaticobiliary Junction Disease. [Expert consensus on endoscopic diagnosis and treatment of the benign pancreaticobiliary junction disease (2023 edition)]. Zhonghua Yi Xue Za Zhi. 2023;103:3174-3179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Cotton PB, Elta GH, Carter CR, Pasricha PJ, Corazziari ES. Rome IV. Gallbladder and Sphincter of Oddi Disorders. Gastroenterology. 2016;S0016-5085(16)00224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 19. | Kamisawa T, Kaneko K, Itoi T, Ando H. Pancreaticobiliary maljunction and congenital biliary dilatation. Lancet Gastroenterol Hepatol. 2017;2:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Mayumi T, Okamoto K, Takada T, Strasberg SM, Solomkin JS, Schlossberg D, Pitt HA, Yoshida M, Gomi H, Miura F, Garden OJ, Kiriyama S, Yokoe M, Endo I, Asbun HJ, Iwashita Y, Hibi T, Umezawa A, Suzuki K, Itoi T, Hata J, Han HS, Hwang TL, Dervenis C, Asai K, Mori Y, Huang WS, Belli G, Mukai S, Jagannath P, Cherqui D, Kozaka K, Baron TH, de Santibañes E, Higuchi R, Wada K, Gouma DJ, Deziel DJ, Liau KH, Wakabayashi G, Padbury R, Jonas E, Supe AN, Singh H, Gabata T, Chan ACW, Lau WY, Fan ST, Chen MF, Ker CG, Yoon YS, Choi IS, Kim MH, Yoon DS, Kitano S, Inomata M, Hirata K, Inui K, Sumiyama Y, Yamamoto M. Tokyo Guidelines 2018: management bundles for acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | ASGE Standards of Practice Committee; Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 587] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 22. | He QB, Xu T, Wang J, Li YH, Wang L, Zou XP. Risk factors for post-ERCP pancreatitis and hyperamylasemia: A retrospective single-center study. J Dig Dis. 2015;16:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Elsebaey MA, Enaba ME, Elashry H, Elbedewy TA, El Nakib AM, Elhadidy AA, Sarhan ME, Elrefaey W, Hagag RY, Alqifari AM, Elsokkary AM, Alabd MAA, Abdulrahim AO, Abo-Amer YE, Abo-Elfetoh AR, Mahfouz MS, Saleh M, Mohamed AA, Ismail AAM. Forgotten Biliary Plastic Stents: Complications, Management, and Clinical Outcomes. Medicina (Kaunas). 2024;60:1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Sohn SH, Park JH, Kim KH, Kim TN. Complications and management of forgotten long-term biliary stents. World J Gastroenterol. 2017;23:622-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 25. | Baron TH. Endoscopic management of biliary disorders: diagnostic and therapeutic. Surg Clin North Am. 2014;94:395-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Hong WD, Chen XW, Wu WZ, Zhu QH, Chen XR. Metal versus plastic stents for malignant biliary obstruction: an update meta-analysis. Clin Res Hepatol Gastroenterol. 2013;37:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2082] [Article Influence: 59.5] [Reference Citation Analysis (1)] |
| 28. | Dumonceau JM, Devière J, Delhaye M, Baize M, Cremer M. Plastic and metal stents for postoperative benign bile duct strictures: the best and the worst. Gastrointest Endosc. 1998;47:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 88] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro M, Hritz I, Mariani A, Paspatis G, Radaelli F, Lakhtakia S, Veitch AM, van Hooft JE. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 581] [Article Influence: 96.8] [Reference Citation Analysis (1)] |
| 30. | Guaglianone E, Cardines R, Vuotto C, Di Rosa R, Babini V, Mastrantonio P, Donelli G. Microbial biofilms associated with biliary stent clogging. FEMS Immunol Med Microbiol. 2010;59:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Vaishnavi C, Samanta J, Kochhar R. Characterization of biofilms in biliary stents and potential factors involved in occlusion. World J Gastroenterol. 2018;24:112-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | ASGE Standards of Practice Committee; Chathadi KV, Chandrasekhara V, Acosta RD, Decker GA, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fanelli RD, Fisher DA, Foley K, Fonkalsrud L, Hwang JH, Jue TL, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The role of ERCP in benign diseases of the biliary tract. Gastrointest Endosc. 2015;81:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 33. | Han S, Obando JV, Bhatt A, Bucobo JC, Chen D, Copland AP, Das KK, Girotra M, Kahn A, Krishnan K, Sakaria SS, Saumoy M, Trikudanathan G, Trindade AJ, Yang J, Law RJ, Lichtenstein DR. Biliary and pancreatic stents. iGIE. 2023;2:240-253. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Blanco-Míguez A, Carloni S, Cardenas C, Dioguardi CC, Lambroia L, Capretti G, Nappo G, Fugazza A, Capogreco A, Armanini F, Asnicar F, Dubois L, Golzato D, Manghi P, Pinto F, Scuderi C, Casari E, Montorsi M, Anderloni A, Rescigno M, Repici A, Zerbi A, Peano C, Tamburini S, Rusconi R, Segata N. Microbial composition associated with biliary stents in patients undergoing pancreatic resection for cancer. NPJ Biofilms Microbiomes. 2024;10:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |