Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.109359
Revised: May 27, 2025
Accepted: September 4, 2025
Published online: October 27, 2025
Processing time: 169 Days and 5.5 Hours
Cholecystectomy, one of the most common surgical procedures worldwide, is generally considered safe and effective. However, emerging evidence suggests a potential link between cholecystectomy and the development or progression of non-alcoholic fatty liver disease. This article examines the current understanding of this association, focusing on mechanisms such as altered bile acid metabolism, gut microbiota dysbiosis, and changes in lipid homeostasis. It addresses resolved challenges, including short-term metabolic effects, and highlights key unresolved questions, such as the long-term impact on liver health and specific at-risk po
Core Tip: Cholecystectomy, a common surgical treatment for gallbladder diseases, may inadvertently increase the risk of non-alcoholic fatty liver disease due to disrupted bile acid metabolism, gut microbiota dysbiosis, and altered lipid regulation. These metabolic disturbances are especially pronounced in patients with obesity or insulin resistance. This review highlights emerging mechanistic insights and underscores the importance of proactive liver health monitoring and lifestyle intervention following cholecystectomy.
- Citation: Liu XY, Ma J, Jiao Y. Cholecystectomy and non-alcoholic fatty liver disease: Exploring the hidden connection and implications. World J Gastrointest Surg 2025; 17(10): 109359
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/109359.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.109359
Cholecystectomy, the surgical removal of the gallbladder, is one of the most frequently performed procedures globally. It is predominantly indicated for symptomatic gallstone disease, acute or chronic cholecystitis, and gallbladder polyps, conditions often associated with metabolic disorders such as obesity and diabetes[1]. The advent of laparoscopic techniques has rendered cholecystectomy a safe and minimally invasive intervention, significantly reducing recovery times and surgical complications.
Despite its established benefits, growing evidence suggests that cholecystectomy may have unintended metabolic consequences, particularly concerning liver health[2]. Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver condition worldwide, affecting approximately 25% of the global population. Characterized by excessive hepatic fat accumulation in individuals with minimal or no alcohol intake, NAFLD is closely associated with metabolic syndrome, including obesity, insulin resistance, and dyslipidemia[3].
It is important to note, however, that a newer term, metabolic dysfunction-associated fatty liver disease (MAFLD), has been introduced to better reflect the association between liver fat accumulation and metabolic dysfunction, such as obesity and insulin resistance. While NAFLD refers to liver fat accumulation without the requirement for metabolic dysfunction, MAFLD specifically incorporates metabolic dysfunction as a prerequisite for its diagnosis[4].
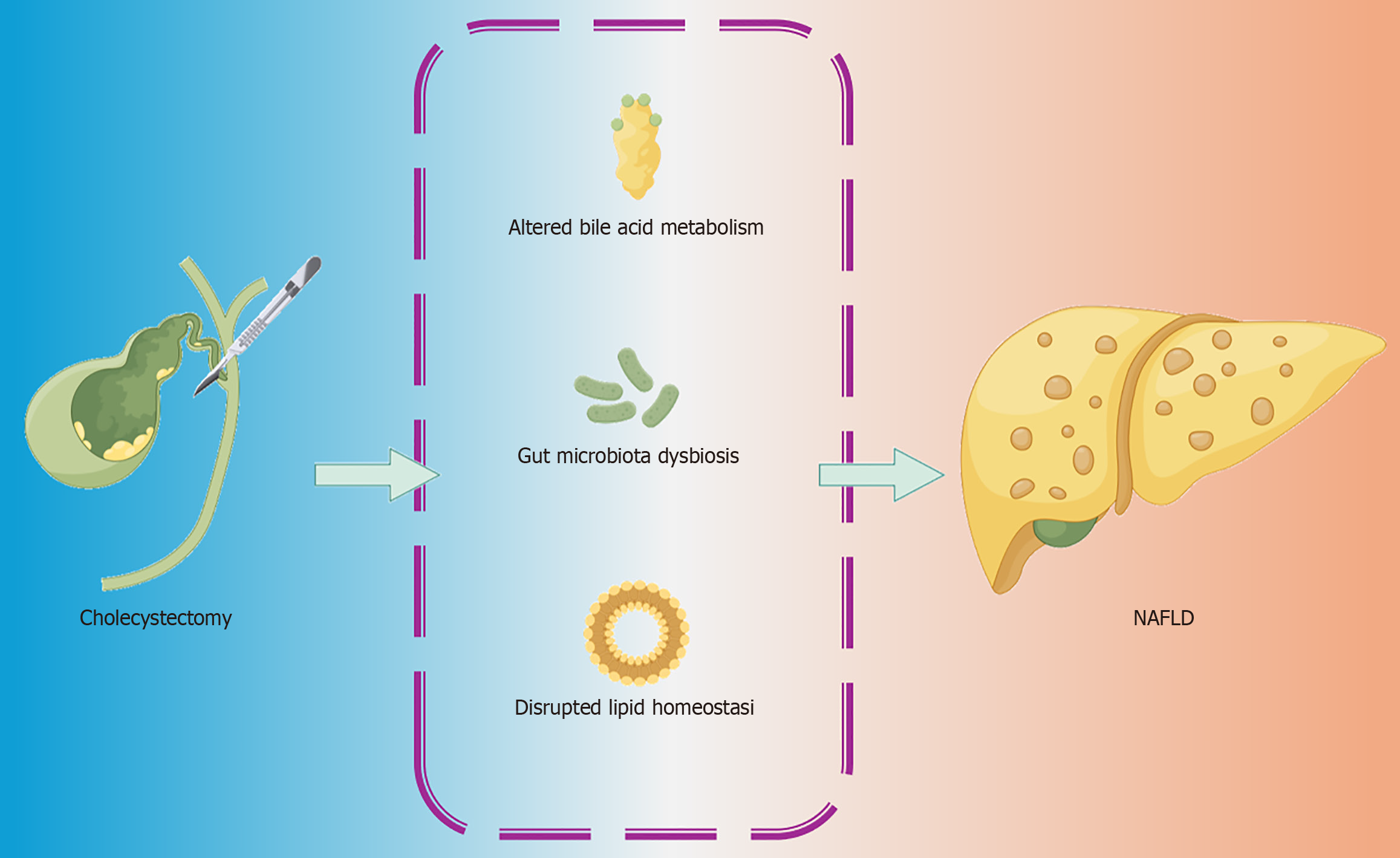
Alarmingly, studies have reported an increased incidence of NAFLD following cholecystectomy, raising concerns about a potential link between gallbladder removal and liver fat accumulation[2,3]. Given the multifactorial nature of NAFLD pathogenesis, disruptions in bile acid metabolism, gut microbiota composition, and lipid homeostasis following cholecystectomy have emerged as key mechanisms warranting further investigation. This review explores the evidence supporting this connection, elucidates the underlying mechanisms, highlights unresolved questions, and provides recommendations for clinical management and future research (Figure 1).
The gallbladder plays a central role in bile acid storage, concentration, and controlled release - critical processes for lipid digestion and metabolic regulation. Bile acids, synthesized in the liver from cholesterol, act not only as emulsifiers for dietary fats but also as key signaling molecules that regulate metabolic pathways.
Under normal physiological conditions, bile acids are stored in the gallbladder and released in a regulated manner in response to dietary fat intake. This controlled release ensures optimal emulsification of dietary lipids, facilitating their digestion and absorption. Moreover, bile acids function as signaling molecules through nuclear and membrane receptors such as the farnesoid X receptor and Takeda G-protein receptor 5. These receptors regulate lipid and glucose metabolism, fatty acid oxidation, and inflammation.
The removal of the gallbladder through cholecystectomy disrupts these functions, leading to continuous bile flow directly from the liver into the intestine. This alteration impairs the enterohepatic circulation of bile acids, resulting in changes in their composition, concentration, and timing of release[1]. Such dysregulation impairs lipid digestion and promotes metabolic changes that favor hepatic fat accumulation, a hallmark of NAFLD.
An additional consequence of altered bile acid dynamics is its effect on the gut microbiota. The continuous, unregulated flow of bile acids into the intestine post-cholecystectomy can disturb the balance of intestinal bacteria, often resulting in dysbiosis. Studies have shown a reduction in beneficial bacteria that produce short-chain fatty acids and an overgrowth of pro-inflammatory bacterial strains[1]. These changes contribute to increased gut permeability, facilitating the translocation of bacterial endotoxins such as lipopolysaccharides into the liver, triggering inflammation and exacerbating NAFLD progression[2].
The association between cholecystectomy and the development of NAFLD has garnered increasing attention. Clinical studies and meta-analyses consistently demonstrate an elevated risk of NAFLD post-cholecystectomy, particularly in individuals with underlying metabolic dysfunction such as obesity, insulin resistance, or metabolic syndrome. However, it is important to note that most of these studies are cross-sectional or retrospective in nature, which makes it difficult to establish a definitive causal relationship between cholecystectomy and NAFLD.
A meta-analysis by Lyu et al[3] reported that cholecystectomy increases the risk of NAFLD, with an odds ratio of 2.14. This association is even stronger in obese individuals, where the odds ratio rises to 2.51, highlighting the amplifying effect of pre-existing metabolic dysfunction on post-surgical outcomes. However, these findings should be interpreted cautiously due to the limitations of the study design, including potential confounding factors such as diet, physical activity, and genetic predispositions. Additionally, cholecystectomy has been identified as an independent risk factor for MAFLD[4].
Cholecystectomy has been associated with an increased prevalence of insulin resistance and metabolic syndrome, both closely linked to NAFLD[5,6]. Insulin resistance contributes to excessive free fatty acid flux into the liver and impaired lipolysis, further driving hepatic steatosis. This association underscores the complex relationship between gallbladder removal and metabolic disturbances, which may exacerbate liver fat accumulation in susceptible individuals.
Retrospective studies indicate significant long-term impacts on liver health. For instance, Xie et al[7] reported a 60% higher risk of liver fibrosis and a 73.3% higher risk of liver cirrhosis in patients who had undergone cholecystectomy. These findings, though compelling, warrant further investigation through prospective studies to establish a more robust temporal relationship. These findings underscore the need for enhanced monitoring of liver health in post-cholecy
The relationship between cholecystectomy and NAFLD is primarily driven by alterations in bile acid metabolism, gut microbiota dysbiosis, and disrupted lipid homeostasis. However, it is important to consider that genetic predispositions and lifestyle factors, such as diet and physical activity, may also play a significant role in modulating the post-cholecy
Post-cholecystectomy, the continuous secretion of bile acids into the intestine disrupts the enterohepatic circulation, altering bile acid composition and signaling functions[8,9]. Disrupted bile acid signaling impairs metabolic processes, promoting hepatic lipid accumulation and increasing susceptibility to NAFLD. Dietary factors, such as a high-fat, high-cholesterol diet, can exacerbate bile acid dysregulation, further increasing NAFLD risk[10]. In addition, lifestyle factors such as lack of physical activity and unhealthy eating habits may worsen bile acid dysregulation, highlighting the need for lifestyle modifications in post-cholecystectomy patients.
Cholecystectomy-induced bile acid dysregulation leads to significant changes in the gut microbiota. The reduction in microbial diversity and the overgrowth of pro-inflammatory bacteria contribute to increased intestinal permeability and the translocation of bacterial endotoxins like lipopolysaccharides into the liver. This triggers inflammation and hepatic fat deposition, key features of NAFLD[1,10]. Genetic predispositions, such as variations in immune function and gut mi
Cholecystectomy affects hepatic lipid metabolism by altering the expression of genes involved in lipid synthesis and storage, such as sterol regulatory element-binding protein-1C and peroxisome proliferator-activated receptor γ[11]. This promotes hepatic lipid accumulation, a defining feature of NAFLD. Additionally, increased insulin resistance post-cholecystectomy enhances lipolysis in adipose tissue, leading to an influx of free fatty acids into the liver and exacerbating steatosis and inflammation[11]. Furthermore, patients with a genetic predisposition to insulin resistance may be at an elevated risk of these metabolic disruptions post-surgery.
Bile acid signaling through receptors like farnesoid X receptor and Takeda G-protein receptor 5 is crucial for metabolic regulation. Dysregulation of these pathways post-cholecystectomy impairs fatty acid oxidation and hepatic triglyceride clearance, contributing to hepatic steatosis[12,13]. Furthermore, fibroblast growth factor receptor 4 dysregulation has been linked to liver damage and fibrosis in cholecystectomized patients, although the precise mechanisms remain under investigation[14]. Incorporating genetic factors and personalized approaches to treatment may be necessary to better address the molecular pathways that are disrupted in high-risk individuals post-cholecystectomy.
While cholecystectomy remains a safe and effective treatment for gallbladder diseases, its long-term impact on liver health presents several clinical challenges and unresolved questions.
Studies utilizing data from the National Health and Nutrition Examination Survey indicate that cholecystectomy significantly increases the odds of liver fibrosis and cirrhosis by 139.3% and 228.7%, respectively, even after adjusting for confounders[7]. This highlights the need for enhanced monitoring of liver fibrosis in post-cholecystectomy patients, particularly those with metabolic comorbidities.
The presence of metabolic syndrome and obesity elevates the risk of liver fibrosis and NAFLD progression post-cholecystectomy[15]. However, distinguishing the direct impact of cholecystectomy from the underlying metabolic conditions remains challenging. Studies like Madeira-Cardoso et al[16] suggest that cholecystectomy may act as a metabolic “trigger”, unmasking or accelerating pre-existing liver dysfunction rather than directly initiating NAFLD[16].
Certain populations, such as elderly individuals and those with significant metabolic dysfunction, appear more vul
Establishing a causal relationship between cholecystectomy and liver disease is complicated by confounding factors such as diet, physical inactivity, and pre-existing conditions. Longitudinal studies are needed to clarify the temporal and causal relationships and to isolate the specific contributions of cholecystectomy to NAFLD progression.
The potential link between cholecystectomy and NAFLD underscores the importance of proactive strategies to monitor liver health and implement sustainable lifestyle changes in post-cholecystectomy patients.
Lifestyle interventions targeting weight loss, dietary modification, and increased physical activity are the first-line treatment for NAFLD management. Evidence indicates that a weight reduction of 7%-10% can significantly improve liver steatosis, reduce inflammation, and reverse early-stage fibrosis[19,20]. Combined diet and exercise interventions are more effective than either strategy alone, synergistically reducing liver enzyme levels and improving insulin sensitivity[20].
Emerging evidence highlights the role of mobile technology in facilitating sustainable lifestyle changes. Digital in
Regular monitoring of liver health is essential for patients at risk of NAFLD progression post-cholecystectomy. Non-invasive tools like vibration-controlled transient elastography provide practical means to assess liver stiffness and fat content, enabling the detection of disease progression without invasive biopsies[21,23].
Adherence to lifestyle interventions remains a significant challenge due to factors such as lack of motivation, limited awareness of benefits, and difficulty sustaining behavioral changes[24,25]. Patient education and structured support systems are crucial to reinforce the importance of lifestyle modifications in preventing liver disease progression.
The absence of approved pharmacological treatments for NAFLD necessitates a focus on lifestyle modifications. Ongoing research into pharmacological therapies is essential, alongside developing supportive care models that motivate patients to adhere to lifestyle changes[26,27].
Incorporating a multidisciplinary approach into clinical practice can enhance patient outcomes. Clinicians should prioritize regular liver function assessments, utilize non-invasive monitoring tools, and develop individualized care plans that include dietary guidance, exercise prescriptions, and behavioral support. Leveraging digital tools can facilitate ongoing engagement and accountability, fostering sustainable lifestyle changes.
Future studies should prioritize long-term, large-scale cohort analyses to clarify the temporal and causal relationships between cholecystectomy and NAFLD. Longitudinal studies are crucial for understanding the long-term consequences of cholecystectomy on liver health and for better establishing the temporal sequence of events leading to NAFLD deve
Cholecystectomy remains an effective and safe treatment for gallbladder disease; however, its potential role in NAFLD progression cannot be overlooked. The disruption of bile acid homeostasis, gut microbiota balance, and lipid metabolism post-surgery may collectively contribute to hepatic fat accumulation and inflammation. Clinicians must adopt a proactive approach to monitoring liver health in post-cholecystectomy patients, particularly those with metabolic risk factors. By implementing lifestyle interventions and targeted management strategies, the adverse metabolic consequences of gallbladder removal can be mitigated, thereby improving long-term liver health outcomes.
| 1. | Amaral Raposo M, Sousa Oliveira E, Dos Santos A, Guadagnini D, El Mourabit H, Housset C, Lemoinne S, Abdalla Saad MJ. Impact of cholecystectomy on the gut-liver axis and metabolic disorders. Clin Res Hepatol Gastroenterol. 2024;48:102370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 2. | Rodríguez-Antonio I, López-Sánchez GN, Garrido-Camacho VY, Uribe M, Chávez-Tapia NC, Nuño-Lámbarri N. Cholecystectomy as a risk factor for non-alcoholic fatty liver disease development. HPB (Oxford). 2020;22:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Lyu J, Lin Q, Fang Z, Xu Z, Liu Z. Complex impacts of gallstone disease on metabolic syndrome and nonalcoholic fatty liver disease. Front Endocrinol (Lausanne). 2022;13:1032557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Qi L, Dai W, Kong J, Tian Y, Chen Y. Cholecystectomy as a risk factor for metabolic dysfunction-associated fatty liver disease: unveiling the metabolic and chronobiologic clues behind the bile acid enterohepatic circulation. J Physiol Biochem. 2021;77:497-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Slouha E, Biput SJ, Kuteyi A, Kalloo AE, Gorantla VR. Non-alcoholic Fatty Liver Disease and Gallstones: A Systematic Review. Cureus. 2023;15:e45027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Qi L, Tian Y, Chen Y. Gall bladder: The metabolic orchestrator. Diabetes Metab Res Rev. 2019;35:e3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Xie ZQ, Li HX, Tan WL, Yang L, Ma XW, Li WX, Wang QB, Shang CZ, Chen YJ. Association of Cholecystectomy With Liver Fibrosis and Cirrhosis Among Adults in the USA: A Population-Based Propensity Score-Matched Study. Front Med (Lausanne). 2021;8:787777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 8. | Lange AH, Pedersen MG, Ellegaard AM, Nerild HH, Brønden A, Sonne DP, Knop FK. The bile-gut axis and metabolic consequences of cholecystectomy. Eur J Endocrinol. 2024;190:R1-R9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Greco M, De Micheli E, Lonardo A. [Multifactorial hepatopathy in a patient with biliopancreatic diversion]. Ann Ital Med Int. 2003;18:99-103. [PubMed] |
| 10. | Xu F, Yu Z, Liu Y, Du T, Yu L, Tian F, Chen W, Zhai Q. A High-Fat, High-Cholesterol Diet Promotes Intestinal Inflammation by Exacerbating Gut Microbiome Dysbiosis and Bile Acid Disorders in Cholecystectomy. Nutrients. 2023;15:3829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 11. | Park S, Zhang T, Yue Y, Wu X. Effects of Bile Acid Modulation by Dietary Fat, Cholecystectomy, and Bile Acid Sequestrant on Energy, Glucose, and Lipid Metabolism and Gut Microbiota in Mice. Int J Mol Sci. 2022;23:5935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Bing H, Li YL. The role of bile acid metabolism in the occurrence and development of NAFLD. Front Mol Biosci. 2022;9:1089359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 13. | Zhang X, Deng R. Dysregulation of Bile Acids in Patients with NAFLD. In: Gad EH, editor. Nonalcoholic Fatty Liver Disease - An Update. London: IntechOpen, 2019. |
