Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.108930
Revised: June 30, 2025
Accepted: August 12, 2025
Published online: October 27, 2025
Processing time: 178 Days and 2.1 Hours
The clinical necessity of routine abdominal drainage following radical gas
To evaluate the necessity of abdominal drainage tube placement following radical gastrectomy in the context of ERAS protocols.
A systematic review and meta-analysis were conducted by searching PubMed, EMBASE, Cochrane Library, Web of Science, China National Knowledge Infra
A total of 21 randomized controlled trials involving 1652 patients were included. Compared with routine abdominal drainage, the ERAS group without drainage showed significantly faster gastrointestinal recovery [standardized mean difference = -1.30, 95% confidence interval (CI): -1.66 to -0.94, P < 0.00001] and shorter hospital stay (standardized mean difference = -1.37, 95%CI: -1.86 to -0.88, P < 0.00001). The incidence of total postoperative complications was also significantly lower (odds ratio = 0.53, 95%CI: 0.40-0.70, P < 0.00001), particularly for anastomotic leakage and pulmonary infection. No significant differences were observed in surgical site infections or urinary tract infections. Sensitivity and subgroup analyses indicated stability of results, although some heterogeneity was noted.
Avoiding routine abdominal drainage under ERAS could lead to faster recovery, reduced complications, and shorter hospital stay following radical gastrectomy, supporting the selective use of drainage rather than routine.
Core Tip: Enhanced recovery after surgery in radical gastrectomy supports early drain removal, faster gastrointestinal recovery, shorter hospitalization, and fewer complications. This review of 21 randomized controlled trials highlights safety and benefits, but also the need for better protocol standardization and region-specific adaptation, as current data stem solely from Asian populations. Strengthening protocol standardization, interdisciplinary coordination, and localized implementa
- Citation: Li HY, Liu Y, Cui WX, Zhao Q. Enhanced recovery after surgery in gastric cancer surgery: Systematic review and meta-analysis of perioperative indwelling drainage tube use. World J Gastrointest Surg 2025; 17(10): 108930
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/108930.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.108930
Gastric cancer (GC) is one of the most common malignant tumors of the digestive system worldwide[1]. According to Global Cancer Observatory estimates, in 2020, the global incidence of GC reached 1.089 million cases[2], ranking fifth among all cancer types, with 480000 new cases reported in China alone[3]. Enhanced recovery after surgery (ERAS), also known as fast-track surgery (FTS), was first proposed by Danish scholar Henrik Kehlet in 1990, and introduced into China by Academician Li and Jiang[4] in 2015. Postoperative complications, particularly bleeding, infection, and anastomotic leakage, remain major concerns after GC surgery, with incidence rates varying by surgical approach, patient profile, and perioperative care, often leading to delayed recovery, extended hospitalization, and higher medical costs[5]. ERAS protocols, developed through multidisciplinary collaboration and evidence-based medicine, aim to shorten hospital stays, reduce complications, improve patient outcomes, and ultimately enhance survival rates. However, the application of ERAS in GC has lagged behind other surgical specialties[6,7]. Traditionally, radical gastrectomy involves extensive lymph node dissection, which can lead to significant wound exudation and therefore requires prophylactic placement of an abdominal drainage tube to drain abdominal fluid and prevent intra-abdominal infections. Although current guidelines recommend minimizing and promoting early removal of drainage tubes to reduce complications such as infection, adherence to these guidelines remains suboptimal[8]. Moreover, some surgeons continue to question the safety and efficacy of this approach, underscoring the need for further high-quality studies to assess the actual impact of ERAS on perioperative management in GC[9].
A particular point of contention between ERAS protocols and traditional surgical practices is the routine placement of an abdominal drainage tube during radical gastrectomy. This systematic review, therefore, aims to evaluate the safety and efficacy of abdominal drainage tube placement under ERAS, providing a more robust and systematic basis for clinical decision-making and offering strong evidence for perioperative nursing care of patients with GC.
This study was prospectively registered in the PROSPERO International Systematic Review Registry (https://www.crd.york.ac.uk/prospero/, accessed on August 31, 2024; registration number CRD42023429639). The study follows the PRISMA guidelines[10].
The search strategy was developed following the Cochrane Handbook (2011) guidelines. The databases screened included China National Knowledge Infrastructure, WanFang, VIP Information, Web of Science, PubMed, and MEDLINE (from January 1, 2000 to August 31, 2024). The search terms included GC, gastric tumor, abdominal drainage, FTS, ERAS, randomized controlled trial (RCT), GC surgery, ERAS, FTS, stomach neoplasms, covering both Chinese and English literature.
Inclusion criteria: “Population”: (1) Patients with GC, with no restrictions on gender, age, nationality, or ethnicity; and (2) The diagnosis of GC was confirmed by postoperative histopathological examination[11]. “Intervention”: In accordance with the 2014 European ERAS Society guidelines for gastrointestinal surgery[12], which define 25 evidence-based perioperative elements across three stages (preoperative, intraoperative, and postoperative), we considered studies eligible if they reported implementing at least 10 of the following validated components: Shortened preoperative fasting, pre
Exclusion criteria: (1) Patients who had undergone preoperative chemotherapy or had severe underlying diseases; (2) Non-RCTs; and (3) Studies for which full text was unavailable or where data were incomplete.
Two researchers (L, L) followed the search strategy, conducted an initial screening of the literature by reading the titles, and performed a secondary screening for eligible studies before downloading the full texts. The researchers indepen
In this study, we used both the Jadad scale and the Cochrane risk of bias tool (RevMan 5.4) to assess methodological quality[16]. The Jadad scale evaluates random sequence generation (0-2 points), implementation of blinding (0-2 points), and reporting of withdrawals and dropouts (0-1 point), yielding a total score between 0 and 5. Studies scoring ≥ 3 points were considered high quality, while those scoring ≤ 2 were classified as low quality. The Cochrane risk of bias tool (RevMan 5.4) assessed seven domains, including selection bias (random sequence generation and allocation conceal
According to the Cochrane Handbook guidelines[17], if post-intervention values with standard deviations are unavai
Review Manager 5.3 was used for data analysis. Heterogeneity was assessed using Q-tests and I2 tests, and forest plots were used to present relative risk/odds ratio (OR), mean difference/standardized mean difference (SMD), and 95% confidence intervals (CIs). Based on P values and I2 values, an appropriate effect model was selected: When I2 < 50%, a fixed-effects model (FEM) was used; when I2 > 50%, a random-effects model (REM) was applied[18].
Subgroup analysis was conducted for time to first flatus, time to first oral intake, time to first ambulation, and urinary catheter removal time. Sensitivity analysis was performed by modifying key influencing factors to assess whether changes occurred in the results, thereby determining the stability and robustness of the findings.
The Cochrane risk of bias tool was used to assess the risk of bias in the included studies. Publication bias was analyzed using funnel plots, and the Grades of Recommendations Assessment, Development and Evaluation (GRADE) rating system was applied to evaluate the results of the traditional meta-analysis. A combined effect analysis was conducted for key and important outcomes in the meta-analysis to integrate both direct and indirect evidence.
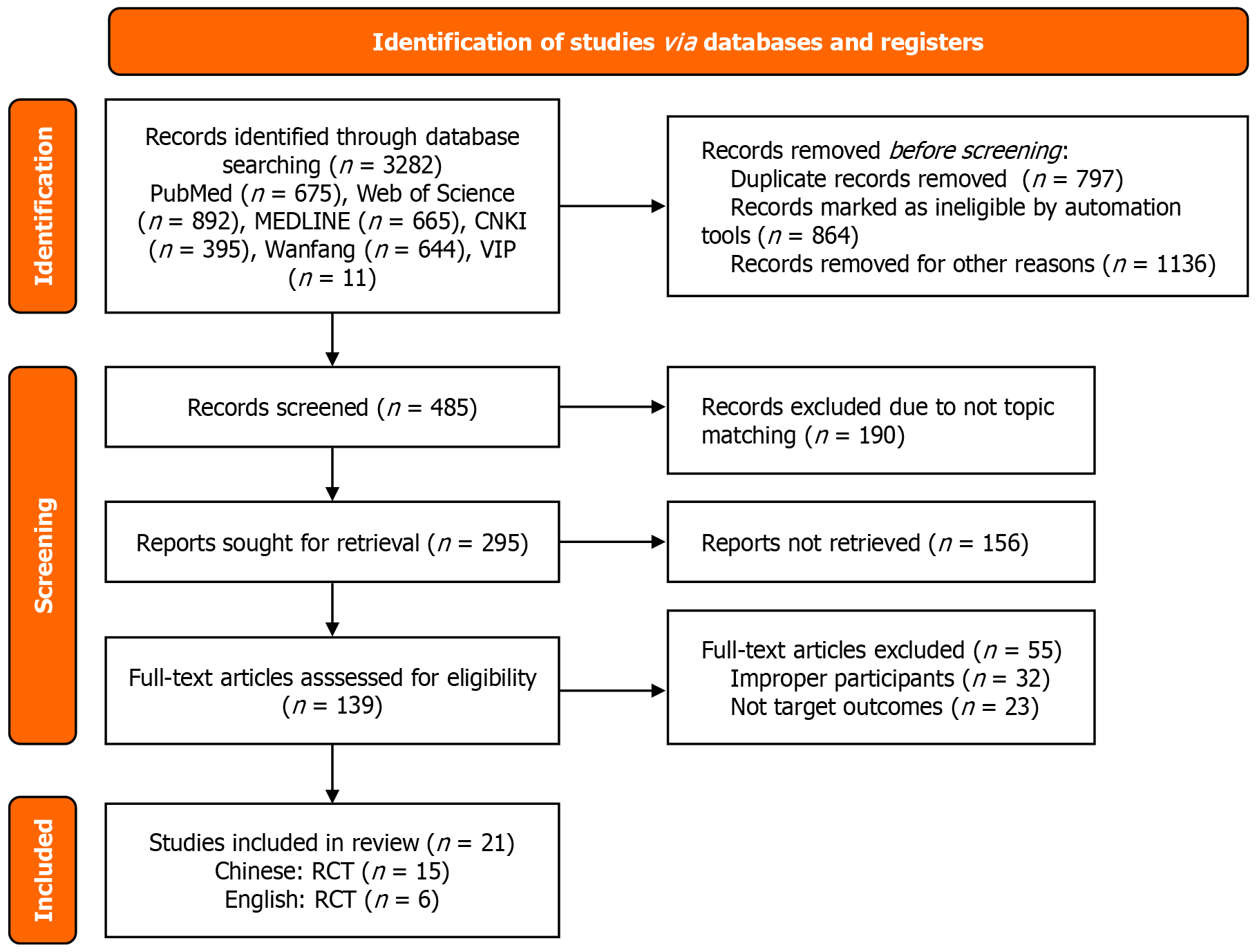
A total of 2232 Chinese-language studies and 1050 English-language studies were initially identified. After removing 2797 duplicate publications, 139 studies were included after screening by title and abstract. Studies without clear outcome indicators or with unavailable full texts were excluded. The literature screening process is shown in Figure 1.
A total of 21 randomized controlled trials were finally included[19-39] (2 from South Korea and 19 from China). The surgical procedures encompassed total gastrectomy or subtotal gastrectomy with D2 or more extensive lymphadenectomy, radical gastrectomy, and laparoscopic-assisted radical gastrectomy. All 21 included studies were two-arm trials, with the intervention group based on the concepts of FTS and ERAS. The basic characteristics of the included studies are shown in Table 1, Figure 2.
| Ref. | Location | Intervention period | Study participants | Surgical method | Sample size | Intervention group | Control group | Outcome indicators |
| Kim et al[19], 2004 | Korea | February 1, 2001-July 31, 2001 | Gastric cancer patients | Total or subtotal gastrectomy with D2 or more lymph node dissection | I = 31, C = 21 | FTS | Conventional care | 1, 2, 13 |
| Wang et al[20], 2019 | China | March 1, 2016-October 31, 2016 | Gastric cancer patients | Radical gastrectomy for gastric cancer | I = 30, C = 30 | ERAS | Conventional care | 1, 4, 6, 7, 8, 10, 11, 12, 13, 14 |
| Kim et al[21], 2012 | Korea | April 1, 2011-January 31, 2012 | Gastric cancer patients | Laparoscopy-assisted radical gastrectomy with D2 Lymph node dissection | I = 22, C = 22 | FTS | Conventional care | 4, 9, 14 |
| Abdikarim et al[22], 2015 | China | June 1, 2010-December 31, 2012 | Advanced gastric cancer patients | Laparoscopic surgery | I = 30, C = 31 | ERAS | Conventional care | 2, 3, 10, 13 |
| Liu et al[23], 2016 | China | September 1, 2014-August 31, 2015 | Elderly gastric cancer patients | Laparoscopic surgery | I = 21, C = 21 | FTS | Conventional care | 1, 4, 5, 10, 11, 13, 14 |
| Chen et al[24], 2012 | China | January 1, 2009-May 31, 2011 | Gastric cancer patients | Laparoscopy-assisted radical distal gastrectomy | I = 19, C = 22 | FTS | Conventional care | 5 |
| Wu[25], 2018 | China | December 1, 2015-August 31, 2017 | Gastric cancer patients | Laparoscopic D2 radical gastrectomy | I = 34, C = 41 | ERAS | Conventional care | 1, 4, 9, 10, 13, 14 |
| Chen[26], 2019 | China | March 1, 2017-February 28, 2018 | Advanced gastric cancer patients | D2 radical gastrectomy | I = 38, C = 37 | ERAS | Conventional care | 1, 4, 5, 9, 10, 12, 13, 14 |
| Wu[27], 2019 | China | June 1, 2016-June 30, 2017 | Malignant gastric tumor patients | Total laparoscopic distal gastrectomy | I = 35, C = 35 | ERAS | Conventional care | 1, 4, 5, 9, 10, 11, 12, 13 |
| Ma[28], 2019 | China | January 1, 2018-January 31, 2019 | Gastric cancer patients | Laparoscopic D2 radical gastrectomy | I = 40, C = 40 | ERAS | Conventional care | 4, 9, 11, 12 |
| Wang[29], 2017 | China | March 1, 2016-October 31, 2016 | Gastric cancer patients | Radical gastrectomy for gastric cancer | I = 30, C = 30 | ERAS | Conventional care | 1, 4, 6, 8, 9, 10, 11, 12, 13, 14 |
| Jiang et al[30], 2007 | China | January 1, 2006-December 31, 2006 | Gastric cancer patients | D2 radical resection | I = 40, C = 40 | FTS | Conventional care | 1, 4, 5, 9 |
| Fan et al[31], 2019 | China | April 1, 2017-May 31, 2018 | Gastric cancer patients | Total gastrectomy or partial gastrectomy | I = 30, C = 30 | FTS | Conventional care | 1, 9, 11, 12 |
| Li et al[32], 2016 | China | July 1, 2013-February 28, 2015 | Gastric adenocarcinoma patients | Laparoscopic radical gastrectomy | I = 67, C = 60 | ERAS | Conventional care | 1, 3, 4, 9, 12, 14 |
| Chen[33], 2019 | China | January 1, 2017-January 31, 2018 | Gastric cancer patients | Laparoscopic surgery | I = 50, C = 50 | FTS | Conventional care | 1, 2, 4, 9, 10, 12, 13 |
| Ruan et al[34], 2019 | China | January 1, 2016-May 31, 2018 | Gastric cancer patients | Laparoscopic radical gastrectomy | I = 65, C = 65 | ERAS | Conventional care | 1, 2, 9 |
| Cheng[35], 2019 | China | January 1, 2017-June 30, 2019 | Gastric cancer patients | Laparoscopic radical gastrectomy | I = 29, C = 29 | ERAS | Conventional care | 1, 4, 6, 9, 10, 12, 13 |
| Liu et al[36], 2018 | China | May 1, 2015-July 31, 2017 | Gastric cancer patients | Laparoscopic radical gastrectomy | I = 75, C = 74 | ERAS | Conventional care | 1, 2, 4, 8, 9, 10, 12, 14 |
| Li and Huang[37], 2021 | China | June 1, 2017-June 30, 2019 | Gastric cancer patients | Laparoscopic radical gastrectomy | I = 41, C = 41 | FTS | Conventional care | 1, 4, 7, 10, 12 |
| Miao[38], 2021 | China | February 1, 2018-March 31, 2019 | Gastric cancer patients | Laparoscopic D2 radical gastrectomy | I = 60, C = 60 | ERAS | Conventional care | 1, 4, 7, 9, 10, 11, 13 |
