Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.108859
Revised: July 31, 2025
Accepted: August 27, 2025
Published online: October 27, 2025
Processing time: 128 Days and 23.3 Hours
Early gastric cancer (EGC) or precancerous lesions (PCLs) are generally small tumors and carry a diminished chance of nodal infiltration. Thus far, very few studies have examined how endoscopic submucosal dissection (ESD) affects post
To evaluate the influence of ESD on postoperative recovery and complications in patients with EGC or PCL.
The study population included patients with EGC and PCL admitted to The First People’s Hospital of Fuyang District between December 2022 and December 2024, who were divided into the research (n = 65) and control (n = 55) groups if they underwent ESD and laparoscopic radical gastrectomy, respectively. Therapeutic outcomes (en bloc and curative resection rates), surgical parameters (incision length, intraoperative bleeding, and operative duration), postoperative recovery indices (time to first ambulation/flatus/first oral intake, and hospital stay dura
Compared with the control group, the research group achieved a significantly higher en bloc resection rate but a notably lower curative resection rate. Addi
When performed for EGC and PCLs, ESD demonstrates advantages such as higher en bloc resection (although lower curative resection rates), surgical trauma minimization, shortened operative duration, and faster recovery. Moreover, it is effective in reducing serum tumor marker levels while maintaining favorable safety.
Core Tip: This study examined how endoscopic submucosal dissection (ESD) influences postoperative recovery and complications in early gastric cancer (EGC) or precancerous lesions (PCLs). Compared with laparoscopic radical gastrectomy, performing ESD for treating EGC and PCLs led to superior en bloc resection, shortened surgical incision length, intraoperative bleeding reduction, and shorter operative duration. Moreover, it effectively accelerated postoperative recovery, lowered complications, and improved serum tumor marker expression.
- Citation: Fang M, Jiang JH, Zhu DD, Yu FX. Endoscopic submucosal dissection for early gastric cancer and precancerous lesions enhances postoperative recovery and mitigates complication risks. World J Gastrointest Surg 2025; 17(10): 108859
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/108859.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.108859
Gastric cancer (GC), a stomach-derived malignancy, is a growing threat to global well-being[1]. The 2020 statistics indicate over 1 million new GC diagnoses worldwide and approximately 800000 related fatalities[2]. Early GC (EGC), characterized by mucosa- or submucosa-confining invasive growths, now makes up to half of cases owing to the effective implementation of screening initiatives, which consequently leads to markedly better clinical outcomes (5- to 10-year survival rate > 90.0%)[3]. Patients with EGC or precancerous lesion (PCL) typically exhibit limited tumor burden and a diminished chance of nodal infiltration. For such patients, early interventions can significantly extend long-term survival and increase the likelihood of complete cure[4,5]. Laparoscopic radical gastrectomy (LRG), while remaining a common lesion excision technique, is challenged by extended operative durations, high hospitalization expenses, and postope
This study enrolled 120 patients with EGC or PCL admitted to our hospital between December 2022 and December 2024. They were divided into the research group who underwent ESD (n = 65) and the control group who received LRG (n = 55).
The eligibility criteria were as follows: Endoscopically identified and biopsy-proven EGC or PCLs[11], initial diagnosis and treatment-naive, surgical candidates for LRG or ESD[12,13], absence of cognitive, communicative, or mental dysfunction, and completeness of clinical data.
The exclusion criteria were as follows: Nodal metastasis identified before operative intervention; coexisting malig
Each patient underwent standard presurgical workups, covering hematologic testing, electrocardiography, abdominal computed tomography, and coagulation function assessments. A fasting period of 8-10 hours was required before the procedure.
Control group (LRG): General anesthesia was induced through endotracheal intubation, followed by urinary catheter placement. The patient was positioned in a 20° reverse Trendelenburg (head-up, feet-down) orientation, with intraoperative end-tidal CO2 maintained between 35 and 45 mmHg. A pneumoperitoneum was created through an umbilical puncture, maintaining a pressure of 12-14 mmHg (1 mmHg = 0.133 kPa). Laparoscopic instruments were introduced following a four-port technique. After identifying the lesion, surrounding veins were dissected using an ultrasonic knife. The right gastroepiploic artery and vein were severed along the transverse colon, and lymph nodes were dissected. The dissection of the right gastroepiploic vessels continued toward the common bile duct. The jejunum was exteriorized through an antecolic approach, and a Roux-en-Y gastrojejunostomy was created 15 cm distal to the ligament of Treitz. Postoperatively, the surgical area was irrigated and sutured, and a drainage tube was placed. Prophylactic antibiotics were administered to prevent infection.
Research group (ESD): After the induction of intravenous general anesthesia, the gastric cavity was examined endoscopically to locate the tumor. The lesion was stained with 0.4% indigo carmine, and marking dots were placed at 2 mm intervals. Then, 0.4% sodium hyaluronate mixed with 1:10000 adrenaline was injected submucosally near the marking points. Once the mucosal layer was elevated by 0.5-0.8 cm, an IT knife was used to incise the mucosa at the edge of the elevation, followed by gradual submucosal dissection. Additional injections of the solution were given as needed to sustain mucosal elevation until the lesion was completely resected. Intraoperative bleeding or perforation was addressed using thermal coagulation or titanium clips. Postoperatively, preventive hemostasis was applied on the wound, which involved using hemostatic forceps for thermal coagulation of exposed vessels or the application of polysaccharide hemostatic bioadhesive to the wound. A drainage tube was routinely placed. Postoperatively, the patient was kept on a 24-hour fasting period. After confirming the absence of bleeding, a gradual transition to a low-residue enteral nutritional solution was initiated (after 48 hours), and dietary intake followed a stepwise progression and small, frequent meals. On the day of surgery, patients were encouraged to engage in limb exercises and regular turning in bed. Ankle pump exercises were encouraged 12 hours postoperatively, and bedside ambulation was initiated after 24 hours. Nursing staff evaluated pain levels, inspected the incision, and provided updates on postoperative recovery to improve patients’ confidence in their rehabilitation.
Treatment efficacy: This was evaluated by comparing the rates of en bloc resection and curative resection between the two groups[14]. En bloc resection refers to the complete removal of suspicious lesions detected during endoscopic examination. Curative resection involves excising the tumor along with cancerous infiltration extending to a depth of ≥ 500 μm beneath the muscularis mucosa.
Surgical parameters: For both groups, the following surgical metrics were monitored and documented: Surgical incision length, intraoperative blood loss, and operative duration.
Postoperative recovery: Postoperative recovery was assessed based on the time to first postoperative ambulation, first flatus, and first oral intake, and duration of hospital stay.
Complication rates: Patients in each group who experienced adverse reactions, such as infection, outflow obstruction, bleeding, and perforation, were observed. Subsequently, the incidence rate was calculated based on these observations.
Tumor markers: Fasting peripheral venous blood samples (5 mL) were collected from patients before treatment and 1 week following treatment. Serum was isolated through centrifugation, and the levels of carcinoembryonic antigen (CEA), carbohydrate antigen (CA)125, and CA19-9 were quantified using enzyme-linked immunosorbent assay.
Continuous variables were summarized as mean ± SE of the mean. Between-group differences were analyzed using independent samples t-tests, whereas paired t-tests were employed to compare pre- and post-treatment values within each group. Categorical variables were presented as percentages, and group comparisons were performed using χ2 tests. Data analysis was conducted using IBM SPSS Statistics version 22.0, and graphical representations were created using GraphPad Prism 7.0. A P value of < 0.05 was deemed significant.
No significant differences were observed between the research and control groups in terms of sex, age, body mass index, tumor diameter, presence of single lesion, ulceration, and tumor location (P > 0.05). Table 1 provides detailed informa
| Data | Research group (n = 65) | Control group (n = 55) | χ2/t value | P value |
| Gender (male/female) | 42/23 | 40/15 | 0.906 | 0.341 |
| Age (years) | 59.72 ± 7.57 | 61.15 ± 9.22 | 0.933 | 0.353 |
| Body mass index (kg/m2) | 23.03 ± 2.42 | 23.25 ± 2.85 | 0.457 | 0.648 |
| Tumor diameter (mm) | 19.88 ± 1.61 | 20.18 ± 1.76 | 0.974 | 0.332 |
| Single lesion presence (yes/no) | 32/33 | 29/26 | 0.146 | 0.703 |
| Ulceration (yes/no) | 22/43 | 25/30 | 1.685 | 0.194 |
| Tumor location (antrum/body/cardia and body) | 36/16/13 | 32/15/8 | 0.629 | 0.730 |
Compared with the control group, the research group demonstrated a markedly higher en bloc resection rate but a notably lower curative resection rate (all P < 0.05). Detailed data are presented in Table 2.
| Treatment outcome | Research group (n = 65) | Control group (n = 55) | χ2 | P value |
| En bloc resection rate | 59 (90.77) | 39 (70.91) | 7.848 | 0.005 |
| Curative resection rate | 6 (9.23) | 16 (29.09) |
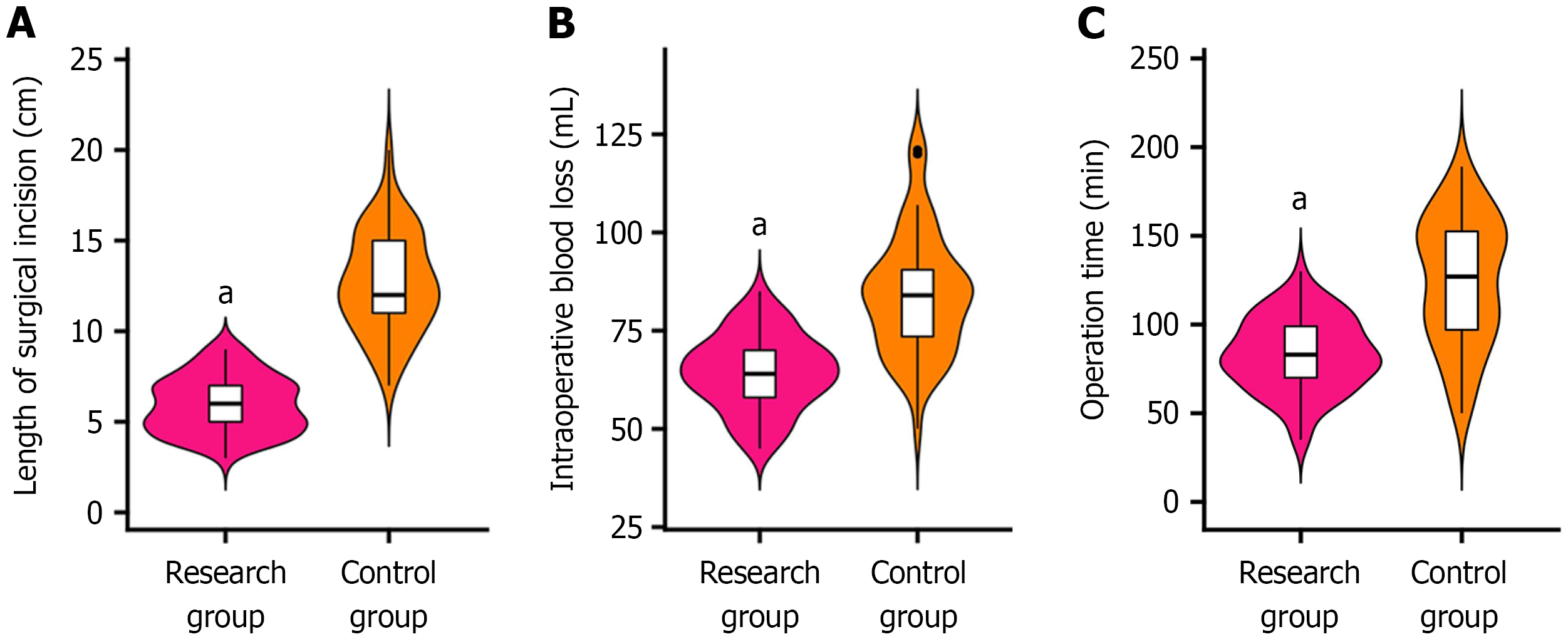
When compared with the control group, in the research group exhibited significant reductions in the surgical incision length, intraoperative blood loss, and operative duration (P < 0.001). These findings are illustrated in Figure 1.
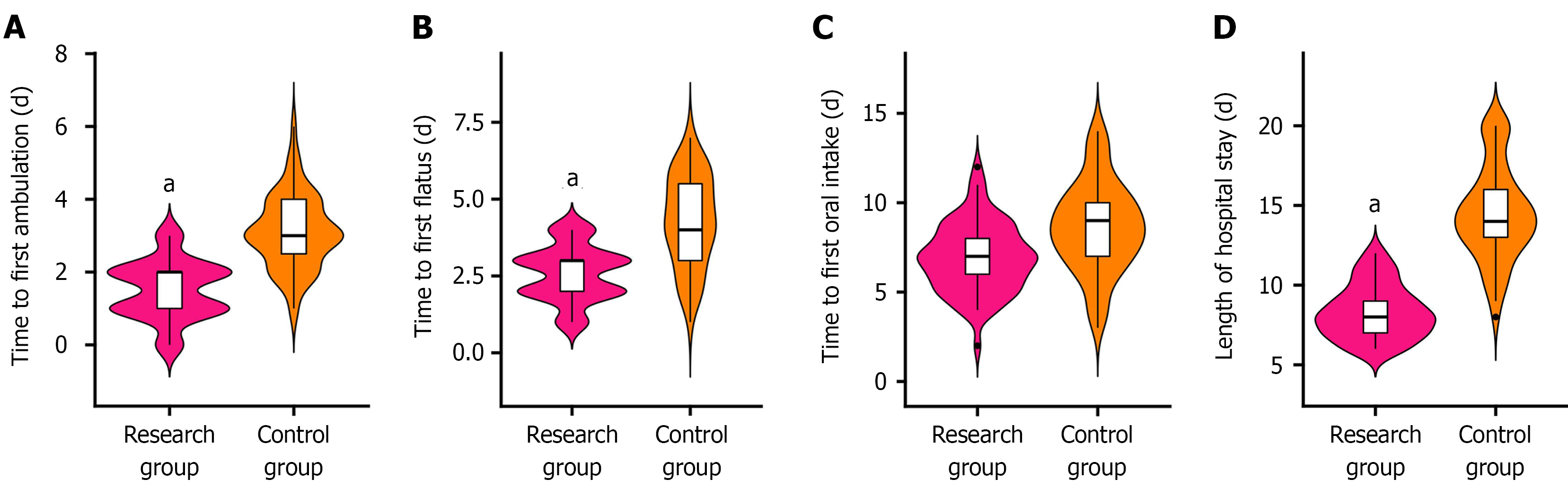
The research group achieved significantly shorter time to first postoperative ambulation, flatus, and oral intake and shorter overall hospital stays than the control group (P < 0.01). This comparison is depicted in Figure 2.
The overall complication rate encompassing infection, outflow obstruction, hemorrhage, and perforation was lower in the research group than in the control group (P < 0.05). Specific details are provided in Table 3.
| Complications | Research group (n = 65) | Control group (n = 55) | χ2 | P value |
| Infection | 0 (0.00) | 3 (5.45) | ||
| Outflow obstruction | 0 (0.00) | 2 (3.64) | ||
| Hemorrhage | 2 (3.08) | 6 (10.91) | ||
| Perforation | 7 (10.77) | 8 (14.55) | ||
| Total | 9 (13.85) | 19 (34.55) | 7.135 | 0.008 |
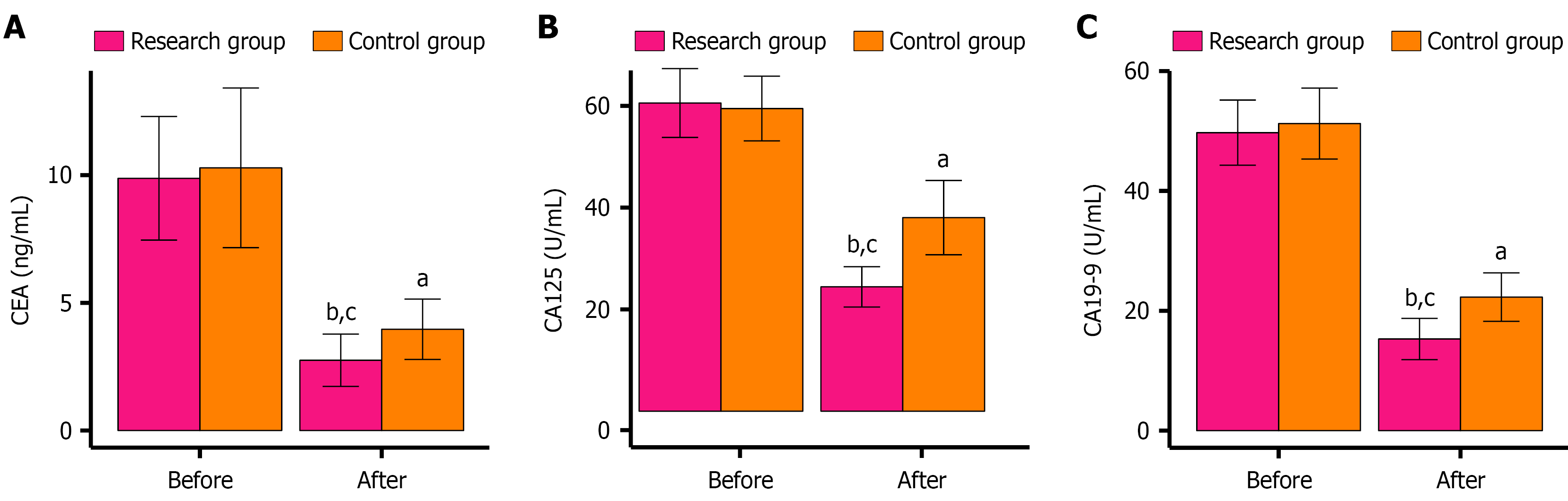
Preoperative assessments revealed no significant disparities in CEA, CA125, and CA19-9 Levels between the two groups (P > 0.05). After surgery, all markers decreased significantly (P < 0.05), with the research group exhibiting even lower levels than the control group (P < 0.05). These trends are captured in Figure 3.
This study involved 120 patients diagnosed with EGC and PCLs to compare the therapeutic outcomes between those undergoing ESD (research group) and those receiving LRG (control group). A notable finding was that ESD yielded a higher en bloc resection rate for EGC and PCLs, albeit with a comparatively lower curative resection rate, than LRG. This could be due to the utilization of a needle knife to excise the mucosa surrounding the lesion in ESD, accompanied by the continuous infusion of a mixed solution during dissection. This method promotes the detachment of the muscular layer from the affected mucosa, proving particularly effective for the removal of extensive and irregularly shaped lesions, thereby increasing the tumor’s en bloc resection rate[15]. Given that malignant lesions may extend beyond the submucosal layer, en bloc resection alone cannot be considered invariably curative[16]. This result was supported by Xu et al[17], who reported that ESD demonstrated a significantly higher curative resection rate and en bloc resection rate than endoscopic mucosal resection (EMR) in patients with EGC and PCLs, closely mirroring our results. Moreover, compared with LRG, ESD for EGC and PCLs was associated with significantly reduced surgical incision length, lesser intraoperative blood loss, and shorter operative duration, implying that ESD imposes less physical trauma on patients, potentially accelerating postoperative recuperation. The postoperative recovery metrics further underscored the superiority of ESD, with patients achieving shorter time to first ambulation, flatus, and oral intake and shorter hospital stays. This finding indicates that ESD effectively aids in the restoration of postoperative gastrointestinal functionality. The precision of ESD in targeting tumor tissue while sparing the normal gastrointestinal architecture likely mitigates surgical stress responses, minimizes collateral damage to adjacent vascular and neural structures, enhances anal exhaust timing, and promotes expedited recovery, culminating in shortened hospital stay[18-20]. Additionally, the minimally invasive nature of ESD, characterized by its procedural simplicity and reduced operative time, further contributes to its recovery benefits[21]. Regarding prevention of complications, ESD demonstrated a clear advantage, significantly lowering the rates of infection, outflow obstruction, hemorrhage, and perforation in patients with EGC and PCL. Although perforation and bleeding are risks associated with ESD, the strategic application of electrocoagulation for hemostasis and the use of clip closures during the procedure have been effective in curtailing these adverse events, thereby diminishing the overall complication rate[22,23]. Furthermore, in this study, ESD was instrumental in significantly decreasing the serum levels of CEA, CA125, and CA19-9 among patients with EGC and PCL. These biomarkers, intimately linked with GC progression, serve as vital indicators for monitoring patient status and disease progression[24,25].
A previous study further highlighted the efficacy of ESD in treating early-stage neoplasms and PCLs. As reported by Tan et al[26], the post-ESD complication rate was merely 12.5% (1/8 cases) in patients with cirrhosis and early-stage cancer or upper gastrointestinal PCLs, with all cases remaining recurrence-free over 45 months of monitoring. Yu et al[27] also demonstrated the effectiveness and safety of ESD in managing early-stage colorectal cancer and PCLs, achieving a 100% local control rate throughout 20 months of postoperative monitoring.
