Published online Feb 15, 2026. doi: 10.4239/wjd.v17.i2.114253
Revised: October 25, 2025
Accepted: December 17, 2025
Published online: February 15, 2026
Processing time: 136 Days and 1.1 Hours
Pre-diabetes is a transitional metabolic stage between health and diabetes, serving as a critical warning signal for disease progression. Early intervention targeting risk factors in prediabetic individuals prevents the progression to type 2 diabetes.
To investigate the predictive value of the triglyceride-glucose (TyG) index and its derived indicators for new-onset diabetes in patients with pre-diabetes.
A prospective community-based cohort study was carried out based on subjects aged over 40 years with pre-diabetes in Dalian, Liaoning Province, China. A total of 1352 subjects with complete follow-up data attended the follow-up survey. Multivariable Cox regression models were performed to assess the association of the TyG index and its derived indicators with risk of diabetes in patients with pre-diabetes. The diagnostic values of the TyG index and derived indicators in predic
During a 3-year follow-up period, 153 cases with incident diabetes were id
The TyG index and its derived indicators were risk factors for the pre-diabetes to diabetes outcome, and may be regarded as predictors of the outcome. The risk of conversion of pre-diabetes to diabetes increased with increases in the TyG index and its derived indicators. The TyG-BMI was better than TyG and TyG-WC in predicting the 3-year outcome for diabetes. Although these indices could aid in the initial risk stratification in primary care, their modest accuracy warrants cautious interpretation.
Core Tip: Pre-diabetes is an abnormal state of glucose metabolism between normal glucose tolerance and diabetes. If early intervention is carried out for risk factors in a pre-diabetic population, the occurrence and development of diabetes can be prevented. The triglyceride-glucose (TyG) index is a new index calculated according to triglycerides and fasting plasma glucose test. The latest research suggests that the TyG index and its derivative indexes, such as the product of TyG index and body mass index and the product of TyG index and waist circumference are novel and surrogate markers for insulin resistance. We prospectively investigated the relationship between TyG index and derived indexes and incident type 2 diabetes using a large sample, community-based cohort, aim at to find a simple indicator predicting the progression of a pre-diabetic population to diabetes, to allow relevant early-stage intervention in a pre-diabetic population and so reduce diabetes incidence.
- Citation: Wang B, Liu MC, Liu XH, Zhu Z, Liu YS, Zhang TT, Li XY, Gao ZN. Using the triglyceride-glucose index and derived indexes to forecast progression from pre-diabetes to diabetes: A 3-year follow-up study. World J Diabetes 2026; 17(2): 114253
- URL: https://www.wjgnet.com/1948-9358/full/v17/i2/114253.htm
- DOI: https://dx.doi.org/10.4239/wjd.v17.i2.114253
Pre-diabetes, also known as impaired glucose regulation, is an abnormal state of glucose metabolism between normal glucose tolerance and diabetes, which is considered an inevitable stage of diabetes and an early warning signal of diabetes[1,2]. If early intervention is carried out for risk factors in a pre-diabetic population, the occurrence and deve
Insulin resistance (IR) is a significant factor in the development from pre-DM to type 2 DM (T2DM)[1,3,4]. The hyper
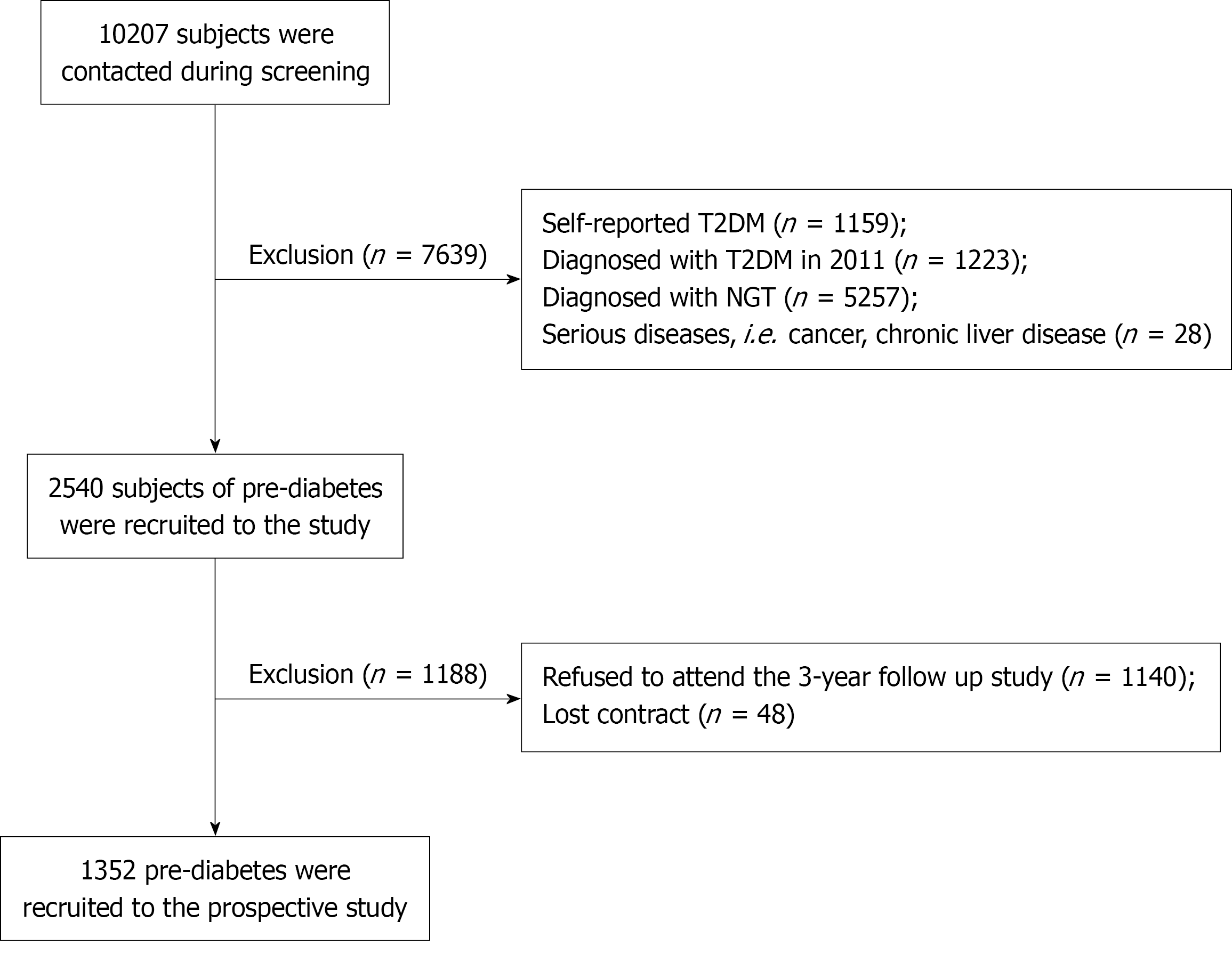
We used all data derived from the China Cardiometabolic Disease and Cancer Cohort study, which is a large prospective cohort study initiated by Ruijin Hospital Affiliated to the Medical School of Shanghai Jiao Tong University. A total of 10207 participants were contacted from two communities in the city of Dalian during screening. Subjects without diabetes history underwent a 75 g OGTT in 2011 and this cohort study was conducted from the baseline survey in 2011 up to 2014. Participants with incomplete follow-up data or missing key variables were excluded from the final analysis. A total of 1352 participants consisting of 341 male and 1011 female residents were eligible for the final analysis, and a flow chart of population exclusion is shown in Figure 1.
All subjects participated in the study voluntarily and provided informed consent prior to enrollment. The study was approved by the Institutional Research Ethics Committee of Ruijin Hospital Affiliated to the Medical School of Shanghai Jiao Tong University.
The following data regarding clinical history, anthropometric measurements and blood analyses were derived at baseline and the follow-up visit. Blood samples were obtained after at least 8 hours of overnight fasting. Levels of FPG, TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured. Subjects without diabetes history underwent a OGTT and tested 2 hours post load plasma glucose (2hPG). Serum for lipid profiles was stored below -20 °C, and whole blood for HbA1c analysis was stored below 4 °C, with sam
BMI = weight (kg)/height2 (m2); waist-hip ratio (WHR) = WC (cm)/hip circumference (HC) (cm); homeostasis model assessment (HOMA)-IR = FPG (mmol/L) × FINS (mU/L)/22.5; HOMA-β = 20 × FINS (mU/L)/(FPG - 3.5) (mmol/L); TyG index = ln[TG (mg/dL) × FPG (mg/dL)/2]; TyG-BMI = TyG index × BMI; TyG-WC = TyG index × WC (cm).
According to 2011 World Health Organization diagnostic criteria, diabetes was defined as FPG ≥ 7.0 mmol/L and/or 2hPG ≥ 11.1 mmol/L, or HbA1c ≥ 6.5%; normal glucose tolerance was defined as FPG < 6.1 mmol/ L and 2hPG < 7.8 mmol/L; impaired fasting glucose (IFG) was defined 6.1 mmol/L ≤ FPG < 7.0 mmol/L and 2hPG < 7.8 mmol/L; impaired glucose tolerance (IGT) was defined FPG < 7.0 mmol/L and 7.8 mmol/L ≤ 2hPG < 11.1 mmol/L.
Subjects (aged ≥ 40 years) who were diagnosed with pre-diabetes (IFG and IGT) at baseline and completed a three-year follow up were selected as the research objects.
Subjects with diagnosed normal glucose tolerance, diabetes, cancer, chronic liver disease, chronic kidney disease and glucocorticoid treatment at the baseline were exclusive; if the subjects didn’t complete the follow up survey, subjects were also exclusive.
Continuous variables are expressed as mean ± SD and qualitative variables as numbers and percentages. Comparisons between the diabetic and non-diabetic groups were performed using two independent samples t-tests and, for categorical variables, the χ2 test was used. Multivariable-adjusted Cox proportional-hazards regression analyses were performed to estimate the hazard ratios (HRs) for the TyG index and its derived indicators in relation to 3-year diabetes incidence. The area under the curve (AUC) of the receiver operating characteristics (ROC) curve was calculated to compare the predictive power of the TyG index and its derivative indexes. Pairwise comparisons of the ROC curves were performed using DeLong’s test. The optimal cut-off points for the TyG index, TyG-BMI and TyG-WC were determined using the Youden index from the ROC curve analysis.
Among the 1352 subjects, the average age was 58.44 ± 8.21 years. The incidence of diabetes was 11.3% (153/1352); 12.6% (43/341) in males and 10.9% (110/1011) in females (χ2 = 0.760, P = 0.375). According to FPG, OGTT or HbA1c results and history of diabetes during follow-up, the subjects were divided into diabetes and non-diabetes groups. All the subjects were analyzed for age, weight, height, BMI, WC, HC, WHR, systolic blood pressure, diastolic blood pressure, FPG, 2hPG, HbA1c, TC, TG, HDL-C, LDL-C, serum uric acid, FINS, HOMA-IR, HOMA-β, TyG index, TyG-BMI, TyG-WC and other clinical characteristics (Table 1).
| Variables | Total | Non-diabetes | Diabetes | P value | Cohen’s d value |
| Type proportion (IFG/IGT) | 282/1070 | 252/947 | 30/123 | 0.752 | |
| Gender (male/female) | 341/1011 | 289/901 | 43/110 | 0.375 | |
| Age (year) | 58.44 ± 8.21 | 58.43 ± 8.35 | 58.58 ± 7.03 | 0.826 | 0.01943 |
| Weight (kg) | 67.52 ± 10.88 | 67.14 ± 10.75 | 70.52 ± 11.43 | 0.000 | 0.30464 |
| Height (m) | 160.73 ± 7.65 | 160.70 ± 7.63 | 160.99 ± 7.92 | 0.661 | 0.03729 |
| BMI (kg/m2) | 26.08 ± 3.38 | 25.94 ± 3.33 | 27.18 ± 3.60 | 0.000 | 0.35759 |
| WC (cm) | 90.73 ± 9.34 | 90.40 ± 9.25 | 93.29 ± 9.64 | 0.000 | 0.30592 |
| HC (cm) | 101.5 ± 7.23 | 101.26 ± 7.13 | 103.37 ± 7.73 | 0.001 | 0.28375 |
| WHR | 0.89 ± 0.06 | 0.89 ± 0.06 | 0.90 ± 0.06 | 0.061 | 0.16667 |
| SBP (mmHg) | 143.29 ± 20.46 | 143.04 ± 20.38 | 145.25 ± 21.00 | 0.069 | 0.1068 |
| DBP (mmHg) | 81.38 ± 11.21 | 81.21 ± 11.25 | 82.68 ± 10.87 | 0.126 | 0.13289 |
| FPG (mmol/L) | 5.91 ± 0.50 | 5.90 ± 0.50 | 6.07 ± 0.49 | 0.001 | 0.34342 |
| 2hPG (mmol/L) | 8.40 ± 1.37 | 8.38 ± 1.36 | 8.62 ± 1.40 | 0.044 | 0.17389 |
| HbA1c (%) | 5.86 ± 0.36 | 5.83 ± 0.34 | 6.06 ± 0.47 | 0.000 | 0.56073 |
| TC (mmol/L) | 5.59 ± 1.07 | 5.58 ± 1.07 | 5.64 ± 1.05 | 0.517 | 0.0566 |
| TG (mmol/L) | 1.64 ± 0.97 | 1.63 ± 0.98 | 1.75 ± 0.87 | 0.139 | 0.1295 |
| HDL-C (mmol/L) | 1.39 ± 0.31 | 1.39 ± 0.32 | 1.33 ± 0.27 | 0.015 | 0.20266 |
| LDL-C (mmol/L) | 3.36 ± 0.89 | 3.35 ± 0.90 | 3.42 ± 0.85 | 0.347 | 0.07997 |
| SUA (μmol/L) | 321.8 ± 72.55 | 319.11 ± 71.28 | 342.82 ± 78.94 | 0.000 | 0.31526 |
| FINS (mU/L) | 9.54 ± 4.71 | 9.41 ± 4.58 | 10.55 ± 5.54 | 0.016 | 0.22429 |
| HOMA-IR | 2.52 ± 1.27 | 2.48 ± 1.26 | 2.84 ± 1.45 | 0.004 | 0.26503 |
| HOMA-β | 81.82 ± 43.3 | 81.26 ± 41.47 | 86.2 ± 55.57 | 0.289 | 0.10076 |
| TyG index (2011) | 8.82 ± 0.51 | 8.80 ± 0.51 | 8.93 ± 0.47 | 0.003 | 0.26509 |
| TyG-BMI (2011) | 230.3 ± 34.98 | 228.64 ± 34.25 | 243.27 ± 37.91 | 0.000 | 0.40497 |
| TyG-WC (2011) | 800.59 ± 100.3 | 796.31 ± 99.25 | 834.08 ± 102.50 | 0.000 | 0.37438 |
| TyG index (2014) | 8.83 ± 0.51 | 8.81 ± 0.50 | 9.00 ± 0.55 | 0.000 | 0.3615 |
| TyG-BMI (2014) | 233.40 ± 96.61 | 230.89 ± 96.30 | 251.49 ± 97.24 | 0.021 | 0.21287 |
| TyG-WC (2014) | 788.41 ± 103.09 | 782.12 ± 98.88 | 833.71 ± 120.50 | 0.000 | 0.46806 |
The grouping by quartile according to the baseline TyG index was group T1 (TyG < 8.46), group T2 (8.46 ≤ TyG < 8.80), group T3 (8.80 ≤ TyG < 9.12) and group T4 (TyG ≥ 9.12). For these quartiles, the corresponding incidences of type 2 diabetes were 7.3%, 11%, 13.4% and 13.5%, showing an increase with increasing quartiles (χ2 = 8.448, P = 0.038; Table 2).
| TyG index quartiles | Non-diabetes | Diabetes | Total | χ2 value | P value |
| T1 group | 305 (92.7) | 24 (7.3) | 329 (100) | 8.448 | 0.038 |
| T2 group | 309 (89.0) | 38 (11) | 347 (100) | ||
| T3 group | 290 (86.6) | 45 (13.4) | 335 (100) | ||
| T4 group | 295 (86.5) | 46 (13.5) | 341 (100) | ||
| Total | 1199 (88.7) | 153 (11.3) | 1352 (100) | ||
| χ2 value for trend = 7.328, P value for trend = 0.007 | |||||
Grouping by quartile according to the baseline TyG-BMI was group B1 (TyG-BMI < 205), group B2 (205 ≤ TyG-BMI < 227), group B3 (227 ≤ TyG-BMI < 252) and group B4 (TyG-BMI ≥ 252). After 3 years, the corresponding incidences of diabetes were 7.8%, 6.8%, 10.6% and 19.9% (χ2 = 36.008, P = 0.000; Table 3).
| TyG-BMI quartiles | Non-diabetes | Diabetes | Total | χ2 value | P value |
| B1 group | 307 (92.2) | 26 (7.8) | 333 (100) | 36.008 | 0.000 |
| B2 group | 314 (93.2) | 23 (6.8) | 337 (100) | ||
| B3 group | 303 (89.4) | 36 (10.6) | 339 (100) | ||
| B4 group | 274 (80.1) | 68 (19.9) | 342 (100) | ||
| Total | 1198 (88.7) | 153 (11.3) | 1351 (100) | ||
| χ2 value for trend = 27.150, P value for trend = 0.000 | |||||
The grouping according to the baseline TyG-WC quartiles was group W1 (TyG-WC < 728), group W2 (728 ≤ TyG-WC < 796), group W3 (796 ≤ TyG-WC < 868) and group W4 (TyG-WC ≥ 868). After 3 years, the corresponding incidences of diabetes were 7.4%, 8.7%, 10.5% and 18.5%, showing an increase with increasing quartiles (χ2 = 25.197, P = 0.000; Table 4).
| TyG-WC quartiles | Non-diabetes | Diabetes | Total | χ2 value | P value |
| W1 group | 312 (92.6) | 25 (7.4) | 337 (100) | 25.197 | 0.000 |
| W2 group | 304 (91.3) | 29 (8.7) | 333 (100) | ||
| W3 group | 306 (89.5) | 36 (10.5) | 342 (100) | ||
| W4 group | 277 (81.5) | 63 (18.5) | 340 (100) | ||
| Total | 1199 (88.7) | 153 (11.3) | 1352 (100) | ||
| χ2 value for trend = 20.821, P value for trend = 0.000 | |||||
Cox regression models were established to evaluate the association of TyG index, TyG-BMI and TyG-WC with diabetes. After adjusting for age, gender, BMI and FINS, TyG index was strongly positively correlated with the risk of diabetes [HR = 1.389, 95% confidence interval (CI): 1.011-1.908, P = 0.043] in pre-diabetic patients. After adjusting for age, gender and FINS, TyG-BMI was strongly positively correlated with the risk of diabetes (HR = 1.010, 95%CI: 1.005-1.015, P = 0.000). After adjusting for age, gender and FINS, TyG-WC was strongly positively correlated with the risk of diabetes (HR = 1.003, 95%CI: 1.001-1.005, P = 0.001) (Table 5).
| Variables | HR | 95%CI | P value |
| TyG index | 1.389 | 1.011-1.908 | 0.043 |
| TyG-BMI | 1.010 | 1.005-1.015 | 0.000 |
| TyG-WC | 1.003 | 1.001-1.005 | 0.001 |
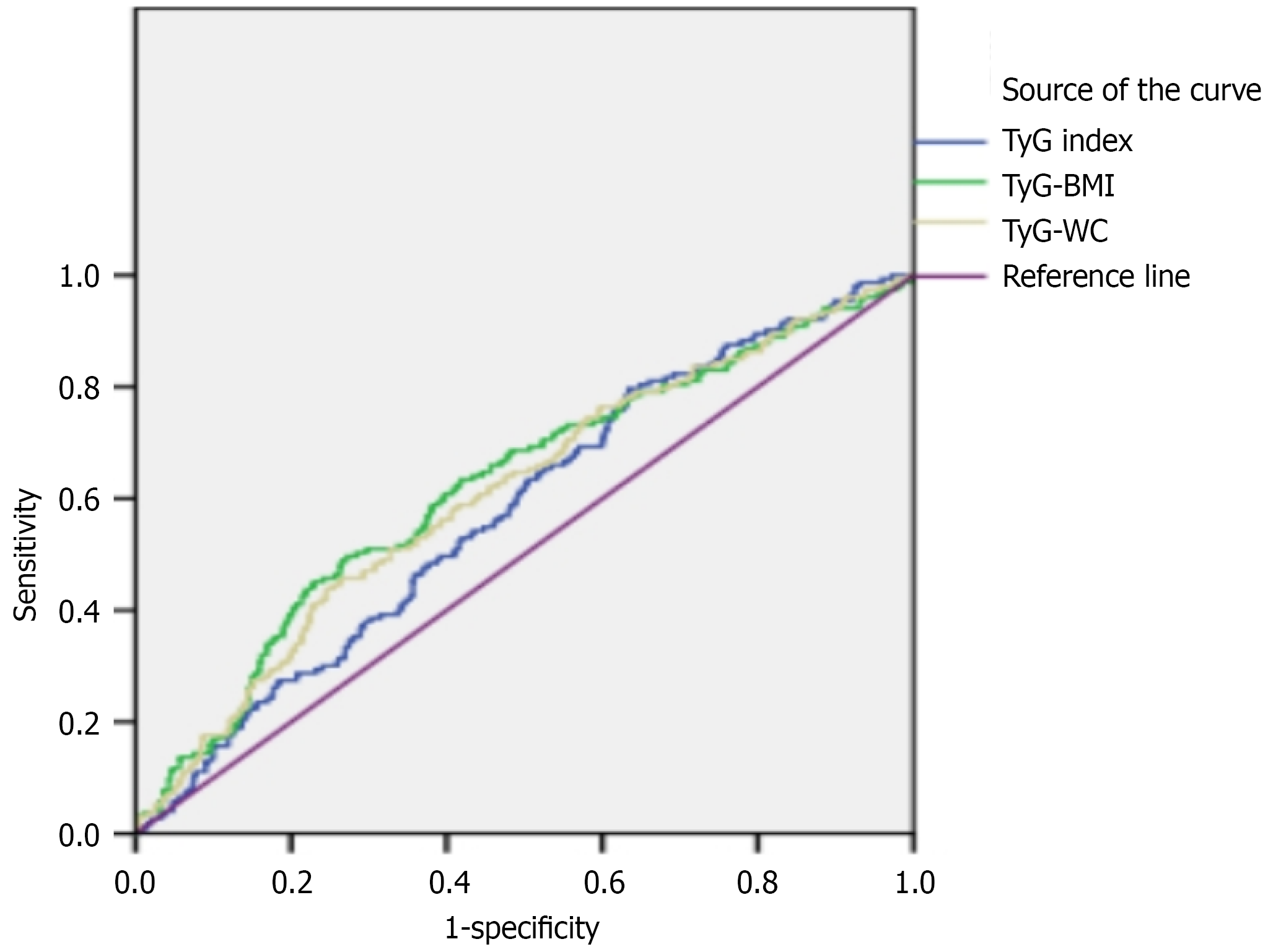
The ROC curves of T2DM for the TyG index, TyG-BMI and TyG-WC are shown in Figure 2. The AUC of the ROC curve for the TyG index was 0.578 (95%CI: 0.533-0.624), for TyG-BMI was 0.622 (95%CI: 0.574-0.670) and for TyG-WC was 0.609 (95%CI: 0.562-0.657). The difference in AUC between TyG-BMI and TyG was significant (P = 0.047), while the differences between TyG-BMI and TyG-WC (P = 0.464) and between TyG-WC and TyG (P = 0.175) were not. Based on the ROC curves, the optimal predictive cut-off for the TyG index was 8.6, with a sensitivity of 79.7% and a specificity of 36.6%. Specifically, the cut-off value of TyG-BMI was 247 (sensitivity = 49.7%, specificity = 72.8%) and for TyG-WC was 860 (sensitivity = 45.8%, specificity = 73.6%).
Pre-diabetes is a state between diabetes and normal blood glucose. The prevalence rate of pre-diabetes in China is experiencing rapid growth. As early as 2011, a flow adjustment result showed that the prevalence rate of pre-diabetes in people over 40 years old in Dalian was as high as 25.1%. The latest flow adjustment data in 2017 showed that the prevalence rate of pre-diabetes in China may currently be 35.2%[10]. Not only does a significantly higher proportion of the pre-diabetic population progress to diabetes compared to the general population, but there is also a significant increase in the risk of cardiac and cerebrovascular diseases. Therefore, diabetes health education and intervention for pre-diabetes patients is important to prevent and treat diabetes and its complications[11-13]. Individuals with pre-diabetes should undergo systematic glycemic surveillance, including periodic measurement of FPG, HbA1c or OGTT, which is critical for early detection of dysglycemia progression, diagnostic confirmation of diabetes onset and timely implementation of therapeutic interventions to prevent microvascular and macrovascular complications. The mean HbA1c in the incident diabetes group was below the diagnostic threshold of 6.5%, underscoring that a significant proportion of new cases were identified through OGTT before their HbA1c had elevated to the diagnostic level, highlighting the complementary role of OGTT in early detection.
