Published online Nov 27, 2025. doi: 10.4240/wjgs.v17.i11.110075
Revised: June 18, 2025
Accepted: September 18, 2025
Published online: November 27, 2025
Processing time: 181 Days and 1.4 Hours
Gastritis cystica profunda (GCP) is a rare submucosal gastric lesion characterized by the extension of cystically dilated gastric mucosal glands into or below the mu
A 37-year-old woman presented with upper abdominal pain. Laboratory tests showed elevated inflammatory markers and carbohydrate antigen 19-9 levels. Gastroscopy revealed a submucosal bulge. Based on enhanced computed tomo
This case provides information on new complications of GCP and emphasizes the diagnostic value of enhanced computed tomography.
Core Tip: Gastritis cystica profunda (GCP) is a rare submucosal lesion of the stomach, and its etiology and pathogenesis remain unclear. It is generally considered benign, and no standardized treatment protocol currently exists. We report a case of GCP complicated by acute inflammation and abscess formation, successfully treated with partial gastrectomy. This case highlights a previously unreported complication of GCP and underscores the diagnostic utility of enhanced computed tomography for GCP.
- Citation: Cui Q, He K, Chen MS, Huang LD. Diagnostic challenge of gastritis cystica profunda with secondary abscess formation: A case report. World J Gastrointest Surg 2025; 17(11): 110075
- URL: https://www.wjgnet.com/1948-9366/full/v17/i11/110075.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i11.110075
Gastritis cystica profunda (GCP) is a rare condition first identified and named by Franzin and Novelli[1] in 1981, because of its histological resemblance to colitis cystica profunda. GCP is characterized by pathological cystic dilation of gastric glands located at or below the muscularis mucosa. These glands are typically regular and intact and may be associated with connective tissue proliferation and inflammatory cell infiltration[2]. GCP primarily affects middle-aged and elderly men, most often occurring in the upper third of the stomach. The etiology and pathogenesis remain unclear. GCP accounts for < 1% of gastric submucosal lesions, typically detected incidentally during endoscopic examinations or postoperative pathological analysis, and its incidence may therefore be underestimated. Clinical manifestations can be asymptomatic or present with nonspecific gastrointestinal symptoms, such as abdominal pain, abdominal discomfort, gastrointestinal bleeding, nausea, vomiting, anorexia, and weight loss[3-6]. In the present case, the patient presented with abdominal pain. Notably, postoperative pathology confirmed concurrent acute inflammation and abscess formation. Abscess formation has not been previously reported in GCP, thereby expanding the recognized spectrum of potential complications associated with this disease.
Due to the rare occurrence and nonspecific manifestations of GCP, diagnosis can be challenging. Endoscopic ultrasonography (EUS) and computed tomography (CT) may aid in the differential diagnosis, but these methods are usually insufficient for definitive diagnosis. At present, histopathological examination is considered the gold standard for diagnosis remains pathological examination. Here, we present a rare case of GCP accompanied by acute inflammation and abscess formation, which was preoperatively misdiagnosed as an ectopic pancreas with cysts on CT. We retro
A 37-year-old woman presented with upper abdominal pain, described as intermittent and distending.
The pain had started after the patient self-administered one tablet of loxoprofen for back pain 2 days prior to admission.
In 2018, the patient had been informed during an examination at another hospital of a gastric mass that was considered a stromal tumor by endoscopic ultrasound. The patient was lost to follow-up until symptoms recurred in 2025.
In 2016, the patient underwent a unilateral salpingectomy due to an ectopic pregnancy. She reported no family history of malignant tumors.
Tenderness was noted in the upper abdomen, without rebound tenderness. The abdomen was soft and flat, and no palpable mass was detected.
Laboratory tests upon presentation revealed the following abnormalities: White blood cell count of 15.5 × 109/L [reference range: (3.5-9.5) × 109/L], neutrophil count of 13.6 × 109/L [reference range: (1.8-6.3) × 109/L], and C-reactive protein (wide range) level of 15.6 mg/L (reference range: 0.2-4 mg/L). Additionally, the tumor marker carbohydrate antigen [car
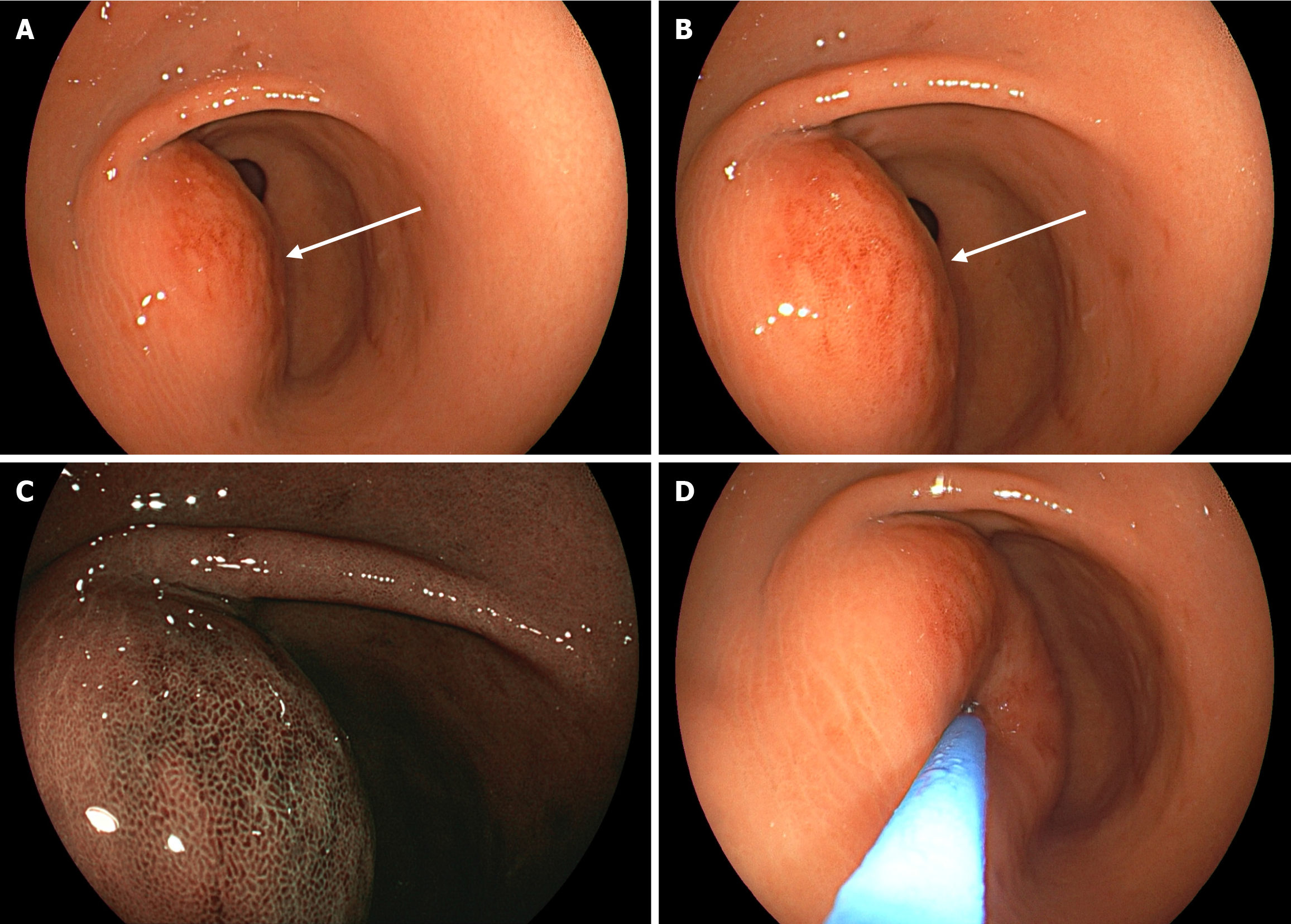
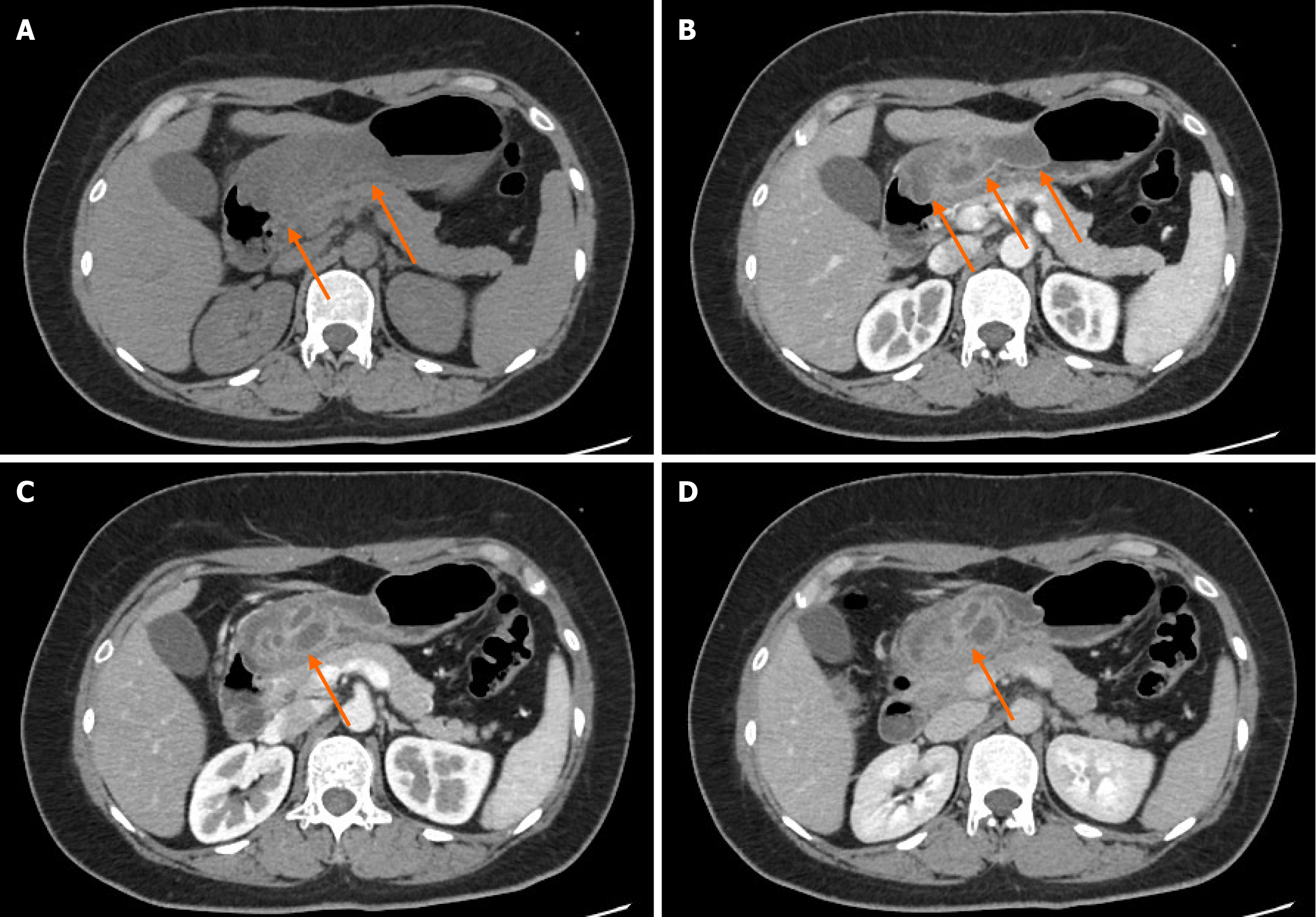
White-light gastroscopy revealed congestion and edema of the gastric antral mucosa, with a submucosal bulge approximately 4.0 cm in diameter on the anterior wall (Figure 1A and B). Optical electronic chromoendoscopy demonstrated the micro surface and microvascular structures of the lesion (Figure 1C). The lesion exhibited a soft texture when pressure was applied using a pair of biopsy forceps (Figure 1D). A plain CT scan revealed considerable thickening of the anterior wall of the gastric body and antrum, with the thickest area measuring approximately 3.2 cm (Figure 2A). The lesion had a density lower than that of muscle, with an average CT value of approximately 35 Hounsfield unit. The corresponding gastric cavity was narrowed; however, the gastric wall was soft rather than rigid. Mild exudation was noted around the gastric antrum, along with several small lymph nodes. An enhanced CT scan revealed a smooth, continuous mucosal line with marked enhancement (Figure 2B), patchy submucosal edema, and a multicystic submucosal mass measuring approximately 3.9 cm × 2.5 cm × 2.6 cm on the anterior wall of the gastric antrum (Figure 2C and D). The cyst walls showed marked enhancement. Based on these findings, the CT diagnosis suggested an ectopic pancreas with cysts.
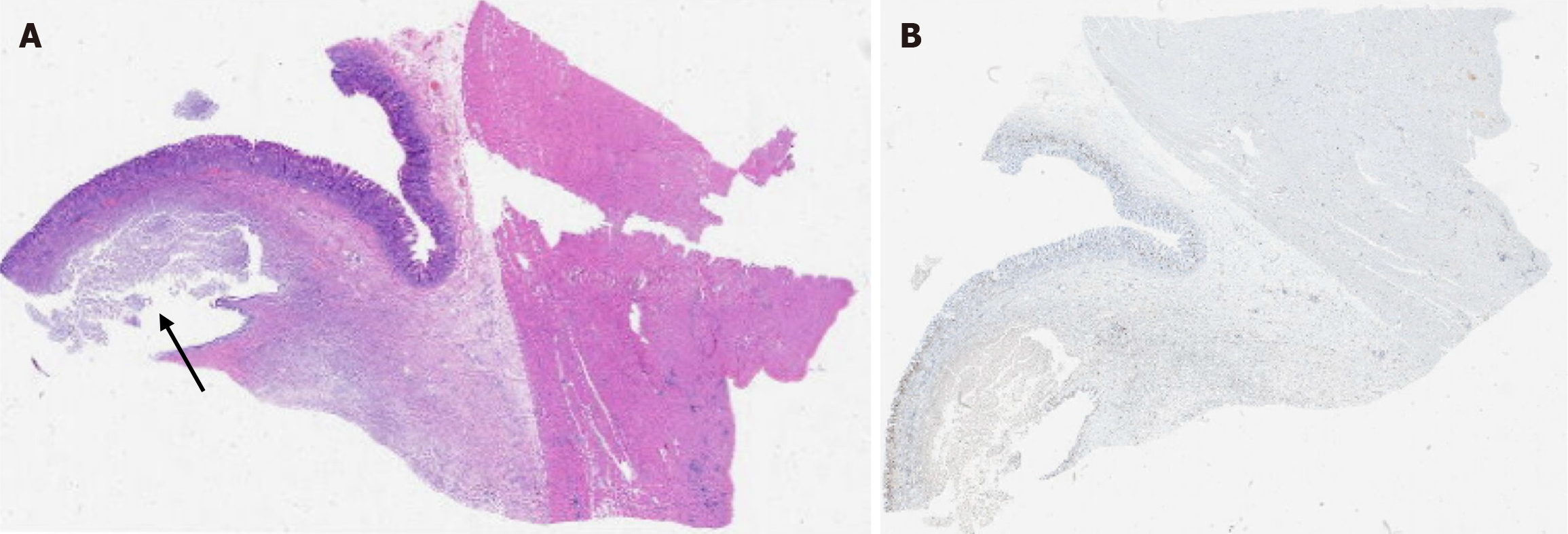
Microscopically, acute inflammation with abscess formation was observed in the gastric submucosa, with the abscess cavity focally lined by gastric mucosal tissue (Figure 3). This finding was attributed to entrapped and dilated glands with abscess formation. The final pathological diagnosis was GCP.
