Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.108938
Revised: May 27, 2025
Accepted: September 1, 2025
Published online: October 27, 2025
Processing time: 181 Days and 16.8 Hours
Acute perforated cholecystitis (APC) is a serious complication of acute cholecy
To evaluate the safety and effectiveness of PTC as an initial treatment modality for APC.
We conducted a retrospective cohort study of patients diagnosed with APC between January 2017 and October 2022 at a single tertiary medical center. All patients underwent PTC as the initial intervention. Data collected included demographics, comorbidities, laboratory and imaging findings, complications, and clinical outcomes over a 24-month follow-up. Patients were stratified into two groups based on whether they subsequently underwent cholecystectomy.
Thirty patients underwent PTC for APC. Half of the patients (n = 15) were stabilized and later underwent cholecystectomy; the remaining 15 were managed non-operatively. Patients in the non-surgical group were significantly older (87.1 ± 6.2 years vs 76.1 ± 7.4 years; P < 0.001). Clinical improvement was observed in 61.4% of non-operated patients, with eventual drain removal or closure. Both groups demonstrated significant reductions in white blood cell count and C-reactive protein levels from admission to discharge. No significant differences were found in hospital stay or complication rates. During follow-up, three deaths occurred due to non-biliary causes. Only one patient required repeat drainage.
PTC is a safe and effective initial treatment for APC, particularly in elderly and comorbid patients for whom surgery poses excessive risk. It provides clinical stabilization and may serve either as a bridge to delayed cho
Core Tip: Percutaneous transhepatic cholecystostomy (PTC) offers a safe, minimally invasive first‐line strategy for acute perforated cholecystitis in patients at high surgical risk. In our retrospective cohort of 30 acute perforated cholecystitis cases, PTC achieved clinical stabilization in 61.4% of non‐operated patients, facilitated elective cholecystectomy in half the cohort, and was associated with low, manageable complication rates. Over a 24-month follow-up, most patients managed with PTC alone maintained durable health improvements, underscoring its potential both as a bridge to surgery and as definitive treatment in select critically ill populations.
- Citation: Mazarieb M, Parvaiz A, Hawashna U, Romanenko Y, Atar E, Bachar GN. Minimally invasive management of acute perforated cholecystitis: The role of percutaneous transhepatic cholecystostomy. World J Gastrointest Surg 2025; 17(10): 108938
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/108938.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.108938
Acute cholecystitis (AC) is one of the most frequent causes of acute abdominal emergencies, affecting approximately 10%-15% of the adult population[1]. While most cases are treated effectively with antibiotics and early cholecystectomy, a subset may progress to acute perforated cholecystitis (APC) - a severe condition characterized by gallbladder wall rupture, bile leakage, peritonitis, and potentially life-threatening sepsis[2-4]. The incidence of gallbladder perforation among AC cases ranges from 2% to 11% and is more common in older males and patients with delayed presentation or multiple comorbidities[2,5].
Emergency cholecystectomy (either open or laparoscopic) has traditionally been the mainstay of treatment for APC[4,5]. However, this approach carries significant perioperative risks in patients who are critically ill, elderly, or with multiple comorbidities reported mortality rates for high-risk patients undergoing emergency cholecystectomy range from 8% to 23%[3,6].
In recent years, percutaneous transhepatic cholecystostomy (PTC) has emerged as a minimally invasive alternative for patients deemed unfit for surgery[4,7-9]. The procedure allows for gallbladder decompression, bile drainage, and infection control, often leading to clinical stabilization and enabling delayed (interval) surgery if needed[10-13]. The Society of Interventional Radiology supports PTC as a temporizing or definitive option in patients where surgery is contraindicated[14].
Several studies have evaluated the outcomes of PTC in patients with complicated cholecystitis, including gallbladder perforation[15]. In a retrospective study by Huang et al[16] PTC was associated with a 100% survival rate compared to 50% in patients undergoing immediate open cholecystectomy. Similarly, Krecko et al[17] analyzed 654 patients with APC and found that those undergoing interval cholecystectomy after initial stabilization had significantly lower 30-day mortality (0% vs 7%) and fewer major complications (14% vs 33%) compared to patients operated during the index admission. Despite this growing body of evidence, the literature on APC remains limited, and most studies focus on uncomplicated AC. Therefore, the present study aims to evaluate PTC’s clinical efficacy and safety in patients diagnosed with APC. By examining complication rates, length of hospital stays, and long-term outcomes, this study seeks to clarify whether PTC can serve as a bridge to surgery and a primary therapeutic strategy in selecting high-risk patients.