| Liu and Li[39], 2021 | China | March 1, 2017-May 31, 2020 | Patients undergoing total gastrectomy for gastric cancer | Radical total gastrectomy | I = 43, C = 43 | ERAS | Conventional care | 4, 7, 8, 9, 12, 13 |
ERAS elements: Table 2 describes the implementation of perioperative ERAS core components in each study. Preope
| Serial number | Phase | Enhanced recovery after surgery core projects | [19] | [20] | [21] | [22] | [23] | [24] | [25] | [26] | [27] | [28] | [29] | [30] | [31] | [32] | [33] | [34] | [35] | [36] | [37] | [38] | [39] |
| 1 | Preoperative | Preoperative education | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2 | Smoking and alcohol cessation before surgery | - | √ | - | - | - | √ | - | - | - | - | √ | - | - | - | - | - | - | - | - | - | √ | |
| 3 | Preoperative visit and assessment | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 4 | Prehabilitation | √ | √ | - | - | - | - | √ | √ | √ | - | √ | √ | √ | - | - | - | √ | - | - | - | - | |
| 5 | Preoperative nutritional support | - | √ | √ | - | - | √ | √ | - | √ | - | - | - | - | - | - | - | - | - | √ | √ | - | |
| 6 | Preventive antithrombotic treatment | - | - | - | - | - | - | - | - | √ | - | - | - | - | - | - | - | - | - | - | √ | √ | |
| 7 | Shortened preoperative fasting and drinking time | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 8 | No routine bowel preparation | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | - | - | - | |
| 9 | Preoperative anesthesia medication | - | √ | - | - | - | - | - | - | - | √ | √ | - | - | - | - | - | - | - | - | - | - | |
| 10 | Prophylactic antibiotics and skin preparation | - | √ | √ | - | - | - | - | - | - | √ | - | - | - | - | √ | - | - | - | - | - | √ | |
| 11 | Anesthesia method, drug selection, and stress control | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | - | - | √ | - | √ | - | √ | √ | √ | √ | |
| 12 | Intraoperative | Multimodal opioid-sparing analgesia | - | √ | √ | - | √ | - | √ | - | √ | √ | √ | - | - | - | - | - | - | - | - | √ | - |
| 13 | Inflammation control | - | √ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | √ | - | - | - | - | |
| 14 | Airway management and lung protection strategy | - | √ | - | - | - | - | - | - | √ | - | - | - | - | - | - | - | - | - | - | - | - | |
| 15 | Brain protection strategy | - | - | √ | - | √ | - | √ | - | √ | √ | √ | - | - | - | √ | - | - | - | - | - | - | |
| 16 | Intraoperative fluid and circulation management | √ | √ | √ | √ | √ | √ | - | √ | - | √ | √ | - | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 17 | Intraoperative temperature management | - | √ | - | - | √ | - | √ | √ | √ | √ | √ | √ | - | √ | - | - | √ | √ | - | √ | - | |
| 18 | Surgical method and quality | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | - | - | - | |
| 19 | Perioperative blood glucose control | - | √ | - | - | - | - | - | - | √ | - | - | - | - | - | - | - | - | - | - | - | - | |
| 20 | No routine nasogastric tube placement | √ | √ | - | √ | √ | √ | √ | - | √ | √ | √ | √ | √ | √ | √ | √ | √ | - | - | - | - | |
| 21 | No routine abdominal drainage | √ | √ | √ | √ | √ | √ | √ | - | √ | √ | √ | √ | √ | √ | √ | - | - | - | - | - | ||
| 22 | Intraoperative urinary catheter placement | √ | √ | √ | √ | √ | √ | √ | - | √ | √ | - | - | - | √ | - | - | √ | - | - | - | - | |
| 23 | Perioperative fluid therapy | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 24 | Postoperative | Postoperative pain management | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 25 | Prevention and treatment of PONV | - | √ | - | - | - | - | - | - | - | √ | - | - | - | - | - | - | - | - | - | - | - | |
| 26 | Early postoperative oral intake | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 27 | Blood management | - | - | - | - | √ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 28 | Early postoperative mobilization | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||
| 29 | Discharge criteria | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 30 | Follow-up and outcome evaluation | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Total | 16 | 27 | 19 | 16 | 20 | 18 | 20 | 15 | 23 | 22 | 21 | 15 | 15 | 16 | 15 | 15 | 17 | 14 | 12 | 15 | 14 | ||
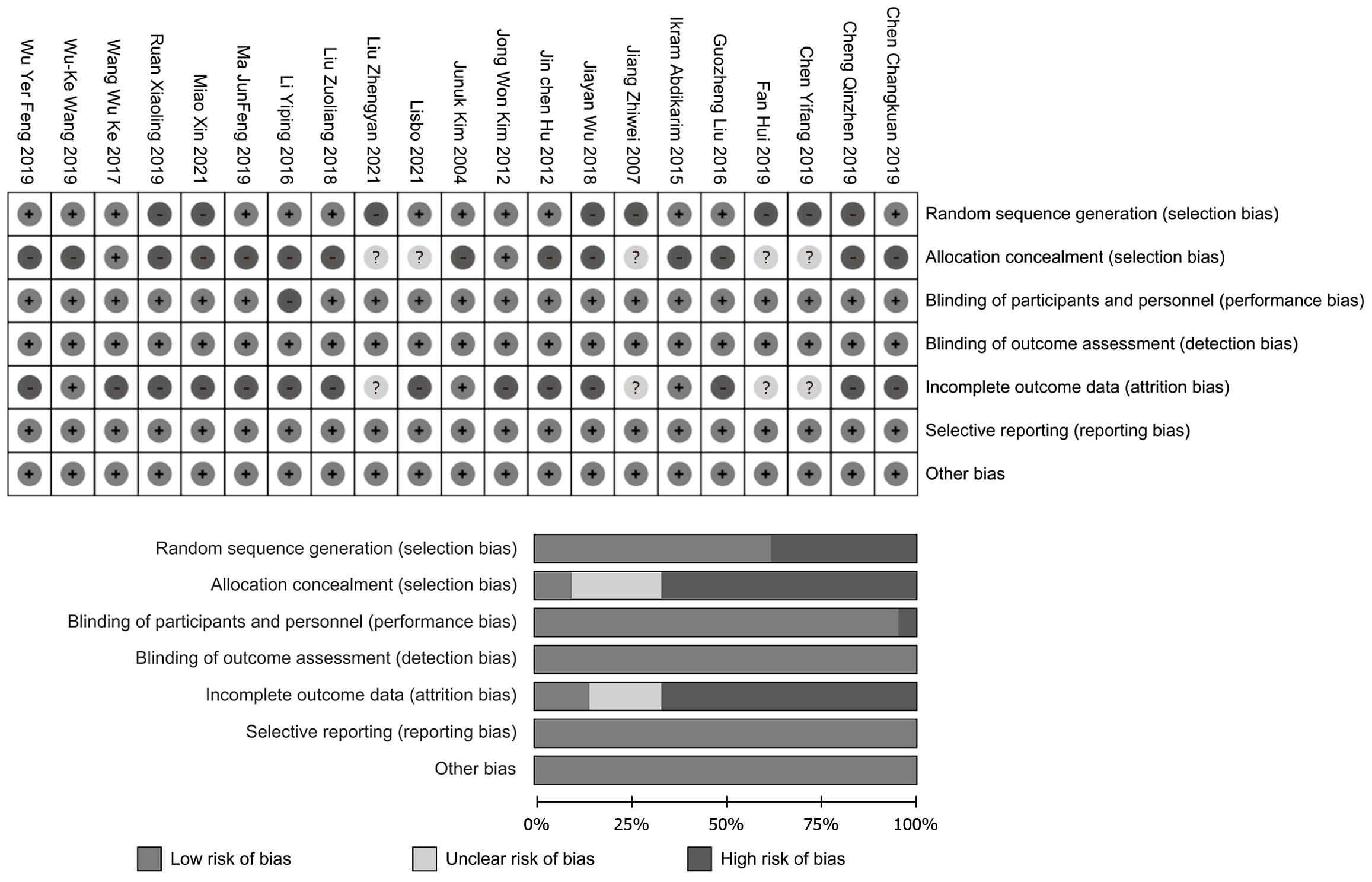
Quality assessment of the included studies based on the Cochrane Handbook is shown in Table 3. The Cochrane + Jadad scale was used to evaluate the quality of the included studies, with an overall rating of moderate to high quality. Two researchers independently assessed the studies, achieving a Kappa consistency of 0.870[40]. Among the 21 included studies[19-39], eight studies had a high risk of bias in the description of randomization methods[25,30,31,33-35,38,39], and 14 studies did not report withdrawal or loss to follow-up[21,23-25,28,34-38].
| Ref. | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | Jadad score | |
| Kim et al[19], 2004 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 7 |
| Wang et al[20], 2019 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 7 |
| Kim et al[21], 2012 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 5 |
| Abdikarim et al[22], 2015 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 5 |
| Liu et al[23], 2016 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 5 |
| Chen et al[24], 2012 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 6 |
| Wu[25], 2018 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 5 |
| Chen[26], 2019 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 5 |
| Wu[27], 2019 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 5 |
| Ma[28], 2019 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 5 |
| Wang[29], 2017 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 6 |
| Jiang et al[30], 2007 | 2 | 3 | 1 | 1 | 3 | 1 | 1 | 4 |
| Fan et al[31], 2019 | 2 | 3 | 1 | 1 | 3 | 1 | 1 | 3 |
| Li et al[32], 2016 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 4 |
| Chen[33], 2019 | 2 | 3 | 1 | 1 | 3 | 1 | 1 | 3 |
| Ruan et al[34], 2019 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 4 |
| Cheng[35], 2019 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 4 |
| Liu et al[36], 2018 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 4 |
| Li and Huang[37], 2021 | 1 | 3 | 1 | 1 | 2 | 1 | 1 | 3 |
| Miao[38], 2021 | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 3 |
| Liu and Li[39], 2021 | 2 | 3 | 1 | 1 | 3 | 1 | 1 | 3 |
Gastrointestinal function recovery: (1) Time to first flatus: Sixteen studies[19,20,23,25-27,29-38] were divided into two subgroups (P ≤ 0.1 and I2 > 50%), showing no statistically significant differences; therefore, a REM was used. Time to first flatus (hours) [weighted mean difference (WMD) = -10.24 (-12.69 to -7.79), P < 0.01]. Time to first flatus (days) [WMD =
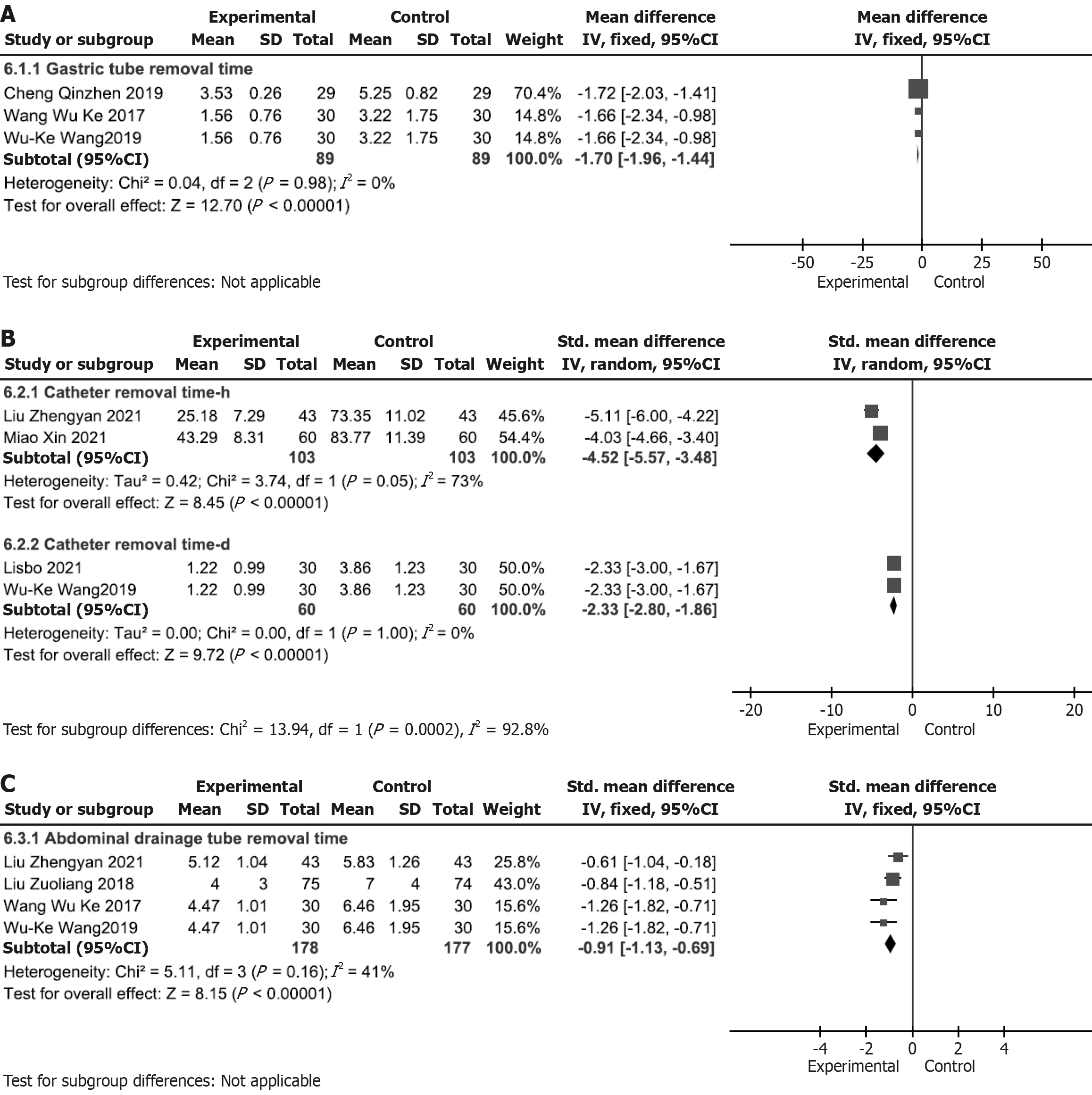
Time to drainage tube removal: (1) Time to nasogastric tube removal: Three studies[20,29,35] (P > 0.1 and I2 < 50%) showed statistically significant differences; therefore, FEM was used. Time to nasogastric tube removal [WMD = -1.70
Incidence of complications: (1) Overall Incidence of complications: Sixteen studies[21,25-39] (P > 0.1 and I2 < 50%) showed statistically significant differences; therefore, FEM was used. Overall incidence of complications [OR = 0.41 (0.30-0.05), P < 0.01], Figure 5A; (2) Incidence of intestinal obstruction: Twelve studies[20,22,23,25-27,29,33,35-38] (P > 0.1 and I2 < 50%) showed statistically significant differences; therefore, FEM was used. Incidence of intestinal obstruction [OR = 0.83 (0.41-1.71), P = 0.62], Figure 5B; (3) Incidence of nausea and vomiting: Six studies[20,23,27,29,31,38] (P > 0.1 and I2 < 50%) showed statistically significant differences; therefore, FEM was used. Incidence of nausea and vomiting [OR = 1.07 (0.52-2.20), P = 0.85], Figure 5C; (4) Incidence of anastomotic leakage: Twelve studies[20,26-29,31-33,35-37,39] (P > 0.1 and I2 < 50%) showed statistically significant differences; therefore, FEM was used. Incidence of anastomotic leakage [OR = 0.37 (0.15-0.89), P = 0.03], Figure 5D; (5) Incidence of surgical site infection: Thirteen studies[19,20,22,23,25-27,29,33,35,36,38,39] (P > 0.1 and I2 < 50%) showed statistically significant differences; therefore, FEM was used. Incidence of surgical site infection [OR = 0.56 (0.27-1.18), P = 0.13], Figure 5E; (6) Incidence of urinary tract infection: Eight studies[20,21,23,25,26,29,32,36] (P > 0.1 and I2 < 50%) showed statistically significant differences; therefore, FEM was used. Incidence of urinary tract infection [OR = 0.39 (0.14-1.06), P = 0.06], Figure 5F; and (7) Incidence of pulmonary infection: Nine studies[20,23,27,29,32,33,35,36,39] (P > 0.1 and I2 < 50%) showed statistically significant differences; therefore, FEM was used. Incidence of pulmonary infection [OR = 0.38 (0.17-0.83), P = 0.02], Figure 5G.
Sensitivity analysis was conducted based on the degree of heterogeneity. When using the REM, the results did not significantly differ from those of the FEM, indicating the stability of the findings. Additionally, after excluding low-quality studies and reanalyzing the data using a traditional meta-analysis approach, the overall effect size remained unchanged, demonstrating the robustness of the results[10].
The time to first flatus outcome was divided into two subgroups, with subgroup 1 including 7 studies and subgroup 2 including 9 studies, all reporting on the impact of ERAS interventions on postoperative flatus time. The meta-analysis showed heterogeneity exceeding 50%. After sequentially removing studies with high weights, the overall meta-analysis results remained unchanged, suggesting good overall stability.
Sensitivity analysis of the time to first flatus subgroup revealed that three studies[25-27] significantly deviated from the X-axis in the forest plot. After excluding these studies, heterogeneity disappeared (P ≤ 0.1 and I2 > 50%), and the difference was not statistically significant, confirming the use of the REM. The results remained statistically significant for subgroup 1 [SMD = -1.11, 95%CI: -1.32 to -0.90, P < 0.01] and subgroup 2 [SMD = -1.34, 95%CI: -1.83 to -0.85, P < 0.01], indicating stable findings.