Clinically, the elevated CA19-9 level raised concerns about the possibility of a tumor. After discussing the risks, the patient consented to a laparoscopic partial gastrectomy. During surgery, intraoperative exploration revealed a small amount of ascitic fluid and adhesions in the upper abdomen. No external invasion was observed on the gastric serosal surface. A distal gastrectomy was performed successfully, and the patient experienced an uneventful postoperative recovery.
The patient was discharged from the hospital 12 days after the operation. A telephone follow-up was conducted 6 months later, and it was found that the patient had a good recovery.
GCP is a rare inflammatory gastric lesion most commonly observed in middle-aged and older men. It predominantly occurs in the gastric body, followed by the cardia, fundus, and antrum[7,8]. Its etiology is generally considered a se
GCP is often asymptomatic but can present with non-specific gastrointestinal symptoms, including abdominal pain, bloating, acid reflux, and vomiting. Severe cases may lead to complications, such as hematemesis, melena, and pyloric obstruction[12,13]. Laboratory findings are typically unremarkable; however, our patient presented with epigastric pain accompanied by acute inflammation, which resulted in elevated white blood cell count, neutrophil count, and C-reactive protein level. Medication may have triggered concurrent acute inflammation. Generally, GCP is not accompanied by elevated tumor markers. However, when it coexists with neoplastic lesions, an increase can occur. In this patient, the lesion was an isolated GCP. Elevated CA19-9 may reflect cyst fluid secretion or systemic inflammation[14].
Gastroscopy typically reveals GCP as a submucosal bulge. However, mucosal-layer biopsies are insufficient for a definitive diagnosis. EUS can reveal submucosal lesions of the digestive tract, observe the layered structure of the gastric wall, and determine the origin of the lesions and their relationship with surrounding tissues, thus playing a crucial role in the diagnosis of GCP. The echotexture of GCP can be homogeneous or heterogeneous, and the echo pattern is classified as hypoechoic, hyperechoic, or mixed echo, which can be summarized into three forms: Heterogeneous echo with mucosal thickening, anechoic cystic cavity in the submucosa, and hypoechoic with small cysts[15]. The typical manifestation is an irregular hypoechoic area originating from the gastric mucosa and gradually extending into the submucosa and even the muscularis propria. However, these findings lack specificity, and EUS localization can be imprecise. Additionally, EUS is an invasive examination that requires anesthesia, which may reduce the willingness of some patients to undergo the examination.
EUS better evaluates layer-by-layer involvement, CT’s advantage lies in assessing adjacent invasion. GCP typically appears on CT as a submucosal cystic or cystic-solid mass with gastric wall thickening and small cysts[16,17]. Key features include cystic components corresponding to glandular dilation, progressive enhancement, and peripheral ring-like enhancement[18]. In some cases, enhanced CT shows a “sandwich-like enhancement” (the surface cyst wall of the lesion is enhanced, the central cystic area is not enhanced, and the underlying muscular layer is enhanced) or a “ho
The incidence of gastric ectopic pancreas in gastrectomy cases is approximately 0.9%, most commonly occurring in the gastric antrum[19]. Typical CT findings include a submucosal flat solid mass with a long diameter/short diameter ratio greater than 1.4, an endoluminal growth pattern, ill-defined borders, and significantly enhanced overlying mucosa[20]. The lobulation sign, duct sign, and central umbilication sign are considered characteristic, but are only observed in a minority of cases. When the ectopic pancreas undergoes cystic changes, it should be differentiated from GCP, as present in Table 1. Ectopic pancreas with cysts is a rare condition attributed to pseudocyst formation, abnormal ductal dilation, or intraductal papillary mucinous neoplasms, mucinous cystadenoma[21-23]. The cyst wall of ectopic pancreas lesions typically exhibits enhancement patterns consistent with that of pancreatic parenchyma. Careful evaluation of these patterns may aid differentiation. Magnetic resonance imaging has unique advantages in the diagnosis of ectopic pancreas. The acinus is rich in protein and glycogen, and it shows a characteristically high signal in T1 weighted images[24]. T2 weighted images and magnetic resonance cholangiopancreatography are helpful in identifying residual ducts.