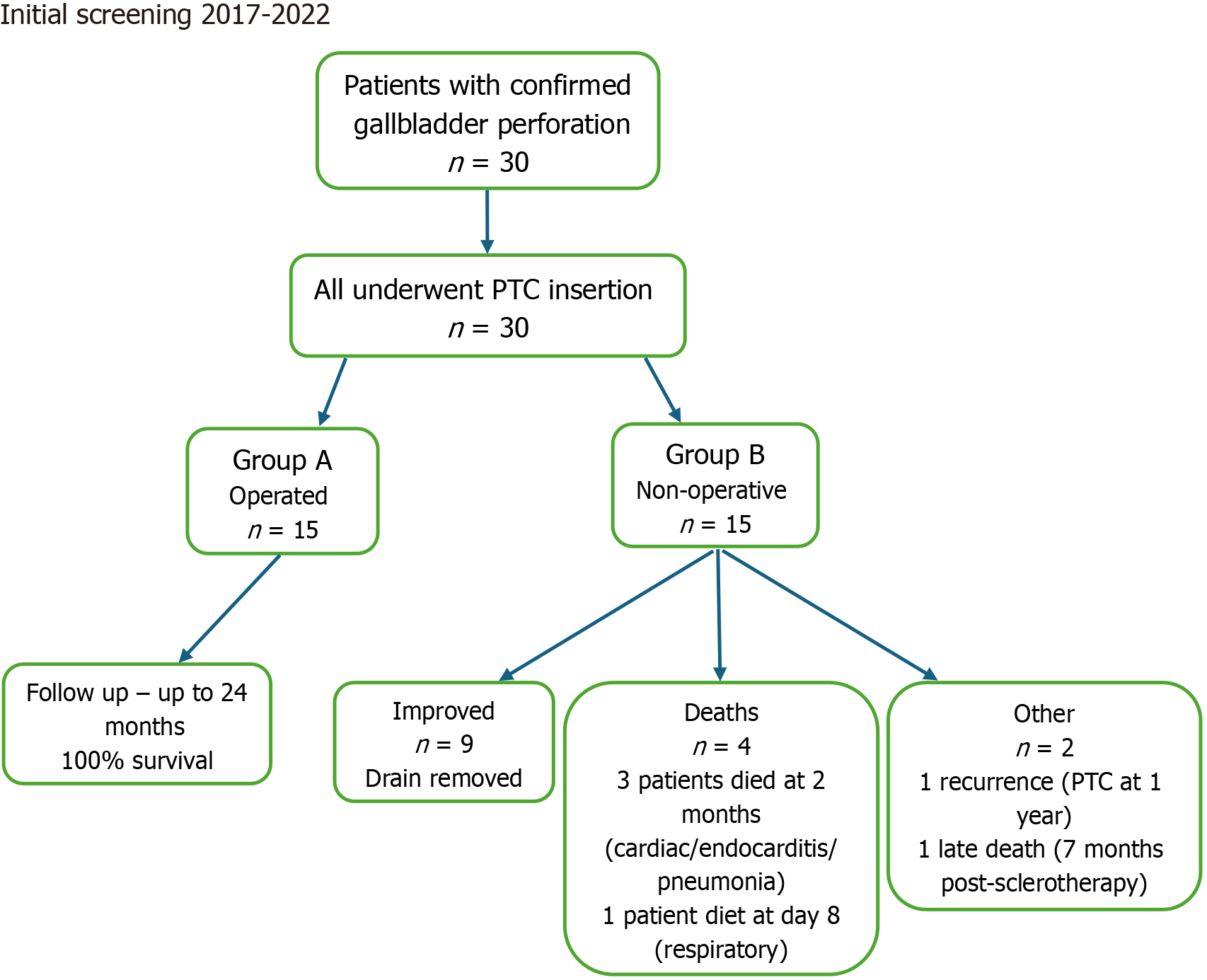
This retrospective cohort study was approved by the Institutional Review Board, approval No. RMC-05010-24. A comprehensive search of the electronic medical records was performed for the period between January 2017 and October 2022 using the terms “cholecystostomy”, “cholecystitis”, and “gallbladder perforation”. Eligible patients included individuals who presented with clinical signs of acute abdomen - abdominal pain, fever, and a positive Murphy’s sign - and who underwent diagnostic imaging with abdominal ultrasound (US) and contrast-enhanced computed tomography. The diagnosis of APC was confirmed based on radiological findings including: Focal or complete gallbladder wall discontinuity, gallbladder wall thickening and edema, gallbladder distension, presence of gallstones or biliary sludge, pericholecystic fluid collection Gas in the gallbladder or biliary system, localized abscess, free intraperitoneal fluid, or pericholecystic inflammatory stranding. Only patients with imaging-confirmed gallbladder perforation diagnosed by a board-certified radiologist were included. Patients under 18 years of age, as well as those with traumatic or iatrogenic gallbladder perforation, were excluded from the study. A flow diagram summarizing the patient selection process, number of patients who underwent surgery, 24-month follow-up, and recorded data on mortality and complications is provided (Figure 1).