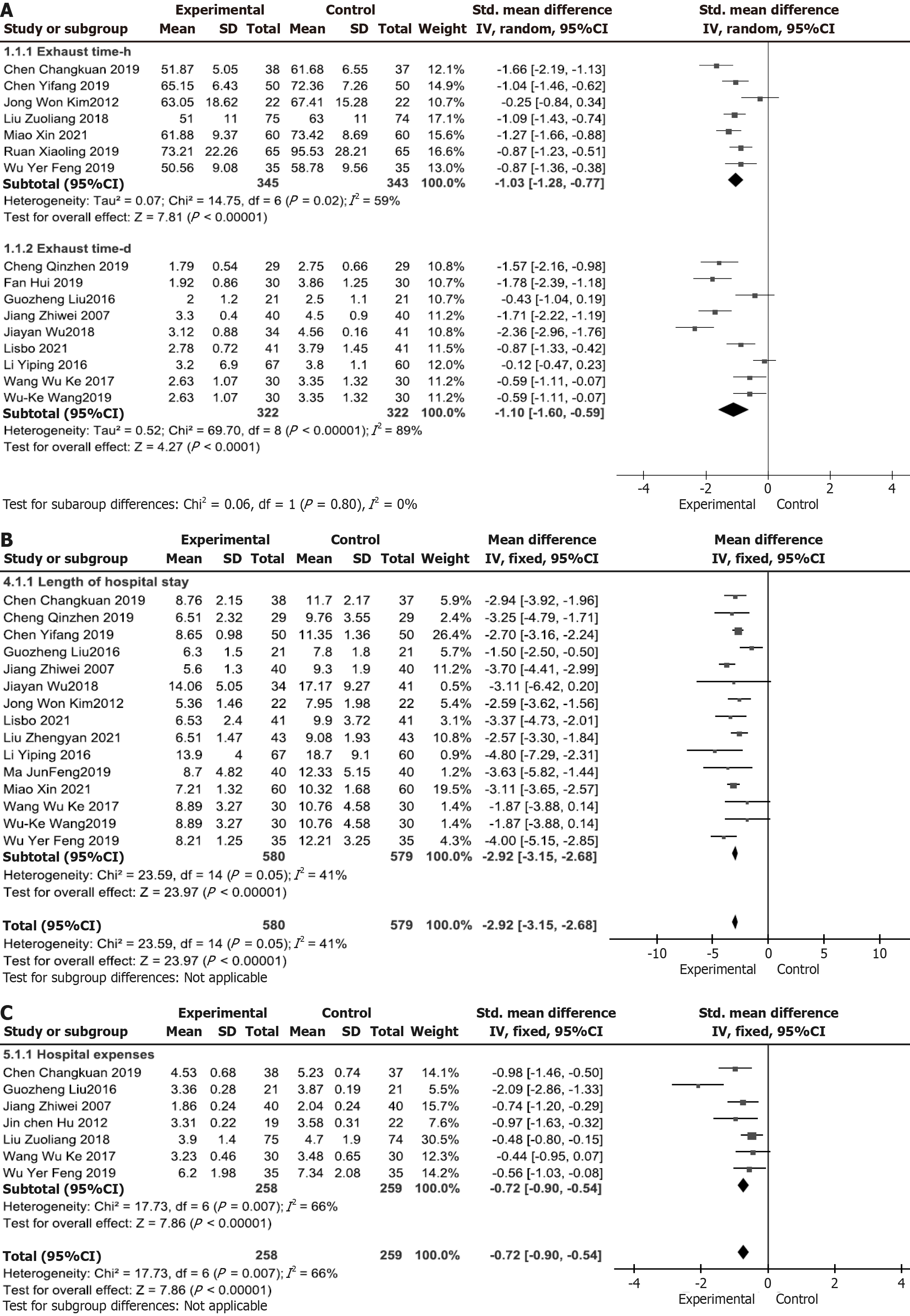
For hospitalization costs, the sensitivity analysis identified one study that significantly deviated from the X-axis in the forest plot. After excluding this study, heterogeneity disappeared (P ≤ 0.1 and I2 > 50%), with no statistically significant difference, supporting the use of the REM. The results remained statistically significant (SMD = -0.64, 95%CI: -0.83 to
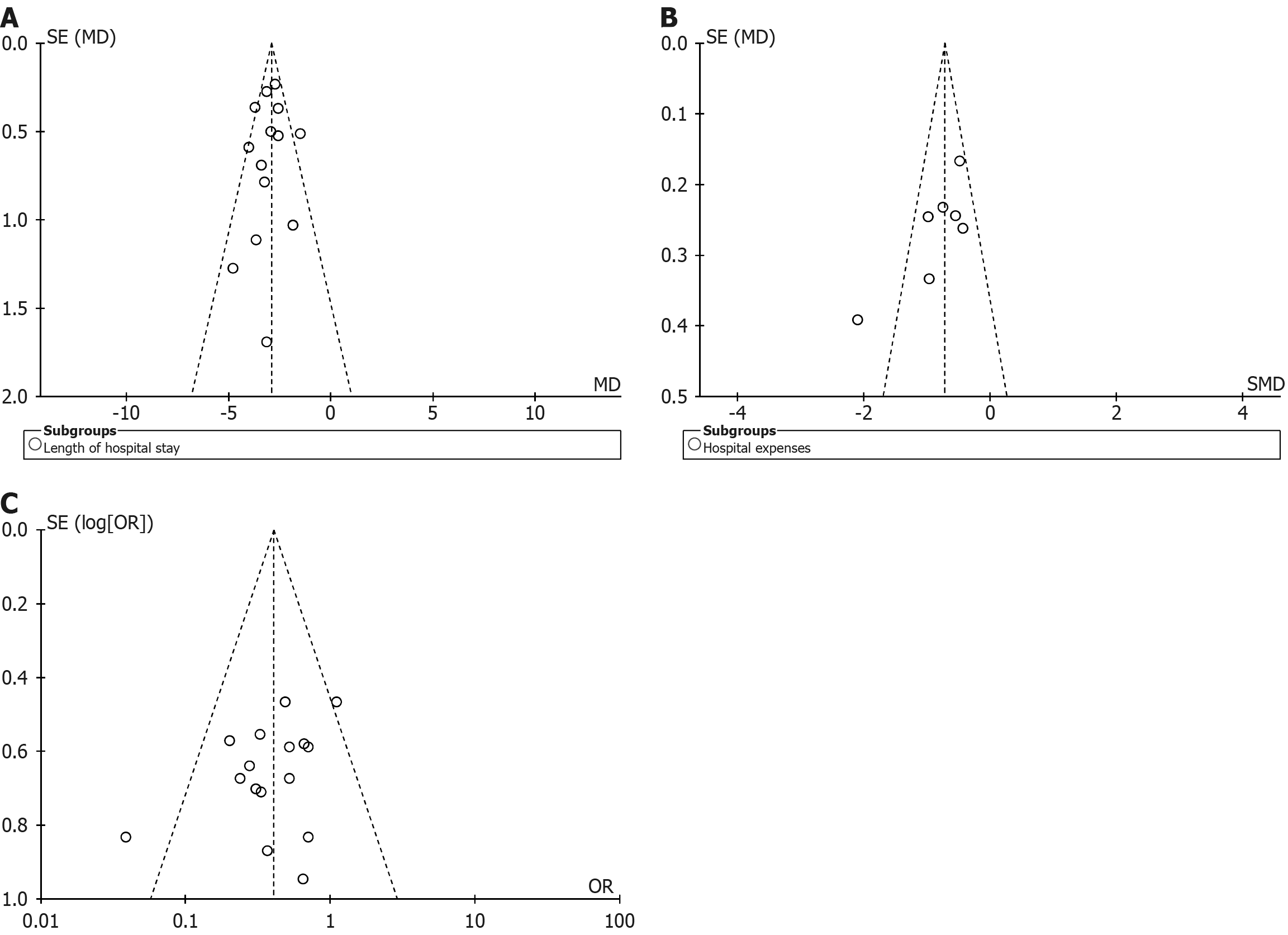
The funnel plot for hospitalization time demonstrated a symmetrical distribution of data points within the funnel boundaries, with most points clustered in the upper and middle regions, suggesting a low likelihood of publication bias (Figure 6A). For hospitalization costs (Figure 6B) and complication rates (Figure 6C), the funnel plots also showed symmetrical distributions, with data points predominantly concentrated in the middle and lower regions, indicating the inclusion of numerous small-sample studies. While this pattern may reflect underlying heterogeneity or selective reporting, the visual inspection did not reveal substantial asymmetry.
