Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.109260
Revised: June 17, 2025
Accepted: September 8, 2025
Published online: October 27, 2025
Processing time: 171 Days and 17.6 Hours
Hemorrhage following pancreaticobiliary surgery is a high-risk complication, with a mortality rate of 16%-38%. At present, minimally invasive endovascular intervention comprising superselective arterial embolization (SAE) and covered stent implantation (CSI) is the treatment of choice. However, in certain cases, both SAE and CSI become infeasible.
To evaluate the effectiveness of coil-assisted N-butyl cyanoacrylate (NBCA) embolization in comparison with that of CSI in managing delayed hemorrhage after hepatobiliary–pancreatic surgery when SAE is infeasible.
Ninety-eight continuous patients (n = 105 cases; mean age, 58.4 years) with de
The technical and clinical success rates in the NBCA group (100% and 93.3%, respectively) were significantly higher than those in the CSI group (88.3% and 73.3%, respectively), with a statistically significant difference between the two groups (P = 0.019 and 0.010, respectively). The 30-day mortality rates and major intervention-related complications were 17.8% and 0%, respectively, in the NBCA group and 18.3% and 1.7% in the CSI group, respectively, with no statistically significant difference between the two groups.
In terms of technical and clinical success, coil-assisted NBCA embolization was more effective than CSI for managing delayed hemorrhage after hepatobiliary–pancreatic surgery when SAE was not feasible.
Core Tip: This relatively large study investigates the safety and efficacy of coil-assisted N-butyl cyanoacrylate (NBCA) embolization and compares it with those of covered stent implantation (CSI) when superselective arterial embolization (SAE) is infeasible. SAE and CSI were performed successfully in only 69% and 68% of patients, respectively. In contrast, we achieved much higher technical and clinical success rates for both the NBCA group (100% and 93.3%, respectively) and the CSI group (88.3% and 73.3%, respectively). In addition, the technical and clinical failure rates of the NBCA group were merely 0% and 6.7%, respectively.
- Citation: He CJ, Wang XD, Ge NJ, Liu X, Huang J, Xu W, Ni CF, Yang YF. Coil-assisted N-butyl cyanoacrylate embolization vs covered stent implantation for delayed hemorrhage in hepatobiliary and pancreatic surgery. World J Gastrointest Surg 2025; 17(10): 109260
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/109260.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.109260
Despite its low incidence rate[1], hemorrhage following major pancreaticobiliary surgery is a complication with a high mortality rate (16%-38%)[2,3]. Based on the time of onset post-surgery, it can be classified as early or delayed hemorrhage[4]. Early hemorrhage, occurring within 24 hours postoperatively, is commonly triggered by a technical failure of appropriate hemostasis or an underlying perioperative coagulopathy, necessitating emergent surgical exploration and management. In contrast, delayed hemorrhage, which occurs > 24 hours postoperatively, is associated with surgical complications, such as pancreatic fistula, biliary fistula, and/or abdominal infection. Some cases of severely delayed hemorrhage are difficult to manage. Minimally invasive endovascular intervention, which potentially preserves blood flow in the parent artery, currently offers optimal treatment[5-7]. It mainly includes superselective arterial embolization (SAE) and covered stent implantation (CSI). In certain special cases, however, SAE is infeasible because of obscure openings, tortuosity, and small targeted arteries. It is also difficult to perform CSI when the parent artery is small or tortuous.
N-butyl cyanoacrylate (NBCA) is a useful liquid embolic agent that polymerizes upon contact with blood or other ion-rich fluids. However, severe reflux and non-target NBCA embolization can potentially induce infarction and organ function injuries. Therefore, the microcatheter used in the procedure should be advanced as close to the bleeding artery as possible to avoid massive non-target embolization. At our center, when SAE and CSI are deemed infeasible, embolization of the parent artery with a coil and subsequent NBCA, referred to as coil-assisted NBCA embolization, is employed. This study aims to evaluate the clinical effectiveness of coil-assisted NBCA embolization in comparison with that of CSI when SAE could not be implemented because of intractable targeted vessels.
This retrospective study was approved by the Ethics Committee of the Third Affiliated Hospital of Naval Medical University, Shanghai, China (ethics review number: EHBHKY2024-K-498) and adhered to the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants for using their data in clinical research. A covered stent (CSI group) was implanted in the parent artery when SAE was infeasible due to the inability of the microcatheter to adequately reach the bleeding site. Coil-assisted NBCA embolization (NBCA group) was performed when CSI was infeasible due to tortuosity or the small size of the parent artery.
The patients were selected based on the following inclusion criteria: (1) Hepatobiliary pancreatic surgery; (2) Delayed massive hemorrhage; and (3) Diagnosis of hemorrhage based on angiography. The exclusion criteria used were as follows: (1) Early hemorrhage; (2) Non-SCI or coil-assisted NBCA embolization; and (3) Age > 80 or < 18 years.
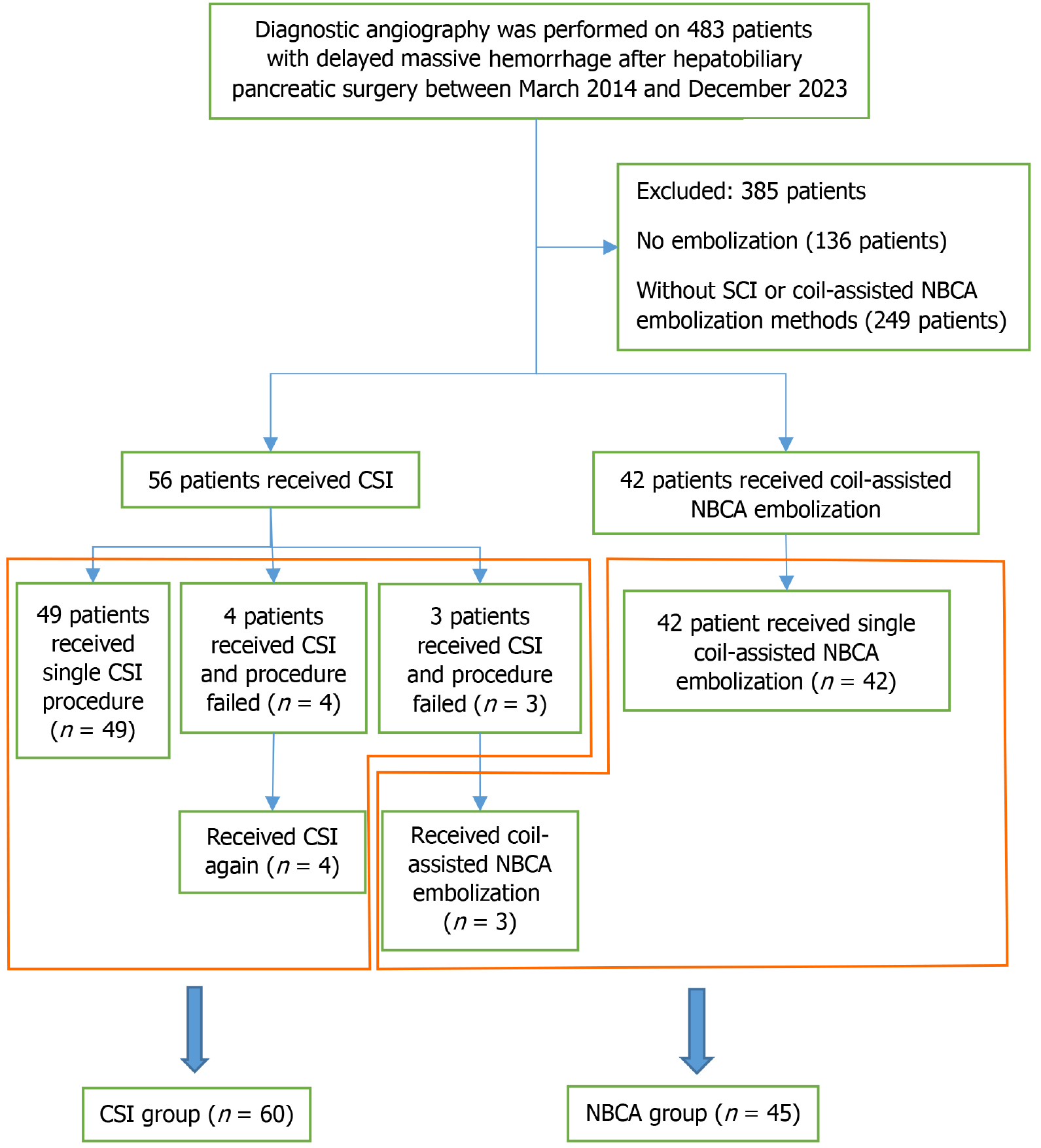
Between March 2014 and December 2023, 483 consecutive patients with delayed massive hemorrhage after hepatobiliary pancreatic surgery at our center received diagnostic angiography. From these 483 patients, 98 consecutive patients (n = 105 cases) were selected to enroll in this study (Figure 1). All patients subsequently underwent endovascular treatment using CSI and/or coil-assisted NBCA embolization after the infeasibility of SAE was confirmed.
Coagulopathy was defined as a prothrombin time of > 15 seconds, an international normalized ratio of > 1.5, and/or a platelet count of < 80000/mm3. Hemodynamic instability was defined as a systolic pressure of < 90 mmHg, heart rate of > 100 beats per minute, and/or a shock index of 1 secondary to blood loss. A pancreatic fistula was defined as the peritoneal amylase concentration of more than five times the serum amylase concentration[8]. Patient demographics, indications for surgery, risk factors for hemorrhage, time of hemorrhagic onset, and hemorrhagic presentation are listed in Table 1.
| Variable | NBCA group (n = 45) | CSI group (n = 60) | P value |
| Sex | 0.494 | ||
| Male | 32 (71.1) | 47 (78.3) | |
| Female | 13 (28.9) | 13 (21.7) | |
| Age (years) | 0.559 | ||
| < 60 | 22 (48.9) | 33 (55.0) | |
| ≥ 60 | 23 (51.1) | 27 (45.0) | |
| Indication for surgery | 0.005 | ||
| Biliary tract cancer | 26 (57.8) | 25 (41.7) | |
| Carcinoma of ampulla | 7 (15.6) | 6 (10.0) | |
| Pancreatic cancer | 4 (8.9) | 9 (15.0) | |
| Hepatocellular carcinoma | 4 (8.9) | 0 (0.0) | |
| Benign | 4 (8.9) | 20 (33.3) | |
| Time of onset (days)1 | 11.3 ± 8.2 | 15.7 ± 8.8 | 0.010 |
| Clinical manifestations | 0.069 | ||
| Gastrointestinal bleeding | 23 (51.1) | 19 (31.7) | |
| Hemorrhage from intra-abdominal | 22 (48.9) | 41 (68.3) | |
| Risk factors for hemorrhage | |||
| Pancreatic fistula | 24 (53.3) | 25 (42.4) | 0.323 |
| Biliary fistula | 6 (13.3) | 3 (5.0) | 0.167 |
| Abdominal infection | 31 (68.9) | 23 (38.3) | 0.003 |
| Coagulopathy2 | 24 (53.3) | 17 (28.8) | 0.015 |
| HB before endovascular treatment (g/L)1 | 65.4 ± 19.8 | 78.7 ± 16.3 | 0.000 |
| Hemodynamic instability3 | 39 (86.7) | 41 (68.3) | 0.037 |
If an arterial hemorrhage was suspected after relevant examinations, angiography was performed to detect the hemorrhage and identify its site, followed by endovascular treatment. All angiographic and embolization procedures were performed under local anesthesia by two interventional radiologists, with one over 5 years of interventional experience. The arteria femoralis was accessed. Angiography of the celiac, hepatic, and splenic arteries, as well as that of the superior and inferior mesenteric arteries, was performed using a 5-F catheter or microcatheter. A hemorrhage was definitively diagnosed and confirmed based on contrast-agent extravasation and pseudoaneurysm detection.
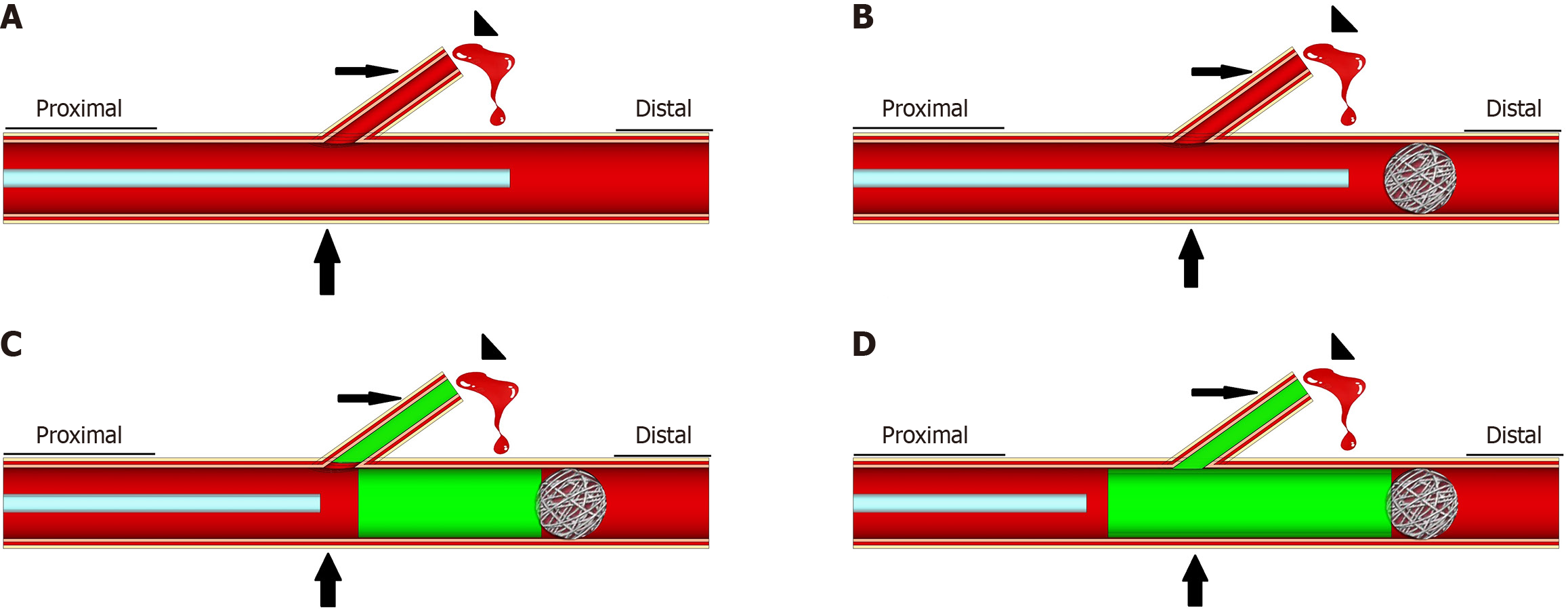
Endovascular treatment was performed based on the bleeding site and targeted artery. Upon confirming the infeasibility of SAE, a covered stent was implanted in the parent artery to exclude contrast-agent extravasation or pseudoaneurysms and to maintain distal organ perfusion. Details of the CSI procedure have been described in previous literature[9].
NBCA was acquired from Compont Medical Devices Beijing (China). The microcatheter (Renegade) was obtained from Boston Scientific (Natick, Massachusetts, United States). The detachable coil (Interlock) was procured from Boston Scientific (United States), while the ethiodized oil (Lipiodol) was from Laboratoire André Guerbet (Aulnay-sous-Bois, France).
Upon confirming that SAE and CSI were infeasible or unsuccessful, coil-assisted NBCA embolization was performed. A microcatheter was inserted and advanced through the parent artery, distal to the origin of the bleeding artery. Sub
Anti-infective treatments were administered based on clinical practice and antibiotic sensitivity tests, and packed red blood cell transfusions were performed as required. Routine blood examinations were performed at 1-3-day intervals. If there were any indications of rebleeding, emergency interventional procedures or surgeries were performed. Bedside ultrasound, routine blood tests, liver function, coagulation function, and amylase levels were examined to identify any potential injuries from target and non-target-organ embolization. An abdominal computed tomography scan was obtained 3-5 days after embolization and whenever any complication was suspected. The clinical effectiveness was evaluated based on technical success (cessation of contrast-agent extravasation and/or disappearance of the pseudoaneurysm at the targeted site), clinical success (defined as technical success and no rebleeding within 7 days of the endovascular procedure), and 30-day mortality. Intervention-related adverse effects (AEs) were evaluated using Common Terminology Criteria of Adverse Events v5.0. Grade 3 and 4 AEs were defined as severe AEs.
Continuous and categorical data were analyzed by utilizing the t-test and the χ2 analysis or Fisher’s exact test, respec
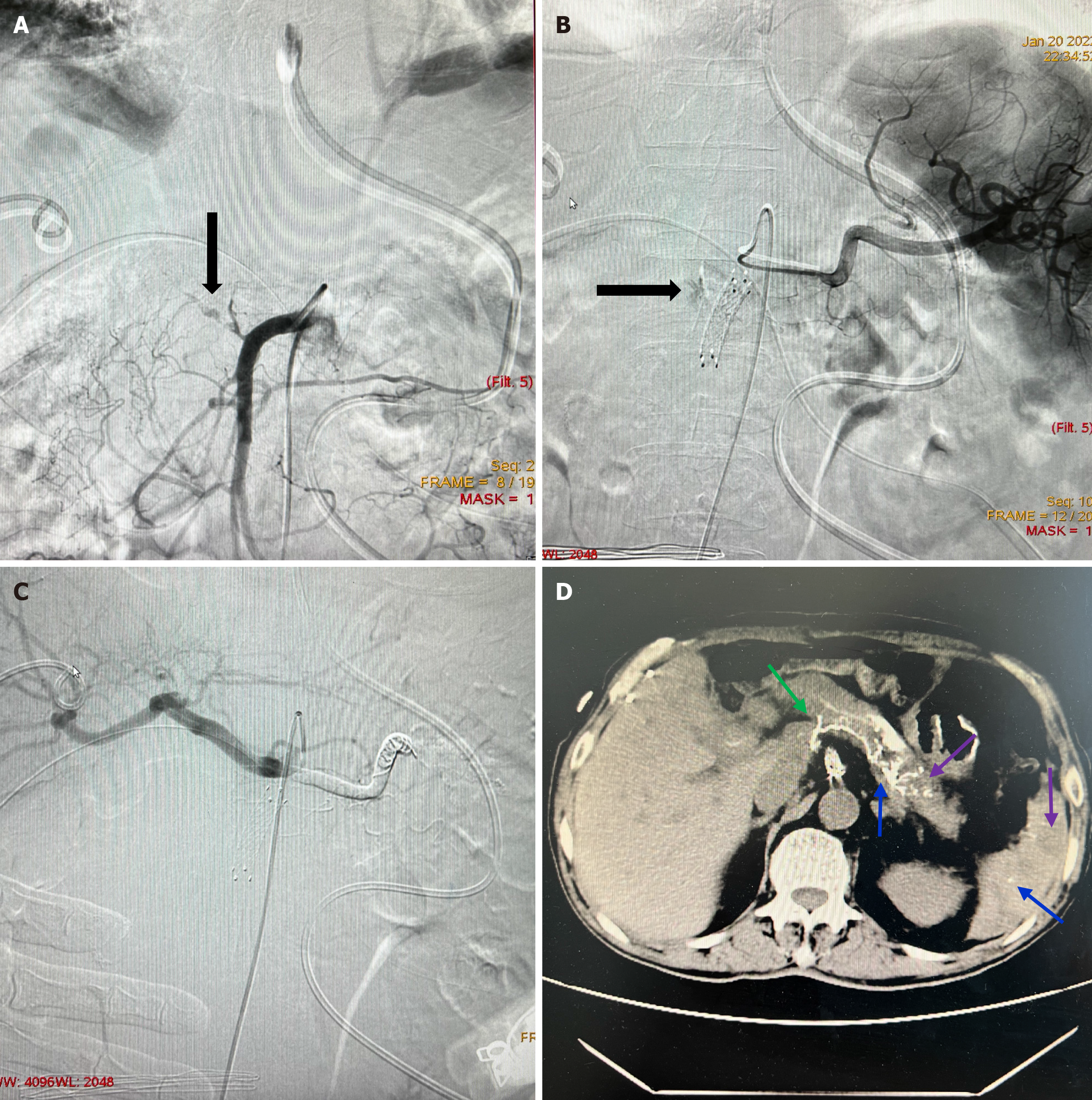
The NBCA group had longer times of onset, more cases of abdominal infection, coagulopathy, hemodynamic instability, fewer benign surgery indications, and hemoglobin before endovascular treatment compared to those in the CSI group (P < 0.05). The NBCA group had significantly higher technical success rate (100%) and clinical success rate (93.3%) than those in the CSI group (88.3% and 73.3%, respectively) (P = 0.019 and 0.010, respectively). The 30-day mortality rates and severe intervention-related AEs were 17.8% and 0%, respectively, in the NBCA group and 18.3% and 1.7%, respectively, in the CSI group. Thus, these two effects were not significantly different between the two groups (P = 1.000 and 1.000, respectively; Table 2). NBCA deposits were observed in the targeted bleeding lesions in all 45 patients of the NBCA group (residual rate, 100%). Additionally, small partial ischemia in target and non-target organs was observed in all patients. In addition, they experienced mild ischemic complications that were asymptomatic and self-limiting, which resolved without specific treatments (Figure 3). However, they did not report any major intervention-related AEs.
| Variable | NBCA group (n = 45) | CSI group (n = 60) | P value |
| Arteriographic findings | 0.351 | ||
| Extravasation of contrast media | 37 (82.2) | 44 (73.3) | |
| Pseudoaneurysm | 8 (17.8) | 16 (26.7) | |
| Parent artery | 0.000 | ||
| Splenic artery | 13 (28.9) | 6 (10.0) | |
| Common hepatic artery | 14 (31.1) | 46 (76.7) | |
| Right hepatic artery | 7 (15.6) | 6 (10.0) | |
| Left hepatic artery | 2 (4.4) | 0 (0.0) | |
| Left gastric artery | 6 (13.3) | 1 (1.7) | |
| Branch of superior mesenteric artery | 3 (6.7) | 1 (1.7) | |
| Technical success | 45 (100) | 53 (88.3) | 0.019 |
| Clinical success | 42 (93.3) | 44 (73.3) | 0.010 |
| Clinical failure and follow-up treatment | 3 (6.7) | 16 (27.6) | |
| Continuous hemorrhage and death | 2 | 5 | |
| Surgical operation | 1 | 4 | |
| CSI | 0 | 4 | |
| Coil-assisted NBCA embolization | 0 | 3 | |
| 30-day mortality | 8 (17.8) | 11 (18.3) | 1.000 |
| Severe intervention-related AEs | 0 (0) | 1 (1.7) | 1.000 |
Over the past decade, the treatment strategy for delayed hemorrhage in hepatobiliary and pancreatic surgery has moved from emergency relaparotomy to endovascular treatment.
The endovascular treatment, which predominantly includes SAE and CSI, aims to preserve the parent arterial flow. The CSI is an ideal choice to preserve the parental arterial inflow in arteries that are straight and relatively wide in diameter. Conversely, most bleeding sites following hepatobiliary and pancreatic surgery involve branches of the proper hepatic artery or the gastroduodenal artery, which tend to be tortuous and narrow in diameter. However, the CSI may often undergo technical failure because of the tortuosity or narrow diameter of the targeted arteries[10,11]. In certain special cases, superselective catheterization and SAE cannot be performed because of obscure arterial openings and tortuosity of the targeted arteries or arterial dissection[12]. Bare stent-assisted coil embolization and perfusion catheter placement with coil embolization, which can preserve parent arterial blood flow, have also been reported[13,14]. However, owing to their complexity and difficulty, the two aforementioned embolization techniques are not used widely.
Parent artery embolization using coils or NBCA has only been reported in cases where the bleeding artery cannot be catheterized directly[12,15]. This embolization method entails the insertion of micro coils from the distal to the proximal end of the parent artery. However, compared to coil-assisted NBCA embolization, it is difficult to achieve tail-end embolization of targeted bleeding arteries and embolization of potential collateral vessels, even with the additional use of NBCA. Because NBCA requires time to polymerize upon contact with blood, parent artery embolization with NBCA alone makes it difficult to achieve distal and proximal end parent artery embolization without NBCA flowing into the distal end of the parent artery, especially when dealing with parent arteries with large diameters and rapid blood flows. Compared to the exclusive use of NBCA, the detachable coil and additional use of NBCA made it possible to achieve complete distal-portion parent artery embolization, which not only prevented NBCA from flowing to the distal end of the parent artery but also compelled it to flow to the end of the targeted artery and bleeding site. To reduce high blood flow and lower the risk of end-organ damage, a few cases of initial coil embolization distal to the lesions before NBCA embolization have also been reported[16,17]. To the best of our knowledge, this is a relatively large-sample study to specially investigate the safety and efficacy of coil-assisted NBCA embolization compared to that of CSI.
A previous systematic review reported successful performance of CSI and SAE in only 69% and 68% of patients, respectively[1]. Approximately 40% of the patients have been reported to experience either continued bleeding or rebleeding after embolization and require additional interventions[18], owing to technical and clinical shortcomings. Technical failure is chiefly attributed to intractable targeted vessels and the misguided choice of embolic methods and materials. Clinical failure, that is, post-procedure rebleeding, is predominantly attributed to persistent fistula erosion and collateral circulation recanalization. However, in this study, the technical and clinical failure rates in the NBCA group were only 0% and 6.7%, respectively, which were significantly lower than those in the CSI group. The possible reasons for such a low failure could be the following: (1) As an effective liquid embolic agent, NBCA can flow into small and tortuous vessels and achieve target-vessel tail-end embolization; (2) It can embolize collateral vessels connected to the bleeding site, potentially prohibiting rebleeding secondary to retrograde collateral flow; (3) NBCA polymerizes rapidly, which is important for life-threatening hemorrhage; (4) It remains effective despite coagulopathy and erosion from pancreatic fistula; (5) When mixed with iodized oil, NBCA becomes perpetually opaque in the blood vessels, which is essential for identifying its deposits in the targeted bleeding site; (6) Superselective catheterization is nonessential in coil-assisted NBCA embolization, rendering this technique easy to perform; and (7) Compared with parent artery embolization using coils or NBCA alone, coil-assisted NBCA embolization potentially lead to more NBCA deposits in the targeted bleeding site and less non-target embolization.
The absence of NBCA in the targeted lesions is reportedly associated with recurrent bleeding[18], possibly because NBCA deposits in the targeted bleeding lesions can theoretically embolize the end of the targeted artery and potential collateral vessels. In our study, NBCA deposits in targeted bleeding lesions and small non-target embolizations occurred in all patients. However, NBCA is a fluid embolic agent. Distal NBCA embolization induces ischemia, which may lead to infarction or abscess formation and impaired organ function. However, other target and non-target embolizations were asymptomatic and self-limited. In theory, complete hepatic arterial embolization is risky for patients who have undergone hepatobiliary surgery because it would dramatically decrease the arterial flow in the remnant liver. However, the complete common hepatic arterial embolization does not cause any liver dysfunction. Hence, it can be suggested that there are sufficient collateral arteries around the liver, such as the inferior phrenic and interlobular arteries, that maintain normal liver function when patients become stable. In addition, the risk of hepatic injury is considered lower because of the two-tier blood supply. Additionally, the spleen, stomach, and pancreas have rich blood supply because of the extensive collateral circulation. Therefore, small partial ischemic complications are asymptomatic and self-limiting in these organs. Kodani et al[19] reported that NBCA arterial embolization of one rectal vein involving more than two branches could also induce ischemic complications. However, the affected patients are typically asymptomatic, with self-limiting complications. Borghese et al[20] reported non-target-vessel embolization in five patients without any compli
The present study has certain limitations too. First, the sample size is quite small. Second, this is a retrospective study lacking randomization. However, we plan to address these limitations in our future studies. Third, baseline characteristics of the patient of the two groups are significantly different. In this study, the CSI and SAE were infeasible in all patients of the NBCA group. Therefore, the conditions of the patients treated with coil-assisted NBCA embolization in this study were more complicated and intractable compared to those in the CSI group. Fourth, because most patients had previously been diagnosed with malignant tumors and had undergone major surgery with severe postoperative compli
Coil-assisted NBCA embolization is a safe and effective endovascular treatment for delayed massive hemorrhage when SAE and CSI are not feasible. Additionally, the small partial ischemic complications in the liver, spleen, stomach, and pancreas that had been induced by NBCA embolization were asymptomatic and self-limited. Coil-assisted NBCA embolization may provide new ideas for the treatment of aneurysms and traumatic bleeding in some specific scenarios. To obtain more persuasive conclusions, our team is conducting a larger-sample prospective study on this project. We will report the results in due time.
| 1. | Floortje van Oosten A, Smits FJ, van den Heuvel DAF, van Santvoort HC, Molenaar IQ. Diagnosis and management of postpancreatectomy hemorrhage: a systematic review and meta-analysis. HPB (Oxford). 2019;21:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, Kaifi J, Schurr PG, Bubenheim M, Nolte-Ernsting C, Adam G, Izbicki JR. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 3. | Wang WG, Fu L, Babu SR, Wang L, Liang CP, Tian BL. Incidence of and Risk Factors and Reinterventions for Post-Pancreatoduodenectomy Hemorrhage: Retrospective Analysis. Dig Surg. 2018;35:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 2072] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 5. | Asari S, Matsumoto I, Toyama H, Yamaguchi M, Okada T, Shinzeki M, Goto T, Ajiki T, Fukumoto T, Ku Y. Recommendation of treatment strategy for postpancreatectomy hemorrhage: Lessons from a single-center experience in 35 patients. Pancreatology. 2016;16:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Khalsa BS, Imagawa DK, Chen JI, Dermirjian AN, Yim DB, Findeiss LK. Evolution in the Treatment of Delayed Postpancreatectomy Hemorrhage: Surgery to Interventional Radiology. Pancreas. 2015;44:953-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Pottier E, Ronot M, Gaujoux S, Cesaretti M, Barbier L, Sauvanet A, Vilgrain V. Endovascular management of delayed post-pancreatectomy haemorrhage. Eur Radiol. 2016;26:3456-3465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Blanc T, Cortes A, Goere D, Sibert A, Pessaux P, Belghiti J, Sauvanet A. Hemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg. 2007;194:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Yu X, Liu X, Huang J, Shen S, Ge N, Yang Y, Wang H. Endovascular therapy choices for different sites of delayed postoperative arterial hemorrhage after hepatobiliary pancreatic surgery: a retrospective study. Gland Surg. 2021;10:2745-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Stoupis C, Ludwig K, Inderbitzin D, Do DD, Triller J. Stent grafting of acute hepatic artery bleeding following pancreatic head resection. Eur Radiol. 2007;17:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Hankins D, Chao S, Dolmatch BL, Jeyarajah RD. Covered stents for late postoperative arterial hemorrhage after pancreaticoduodenectomy. J Vasc Interv Radiol. 2009;20:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Lee CW, Liu KL, Wang HP, Chen SJ, Tsang YM, Liu HM. Transcatheter arterial embolization of acute upper gastrointestinal tract bleeding with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol. 2007;18:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Hasegawa T, Ota H, Matsuura T, Seiji K, Mugikura S, Motoi F, Unno M, Takase K. Endovascular Treatment of Hepatic Artery Pseudoaneurysm after Pancreaticoduodenectomy: Risk Factors Associated with Mortality and Complications. J Vasc Interv Radiol. 2017;28:50-59.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Hartnell GG, Gates J. Treatment of gastroduodenal artery hemorrhage with a conventional stent. J Vasc Interv Radiol. 1999;10:172-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Hur S, Yoon CJ, Kang SG, Dixon R, Han HS, Yoon YS, Cho JY. Transcatheter arterial embolization of gastroduodenal artery stump pseudoaneurysms after pancreaticoduodenectomy: safety and efficacy of two embolization techniques. J Vasc Interv Radiol. 2011;22:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Shi Y, Chen L, Zhao B, Huang H, Lu Z, Su H. Transcatheter arterial embolization for massive hemobilia with N-butyl cyanoacrylate (NBCA) Glubran 2. Acta Radiol. 2022;63:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Yata S, Ihaya T, Kaminou T, Hashimoto M, Ohuchi Y, Umekita Y, Ogawa T. Transcatheter arterial embolization of acute arterial bleeding in the upper and lower gastrointestinal tract with N-butyl-2-cyanoacrylate. J Vasc Interv Radiol. 2013;24:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Huang YS, Chang CC, Liou JM, Jaw FS, Liu KL. Transcatheter arterial embolization with N-butyl cyanoacrylate for nonvariceal upper gastrointestinal bleeding in hemodynamically unstable patients: results and predictors of clinical outcomes. J Vasc Interv Radiol. 2014;25:1850-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Kodani M, Yata S, Ohuchi Y, Ihaya T, Kaminou T, Ogawa T. Safety and Risk of Superselective Transcatheter Arterial Embolization for Acute Lower Gastrointestinal Hemorrhage with N-Butyl Cyanoacrylate: Angiographic and Colonoscopic Evaluation. J Vasc Interv Radiol. 2016;27:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Borghese O, Ganimede MP, Briatico Vangosa A, Pisani A, Vidali S, Di Stasi C, Burdi N, Semeraro V. The Minimally Invasive Treatment of Visceral Artery Pseudoaneurysms: A Retrospective Observational Single Centre Cohort Study on Glue Embolization. Vasc Endovascular Surg. 2021;55:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/