In accordance with departmental protocol, all patients diagnosed with APC underwent initial PTC. Cholecystectomy was considered only after clinical stabilization. Following informed consent, the procedure was performed under local anesthesia in either the intensive care unit or the interventional radiology suite. A board-certified interventional radiologist carried out the procedure using real-time US guidance and standard aseptic technique. Vital signs - including blood pressure, heart rate, and oxygen saturation - were continuously monitored throughout. The gallbladder was accessed via a transhepatic approach using a 21-gauge Chiba needle (Cook Medical, Bloomington, IN, United States). Bile was aspirated and sent for culture. The Seldinger technique was then employed, using a 0.018-inch guidewire and Neff set, followed by exchange over a 0.038-inch guidewire. After tract dilation, an 8.3 or 10 French multipurpose pigtail catheter (Cook Medical) was inserted into the gallbladder. Closed-system drainage was performed using a 20 mL syringe and 3-way stopcock until near-complete evacuation was confirmed sonographically. The catheter was then connected to a gravity-assisted drainage bag and flushed twice daily with 5 mL of normal saline to prevent obstruction. The catheter remained in place until drainage output declined to ≤ 10 mL over 24 hours and the patient demonstrated clinical improvement, defined by resolution of abdominal tenderness and fever, along with normalization of leukocyte count. Antibiotics were continued and adjusted according to culture sensitivity results. For patients who underwent subsequent cholecystectomy, the PTC drain was removed intraoperatively.
Data were collected retrospectively from the patients’ medical records. Variables included demographic information, comorbidities, clinical presentation, and laboratory results [visual analog scale pain score, body temperature, pulse, white blood cell (WBC) count, and C-reactive protein (CRP) level]. Additional variables included the American Society of Anesthesiologists physical status classification, hemodynamic stability at presentation, length of hospital stay following PTC insertion, and time interval from PTC to cholecystectomy in operated patients. Complications during hospitalization and follow-up (up to 24 months) were recorded.
All statistical analyses were conducted using IBM SPSS Statistics version 29.0 (IBM Corp., Armonk, NY, United States). Continuous variables were reported as means ± SD, and categorical variables as frequencies and percentages. Com
A total of 30 patients diagnosed with gallbladder perforation underwent PTC. Of these, 15 patients (50%) achieved clinical stabilization and subsequently underwent cholecystectomy. The remaining 15 were managed conservatively until clinical improvement allowed discharge without surgery.