This study assessed 18 outcome indicators, with hospitalization costs and urinary catheter removal time classified as moderate-quality evidence, while all other indicators were classified as high-quality evidence. These included 13 key outcomes and 5 important outcomes. The GRADE system assessed the level of evidence based on risk of bias, inconsistency, indirectness, and imprecision, as detailed in Table 4.
| Certainty assessment | Number of patients | Effect | |||||||||||
| Outcome | n | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Intervention | Control | Relative (95%CI) | Absolute (95%CI) | Certainty | Importance |
| Time to first flatus (hour) | 7 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 345 | 343 | - | MD = 10.24 Lower (12.69 Lower, 7.79 Lower) | ++++ high | Critical |
| Time to first flatus (day) | 9 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 322 | 322 | - | MD = 1.09 SD: Lower (1.36 Lower, 0.83 Lower) | ++++ high | Critical |
| Time to oral intake (hour) | 3 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 190 | 189 | - | MD = 21.06 Lower (23.18 Lower, 18.94 Lower) | ++++ high | Critical |
| Time to oral intake (day) | 2 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 61 | 52 | - | MD = 0.56 Lower (0.92 Lower, 0.2 Lower) | ++++ high | Critical |
| Time to first ambulation (day) | 2 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 97 | 91 | - | SMD = 0.45 SD: Lower (0.74 Lower, 0.16 Lower) | ++++ high | Critical |
| Length of hospital stay | 15 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 580 | 579 | - | MD = 2.92 Lower (3.15 Lower, 2.68 Lower) | ++++ high | Important |
| Hospitalization cost | 7 | Randomized trials | Not serious | Not serious | Serious a | Not serious | None | 258 | 259 | - | SMD = 0.72 SD: Lower (0.9 Lower, 0.54 Lower) | +++-moderate a | Important |
| Nasogastric tube removal time (day) | 3 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 89 | 89 | - | MD = 1.7 Lower (1.96 Lower, 1.44 Lower) | ++++ high | Critical |
| Urinary catheter removal time (hour) | 2 | Randomized trials | Not serious | Not serious | Serious b | Not serious | None | 103 | 103 | - | SMD = 4.52 SD: Lower (5.57 Lower, 3.48 Lower) | +++-moderate a | Critical |
| Urinary catheter removal time (day) | 2 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 60 | 60 | - | SMD = 2.33 SD: Lower (2.8 Lower, 1.86 Lower) | ++++ high | Critical |
| Abdominal drain removal time | 4 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 178 | 177 | - | SMD = 0.91 SD: Lower (1.13 Lower, 0.69 Lower) | ++++ high | Critical |
| Total complication rate | 16 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 88/699 (12.6%) | 173/697 (24.8%) | OR = 0.41 (0.30-0.55) | 129 fewer per 1000 (from 158 fewer to 95 fewer) | ++++ high | Critical |
| Incidence of intestinal obstruction | 12 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 13/473 (2.7%) | 16/479 (3.3%) | OR = 0.83 (0.41-1.71) | 6 fewer per 1000 (from 19 fewer to 22 more) | ++++ high | Critical |
| Incidence of nausea and vomiting | 6 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 16/206 (7.8%) | 15/206 (7.3%) | OR = 1.07 (0.52-2.20) | 5 more per 1000 (from 34 fewer to 75 more) | ++++ high | Important |
| Incidence of anastomotic leakage | 12 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 6/508 (1.2%) | 17/499 (3.4%) | OR = 0.37 (0.15-0.89) | 21 fewer per 1000 (from 29 fewer to 4 fewer) | ++++ high | Critical |
| Incidence of surgical site infection | 13 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 10/506 (2.0%) | 18/502 (3.6%) | OR = 0.56 (0.27-1.18) | 15 fewer per 1000 (from 26 fewer to 6 more) | ++++ high | Critical |
| Incidence of urinary tract infection | 8 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 3/317 (0.9%) | 11/315 (3.5%) | OR = 0.39 (0.14-1.06) | 21 fewer per 1000 (from 30 fewer to 2 more) | ++++ high | Important |
| Incidence of pulmonary infection | 9 | Randomized trials | Not serious | Not serious | Not serious | Not serious | None | 8/380 (2.1%) | 21/372 (5.6%) | OR = 0.38 (0.17-0.83) | 34 fewer per 1000 (from 46 fewer to 9 fewer) | ++++ high | Important |
The GRADE analysis included 13 key outcome indicators, with high-quality evidence indicating that ERAS interventions had a significant impact on these key outcomes, making them highly valuable for clinical decision-making. Additionally, 5 important outcome indicators were assessed, where the evidence quality was slightly lower than that of key outcome indicators, but still played a crucial role in overall patient recovery and economic burden. Postoperative nausea and vomiting not only affects patient comfort and satisfaction but may also lead to prolonged hospitalization and increased medical costs. Therefore, these important outcome indicators should also be given attention in clinical practice, with appropriate preventive and management strategies implemented to control them effectively[41].
Gastrointestinal function recovery is a key target of ERAS protocols in abdominal surgery. Traditionally, preoperative mechanical bowel preparation has been used to reduce the bacterial load within the digestive tract, thereby lowering the risk of postoperative infections and anastomotic leakage[42]. However, this practice is being increasingly reconsidered under ERAS principles, which emphasize minimizing surgical stress and promoting early functional recovery.
In this meta-analysis, sixteen studies assessed the effect of ERAS protocols on time to first flatus. Although subgroup analyses revealed statistically significant reductions in both hours and days, considerable heterogeneity was observed among the studies. Therefore, while the results suggest a potential association between ERAS interventions and earlier return of bowel function, these findings should be interpreted with caution. The variation in intervention protocols, patient characteristics, and outcome definitions may have influenced the overall estimates.
Beyond bowel recovery, ERAS protocols were associated with shorter time to first ambulation and reduced hospital length of stay. These outcomes are likely attributable to coordinated perioperative strategies, such as early oral intake, limited use of intravenous fluids, and minimized reliance on routine mechanical bowel preparation and nasogastric decompression[43]. Furthermore, individualized management of postoperative drainage contributes to earlier mobilization and discharge readiness. Evidence from the included studies indicated that ERAS groups experienced a mean reduction in hospital stay by 1.12 days compared to conventional care[44,45]. In summary, while the overall trends support the beneficial effects of ERAS on postoperative gastrointestinal recovery, the observed interstudy heterogeneity underscores the need for further standardized, high-quality trials to confirm these findings.
ERAS guidelines recommend avoiding routine nasogastric decompression and not placing prophylactic intra-abdominal drainage tubes in elective abdominal surgeries[46]. This approach helps reduce the incidence of postoperative atelectasis and pneumonia[47]. ERAS nursing focuses on minimizing surgical trauma while maximizing therapeutic efficacy, gaining increasing acceptance among medical professionals. Studies have demonstrated that ERAS patients have shorter postoperative hospital stays compared to traditional care groups, with a 34% lower risk of postoperative complications[48]. Additionally, ERAS has been shown to reduce postoperative inflammatory responses and enhance immune function, further promoting faster recovery. For future clinical applications, it is recommended to establish an ERAS assessment system (ERAS Interactive Audit System). This system should use web-based data input and analysis to monitor pathway adherence, evaluate its impact on clinical outcomes, and implement a feedback mechanism to continuously improve ERAS protocols.
ERAS protocols emphasize early postoperative oral intake and hydration in elective abdominal surgery to promote gastrointestinal function recovery and maintain the integrity of the intestinal mucosal barrier, which may reduce infection risk and contribute to shorter hospital stays[49]. In parallel, early mobilization under ERAS facilitates systemic recovery across multiple organ systems, including the respiratory, gastrointestinal, and musculoskeletal systems, thereby decreasing the incidence of pulmonary complications, pressure ulcers, and venous thromboembolism.
In this meta-analysis, ERAS interventions were associated with a significantly lower overall rate of postoperative complications. Specifically, reductions were observed in the incidence of anastomotic leakage and pulmonary infection, while no significant differences were found for intestinal obstruction, nausea and vomiting, surgical site infections, or urinary tract infections. These results align with prior findings, such as those reported by Zhang[50], whose meta-analysis indicated that drainage tube placement prolonged postoperative hospitalization and increased the likelihood of drainage-related complications. Similarly, another meta-analysis demonstrated that omitting prophylactic drainage after gastrectomy was associated with a lower complication rate and reduced length of stay[51]. Furthermore, the routine use of drainage tubes may exacerbate patient discomfort and hinder early ambulation, thereby delaying recovery and increasing the risk of secondary injury[52].
Taken together, these findings support the ERAS guideline recommendation to avoid routine drainage following gastrectomy. The evidence suggests that minimizing unnecessary drainage tube use can enhance recovery while reducing complication rates. To ensure safe and effective implementation of this approach, institutions should adopt standardized postoperative nursing protocols, invest in staff training, and establish robust early surveillance systems. These strategies are essential for improving ERAS adherence and optimizing postoperative outcomes.
All 21 included studies were from Asia, with 19 conducted in China and 2 in South Korea, which limits the extrapolation of the findings to non-Asian populations. GC presents with distinct epidemiological characteristics across regions, including differences in tumor distribution, Helicobacter pylori infection rates, genetic background, and dietary habits, with nearly 50% of all cases originating from China. These variations may influence perioperative risk profiles and potentially modify the effectiveness of specific ERAS components, particularly those related to nutritional management and recovery pathways. The predominance of Asian studies in this meta-analysis likely reflects the greater availability of randomized controlled trials meeting inclusion criteria from Chinese databases and a potential increasing emphasis on ERAS implementation. For instance, the integration of ERAS protocols into Chinese hospital evaluation systems and research agendas has contributed to a growing body of literature on this topic. European ERAS studies may emphasize long-term outcomes, involve multicenter designs, or appear in publication formats outside our search parameters, potentially explaining their lower visibility. These contextual variations highlight the necessity of validating ERAS protocols within a range of healthcare settings.