However, this study has several limitations, highlighting the need for further exploration: (1) Validation through larger, multicenter studies is needed, given the restricted sample size that could possibly limit the generalizability and analytical rigor of the study; (2) Owing to the relatively brief observation period, ESD‘s long-term effectiveness and recurrence risks might not be adequately captured, underscoring the need for prolonged surveillance to assess durable outcomes; (3) Differences in biological behaviors among undifferentiated GC subtypes (e.g., pure signet ring cell carcinoma vs mixed forms) and between differentiated and undifferentiated types may remain unclear owing to the lack of stratified research; (4) An in-depth evaluation of ESD and alternative minimally invasive approaches (e.g., EMR) in the removal of sizable lesions remains imperative. Supplementing such analysis would provide clearer insights into optimal patient selection for these interventions; and (5) Given that all procedures were performed by highly skilled endoscopists, the outcomes may reflect expertise-dependent results. Such future analyses could deepen our knowledge of the the
In summary, ESD performed to treat EGC and PCLs markedly enhances the en bloc resection rate, minimizes surgical incision length, reduces intraoperative blood loss, and shortens operative duration. It also facilitates smoother postope
| 1. | Huang Y, Shao Y, Yu X, Chen C, Guo J, Ye G. Global progress and future prospects of early gastric cancer screening. J Cancer. 2024;15:3045-3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 3314] [Article Influence: 662.8] [Reference Citation Analysis (9)] |
| 3. | Chiarello MM, Fico V, Pepe G, Tropeano G, Adams NJ, Altieri G, Brisinda G. Early gastric cancer: A challenge in Western countries. World J Gastroenterol. 2022;28:693-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (2)] |
| 4. | Wang P, Li P, Chen Y, Li L, Lu Y, Zhou W, Bian L, Zhang B, Yin X, Li J, Chen J, Zhang S, Shi Y, Tang X. Chinese integrated guideline on the management of gastric precancerous conditions and lesions. Chin Med. 2022;17:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Gullo I, Grillo F, Mastracci L, Vanoli A, Carneiro F, Saragoni L, Limarzi F, Ferro J, Parente P, Fassan M. Precancerous lesions of the stomach, gastric cancer and hereditary gastric cancer syndromes. Pathologica. 2020;112:166-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 6. | Dao Q, Chen K, Zhu L, Wang X, Chen M, Wang J, Wang Z. Comparison of the clinical and prognosis risk factors between endoscopic resection and radical gastrectomy for early-stage gastric cancer. World J Surg Oncol. 2023;21:147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Zizzo M, Zanelli M, Sanguedolce F, Torricelli F, Morini A, Tumiati D, Mereu F, Zuliani AL, Palicelli A, Ascani S, Giunta A. Robotic versus Laparoscopic Gastrectomy for Gastric Cancer: An Updated Systematic Review. Medicina (Kaunas). 2022;58:834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Li C, Xie S, Chen D, Zhang J, Zhang N, Mu J, Gong A. Clinicopathological characteristics of early gastric cancer with different level of undifferentiated component and nomogram to predict lymph node metastasis. Front Surg. 2023;10:1097927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Ryu DG, Kim SJ, Choi CW, Park SB, Nam HS, Lee SH, Hwang SH. Local Recurrence after Endoscopic Submucosal Dissection of Early Gastric Cancer. J Clin Med. 2023;12:2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 10. | Costa LCDS, Santos JOM, Miyajima NT, Montes CG, Andreollo NA, Lopes LR. Efficacy analysis of endoscopic submucosal dissection for the early gastric cancer and precancerous lesions. Arq Gastroenterol. 2022;59:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Conti CB, Agnesi S, Scaravaglio M, Masseria P, Dinelli ME, Oldani M, Uggeri F. Early Gastric Cancer: Update on Prevention, Diagnosis and Treatment. Int J Environ Res Public Health. 2023;20:2149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 111] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 12. | Liang XQ, Wang Z, Li HT, Ma G, Yu WW, Zhou HC, Liu HB. Indication for endoscopic treatment based on the risk of lymph node metastasis in patients with undifferentiated early gastric cancer. Asian J Surg. 2020;43:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Yoon J, Yoo SY, Park YS, Choi KD, Kim BS, Yoo MW, Lee IS, Yook JH, Kim GH, Na HK, Ahn JY, Lee JH, Jung KW, Kim DH, Song HJ, Lee GH, Jung HY. Reevaluation of the expanded indications in undifferentiated early gastric cancer for endoscopic submucosal dissection. World J Gastroenterol. 2022;28:1548-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (2)] |