| Gastritis cystica profunda | Ectopic pancreas | |
| Age | Elderly | Middle-aged |
| Location | Gastric body | Antrum |
| Morphology | Sphere, hemisphere, diffuse | Flat ovoid, long diameter/short diameter > 1.4 |
| Endoscope | Soft texture, submucosal bulge, polypoid lesion, hypertrophic fold | Toughness, submucosal bulge, umbilicated depression on the surface |
| Endoscopic ultrasonography | Anechoic, mixed echoic with thickened mucosa, hypoechoic with microcysts | Heterogeneous hypoechoic, mixed cystic and solid, anechoic ductal structure |
| Computed tomography | Cystic change in submucosa, progression enhancement, peripheral rim-like enhancement | Endoluminal growth, lobulation sign, ill-defined border, prominent enhancement of overlying mucosa |
GCP is considered a benign lesion; however, some cases may exhibit dysplasia or malignant transformation. In
At present, there are no clear guidelines and conventions for the treatment of GCP. Because of the uncertainty of preoperative diagnosis, treatment options for GCP include endoscopic and surgical approaches. When the lesion mea
This case highlights abscess formation as a novel GCP complication, necessitating pathological confirmation despite advanced imaging. Although the malignant potential of GCP remains uncertain, active treatment is recommended, with ESD considered a safe and effective therapeutic option.
| 1. | Franzin G, Novelli P. Gastritis cystica profunda. Histopathology. 1981;5:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Tsuji T, Iwahashi M, Nakamori M, Ueda K, Ishida K, Naka T, Ojima T, Akamatsu H, Yamaue H. Multiple early gastric cancer with gastritis cystica profunda showing various histological types. Hepatogastroenterology. 2008;55:1150-1152. [PubMed] |
| 3. | Li Y, Liu R, Wang Z, Mou Y. Gastritis cystica profunda presenting as an ulcerated lesion. Dig Liver Dis. 2024;56:1249-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Zimmer V, Heinrich C. Pedunculated gastritis cystica profunda polyp presenting with upper GI bleeding and volcano-like surface alteration. Dig Liver Dis. 2022;54:1267-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Shimizu S, Hara H, Muto Y, Kido T, Miyata R. Gastritis cystica profunda in an unoperated stomach mimicking a pyloric submucosal tumor and causing anorexia: A case report and literature review. Medicine (Baltimore). 2024;103:e37652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Carvalho JR, Quadros AC, Meireles L, Alves I, Moura Dos Santos P, Serejo F, Ferreira C, Freire JP, Velosa J. Gastritis cystica profunda mimicking a GIST - A diagnostic challenge. Gastroenterol Hepatol. 2018;41:448-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 7. | Deng S, Cao Y, Shen L, Wang J, Tao K, Wang G, Li J, Cai K. Bile reflux gastritis cystica profunda: A case report and literature review. Medicine (Baltimore). 2019;98:e15295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ichinoe M, Mikami T, Hara A, Tsuruta T, Okayasu I. Background submucosal cysts in early gastric cancer cases have unique clinicopathologic features suggestive of postgastritis and significant smoking association. Am J Clin Pathol. 2007;128:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Huang D, Zhan Q, Yang S, Sun Q, Zhou Z. Synchronous double superficial mixed gastrointestinal mucus phenotype gastric cancer with gastritis cystica profunda and submucosal lipoma: A case report. Medicine (Baltimore). 2018;97:e10825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Itami H, Morita K, Nakai T, Uchiyama T, Sugimoto S, Sasaki S, Matsuoka M, Myojin T, Nitta Y, Okabe F, Fujii T, Hatakeyama K, Mitoro A, Sho M, Ohbayashi C. Gastritis cystica profunda is associated with aberrant p53 and Epstein-Barr virus in gastric cancer: A clinicopathological, immunohistochemical and in situ hybridization study. Pathol Int. 2021;71:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 11. | Cho H, Hashimoto T, Naka T, Yatabe Y, Oda I, Saito Y, Yoshikawa T, Sekine S. Activating KRAS and GNAS mutations in heterotopic submucosal glands of the stomach. J Gastroenterol. 