Despite variability in implementation rates of individual ERAS components, our analysis confirms that all included studies met the ERAS framework by integrating at least 10 guideline-based elements[12]. Components with implemen
To adapt perioperative strategies to diverse patient populations, clinical practices, and healthcare settings, future international multicenter trials should incorporate both patient-reported outcomes and standardized ERAS adherence metrics. The use of tools such as adherence scores or the ERAS Interactive Audit System would help quantify protocol compliance and improve the reliability of outcome comparisons across studies. In parallel, the establishment of a global ERAS registry platform could facilitate international data sharing and support the development of region-specific, evidence-based care pathways for GC surgery.
This study has several limitations. First, it included only RCTs published in Chinese and English since 2000, which may have excluded relevant studies and reduced the comprehensiveness of the evidence base. Second, some included trials were conducted by the same surgical teams, potentially introducing bias due to lack of independent validation. Third, although the overall methodological quality was rated as moderate to high, eight studies had a high risk of bias in randomization, and fourteen did not report withdrawal or loss to follow-up, raising concerns about performance and detection bias. While sensitivity analyses excluding high-risk studies demonstrated consistent effect directions, the absence of blinding in many trials may have inflated effect estimates, leading to downgrading of the recommendation strength for several outcomes in the GRADE assessment. Fourth, although all studies met the minimum requirement of implementing at least ten ERAS components, substantial variation was observed in the selection and reporting of these components. Measures such as opioid-sparing analgesia and nutritional support were applied in fewer than 40% of studies, and elements like blood glucose control and antithrombotic prophylaxis in fewer than 15%, reflecting notable heterogeneity in ERAS implementation and limiting the generalizability of pooled results. Lastly, national surveys indicate that even tertiary hospitals in China often implement only a limited number of ERAS components due to staffing shortages, limited interdisciplinary coordination, and inadequate training. Future studies should address these limi
This meta-analysis of 21 randomized controlled trials suggests that ERAS protocols in GC surgery can enhance gas
| 1. | Wang SL. The role and mechanism of long non-coding RNA KRT7-AS in the progression of gastric cancer. M.Sc. Thesis, Tongji University. 2019. Available from: https://d.wanfangdata.com.cn/thesis/CiBUaGVzaXNOZXdTMjAyNTA2MTMyMDI1MDYxMzE2MTkxNhIJRDAyMjQ1NjA3GghqbTM4bjhoMQ%3D%3D. |
| 2. | Cao MM, Chen WQ. [Interpretation on the global cancer statistics of GLOBOCAN 2020]. Zhongguo Yixue Qianyan Zazhi. 2021;13:63-69. [DOI] [Full Text] |
| 3. | Ren HM. Research on the mechanism of a novel phenothiazine LSD1 inhibitor in downregulating PD-L1 and inhibiting the proliferation of gastric cancer. M.Sc. Thesis, Zhengzhou University. 2021. Available from: https://d.wanfangdata.com.cn/thesis/ChhUaGVzaXNOZXdTMjAyNDA5MjAxNTE3MjUSCFkzODQxNzg4GghxY3h2enNveg%3D%3D. |
| 4. | Li JS, Jiang ZW. [Clinical significance of enhanced recovery after surgery is not only to shorten the duration of hospital stay]. Zhonghua Xiaohua Waike Zazhi. 2015;14:22-24. [DOI] [Full Text] |
| 5. | Wang D, Kong Y, Zhong B, Zhou X, Zhou Y. Fast-track surgery improves postoperative recovery in patients with gastric cancer: a randomized comparison with conventional postoperative care. J Gastrointest Surg. 2010;14:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Zhang ZY, Gao ZL, Song XN, Li KZ, Shi L. [The standardized management process of enhanced recovery after surgery in the perioperative clinical practice of urology]. Zhonghua Qiangjing Miniao Waike Zazhi. 2017;11:359-363. [DOI] [Full Text] |
| 7. | Xu X, Chen B. [Application and new progress of enhanced recovery after surgery in perioperative period of gastric cancer patients]. Guoji Zhongliuxue Zazhi. 2020;47:43-45. [DOI] [Full Text] |
| 8. | Robotics and Laparoscopic Surgery Professional Committee of the Chinese Society of Research Hospitals. [Expert consensus on enhanced recovery after gastrectomy for gastric cancer (2016 edition)]. Zhonghua Xiaohua Waike Zazhi. 2017;16:14-17. [DOI] [Full Text] |
| 9. | Zhou YB. [Thinking and suggestions on pathway management of perioperative enhanced recovery after surgery in gastrointestinal tumors in China]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11319] [Article Influence: 665.8] [Reference Citation Analysis (0)] |
| 11. | Ma ZT. Meta-analysis of the efficacy and safety of enhanced recovery after surgery in laparoscopic radical gastrectomy during the perioperative period. M.Sc. Thesis, Dalian Medical University. 2020. Available from: https://d.wanfangdata.com.cn/thesis/ChhUaGVzaXNOZXdTMjAyNDA5MjAxNTE3MjUSCUQwMjEyMjI5NhoIZXR5N3o3YmE%3D. |
| 12. | Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, Carli F, Demartines N, Griffin SM, Lassen K; Enhanced Recovery After Surgery (ERAS®) Group. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg. 2014;101:1209-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 547] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 13. | Liu YM. Meta-analysis of the safety and effectiveness of enhanced recovery after surgery in the perioperative period of gastric cancer. M.Sc. Thesis, Nanchang University (Medical School). 2020. Available from: https://d.wanfangdata.com.cn/thesis/CiBUaGVzaXNOZXdTMjAyNTA2MTMyMDI1MDYxMzE2MTkxNhIJRDAyMDEyMjQwGghsazltYmFuag%3D%3D. |
| 14. | Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, MacFie J, Liberman AS, Soop M, Hill A, Kennedy RH, Lobo DN, Fearon K, Ljungqvist O; Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:783-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 474] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 15. | Yao RW, Bi XG. [Application of Enhanced Recovery after Surgery Combined with Traditional Chinese Medicine Therapy in Gastric Cancer: a Meta-analysis]. Zhongguo Zhongxiyi Jiehe Waike Zazhi. 2021;27:374-381. [DOI] [Full Text] |
| 16. | Yan H, Hao YF. Evidence-Based Nursing. Beijing: People’s Medical Publishing House, 2018.. |
| 17. | The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0. March, 2011. [cited 29 April 2025]. Available from: extension://ngbkcglbmlglgldjfcnhaijeecaccgfi/https://www.cochrane.org/authors/handbooks-and-manuals/handbook/archive/v5.1.0. |
| 18. | Wang LH. Optimization of the clinical pathway for enhanced recovery after surgery in gastric cancer patients during the perioperative period. M.Sc. Thesis, Qingdao University. 2018. Available from: https://med.wanfangdata.com.cn/Paper/Detail?id=DegreePaper_D01259077&dbid=WF_XW. |
| 19. | Kim J, Lee J, Hyung WJ, Cheong JH, Chen J, Choi SH, Noh SH. Gastric cancer surgery without drains: a prospective randomized trial. J Gastrointest Surg. 2004;8:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Wang WK, Tu CY, Shao CX, Chen W, Zhou QY, Zhu JD, Xu HT. Impact of enhanced recovery after surgery on postoperative rehabilitation, inflammation, and immunity in gastric carcinoma patients: a randomized clinical trial. Braz J Med Biol Res. 2019;52:e8265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Kim JW, Kim WS, Cheong JH, Hyung WJ, Choi SH, Noh SH. Safety and efficacy of fast-track surgery in laparoscopic distal gastrectomy for gastric cancer: a randomized clinical trial. World J Surg. 2012;36:2879-2887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Abdikarim I, Cao XY, Li SZ, Zhao YQ, Taupyk Y, Wang Q. Enhanced recovery after surgery with laparoscopic radical gastrectomy for stomach carcinomas. World J Gastroenterol. 2015;21:13339-13344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Liu G, Jian F, Wang X, Chen L. Fast-track surgery protocol in elderly patients undergoing laparoscopic radical gastrectomy for gastric cancer: a randomized controlled trial. Onco Targets Ther. 2016;9:3345-3351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Chen Hu J, Xin Jiang L, Cai L, Tao Zheng H, Yuan Hu S, Bing Chen H, Chang Wu G, Fei Zhang Y, Chuan Lv Z. Preliminary experience of fast-track surgery combined with laparoscopy-assisted radical distal gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1830-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Wu JY. The application of fast track surgery combined with laparoscopic D2 radical operation for gastric carcinoma. M.Sc. Thesis, Ningbo University. 2018. Available from: https://med.wanfangdata.com.cn/Paper/Detail?id=DegreePaper_D01683452&dbid=WF_XW. |