| 14. | Dalal I, Andalib I. Advances in endoscopic resection: a review of endoscopic submucosal dissection (ESD), endoscopic full thickness resection (EFTR) and submucosal tunneling endoscopic resection (STER). Transl Gastroenterol Hepatol. 2022;7:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Aihara H, Othman MO, Jawaid SA, Gorgun E, Sharma NR, Siddiqui UD, Peetermans JA, Rousseau MJ, Nishimura M. A multicenter, retrospective study of a through-the-needle injection-capable electrosurgical knife for endoscopic submucosal dissection. Gastrointest Endosc. 2024;100:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Kim JH, Kim YI, Ahn JY, Shin WG, Yang HJ, Nam SY, Min BH, Jang JY, Lim JH, Lee WS, Lee BE, Joo MK, Park JM, Lee HL, Gweon TG, Park MI, Choi J, Tae CH, Kim YW, Park B, Choi IJ. Long-term outcomes of endoscopic resection followed by additional surgery after non-curative resection in undifferentiated-type early gastric cancer: a nationwide multi-center study. Surg Endosc. 2022;36:1847-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 17. | Xu WS, Zhang HY, Jin S, Zhang Q, Liu HD, Wang MT, Zhang B. Efficacy and safety of endoscopic submucosal dissection for early gastric cancer and precancerous lesions in elderly patients. World J Gastrointest Surg. 2024;16:511-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Cecinato P, Sinagra E, Laterza L, Pianigiani F, Grande G, Sassatelli R, Barbara G. Endoscopic removal of gastrointestinal lesions by using third space endoscopy techniques. Best Pract Res Clin Gastroenterol. 2024;71:101931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Wang P, Zhao X, Wang R, Xu D, Yang H. Risk factors for pathological upgrading and noncurative resection in patients with gastric mucosal lesions after endoscopic submucosal dissection. BMC Gastroenterol. 2024;24:253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Chinda D, Shimoyama T. Assessment of physical stress during the perioperative period of endoscopic submucosal dissection. World J Gastroenterol. 2022;28:4508-4515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 21. | Jiang Y, Bu BL, Yang W, Zhi Y, Ye HY. Humanistic and graded psychological nursing care for patients undergoing endoscopic submucosal dissection of gastrointestinal tumors. World J Gastrointest Surg. 2025;17:100322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Chen R, Zhang Q, Hong S, Chen F, Huang X, Bao X, Ni Z, Zhang R. The clinical efficacy of "water-jet" hemostasis in gastrointestinal endoscopic submucosal dissection. Gastroenterol Rep (Oxf). 2024;12:goae088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Yan B, Li X, Qiao Y, Zhou L, Shen L. Clinical Efficacy of Endoscopic Submucosal Dissection for the Treatment of Duodenal Lesions in Terms of Operative Technique and Management of Complications. J Laparoendosc Adv Surg Tech A. 2022;32:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Zhu XD, Zhang LX, Luo PQ, Zhu H, Wei ZJ, Xu AM. Prognostic significance of post-preoperative tumor markers increments in patients with non-metastatic gastric cancer. J Cancer Res Clin Oncol. 2023;149:12191-12201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Yanan Z, Juan W, Jun W, Xin M, Kejian W, Fangyu W. Application of serum gastric function markers and digestive tumor indices to the diagnosis of early gastric cancer and precancerous lesions. Saudi Med J. 2023;44:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Tan Y, Qing Y, Liu D, Gong J. Endoscopic Submucosal Dissection for Treatment of Early-Stage Cancer or Precancerous Lesion in the Upper Gastrointestinal Tract in Patients with Liver Cirrhosis. J Clin Med. 2023;12:6509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Yu J, Zhang Y, Qian J. Endoscopic submucosal dissection in the treatment of patients with early colorectal carcinoma and precancerous lesions. J Gastrointest Oncol. 2020;11:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/