While OGTT remains the reference standard for identifying pre-diabetes and DM, its clinical utility in population-based screening is constrained by limited reproducibility and labor-intensive procedures. This underscores the need to develop simplified yet precise glycemic assessment protocols for high-risk populations undergoing diabetes surveillance.
IR and β-cell dysfunction constitute the dual core pathogenesis of T2DM from pre-diabetes[8], and this metabolic derangement is exacerbated by lipid metabolism dysregulation. The pathological accumulation of TG within pancreatic β-cells has been shown to induce apoptotic cascades while simultaneously compromising their regenerative potential. The impaired oxidation of free fatty acids in non-adipose tissue causes cytosolic TG accumulation in non-adipose tissues, leading to IR and β-cell dysfunction[14]. The hyperinsulinemic-euglycemic clamp is universally accepted as the reference technique for assessing insulin-mediated glucose uptake and defining IR phenotypes, but is invasive and cumbersome, and only suitable for evaluation in a small number of studies and investigations.
The steady-state model evaluation HOMA-IR, an index for evaluating IR[15], is widely used in Asia. The results of this study showed that the baseline HOMA-IR value was higher in patients with T2DM (2.84 ± 1.45 vs 2.48 ± 1.26). According to the European Group for the Study of IR recommendation, HOMA-IR index ≥ 2.8 is tentatively defined as IR[16]. This suggests that IR is present at baseline, i.e. in pre-diabetic status, in subjects progressing to diabetes after 3 years. However, fasting insulin level should be measured when calculating HOMA-IR, which is difficult to achieve in most primary medical institutions[17].
There are many reasons for IR, with lipid metabolism disorder, oxidative stress, mitochondrial dysfunction and inflammatory reaction all closely related to IR[18]. Studies have shown that abnormal blood glucose and blood lipid (especially increased TG level), high BMI and WC are all risk factors for IR-related metabolic diseases. If multiple risk factors are present in the same individual, the incidence of IR will be greatly increased[19].
The TyG index combined with blood glucose and TG is likely related to IR-associated metabolic diseases[20]. The TyG index can be used as an index to diagnose IR in normal people and those with metabolic disorder[21,22]. This index only requires FPG and TG levels, which is more suitable for clinical practice. Simental-Mendía et al[23] found for the first time that the TyG index of healthy people is correlated with IR evaluated by HOMA-IR. Similar results were found in obese children and adolescents in South America[24]. Some scholars have compared the TyG index with the normal blood glucose hyperinsulinemia clamp test, and found that the TyG index had higher sensitivity and specificity in distinguishing insulin sensitivity reduction[25]. Vasques et al[26] in 2011 showed that the TyG index was better than HOMA-IR in evaluating IR of a Brazilian population. Many studies have confirmed that the TyG index is closely related to IR, and it is a simple and reliable index for diagnosing IR[24,27]. A follow-up study of the middle-aged general population in Korea shows that the increase of the TyG index is related to increased risk of diabetes[28,29]. According to the correlation between the TyG index and IR, its mechanism may be related to two main components of TyG index (FPG and TG), and these two elements play key roles in the occurrence and development of IR[30]. Excessive serum TG may lead to accumulation of fatty acids in non-adipose tissues such as liver, muscle and heart, leading to ectopic lipid deposition. “Lipotoxicity” is considered another mechanism of IR. When the body is in IR state, the decomposition of peripheral adipose tissue is enhanced, and the excessive free fatty acids produced enter the liver through the portal vein system, and accumulate abnormally in the liver, resulting in a corresponding increase in TG synthesis in the liver[31].
The TyG-BMI is the product of TyG and BMI, and was first reported in 2016[32]. The TyG-BMI is better at predicting IR than the TyG index alone[33]. Study showed that TyG-BMI is a simple, effective and clinical substitute marker for evaluating IR[32,34]. Lim et al[33] pointed out, in a cross-sectional study involving 11149 Korean subjects, that the ability of TyG-BMI to predict IR is significantly greater than that of the TyG index. A cohort study from China also shows that TyG-WC is also a novel index to evaluate IR, which can be used to early identify the pre-diabetes and diabetes risk of first-degree relatives of T2DM patients[35]. A study from India also confirmed that the TyG index and TyG-WC could predict incidence of pre-diabetes[36].