| 26. | Chen CK. Clinical study on the application of enhanced recovery after surgery in laparoscopic-assisted radical gastrectomy for gastric cancer. M.Sc. Thesis, Zhengzhou University. 2019. Available from: https://med.wanfangdata.com.cn/Paper/Detail?id=DegreePaper_Y3568326&dbid=WF_XW. |
| 27. | Wu YF. Clinical application of enhanced recovery after surgery concepts in totally laparoscopic distal gastrectomy for gastric cancer. M.Sc. Thesis, Zhejiang University. 2019. Available from: https://d.wanfangdata.com.cn/thesis/ChhUaGVzaXNOZXdTMjAyNDA5MjAxNTE3MjUSCFkzNjU3MzY1Ggh0MWJrdGFwMw%3D%3D. |
| 28. | Ma JF. Randomized controlled study on the impact of enhanced recovery after surgery treatment on immune function after laparoscopic gastric cancer radical surgery. M.Sc. Thesis, Lanzhou University. 2019. Available from: https://d.wanfangdata.com.cn/thesis/D01766592. |
| 29. | Wang WW. Impact of enhanced recovery after surgery on postoperative recovery, inflammation, and immune indicators in gastric cancer. M.Sc. Thesis, Wenzhou Medical University. 2017. Available from: https://kms.wmu.edu.cn/handle/3ETUA0LF/113277. |
| 30. | Jiang ZW, Li JS, Wang ZM, Li N, Li WY, Diao YQ, Nai YJ, Huang XJ. [The safety and efficiency of fast track surgery in gastric cancer patients undergoing D2 gastrectomy]. Zhonghua Waike Zazhi. 2007;45:1314-1317. [DOI] [Full Text] |
| 31. | Fan H, Qiao LN, Zhang HM, Jin XZ, Liao CY. [Application effect of fast track surgery on perioperative nursing of patients with gastric cancer]. Huli Yanjiu. 2019;33:503-506. [DOI] [Full Text] |
| 32. | Li YP, Qiu JF, Cao H. [Application of enhanced recovery after surgery in laparoscopic radical gastrectomy for gastric cancer during the perioperative period]. Zhonghua Waike Zazhi. 2016;19:269-273. [DOI] [Full Text] |
| 33. | Chen YF. [Effect of fast track surgery nursing on gastrointestinal function recovery and incidence of postoperative complications in gastrointestinal surgery patients]. Zhongguo Jiceng Yiyao. 2019;26:373-376. [DOI] [Full Text] |
| 34. | Ruan XL, Peng C, Liu HX, Wang GH. [Impact of enhanced recovery after surgery on prognosis, negative emotions, and psychological stress in patients undergoing laparoscopic radical gastrectomy for gastric cancer]. Zhongguo Yixue Qianyan. 2019;11:76-80. [DOI] [Full Text] |
| 35. | Cheng QZ. [Impact of enhanced recovery after surgery concept intervention on postoperative gastrointestinal function recovery and prognosis in patients undergoing laparoscopic radical gastrectomy for gastric cancer]. Linchuang Heli Yongyao Zazhi. 2019;12:131-132. [DOI] [Full Text] |
| 36. | Liu ZL, Xie XH, Tian HP, Li LF, Hou HF, Zhang KJ, Zhou T, Liang XB. [Enhanced recovery after surgery in laparoscopic radical gastrectomy for gastric cancer]. Zhonghua Putong Waike Zazhi. 2018;33:1026-1029. [DOI] [Full Text] |
| 37. | Li SB, Huang L. [Effect of fast track surgery concept on postoperative rehabilitation and perioperative stress index in patients undergoing laparoscopic radical gastrectomy]. Zhongguo Yiyao Daobao. 2021;18:9-93. [DOI] [Full Text] |
| 38. | Miao X, Liu PF, J YB, Xi HQ, Chen L. [Enhanced recovery after surgery in total laparoscopic D2 radical resection for gastric cancer]. Jiefanjun Yixueyuan Xuebao. 2021;42:383-386. [DOI] [Full Text] |
| 39. | Liu ZY, Li TJ. [Effect of ERAS combined with nutritional support on nutritional parameters and inflammatory stress levels in patients with gastric cancer after total gastrectomy]. Hainan Yixue. 2021;32:1400-1404. [DOI] [Full Text] |
| 40. | Xia CC. Clinical study on early mobilization and postoperative intestinal function recovery in patients after gastric cancer surgery under enhanced recovery after surgery. M.Sc. Thesis, Bengbu Medical College. 2016. Available from: https://d.wanfangdata.com.cn/thesis/ChhUaGVzaXNOZXdTMjAyNDA5MjAxNTE3MjUSCUQwMTA1MDAwNBoINWJ0cTh3NmM%3D. |
| 41. | Yang XT, Huang ZH. [Influencing Factors and Prevention Measures of Postoperative Nausea and Vomiting]. Yazhou Jizhen Yixue Bingli Yanjiu. 2024;12:88-93. [DOI] [Full Text] |
| 42. | Chen L, Chen YJ, Dong HL, Fang Y, Gu XP, Huang YG, Jiang ZW, Lou WH, Liu LX, Mi WD, Ma ZL, Min S, Peng SL, Tian JD, Wang TL, Xu ZK, Xue ZG, Yao HW, Yang YM, Zhang KC, Zhu SM. [Consensus and pathway management guidelines of Enhanced Recovery After Surgery in China (2018)]. Zhongguo Shiyong Waike Zazhi. 2018;38:8-13. [DOI] [Full Text] |
| 43. | Xia GF. Study on the application of the concept of fast-track surgery in the perioperative period of laparoscopic-assisted radical gastrectomy for gastric cancer. M.Sc. Thesis, Nanchang University. 2017. Available from: https://d.wanfangdata.com.cn/thesis/ChhUaGVzaXNOZXdTMjAyNDA5MjAxNTE3MjUSCUQwMTI3MDQzNBoIc3pndGNkaGg%3D. |
| 44. | Béguin P, Hasler U, Staub O, Geering K. Endoplasmic reticulum quality control of oligomeric membrane proteins: topogenic determinants involved in the degradation of the unassembled Na,K-ATPase alpha subunit and in its stabilization by beta subunit assembly. Mol Biol Cell. 2000;11:1657-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Sierzega M, Choruz R, Pietruszka S, Kulig P, Kolodziejczyk P, Kulig J. Feasibility and outcomes of early oral feeding after total gastrectomy for cancer. J Gastrointest Surg. 2015;19:473-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Chinese Society of Surgery, Chinese Society of Anesthesiology. [Clinical Practice Guidelines for ERAS in China (2021)(Ⅰ)]. Xiehe Yixue Zazhi. 12:624-631. [DOI] [Full Text] |
| 47. | Chan MY, Foo CC, Poon JT, Law WL. Laparoscopic colorectal resections with and without routine mechanical bowel preparation: A comparative study. Ann Med Surg (Lond). 2016;9:72-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Noba L, Rodgers S, Doi L, Chandler C, Hariharan D, Yip V. Costs and clinical benefits of enhanced recovery after surgery (ERAS) in pancreaticoduodenectomy: an updated systematic review and meta-analysis. J Cancer Res Clin Oncol. 2023;149:6639-6660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Yang R, Tao W, Chen YY, Zhang BH, Tang JM, Zhong S, Chen XX. Enhanced recovery after surgery programs versus traditional perioperative care in laparoscopic hepatectomy: A meta-analysis. Int J Surg. 2016;36:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Zhang HC. Meta-analysis of randomized controlled trials on the application of Enhanced Recovery After Surgery (ERAS) in radical gastrectomy for gastric cancer. M.Sc. Thesis, Guangzhou Medical University. 2015. Available from: https://d.wanfangdata.com.cn/thesis/CiBUaGVzaXNOZXdTMjAyNTA2MTMyMDI1MDYxMzE2MTkxNhIHRDcxNTA0ORoIaGhrc241amo%3D. |
| 51. | Qi X, Liu Y, Wang W, Cai D, Li W, Hui J, Liu C, Zhao Y, Li G. Management of advanced gastric cancer: An overview of major findings from meta-analysis. Oncotarget. 2016;7:78180-78205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Wee IJY, Syn NL, Shabbir A, Kim G, So JBY. Enhanced recovery versus conventional care in gastric cancer surgery: a meta-analysis of randomized and non-randomized controlled trials. Gastric Cancer. 2019;22:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/