2022;57:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Yoshikawa M, Kinoshita H, Nishimura N, Takai R, Matsuda T, Nakatani S, Shioyama E, Takeda K, Yoshiji H. A surgically treated case of severe upper gastrointestinal hemorrhage with gastritis cystica polyposa. BMC Gastroenterol. 2021;21:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Butt MO, Luck NH, Hassan SM, Abbas Z, Mubarak M. Gastritis profunda cystica presenting as gastric outlet obstruction and mimicking cancer: A case report. J Transl Int Med. 2015;3:35-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 14. | Park CH, Park JM, Jung CK, Kim DB, Kang SH, Lee SW, Cho YK, Kim SW, Choi MG, Chung IS. Early gastric cancer associated with gastritis cystica polyposa in the unoperated stomach treated by endoscopic submucosal dissection. Gastrointest Endosc. 2009;69:e47-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kurland J, DuBois S, Behling C, Savides T. Severe upper-GI bleed caused by gastritis cystica profunda. Gastrointest Endosc. 2006;63:716-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Lin SH, Liu W, Yan XL. Gastritis cystica profunda. J Gastrointest Surg. 2024;28:592-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Zeng L, Zheng L, Hu B, Ye L. Gastritis cystica profunda mimicking submucosal tumor. Endoscopy. 2023;55:E1180-E1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Wang R, Lu H, Yu J, Huang W, Li J, Cheng M, Liang P, Li L, Zhao H, Gao J. Computed tomography features and clinical characteristics of gastritis cystica profunda. Insights Imaging. 2022;13:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Nambu N, Yamasaki T, Nakagomi N, Kumamoto T, Nakamura T, Tamura A, Tomita T, Miwa H, Shinohara H, Hirota S. A case of ectopic pancreas of the stomach accompanied by intraductal papillary mucinous neoplasm with GNAS mutation. World J Surg Oncol. 2021;19:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Kim JY, Lee JM, Kim KW, Park HS, Choi JY, Kim SH, Kim MA, Lee JY, Han JK, Choi BI. Ectopic pancreas: CT findings with emphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology. 2009;252:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Rocha HL, Bueno FK, Faraco J, Assef MS, Araki OM, Nakao F, Rossini LG. Heterotopic pancreas complicated by pseudocyst in the gastric wall diagnosed by endoscopic ultrasound-guided fine needle aspiration. Endosc Ultrasound. 2013;2:159-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Pang Y, Liu Y, Liu Q, Hou G. Intraductal Papillary Mucinous Neoplasm Arising from Heterotopic Pancreas in Stomach: A Case Report and Review of Literature. Int J Surg Pathol. 2023;31:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Parra V, Acero F, Alvarez E, Aponte DM, Sabbagh LC. A case of mucinous cystic neoplasm from a gastric ectopic pancreas. Gastrointest Endosc. 2017;85:1096-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Braga M, Matos AP, Marques PP, Ramalho M. Gastric ectopic pancreas in magnetic resonance imaging: A review of 2 cases. Radiol Case Rep. 2023;18:1181-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Kim DH, Kim KM, Oh SJ, Oh JA, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S. Early gastric cancer arising from heterotopic gastric mucosa in the gastric submucosa. J Korean Surg Soc. 2011;80 Suppl 1:S6-S11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Kim JY, Ahn S, Kim KM, Chang SH, Kim HS, Lee JH, Kim JJ, Sohn TS, Kang HJ, Joo M. Gastric Inverted Polyps-Distinctive Subepithelial Lesions of the Stomach: Clinicopathologic Analysis of 12 Cases With an Emphasis on Neoplastic Potential. Am J Surg Pathol. 2021;45:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Goto O, Higuchi K, Koizumi E, Iwakiri K. Advancements in Endoscopic Treatment for Gastric Subepithelial Tumors. Gut Liver. 2025;19:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Xu G, Peng C, Li X, Zhang W, Lv Y, Ling T, Zhou Z, Zhuge Y, Wang L, Zou X, Zhang X, Huang Q. Endoscopic resection of gastritis cystica profunda: preliminary experience with 34 patients from a single center in China. Gastrointest Endosc. 2015;81:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |