INTRODUCTION
Colorectal cancer (CRC) is a common malignant tumor, originating from the mucosal epithelium of the colorectum. Minimally invasive surgery (MIS) is a common method for the treatment of CRC due to its technical advantages and favorable postoperative outcomes[1]. With the development of robotic-assisted laparoscopic surgery in recent years, the number of possible surgical modalities for gastrointestinal tumor dissection has increased, but conventional laparoscopic surgery (CLS) and hand-assisted laparoscopic surgery (HALS) still have unique advantages. In addition, according to Globocan 2022, CRC is the 3rd most common malignant tumor in terms of morbidity and the 2nd most common in terms of mortality globally, with 1926000 new cases of CRC and 904000 deaths globally in 2022, accounting for 9.6% and 9.3% of all malignant neoplasms and associated deaths, respectively. The standardized incidence (22.0/100000) and mortality (9.9/100000) rates for CRC in men are approximately 1.5 times higher than those (15.2/100000 and 6.5/100000, respectively) in women[2]. In China, research study has pointed out that the incidence of CRC is increasing in both men and women, and there is a strong correlation between men and CRC[3]. Therefore, the work of comparing different minimally invasive procedures seems imminent. The right half of the colon includes the cecum, ascending colon and proximal 2/3 of the transverse colon, and the left half of the colon includes the distal 1/3 of the transverse colon, the descending colon, the sigmoid colon and the rectum[4]. However, as the embryonic, anatomical and tumor microenvironments of patients with CRC differ substantially, these differences needs to be fully considered in research and discussion. The surgical patients included in this study had only left-sided colon cancer (LCC), defined as a malignant tumor of the splenic flexure and descending colon alone; patients with malignant tumors of the sigmoid colon and rectum were not included. The clinical efficacies of HALS, CLS and robotic-assisted surgery (RAS) for the dissection of LCC and the effects of these procedures on long-term patient prognosis were compared. HALS involves the surgeon inserting a hand inside the abdomen through a special port to facilitate dissection without disturbing the pneumoperitoneum[5]. Moreover, HALS enables tactile feedback and proprioception for blunt dissection, allowing rapid control of unexpected bleeding incidents[6]; it also fully combines the advantages of open surgery with a reduced difficulty of cooperation during surgery and easier exposure due to the use of a 30° mirror for a high-definition view. HALS is commonly leveraged to the patients with severe intestinal adhesions or complex masses in the abdominal cavity. But it has also been effective in the treatment of single tumors[7,8], However, there are disadvantages to HALS, such as increased risks of surgical glove tears and gas leakage[9]. CLS has been demonstrated to be an effective and safe treatment for CRC, it can reduce trauma and surgery-related complications[10]. Recent years have seen an increase in the use of RAS, whose most significant technological advantage over CLS is the ability to perform fine and flexible maneuvers in confined spaces[11], also, many studies have compared the postoperative outcomes between RAS and CLS for CRC[12-18]. While CLS and HALS still play indispensable clinical roles, few studies have compared HALS with CLS and RAS for the dissection of LCC.
This retrospective analysis used clinical data from patients with LCC admitted to our hospital to compare these surgical procedures in terms of long-term prognosis, clinical efficacy, postoperative recovery, and health economics to provide an effective resource to support clinical applications.
DISCUSSION
With advancements in science and technology, patients have high expectations of what surgery can achieve, but patients have unique individual characteristics, making it important to consider a patient's tumor stage, physical condition, and financial situation when selecting a surgical procedure. Technological advances with today's da Vinci robots have increased the accuracy and precision of MIS procedures for dissection and reconstruction, especially in deep, limited or narrow cavities such as the chest and abdomen, allowing the use of more complex MIS procedures than those used in traditional minimally invasive approaches[19-22]. Although the safety and efficacy of robotic colectomy have been proven in previous studies, socioeconomic benefits need to be considered. In this study, the hospitalization costs for RAS were significantly greater than those for CLS and HALS, and the differences were statistically significant. The benefits to patients of these surgical procedures still need to be compared, as not all patients are eligible for the relatively expensive da Vinci robotic surgery. HALS is a versatile, minimally invasive technique for colorectal resection for which patient eligibility is independent of BMI[23]. HALS also provides anatomical and reconstructive accuracy and precision and allows for manual palpation, which improves depth perception. Moreover, this procedure permits manual dissection and better controls intraoperative bleeding[24], enabling the surgeon to quickly adapt to changes during colonic surgery, particularly during surgeries involving dense adhesions, such as colonic surgery to treat inflammatory bowel disease or complex surgery to treat diverticulosis with phlegm[25,26]. Traditional laparoscopic surgery is the mainstay of clinical practice, so it is necessary to study and investigate procedural variations.
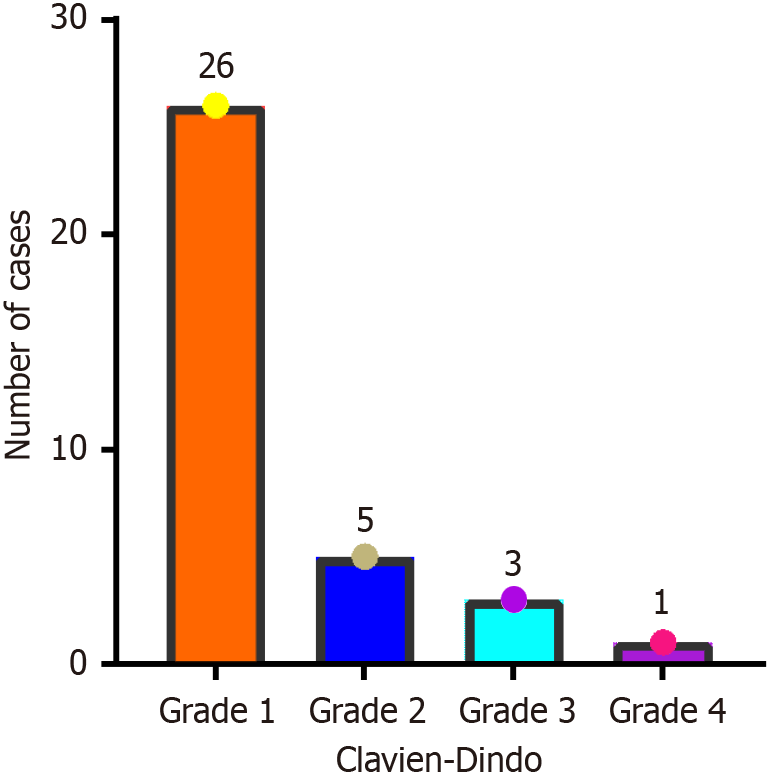
In this study, the mean BMI of the patients was 22.76 ± 3.09 kg/m2, and all patients had normal BMI values, thereby reducing the impact of obesity on surgical operations. Differences in baseline patient characteristics, such as patient age and tumor stage, were not significant (P > 0.05), so PSM was not required. A total of 35 (5.8%) patients experienced postoperative complications of grade I or above, including 6 (17.6%) patients who underwent HALS, 14 (14.9%) who underwent CLS and 15 (16.0%) who underwent RAS. The severity of postoperative complications was relatively mild, and most were effectively controlled postoperatively. Differences in the incidence of postoperative complications were not significant (P > 0.05), but in another study, Samalavicius et al[27] reported that 10.7% of patients who underwent hand-assisted colorectal surgery experienced postoperative complications. Moreover, a comparative study by Park et al[28] on different surgical procedures for the colon cancer dissection revealed that the incidence of postoperative complications was 13.6% among patients who underwent open surgery, 18.2% among those who underwent robotic surgery and 12.1% among those who underwent CLS, but the differences were not statistically significant. These results roughly agree with those of this study but are affected by sample size; the overall incidence of postoperative complications is expected to be higher than that reported by Park et al[28] Another study comparing robotic and laparoscopic surgical procedures for the treatment of colorectal disease via systematic review and meta-analysis of randomized controlled trials reported an incidence of short-term postoperative complications of 21.05% for robotic surgery and 22.73% for laparoscopic surgery[16]. Therefore, the incidence of postoperative complications should still be considered throughout the treatment process, including that of complications related to preoperative chemotherapy and radiotherapy and complications related to postoperative infections, such as incision infections, lung infections, and anastomotic fistulas. Particular attention should be given to incisional infections in patients undergoing HALS, as the rate of intraoperative contamination is relatively high for this procedure, making awareness of asepsis and postoperative antibiotic prophylaxis critical.
In terms of short-term postoperative outcomes, the median length of postoperative hospital stay among patients was 7.00 days (IQR, 6.00–8.00 days) after HALS, 9.00 days (IQR, 8.00–11.00 days) after CLS, and 8.00 days (IQR, 7.00-10.00 days) after RAS, and the differences were statistically significant (P < 0.001). These results contrast those of Park et al[28], whose reported the longest postoperative hospital stay after open surgery (10.5 ± 3.6 days) and the shortest after CLS (8.6 ± 3.9 days), with that after robotic surgery (9.9 ± 7.3 days) falling in between, but the differences were not statistically significant. The reasons for these differing results may be related to sample size and type. Moreover, Park et al[28] studied a broader group of colon cancers, including transverse and right hemicolon cancers, and radical resection of the right hemicolon often requires clearance of the D3 Lymph nodes and more complex mesenteric dissection. The results of this study indicate that the shortest postoperative hospital stay occurred after HALS and the longest occurred after CLS, which may be closely related to the degree of surgical trauma and perioperative management strategies used. HALS is performed manually through small incisions, retaining the advantages of minimal invasiveness while avoiding the complex in vivo anastomosis step of total laparoscopic surgery, potentially reducing tissue trauma and decreasing the postoperative recovery time of gastrointestinal function. The duration of surgery was 175.23 ± 36.29 minutes for HALS, 184.10 ± 42.83 minutes for CLS, and 172.42 ± 45.12 minutes for RAS, and the differences were not statistically significant (P = 0.166). Notably, the relatively long duration of CLS this may have contributed to a relatively slower postoperative recovery process. In a retrospective propensity score-matched study, robotic-assisted colon cancer surgery resulted in shorter recovery milestones than did laparoscopic surgery (length of postoperative hospital stay: 6.5 days vs 10.2 days)[29], which is agrees with the result of our study. However, another study reported that the median length of postoperative hospital stay for elderly patients who underwent HALS for CRC liver metastases was 6 (5:7) days[30] and that, compared with patients without liver metastases, those with them underwent a longer and more invasive surgery but had a shorter postoperative hospital stay. These results together with those of our study, which revealed that the postoperative hospital stay after HALS was shorter than that after RAS, suggest additional factors that may be related to the learning curve of the robot. In a study of laparoscopic gastric cancer surgery, a learning curve of > 40 cases was required for the robot to reach a steady state[31]. While this may be a factor in our study of left hemicolon surgery, there is undeniable heterogeneity in the length of postoperative hospital stay after the different surgical procedures.
In addition to the length of postoperative hospital stay, the postoperative recovery time of gastrointestinal function is an indispensable observable outcome for assessing short-term efficacy. In this study, the median postoperative recovery time of gastrointestinal function was 4.00 days (IQR, 3.00–5.00 days) after HALS, 5.00 days (IQR, 4.50–6.00 days) after CLS, and 5.00 days (IQR, 4.00–6.00 days) after RAS, and the differences were statistically significant (P < 0.001). The time from postoperative Day 1 to the resumption of a liquid diet was defined as the time to functional recovery, and the results were roughly synchronized with those of the length of postoperative hospital stay. The postoperative recovery time of gastrointestinal function as a factor influencing the length of postoperative hospital stay is supported. Cuk et al[32] reported no statistically significant differences between robotic and laparoscopic colon surgeries in terms of time to postoperative defecation and length of postoperative hospital stay. Thus, the postoperative recovery time of gastrointestinal function could be influenced by a number of factors,trointestinal function could be influenced by a number of factors, including surgical procedure, anesthesia, intraoperative fluid replacement and postoperative pain management. Surgeons performing HALS have direct access to the abdominal organs through a small incision in the middle of the operation, which may reduce intraoperative intestinal pulling and thermal damage, thereby reducing intestinal edema and decreasing the postoperative recovery time of gastrointestinal function. This advantage over CLS and RAS was not significant in our study. Perioperative administration of opioids at high cumulative doses is an independent risk factor for poor recovery of gastrointestinal function and postoperative intestinal obstruction[33]. In the present study, the duration of postoperative use of pain medication did not significantly differ between procedures, which may be due to the effects of medication dose and type.
Control of postoperative inflammation, whose incidence and severity vary based on surgical procedure, is critical. In this study, the median leukocyte counts on postoperative day 4 were 5.76 × 109/L (IQR, 5.03 × 109-7.52 × 109/L) after HALS, 6.58 × 109/L (IQR, 5.47 × 109-7.98 × 109/L) after CLS, and 7.27 × 109/L (IQR, 6.23 × 109-9.44 × 109/L) after RAS, and the differences were statistically significant (P = 0.003) In addition, the median absolute neutrophil counts on postoperative Day 4 were 5.03 × 109/L (IQR, 3.78 × 109-6.06 × 109/L), 5.67 × 109/L (IQR, 3.78 × 109-6.06 × 109/L), and 5.67 × 109/L (IQR, 4.74 × 109-7.72 × 109/L), respectively, and the differences were statistically significant (P = 0.002). The lymphocyte counts and CRP levels on postoperative Days 1 and 4 were not significantly different. However, in the study by Cuk et al[32], among patients with colon cancer, the CRP levels on postoperative Day 1 after were significantly greater after CLS than after robotic surgery, and the differences were statistically significant; however, these differences on postoperative Days 2 and 3 were not significant. Ingham et al[34] studied the severity of postoperative inflammation after different surgical procedures and reported that the CRP levels after robotic surgery were significantly different from those after CLS in PODs 1, 2 and 3 (P < 0.001, P = 0.001, and P = 0.037, respectively). In addition, MIS has been reported to elicit a lower postoperative inflammatory response than that after open surgery[35,36], although the results of this study are diametrically opposed to those of other studies. The heterogeneity of the above studies suggests that the regulatory mechanism underlying the effects of different surgical procedures on the inflammatory response is multidimensional, considering surgical details, the sensitivity of examination methods, and postoperative anti-inflammatory medications.
Although no significant differences in intraoperative lymph node clearance and intraoperative bleeding were found in this study, Rein et al[18] found in a national cohort study comparing robotic and laparoscopic surgeries for the treatment of left hemicolonic cancer that the intraoperative lymph nodes clearance was significantly higher for robotic surgery than for laparoscopic surgery. de Almeida Leite et al[37] similarly reported significantly higher intraoperative lymph node clearance for robotic colon surgery, as well as a significantly decreased need for intra- or postoperative blood transfusion among patients undergoing robotic surgery. However, this study and that of Park et al[28] report no statistically significant difference in the number of lymph nodes cleared.
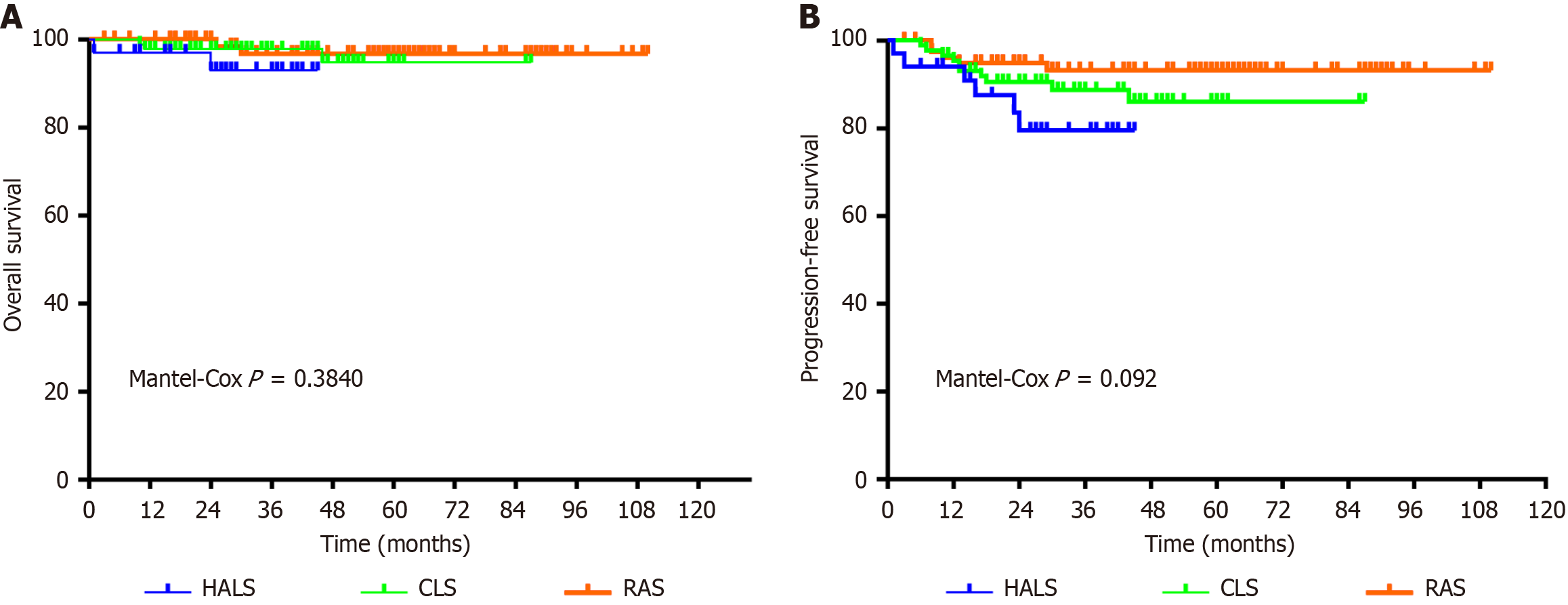
The degree of lymph node dissection may be related to the long-term prognosis of patients undergoing a left hemicolectomy. Notably, robotic surgery allows for more complete lymph node dissection with higher rates of complete colonic mesocolic resection. The relevance of this finding is that complete colonic mesocolic resection allows assessment through the pelvic lymph nodes, which may be associated with improved PFS[37,38]. In this study, there were no statistically significant differences in OS or PFS. These findings indicate that all three surgical procedures were able to achieve radical dissection of the malignant tumors. This result has also been reported in other study, where postoperative morbidity and mortality rates were similar between robot-assisted and laparoscopic left hemicolectomies[18]. Rein et al[18] reported that in the PSM-adjusted analysis, the 30-day postoperative mortality rate for CLS and RAS was 0.4% vs 0.9% (P = 0.369) and the 90-day postoperative mortality rate was 0.9% vs 1.9% (P = 0.08), respectively. This is the result of a short-term survival follow-up, and both have similar mortality rates. However, among the long-term survival prognosis, Cuk et al[39] reported that patients undergoing CLS had a significantly increased risk of cancer recurrence (CLS: 17.1% vs RAS: 12.4%), P = 0.002, with a mean follow-up time of 4.93 years. No associations between the 2 surgical platforms were evident regarding all-cause P = 0.783, or colon cancer-specific mortality P = 0.405. Analyzing the results of these studies in conjunction with our findings of the present study, although there was no statistical difference in the results of the three groups of surgeries, we were still able to clearly understand through the graphs that RAS may have an impact on OS and PFS to a certain extent, which includes the fact that RAS can reduce the amount of intraoperative blood loss, reduce the probability of intermediate open surgery, and improve the rate of recovery. In contrast, fewer comparisons have been made between HALS and CLS. A single-center study reported postoperative survival of HALS compared to conventional open surgery. In the study, there was a statistically significant 5-year postoperative OS for all stage I-III patients in the HALS vs conventional open group (HALS: 90.5% vs conventional open: 80.5%), P = 0.042[8]. Although this study did not compare the postoperative long-term prognosis with CLS, it was able to demonstrate the unique advantages of HALS compared to open surgery. Besides, in conjunction with the data from our study, it can be found that the results of OS and PFS were better in the CLS group compared to HALS. One reason for this may be because the HALS surgical group is earlier than CLS and RAS, and the surgeons in charge do not have as much surgical experience as in CLS group and RAS group. Of course there may be some other reasons, such as the number of lymph nodes cleared. In other studies where the number of lymph nodes cleared was higher, the postoperative recurrence and survival rates were the same as those in the present study, with no significant differences. Therefore, the 1992 American Joint Committee on Cancer Cancer Staging Manual, 4th edition, recommended that at least 12 Lymph nodes should be detected for pathological examination in radical colon cancer samples[40]. A clearance rate of ≥ 12 Lymph nodes allows for a better determination of the patient's tumor stage, but further studies are needed to determine whether it impacts patient survival.
We can find that different surgeries may affect the length of hospitalization of patients, and the inflammatory response varies with different surgeries, which may be one of the important factors affecting the short-term prognosis in future studies. We need to implement different surgical procedures for different conditions of patients, for example, for patients who have had major abdominal surgery, we prefer HALS, which not only can have faster postoperative recovery time of gastrointestinal function, but also avoids the surgical impact of severe adhesions in the abdominal cavity, for families with a high financial burden we favor the CLS, which costs less money. And, there are several potential limitations to this study. First, this was a single-center retrospective analysis, the patients enrolled were in the same hospital, and there was selection bias related to the nonrandom distribution of patients. Second, most of the patients were elderly, and we did not stratify the elderly patients in this study, which may have contributed to the results of this study. Third, we do not have data on the surgical experience of the operating surgeons, which is a parameter that affects patient prognosis. Therefore, in the follow-up study, we would like to launch a multicenter stratified study to better address these shortcomings and problems.