The operated group tended to be younger (76.1 ± 7.4 years) than the non-operated group (87.1 ± 6.2 years, P < 0.001). Most patients in both groups were male (73.3% vs 60.0%, P = 0.493). Diabetes mellitus was significantly more prevalent in the operated group (73.3% vs 30.8%, P = 0.024), while ischemic heart disease and other comorbidities showed no sig
| Variable | Non-operated (n = 15) | Operated (n = 15) | P value |
| Age (year), mean ± SD | 87.1 ± 6.2 | 76.1 ± 7.4 | < 0.001 |
| Male sex (%) | 60.00% | 73.30% | 0.493 |
| Diabetes mellitus (%) | 30.80% | 73.30% | 0.024 |
| Ischemic heart disease (%) | 38.50% | 50.00% | 0.547 |
| Chronic kidney disease (%) | 35.70% | 26.70% | 0.599 |
| Interstitial COPD (%) | 30.80% | 6.70% | 0.097 |
| Variable | Non-operated (n = 15) | Operated (n = 15) | P value |
| VAS score (0-10) | 1.8 ± 2.3 | 3.9 ± 2.8 | 0.031 |
| Body temperature (°C) | 38.0 ± 1.2 | 37.2 ± 1.0 | 0.051 |
| Pulse (bpm) | 99.7 ± 26.7 | 87.0 ± 23.4 | 0.384 |
| WBC (K/μL) | 15.7 ± 8.0 | 15.1 ± 4.7 | 0.811 |
| CRP (mg/dL) | 24.8 ± 17.7 | 15.3 ± 10.1 | 0.265 |
| Hemodynamic stability (%) | 66.70% | 93.30% | 0.068 |
| ASA II (%) | 33.30% | 14.30% | 0.49 |
| ASA III (%) | 66.70% | 85.70% | 0.62 |
| Anticoagulant use (%) | 57.10% | 66.70% | 0.597 |
Diagnostic imaging included US, computed tomography, or both. The use of both modalities was higher in the ope
| Variable | Non-operated | Operated | P value |
| US performed (%) | 71.40% | 93.30% | 0.119 |
| CT performed (%) | 76.90% | 85.70% | 0.557 |
| Both US and CT performed (%) | 53.80% | 80.00% | 0.139 |
| Bile culture obtained (%) | 92.90% | 100.00% | 0.292 |
| Cholelithiasis diagnosed (%) | 86.70% | 93.30% | 1 |
| Sepsis present (%) | 64.30% | 40.00% | 0.191 |
In both groups, WBC and CRP levels significantly decreased from admission to discharge. Among non-operated patients, WBC decreased from 14.6 ± 6.8 K/μL to 8.9 ± 2.9 K/μL (P = 0.004), and CRP from 26.9 ± 14.0 mg/dL to 6.2 ± 7.9 mg/dL (P < 0.001). Similar trends were observed in the operated group (WBC: 15.1 ± 4.7 K/μL to 9.2 ± 2.9 K/μL, P < 0.001; CRP: 19.8 ± 8.8 mg/dL to 3.7 ± 3.4 mg/dL, P = 0.005). However, the changes between admission and discharge values did not differ significantly between the groups (Table 4). Mean hospitalization duration following PTC was comparable between groups (10.9 ± 6.5 days vs 10.3 ± 3.6 days, P = 0.783). Early complications within 30 days included subcutaneous hemorrhage related to the placement of the PTC drain that resolved spontaneously without clinical significance with no significant intergroup differences (50.0% vs 42.9%; P = 0.705).
| Variable | Non-operated | P value | Operated | P value | |
| WBC (K/μL) | Admission | 14.6 ± 6.8 | 0.004 | 15.1 ± 4.7 | < 0.001 |
| Discharge | 8.9 ± 2.99 | 9.2 ± 2.9 | |||
| CRP (mg/dL) | Admission | 26.9 ± 14.1 | < 0.001 | 18.8 ± 6.8 | 0.005 |
| Discharge | 6.2 ± 7.9 | 3.78 ± 3.4 | |||
Among non-operated patients, 61.4% showed sufficient clinical improvement to allow drain removal or closure and were discharged to rehabilitation. Over the 24-month follow-up, one patient developed recurrent cholecystitis requiring repeat PTC. Three patients died within two months post-procedure from unrelated complications (cardiac event, endocarditis, aspiration pneumonia). Two patients underwent sclerotherapy; one of them died eight days post-procedure due to respiratory failure despite resolution of biliary symptoms, and the other died seven months later.
PTC is a minimally invasive, image-guided procedure designed to decompress the gallbladder and control infection, particularly in critically ill or high-risk patients. While PTC is not considered a definitive treatment for gallbladder perforation, it is frequently used as a bridge to surgery once the acute inflammatory process subsides and the patient’s clinical status stabilizes[18-22]. According to the World Society of Emergency Surgery, PTC is recommended in high-risk patients due to its ability to minimize bile leakage and provide effective drainage[23]. The procedure demonstrates high technical success and clinical improvement rates, approaching 90% in some series[18]. Nonetheless, it carries procedural risks, including bile leak, infection, and catheter dislodgement, which can occur in up to 25% of patients[18,19]. Despite these potential complications, PTC remains a key therapeutic option in patients unfit for immediate cholecystectomy[21,22]. At our institution, PTC is routinely performed in all patients diagnosed with APC, regardless of age or comorbidity profile. In this retrospective analysis of 30 such cases, half of the patients were stabilized with PTC and subsequently underwent delayed cholecystectomy, while the remainder were managed non-surgically. Notably, 61.4% of non-operated patients demonstrated sustained clinical improvement and were discharged following drain removal or closure.
While PTC is typically viewed as an adjunct to surgery, there is limited literature examining its use as a standalone treatment in APC. One notable exception is the study by Huang et al[16], which compared outcomes in patients with gallbladder perforation treated with PTC alone vs emergency cholecystectomy. Although the overall mortality rate in their cohort was 24.2%, no deaths occurred in the PTC-only group, which also demonstrated a significantly higher survival rate (100% vs 50%, P = 0.001). Most patients treated solely with PTC were elderly or had multiple comorbidities, factors that precluded surgical intervention during the index hospitalization. Krecko et al[17] similarly evaluated outcomes in 654 patients with APC, comparing immediate vs delayed cholecystectomy following stabilization. Delayed surgery was associated with significantly lower 30-day mortality (0% vs 7%) and fewer major complications (14% vs 33%), supporting the strategy of initial non-operative management followed by elective surgery. Our findings align with these reports, suggesting that PTC is a viable initial treatment strategy in selected high-risk patients with gallbladder perforation. While elective cholecystectomy remains the definitive treatment, PTC can offer substantial clinical benefit in stabilizing the acute phase and, in some cases, may obviate the need for surgery altogether. Its minimally invasive nature, ease of performance, and favorable safety profile make it particularly attractive for elderly or comorbid populations. However, this study has several limitations. First, it is based on a retrospective review conducted at a single medical center, which may limit generalizability. Second, the sample size is relatively small. Third, due to institutional protocol, all patients with APC were treated initially with PTC, and no direct surgical interventions were performed without prior drainage. This introduces a potential selection bias. Lastly, we did not classify the type of gallbladder perforation (e.g., free, localized, or encapsulated), as this distinction did not influence our management approach; all patients were treated uniformly with PTC regardless of perforation subtype.
Our findings suggest that PTC is an effective initial treatment modality in patients with acute gallbladder perforation, particularly those who are elderly or have significant comorbidities. Most patients experienced clinical improvement within a 24-month follow-up period, and a considerable proportion avoided surgery altogether. While further pro
| 1. | Di Ciaula A, Wang DQ, Portincasa P. An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol. 2018;34:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 2. | Quiroga-Garza A, Alvarez-Villalobos NA, Angeles-Mar HJ, Garcia-Campa M, Muñoz-Leija MA, Salinas-Alvarez Y, Elizondo-Omaña RE, Guzmán-López S. Localized gallbladder perforation: a systematic review of treatment and prognosis. HPB (Oxford). 2021;23:1639-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Puglisi M, Peter M, Egger B. Perforated Gallbladder into the Abdominal Wall. Case Rep Surg. 2022;2022:4782539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Arkoudis NA, Moschovaki-Zeiger O, Grigoriadis S, Palialexis K, Reppas L, Filippiadis D, Alexopoulou E, Brountzos E, Kelekis N, Spiliopoulos S. US-guided trocar versus Seldinger technique for percutaneous cholecystostomy (TROSELC II trial). Abdom Radiol (NY). 2023;48:2425-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Krishnamurthy G, Ganesan S, Ramas J, Damodaran K, Khanna A, Patta R. Early laparoscopic cholecystectomy in acute gallbladder perforation: Single-centre experience. J Minim Access Surg. 2021;17:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Van Steenbergen W, Ponette E, Marchal G, Pelemans W, Aerts R, Fevery J, De Groote J. Percutaneous transhepatic cholecystostomy for acute complicated cholecystitis in elderly patients. Am J Gastroenterol. 1990;85:1363-1369. [PubMed] |