| 14. | Pal SC, Castillo-Castañeda SM, Díaz-Orozco LE, Ramírez-Mejía MM, Dorantes-Heredia R, Alonso-Morales R, Eslam M, Lammert F, Méndez-Sánchez N. Molecular Mechanisms Involved in MAFLD in Cholecystectomized Patients: A Cohort Study. Genes (Basel). 2023;14:1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Latenstein CSS, Alferink LJM, Darwish Murad S, Drenth JPH, van Laarhoven CJHM, de Reuver PR. The Association Between Cholecystectomy, Metabolic Syndrome, and Nonalcoholic Fatty Liver Disease: A Population-Based Study. Clin Transl Gastroenterol. 2020;11:e00170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Madeira-Cardoso MJ, Alexandrino H, Oliveira P, Rodrigues F, Oliveira AS, Vieira V, Oliveiros B, Tralhão JG, Carvalho H, Furtado E. Is Cholecystectomy Really Harmful? A Long-Term Quality of Life Study in Living Donor Liver Transplantation. Transplant Proc. 2020;52:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Ambartsumyan AR, Kozlov KL, Pyatibrat ED, Pyatibrat AO. [Prognosis of a high risk of non-alcoholic fatty liver disease in the long-term period of laparoscopic cholecystectomy in the elderly and senile]. Adv Gerontol. 2024;37:102-110. [PubMed] |
| 18. | Ambartsumyan AR, Chumak BA, Deryagina LE, Batskov SS, Pyatibrat ED. Criteria of Formation of Fatty Liver Disease in Individuals of Different Age Groups in the Long-Term Period after Minimally Invasive Cholecystectomy. I P Pavlov Pavlov Russ Med Biol. 2023;31:231-242. [DOI] [Full Text] |
| 19. | Fernández-Mincone T, Viñuela Morales M, Jiménez MA, Arab Verdugo JP, Cabrera D, Martínez FB. Lifestyle Changes In Patients With Non-Alcoholic Fatty Liver Disease: A Systematic Review Protocol. 2020 Preprint. Available from: bioRxiv: 2020.06.22.20137430. [DOI] [Full Text] |
| 20. | Fernández T, Viñuela M, Vidal C, Barrera F. Lifestyle changes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. PLoS One. 2022;17:e0263931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 21. | Tincopa MA, Patel N, Shahab A, Asefa H, Lok AS. Implementation of a randomized mobile-technology lifestyle program in individuals with nonalcoholic fatty liver disease. Sci Rep. 2024;14:7452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Bischoff M, Zimny S, Feiner S, Sauter J, Sydor S, Denk G, Nagel JM, Bischoff G, Rust C, Hohenester S. Multidisciplinary lifestyle intervention is associated with improvements in liver damage and in surrogate scores of NAFLD and liver fibrosis in morbidly obese patients. Eur J Nutr. 2022;61:2725-2735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Mundi MS, Velapati S, Patel J, Kellogg TA, Abu Dayyeh BK, Hurt RT. Evolution of NAFLD and Its Management. Nutr Clin Pract. 2020;35:72-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 24. | Zhang XL, Wang TY, Targher G, Byrne CD, Zheng MH. Lifestyle Interventions for Non-Obese Patients Both with, and at Risk, of Non-Alcoholic Fatty Liver Disease. Diabetes Metab J. 2022;46:391-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Arredouani A. Effectiveness of Lifestyle Interventions for Nonalcoholic Fatty Liver Disease Treatment. In: Shiomi N, editor. Lifestyle-Related Diseases and Metabolic Syndrome. London: IntechOpen, 2022. |
| 26. | Lv H, Liu Y. Management of non-alcoholic fatty liver disease: Lifestyle changes. World J Gastroenterol. 2024;30:2829-2833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 27. | Hekmatdoost A. Prevention of Nonalcoholic Fatty Liver Disease (NAFLD) Progression to Nonalcoholic Steatohepatitis (NASH) by Modification of Lifestyle and Dietary Supplements. J Clin Nutr Diet. 2016;2. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/