Published online Oct 27, 2025. doi: 10.4240/wjgs.v17.i10.109062
Revised: June 28, 2025
Accepted: September 1, 2025
Published online: October 27, 2025
Processing time: 178 Days and 23.4 Hours
Hepatic portal venous gas (HPVG) is a rare yet clinically significant radiological manifestation characterized by diverse etiologies, unclear pathophysiology, and nonspecific clinical presentations. Its appearance frequently heralds a severe underlying condition, with a reported mortality rate as high as 75%, earning it the moniker “harbinger of death”. In recent years, with advancements in diagnosis and treatment of HPVG, its mortality rate has been reduced to below 40%. Never
Core Tip: Hepatic portal venous gas (HPVG) represents a rare yet clinically significant radiological finding, underpinned by complex etiologies. Early and comprehensive diagnosis is important, and abdominal contrast-enhanced computed tomography scans can identify the underlying causes and assess the severity of the condition. It is essential to adopt individualized treatment strategies on the basis of the specific etiology of HPVG. Dynamic evaluation of patients’ conditions is important, and timely surgical intervention is warranted when intestinal necrosis occurs. Enterostomy may be considered instead of one-stage anastomosis after bowel resection. Clinicians must enhance their awareness of HPVG to elevate their diagnostic and therapeutic capabilities, ultimately improving patient outcomes.
- Citation: Zhou QY, Zheng ZH, Lv XL, Guo JQ, Zhang K, Xu HT. Advancement in the diagnosis and treatment of hepatic portal venous gas. World J Gastrointest Surg 2025; 17(10): 109062
- URL: https://www.wjgnet.com/1948-9366/full/v17/i10/109062.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i10.109062
Hepatic portal venous gas (HPVG) is a rare imaging finding characterized by the accumulation of gas within the portal vein and its intrahepatic branches, typically manifesting as branching or tubular gas distributions extending from the porta hepatis to the liver capsule. First reported by Wolfe and Evans in 1955[1,2], HPVG has traditionally been regarded as a “harbinger of death”, which was indicative of severe underlying pathology and reported to have a mortality rate of up to 75%[3]. However, recent advancement in diagnostic techniques and therapeutic strategies have reduced this mor
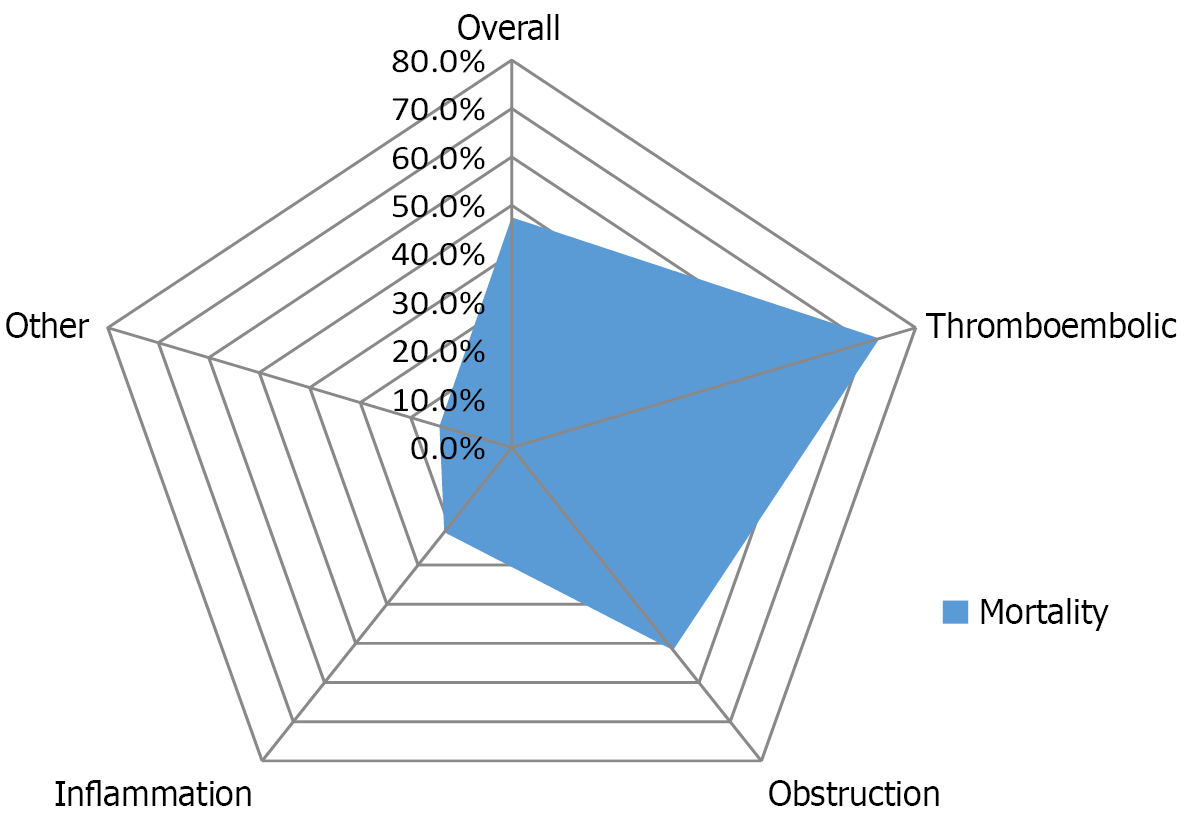
Koizumi et al[6] reported that the incidence of HPVG was 0.0045%, the average onset age was 79.3 years, the male-to-female ratio was approximately 1.16:1, and the incidence of intestinal ischemia was 53%. Daneshmand et al[7] found that the average age of patients was 65.6 years, with a male-to-female ratio of about 1.2:1. The etiologies were broadly categorized as follows: Intestinal thromboembolic diseases (42.6%), mechanical bowel obstruction (17.9%), inflammatory diseases (22.6%), and other conditions (17.1%). The overall mortality rate stood at 47.5%, varying significantly based on underlying causes (ranging from 14.3% to 72.8%) (Figure 1). In summary, HPVG is characterized by a low incidence, predominantly affecting middle-aged and elderly individuals, with a slight male predominance. It is commonly associated with the following types of conditions[8-11]: (1) Intestinal ischemia, such as mesenteric vascular embolism, vascular spasms, aneurysm formation, etc.; (2) Increased pressure in the gastrointestinal lumen, including bowel obstruction of various causes, gastric distension, abdominal trauma, and gastrointestinal tumors; (3) Abdominal infection, such as inflammatory bowel disease, abdominal abscesses, diverticulitis, pancreatitis, etc; and (4) Iatrogenic interventions, including colonoscopy, endoscopic procedures, and hemodialysis, pharmacotherapy. Among these, intestinal ischemia and increased pressure in the gastrointestinal lumen are most prevalent. Recent advancement in diagnostic technology have led to an increase in the reported incidence of HPVG. The mortality rates were closely linked to underlying etiologies, which were notably higher in ischemic conditions compared to non-ischemic ones[12]. Consequently, we classify the etiologies of HPVG into high-risk, moderate-risk, and low-risk categories based on their severities and threats (Table 1). The clinical manifestations of HPVG are generally non-specific, which depend on the severity of the underlying etiologies. Over 90% of patients presented with varying degrees of nausea, vomiting, abdominal pain, and diarrhea[13]. Some patients may exhibit infectious shock at early stage, indicating a poor prognosis[14].
| Classification | Etiology |
| Low-risk | Ulcerative disease: Esophageal ulcer, non-perforated gastric ulcer, non-perforated intestinal ulcer |
| Inflammatory disease: Pancreatitis, ulcerative colitis, Crohn’s disease, appendicitis, enteritis, cholangitis, diverticulitis | |
| Iatrogenic interventions: Liver transplantation surgery, biliary tract surgery, gastrointestinal surgery, colonoscopy, cardiac surgery, spinal surgery, endoscopic procedures, hemodialysis, and interventional operation | |
| Others: Diving, chronic obstructive pulmonary disease, trauma, poisoning, drugs, etc. | |
| Middle-risk | Perforation: Esophageal perforation, gastric ulcer perforation, duodenal ulcer perforation, jejunal ulcer perforation, intestinal diverticular disease with perforation, intestinal perforation |
| Obstruction or dilation: Pyloric stenosis, duodenal obstruction, acute cholelytic intestinal obstruction, paralytic intestinal obstruction | |
| Tumor: Oral and pharyngeal malignant tumor, esophageal malignant tumor, gastric cancer, small intestinal malignant tumor, colon cancer, rectosigmoid colon malignant tumor, malignant tumors of the anus and anal canal, malignant tumors of the liver and intrahepatic bile ducts, gallbladder cancer, malignant tumors of other unknown parts of the biliary tract, pancreatic cancer, malignant tumors of other digestive organs, lip malignant tumor, lung cancer, breast cancer, malignant tumors of bone and articular cartilage, skin cancer, mesothelial and soft tissue tumors, malignant tumors of reproductive organs, urinary system tumors, lymphoma, leukemia, etc. | |
| Infectious diseases: Clostridium perfringens infection (hemorrhagic enteritis, necrotizing enteritis, gas gangrene, food poisoning) | |
| Sepsis: Escherichia coli sepsis, streptococcal sepsis, Listeria sepsis, meningococcal septicemia, Candida sepsis, enterogenous infection, abdominal abscess, anastomotic fistula | |
| High-risk | Intestinal ischemia/necrosis: Mesenteric vascular embolism, vascular spasms, aneurysm formation, bowel obstruction, intestinal volvulus |
It is indicated that HPVG is not a distinct disease in itself but rather a secondary manifestation resulting from various underlying etiologies, such as intestinal necrosis, bowel obstruction, intra-abdominal abscesses, ulcerative colitis, gastric ulcers, Crohn’s disease, complications from endoscopic procedures, intra-abdominal tumors and so on[15,16]. The pathogenesis of HPVG remains incompletely understood, although two primary hypotheses have been proposed: (1) Mechanical theory posits that high-pressure gas from the intestinal lumen enters the portal venous system when endothelial injury is caused by a variety of factors (such as abdominal trauma or intestinal ischemia) or when mucosal rupture is caused by increased intraluminal gas pressure. Statistics show that 85% of HPVG cases are associated with intestinal mucosal injury, bowel distension, and increased intraluminal pressure; and (2) Bacterial theory, which suggests that gas-producing bacteria escape through damaged intestinal mucosa into the submucosa and vascular system, subsequently entering the portal venous system. The most commonly implicated bacteria include Escherichia coli and Clostridium perfringens. Gas-producing organisms such as Clostridium perfringens, Escherichia coli, Citrobacter, Candida, and anaerobes can be isolated from blood cultures and surgical specimens in some patients with HPVG, which are associated with intestinal ischemia and sepsis. Some patients exhibit favorable prognosis following antibiotic treatment[17,18].
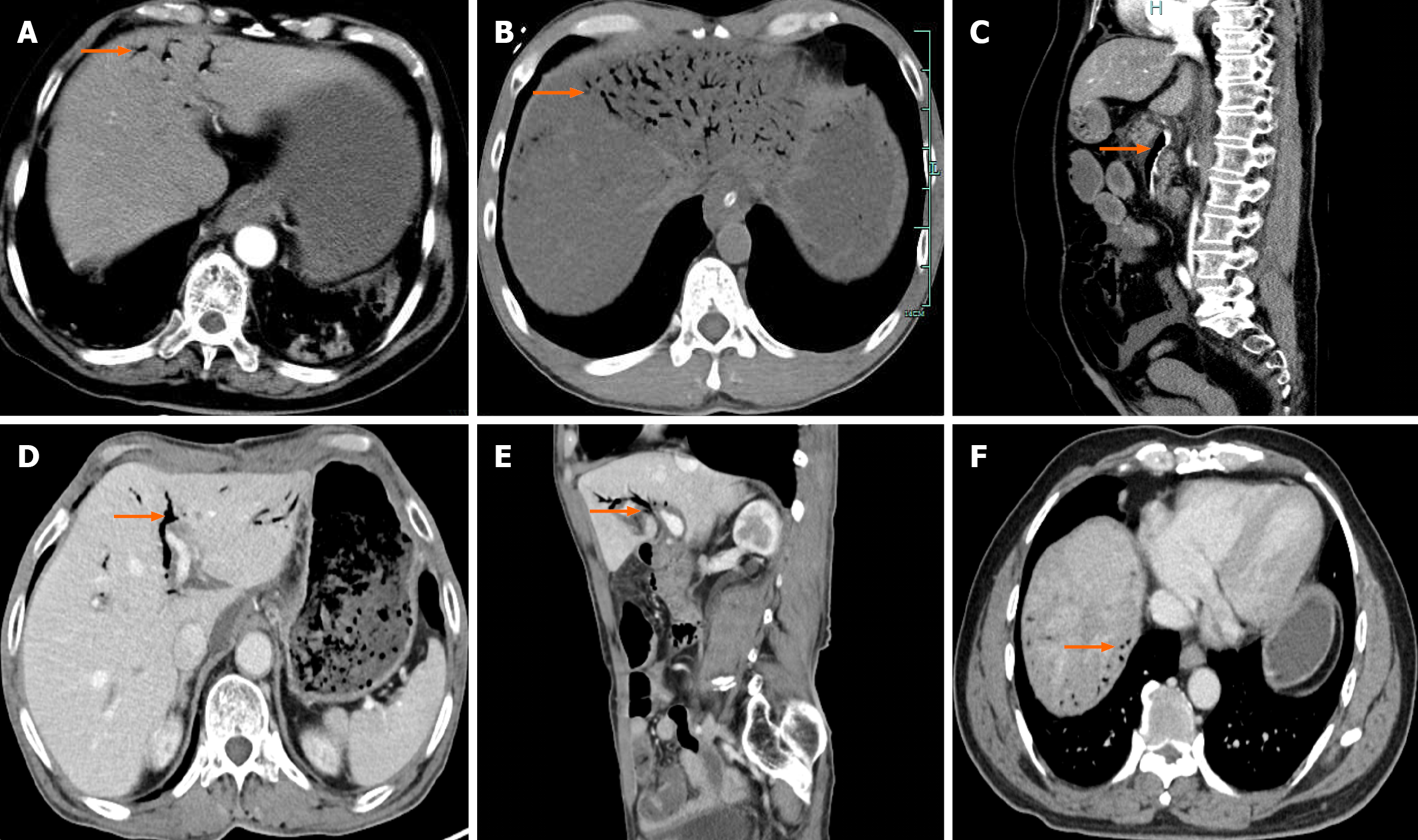
At early stage, the detection of HPVG primarily relied on X-ray imaging, which typically revealed characteristic bran
Currently, there is no consensus on the standardized treatment strategies for HPVG. The management for HPVG is dependent on patient’s clinical manifestation, imaging findings, and state analysis, including surgical approach and conservative approach. The progression of patients with HPVG often is acute and rapid, with some patients succumbing within 24 hours of onset. However, HPVG is not an indication for surgery. The treatment strategies should focus on the underlying etiology, and patient’s disease severity determines whether surgery is necessary. The consensus on surgical indications has not yet been reached[27]. Moser et al[28] found that the mortality rate exceeded 50% when HPVG coexisted with intestinal necrosis. A retrospective study involving 182 patients with HPVG reported an overall mortality rate of 39%, which was soaring to 75% among those with intestinal necrosis. Thus exploratory laparotomy was recom
Conservative management is suitable for HPVG patients with stable vital signs, non-ischemic disease, and mild or no signs of peritonitis[35]. Combining with literature review and clinical experience, we summarize the following insights: (1) Aggressive treatment of underlying etiologies is important. HPVG is a secondary phenomenon arising from primary diseases, and therefore treatment should aim at the primary etiology. Successful management of the underlying etiology can lead to the spontaneous resolution of HPVG and improved prognosis[36]; (2) Antibiotics should be administered in the early stage. Timely and appropriate antibiotic therapy is crucial in preventing the occurrence of infectious shock and necrotizing enterocolitis[37]. Patients with HPVG are at risk of bacteremia, and a recent study reported that appro
It is worth noting that perioperative management plays a crucial role in improving patient survival, and it often runs through the entire treatment process of the patient. We summarize the key points in perioperative management as follows: (1) Fluid resuscitation protocols: The administration of balanced crystalloid over normal saline is suggested in the initial resuscitation of shock, as administering a large dose of normal saline may lead to hyperchloremic metabolic acidosis and increase the risk of acute kidney injury[43]. Balanced crystalloid fluids, whose chloride concentration is similar to plasma, should be used instead of normal saline in adult patients with sepsis or septic shock. For patients who do not respond to the standard treatment with crystalloid fluids, isotonic albumin solutions may be considered[44]; (2) Antibiotics selection criteria: Broad-spectrum antibiotics, such as piperacillin-tazobactam, cefperazone-sulbactam and imipenem, should be empirically employed on the basis of the features of most commonly implicated bacteria[17,18]. In the subsequent treatment process, bacterial culture and drug sensitivity detection are able to guide the use of antibiotic therapy[13]. Metronidazole can be used for the treatment of anaerobic bacterial infections. Another proposed alternative therapy is hyperbaric oxygen, which can reduce the partial pressure of gas in the venous system and act as a toxin for anaerobic bacteria[45]. When there is a concern about fungal sepsis, a reliable approach is to increase empirical antifungal coverage and perform a beta-d-glucan testing[46]; (3) Vasopressor strategies for septic shock complicating HPVG: We recommend early administration of vasopressors with resuscitation fluids during the initial resuscitation of septic shock complicating HPVG. Norepinephrine is used as a first-line vasopressor for septic shock, and vasopressin is used as a second-line vasopressor for septic shock. Patients with septic shock who do not respond to initial fluid resuscitation are given low-dose hydrocortisone (200-300 mg/day) and vasopressors for recovery from shock[44]; and (4) Differentiating between infectious and thrombotic causes: A retrospective study involving 21 patients with HPVG found that C-reactive protein, procalcitonin (PCT), and D-dimer (DD-I) in patients with HPVG were higher than normal levels. The levels of PCT and C-reactive protein can indicate the degree of inflammation in patients[47]. As a degradation product of cross-linked fibrin, DD-I mainly reflects the fibrinolytic function and is used in the diagnosis of venous thromboembolism, deep vein thrombosis and pulmonary embolism. These biomarkers appear to be useful in distinguishing infectious cause from thrombotic cause. DD-I is more prone to thrombotic causes, while PCT is more prone to infectious causes. However, as the disease progresses, ischemia is often complicated with infection, which is easy to be confused. Therefore, they should be used in combination to improve the differential efficiency, but the final diagnosis depends on imaging and clinical evaluation[26].
In recent years, with the improvement of imaging techniques and treatment methods, the mortality rate has decreased from 75% to 40%[4]. Nevertheless, HPVG still has the characteristics of insidious onset, rapid progression and high mortality rate[48]. Many aspects such as exploration of biomarkers related to intestinal necrosis, artificial intelligence-assisted CT interpretation and prognostic related factors (age, comorbidities, and etiology) still require further research and verification. More prospective multicenter clinical studies should be conducted to provide reliable evidence for the diagnosis and treatment of HPVG in the future, thereby improving the prognosis of patients.
In summary, HPVG is a rare yet clinically significant imaging manifestation characterized by diverse etiologies, unclear pathophysiology, and nonspecific clinical presentations. Recent advancements in imaging technology have markedly improved the early diagnosis rates of HPVG and contributed to a reduction in mortality. However, it remains a perilous condition. Comprehensive imaging examinations, particularly contrast-enhanced abdominal CT, contribute to the early identification of underlying causes and thorough assessment of the clinical status. Treatment for HPVG should involve an adequate evaluation of the primary disease and its severity, and individualized therapeutic strategies while dynamically evaluating the patients’ conditions. Timely surgical intervention is warranted when intestinal necrosis occurs. Entero
| 1. | Wolfe JN, Evans WA. Gas in the portal veins of the liver in infants; a roentgenographic demonstration with postmortem anatomical correlation. Am J Roentgenol Radium Ther Nucl Med. 1955;74:486-488. [PubMed] |
| 2. | Kinoshita H, Shinozaki M, Tanimura H, Umemoto Y, Sakaguchi S, Takifuji K, Kawasaki S, Hayashi H, Yamaue H. Clinical features and management of hepatic portal venous gas: four case reports and cumulative review of the literature. Arch Surg. 2001;136:1410-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Cambria RP, Margolies MN. Hepatic portal venous gas in diverticulitis: survival in a steroid-treated patient. Arch Surg. 1982;117:834-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Yoo SK, Park JH, Kwon SH. Clinical outcomes in surgical and non-surgical management of hepatic portal venous gas. Korean J Hepatobiliary Pancreat Surg. 2015;19:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Fujii M, Yamashita S, Tanaka M, Tashiro J, Takenaka Y, Yamasaki K, Masaki Y. Clinical features of patients with hepatic portal venous gas. BMC Surg. 2020;20:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Koizumi C, Michihata N, Matsui H, Fushimi K, Yasunaga H. In-Hospital Mortality for Hepatic Portal Venous Gas: Analysis of 1590 Patients Using a Japanese National Inpatient Database. World J Surg. 2018;42:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Daneshmand A, Parys S, Rao S, Watanabe Y, Sieunarine K. Portal venous gas: different aetiologies and their respective outcomes. ANZ J Surg. 2020;90:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Lin L, Zhou Q, Gao J, Zhang H. Hepatic portal venous gas associated with ischemic colitis: a case report. Int J Emerg Med. 2025;18:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Yu ZX, Bin Z, Lun ZK, Jiang XJ. Portal venous gas complication following coronary angiography: A case report. World J Radiol. 2024;16:586-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Furuta T, Fujiwara M, Motonaga T, Matsufuji H, Tateishi H, Nakada S, Kanagawa T, Uchida M. Ultrasound and computed tomography findings of hepatic portal venous gas associated with acute appendicitis in a paediatric patient: A case report. Ultrasound. 2024;32:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Iskander OA. Unraveling the Mystery of Hepatic Portal Vein Gas: Exploring Its Benign Nature and Surgical Implications. Cureus. 2023;15:e41231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Zhang C, Liu Z, Zhang JY, Wang SF, Wang Z, Liu C. [Clinical diagnosis and treatment strategies for adult portal venous gas]. Zhonghua Xiaohua Waike Zazhi. 2024;23:1403-1409. [DOI] [Full Text] |
| 13. | Liu C, Wu CH, Zheng XD, Liu JP, Li CL, Zhao JY, Lan Q, Zhou WL, Li WB. Hepatic portal venous gas: A case report and analysis of 131 patients using PUBMED and MEDLINE database. Am J Emerg Med. 2021;45:506-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Tian C, Bai Y, Ma QB, Ge HX. [Clinical characteristics of 7 cases of hepatic portal venous gas]. Beijing Da Xue Xue Bao Yi Xue Ban. 2023;55:743-747. [PubMed] [DOI] [Full Text] |
| 15. | Chen H, Wu Q, Fang H, Liang B, Fang L. Intestinal necrosis cannot be neglected in a patient with hepatic portal vein gas combined with appendicitis: a rare case report and literature review. BMC Surg. 2019;19:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Denis C, Hantson P. Hepatic portal venous gas and combined colchicine-bortezomib toxicity. Clin Toxicol (Phila). 2021;59:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Ghoz HM, Sheikh SM, Khandelwal K, Fiore J, James N, Weinstock J. A Case of Hepatic Portal Venous Gas: Hypothesis of a Transient Direct Communication between a Penetrating Antral Gastric Ulcer and Mesenteric Varices. Case Rep Gastrointest Med. 2017;2017:8185132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Rankin I, Sheth H. Hepatic portal venous gas: comparison of two cases. Case Rep Surg. 2013;2013:637951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Liebman PR, Patten MT, Manny J, Benfield JR, Hechtman HB. Hepatic--portal venous gas in adults: etiology, pathophysiology and clinical significance. Ann Surg. 1978;187:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 322] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Sebastià C, Quiroga S, Espin E, Boyé R, Alvarez-Castells A, Armengol M. Portomesenteric vein gas: pathologic mechanisms, CT findings, and prognosis. Radiographics. 2000;20:1213-1224; discussion 1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Cheong I, Tamagnone FM. The role of different ultrasound modes in hepatic portal venous gas diagnosis, including a novel method using color M-mode. J Ultrasound. 2024;27:1009-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Liang KW, Huang HH, Tyan YS, Tsao TF. Hepatic Portal Venous Gas: Review of Ultrasonographic Findings and the Use of the "Meteor Shower" Sign to Diagnose It. Ultrasound Q. 2018;34:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Trenker C, Görg C, Dong Y, Cui XW, Zadeh ES, Alhyari A, Kripfgans O, Dietrich CF. Portal venous gas detection in different clinical situations. Med Ultrason. 2023;25:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Dogaru IA, Gheoca Mutu DE, Ursuț BM, Filipoiu FM, Tulin AD. Decoding Hepatic Portal Venous Gas: A Case Report. Cureus. 2024;16:e54050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Wang C, Jin H, Xue H, Zhang Y. Intrahepatic Gas Caused by Acute Gastroenteritis: Hepatic Portal Venous Gas or Biliary Tract Gas? Int Med Case Rep J. 2024;17:589-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Zhang Y, Liu HL, Tang M, Wang H, Jiang HH, Lin MB. Clinical features and management of 20 patients with hepatic portal venous gas. Exp Ther Med. 2022;24:525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Koami H, Isa T, Ishimine T, Kameyama S, Matsumura T, Yamada KC, Sakamoto Y. Risk factors for bowel necrosis in patients with hepatic portal venous gas. Surg Today. 2015;45:156-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Moser A, Stauffer A, Wyss A, Schneider C, Essig M, Radke A. Conservative treatment of hepatic portal venous gas consecutive to a complicated diverticulitis: A case report and literature review. Int J Surg Case Rep. 2016;23:186-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Bani Hani M, Kamangar F, Goldberg S, Greenspon J, Shah P, Volpe C, Turner DJ, Horton K, Fishman EK, Francis IR, Daly B, Cunningham SC. Pneumatosis and portal venous gas: do CT findings reassure? J Surg Res. 2013;185:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Gonda M, Osuga T, Ikura Y, Hasegawa K, Kawasaki K, Nakashima T. Optimal treatment strategies for hepatic portal venous gas: A retrospective assessment. World J Gastroenterol. 2020;26:1628-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Pan W, Xiang S, Zhang J, Gao Y, Liu S. Chemotherapy-induced pneumatosis intestinalis followed by hepatic portal venous gas. A case report. J Int Med Res. 2024;52:3000605241239276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Higashizono K, Yano H, Miyake O, Yamasawa K, Hashimoto M. Postoperative pneumatosis intestinalis (PI) and portal venous gas (PVG) may indicate bowel necrosis: a 52-case study. BMC Surg. 2016;16:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Moussa M, Marzouk I, Abdelmoula K, Manamani A, Dali N, Farhat LC, Hendaoui L. Role of Computed tomography in predicting prognosis of Hepatic portal venous gas. Int J Surg Case Rep. 2017;30:177-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Liot E, Assalino M, Buchs NC, Schiltz B, Douissard J, Morel P, Ris F. Does near-infrared (NIR) fluorescence angiography modify operative strategy during emergency procedures? Surg Endosc. 2018;32:4351-4356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Dibra R, Picciariello A, Trigiante G, Labellarte G, Tota G, Papagni V, Martines G, Altomare DF. Pneumatosis Intestinalis and Hepatic Portal Venous Gas: Watch and Wait or Emergency Surgery? A Case Report and Literature Review. Am J Case Rep. 2020;21:e923831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | He C, Zhang J, Yuan B, Pang Y. Is reoperation required for patients presenting with hepatic portal venous gas after gastrointestinal surgery: a review of the literature. Ann Med. 2024;56:2389293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Zhou C, Kilpatrick MD, Williams JB, Rivers JF, Miller TA. Hepatic Portal Venous Gas: A Potentially Lethal Sign Demanding Urgent Management. Am J Case Rep. 2022;23:e937197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Ling XJ, Wang YS, Pan SH. [Death-related risk factors in adult patients with portal venous gas: Case reports and literature review]. Gandanyi Waike Zazhi. 2023;35:483-488. [DOI] [Full Text] |
| 39. | Liu A, Shen J, Long L, Shi X, Wen Q, Pan Z. Hepatic portal venous gas initially manifesting as severe shock: a case series. J Int Med Res. 2024;52:3000605241239469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Ma AS, Ewing I, Murray CD, Hamilton MI. Hepatic portal venous gas and portal venous thrombosis following colonoscopy in a patient with terminal ileal Crohn's disease. BMJ Case Rep. 2015;2015:bcr2014206854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Tafur A, Douketis J. Perioperative management of anticoagulant and antiplatelet therapy. Heart. 2018;104:1461-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Arai M, Kim S, Ishii H, Takiguchi T, Yokota H. Portal Venous Gas in Adults: Clinical Significance, Management, and Outcomes of 25 Consecutive Patients. J Nippon Med Sch. 2021;88:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 43. | Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 788] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 44. | Shime N, Nakada TA, Yatabe T, Yamakawa K, Aoki Y, Inoue S, Iba T, Ogura H, Kawai Y, Kawaguchi A, Kawasaki T, Kondo Y, Sakuraya M, Taito S, Doi K, Hashimoto H, Hara Y, Fukuda T, Matsushima A, Egi M, Kushimoto S, Oami T, Kikutani K, Kotani Y, Aikawa G, Aoki M, Akatsuka M, Asai H, Abe T, Amemiya Y, Ishizawa R, Ishihara T, Ishimaru T, Itosu Y, Inoue H, Imahase H, Imura H, Iwasaki N, Ushio N, Uchida M, Uchi M, Umegaki T, Umemura Y, Endo A, Oi M, Ouchi A, Osawa I, Oshima Y, Ota K, Ohno T, Okada Y, Okano H, Ogawa Y, Kashiura M, Kasugai D, Kano KI, Kamidani R, Kawauchi A, Kawakami S, Kawakami D, Kawamura Y, Kandori K, Kishihara Y, Kimura S, Kubo K, Kuribara T, Koami H, Koba S, Sato T, Sato R, Sawada Y, Shida H, Shimada T, Shimizu M, Shimizu K, Shiraishi T, Shinkai T, Tampo A, Sugiura G, Sugimoto K, Sugimoto H, Suhara T, Sekino M, Sonota K, Taito M, Takahashi N, Takeshita J, Takeda C, Tatsuno J, Tanaka A, Tani M, Tanikawa A, Chen H, Tsuchida T, Tsutsumi Y, Tsunemitsu T, Deguchi R, Tetsuhara K, Terayama T, Togami Y, Totoki T, Tomoda Y, Nakao S, Nagasawa H, Nakatani Y, Nakanishi N, Nishioka N, Nishikimi M, Noguchi S, Nonami S, Nomura O, Hashimoto K, Hatakeyama J, Hamai Y, Hikone M, Hisamune R, Hirose T, Fuke R, Fujii R, Fujie N, Fujinaga J, Fujinami Y, Fujiwara S, Funakoshi H, Homma K, Makino Y, Matsuura H, Matsuoka A, Matsuoka T, Matsumura Y, Mizuno A, Miyamoto S, Miyoshi Y, Murata S, Murata T, Yakushiji H, Yasuo S, Yamada K, Yamada H, Yamamoto R, Yamamoto R, Yumoto T, Yoshida Y, Yoshihiro S, Yoshimura S, Yoshimura J, Yonekura H, Wakabayashi Y, Wada T, Watanabe S, Ijiri A, Ugata K, Uda S, Onodera R, Takahashi M, Nakajima S, Honda J, Matsumoto T. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2024. J Intensive Care. 2025;13:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 45. | Erdem S, Patel SV, Patel D, Patel S, Patel S, Chaudhary AJ. Understanding the Nuances of Hepatic Portal Venous Gas in Pneumatosis Intestinalis: An Indication of Bowel Ischemia? Cureus. 2023;15:e45330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 46. | Jenkins JK, Georgiou A, Laugharne M, Meisner S, Cook T. Emphysematous gastritis in a patient with neutropenic sepsis: A case report and literature review with comment on management. J Intensive Care Soc. 2023;24:328-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 47. | Wei HR, Shan YQ, Dong FH, Zhou LP, Yang YB, Shi J, Ji CH, Kong WC. Clinical characteristics and treatment of hepatic portal venous gas: case series and literature review. Front Med (Lausanne). 2025;12:1540418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Mollah T, Zhang X, Kuany T, Onasanya O, Knowles B. Hepatic Portal Venous Gas in Acute Pancreatitis-A Critical Finding: A Systematic Review. Am Surg. 2025;91:1203-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/