| 7. | Horn T, Christensen SD, Kirkegård J, Larsen LP, Knudsen AR, Mortensen FV. Percutaneous cholecystostomy is an effective treatment option for acute calculous cholecystitis: a 10-year experience. HPB (Oxford). 2015;17:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Aroori S, Mangan C, Reza L, Gafoor N. Percutaneous Cholecystostomy for Severe Acute Cholecystitis: A Useful Procedure in High-Risk Patients for Surgery. Scand J Surg. 2019;108:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Jones MW, Santos G, Patel PJ, O'Rourke MC. Acute Cholecystitis. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 10. | Pang KW, Tan CH, Loh S, Chang KY, Iyer SG, Madhavan K, Kow WC. Outcomes of Percutaneous Cholecystostomy for Acute Cholecystitis. World J Surg. 2016;40:2735-2744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Bundy J, Srinivasa RN, Gemmete JJ, Shields JJ, Chick JFB. Percutaneous Cholecystostomy: Long-Term Outcomes in 324 Patients. Cardiovasc Intervent Radiol. 2018;41:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Fleming CA, Ismail M, Kavanagh RG, Heneghan HM, Prichard RS, Geoghegan J, Brophy DP, McDermott EW. Clinical and Survival Outcomes Using Percutaneous Cholecystostomy Tube Alone or Subsequent Interval Cholecystectomy to Treat Acute Cholecystitis. J Gastrointest Surg. 2020;24:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Little MW, Briggs JH, Tapping CR, Bratby MJ, Anthony S, Phillips-Hughes J, Uberoi R. Percutaneous cholecystostomy: the radiologist's role in treating acute cholecystitis. Clin Radiol. 2013;68:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Devane AM, Annam A, Brody L, Gunn AJ, Himes EA, Patel S, Tam AL, Dariushnia SR. Society of Interventional Radiology Quality Improvement Standards for Percutaneous Cholecystostomy and Percutaneous Transhepatic Biliary Interventions. J Vasc Interv Radiol. 2020;31:1849-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (2)] |
| 15. | Wadhwa V, Trivedi PS, Makary MS, Strain DV, Ahmed O, Chick JFB, Charalel RA. Utilization and Outcomes of Cholecystostomy and Cholecystectomy in Patients Admitted With Acute Cholecystitis: A Nationwide Analysis. AJR Am J Roentgenol. 2021;216:1558-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Huang CC, Lo HC, Tzeng YM, Huang HH, Chen JD, Kao WF, Yen DH, Huang CI, Lee CH. Percutaneous transhepatic gall bladder drainage: a better initial therapeutic choice for patients with gall bladder perforation in the emergency department. Emerg Med J. 2007;24:836-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Krecko LK, Hoyos Gomez T, Scarborough JE, Jung HS. Postoperative Outcomes after Index vs Interval Cholecystectomy for Perforated Cholecystitis. J Am Coll Surg. 2021;232:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Baron TH, Grimm IS, Swanstrom LL. Interventional Approaches to Gallbladder Disease. N Engl J Med. 2015;373:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Baron TH, Jorge I, Husnain A, Benias PC, Reames BN, Bhanushali A, Docimo S Jr, Bloom M, Salem R, Murphy P, Singh H, Varadarajulu S, Riaz A. Comprehensive Review of the Management of Patients with Acute Cholecystitis Who Are Ineligible for Surgery. Ann Surg. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Hasbahceci M, Cengiz MB, Malya FU, Kunduz E, Memmi N. The impact of a percutaneous cholecystostomy catheter in situ until the time of cholecystectomy on the development of recurrent acute cholecystitis: a historical cohort study. Rev Esp Enferm Dig. 2018;110:629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Iino C, Shimoyama T, Igarashi T, Aihara T, Ishii K, Sakamoto J, Tono H, Fukuda S. Perforated emphysematous cholecystitis managed by endoscopic transpapillary gallbladder drainage. Clin J Gastroenterol. 2017;10:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Doi S, Yasuda I, Mabuchi M, Iwata K, Ando N, Iwashita T, Uemura S, Okuno M, Mukai T, Adachi S, Taniguchi K. Hybrid procedure combining endoscopic gallbladder lavage and internal drainage with elective cholecystectomy for acute cholecystitis: A prospective pilot study (The BLADE study). Dig Endosc. 2018;30:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Pisano M, Ceresoli M, Cimbanassi S, Gurusamy K, Coccolini F, Borzellino G, Costa G, Allievi N, Amato B, Boerma D, Calcagno P, Campanati L, Campanile FC, Casati A, Chiara O, Crucitti A, di Saverio S, Filauro M, Gabrielli F, Guttadauro A, Kluger Y, Magnone S, Merli C, Poiasina E, Puzziello A, Sartelli M, Catena F, Ansaloni L. 2017 WSES and SICG guidelines on acute calcolous cholecystitis in elderly population. World J Emerg Surg. 2019;14:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/