In this study, we focused on TyG-BMI and TyG-WC as they are among the most established and commonly reported TyG-derived indices for predicting diabetes and IR. The BMI is a general measure of overall adiposity, while WC more specifically reflects central (visceral) adiposity, which is strongly linked to IR and metabolic dysfunction. We chose these two to investigate whether combining the TyG index with a general (BMI) or a central (WC) adiposity measure would yield different predictive power for diabetes progression in pre-diabetes. While other indices like the TyG-waist-to-height ratio also exist and may have merit, our primary aim was to validate and compare the performance of these two foundational combined indices in our prospective cohort. Future studies could include a broader range of derived indices for a more comprehensive comparison.
The results of this study showed that baseline body weight, BMI, WC, HC, blood glucose indexes (FPG, 2hPG and HbA1c), blood lipid levels (TC, TG and LDL-C) and HOMA-IR were higher in the diabetic group. Moreover, the TyG index, TyG-BMI and TyG-WC were significantly higher in diabetic than non-diabetic subjects. This shows that although all the subjects in this study were in pre-diabetic state at baseline, there were some differences in their weight, WC, blood lipid, blood glucose and other indicators, which also indicate the development direction of blood glucose of these subjects. The incidence of diabetes increased across the quartiles of the TyG index, TyG-BMI and TyG-WC. Further research showed that the increase of the TyG index, TyG-BMI and TyG-WC were independent risk factors for the progression of a pre-diabetic population to diabetes. The focus of this study was whether the progression of blood glucose in a pre-diabetic population could be preliminarily determined by simple biochemical indicators and physical measurement indicators. Then the ROC curve was used to analyze and compare the efficacy of the TyG index, TyG-BMI and TyG-WC in predicting early onset of diabetes in a pre-diabetic population. The AUC of these three indexes to predict the prognosis of diabetes was 0.578-0.622, which has certain diagnostic value, but could not directly diagnose diabetes like blood glucose or HbA1c testing, but it can serve as a preliminary tool for risk-association indication. Its core clinical significance does not lie in accurately determining whether an individual will experience the outcome, but in identifying potentially high-risk populations and assisting in the initial screening for clinical decision-making. In our cohort, the AUC for HOMA-IR was 0.575 and for baseline HbA1c was 0.640. The performance of TyG-BMI (AUC = 0.622) was comparable to that of HbA1c. This indicates that TyG-BMI, a simple calculated index, performs similarly to a standard glycemic marker like HbA1c in this context. The TyG-BMI is a comprehensive statistic that includes TGs, fasting blood glucose, weight and height all easily obtainable indicators at primary service centers, straight forward to compute and not limited by time or cost. This makes TyG-BMI particularly suitable for large populations, and it could be used as a pre-judgment providing clues concerning high-risk groups. When TyG-BMI was 247, its sensitivity and specificity were 49.7% and 72.8%, respectively, suggesting that it could be a simple tool to predict diabetes progression in a pre-diabetic population.
The innovation of this project is the first analysis of the relationship between the TyG index and its derivative indexes of pre-diabetic patients and their 3-year risk of diabetes. Because of the tedious OGTT procedure, most large-scale screening at home and abroad rarely uses OGTT to evaluate blood glucose status, but only uses FPG to screen diabetes prevalence, which cannot evaluate the situation of some specific people, such as those who are pre-diabetic. In this project, OGTT was used to evaluate pre-diabetes, making the target population more representative. In addition, research on the follow-up data of community populations using a large sample size offers great value for promoting the whole-process health management of T2DM.
However, there are still some limitations in this study. The male to female ratio was unbalanced, and the population was mainly urban. Collecting data through community recruitment will inevitably miss some hidden diabetic patients. There was a certain rate of missing visits and, due to this bias, the research results may be overestimated or underestimated. Although we adjusted for key confounders, data on physical activity, dietary patterns and medication use were not available and may represent residual confounding. The use of these indices in screening may lead to false positives, potentially resulting in unnecessary anxiety or overtreatment. Therefore, they should be used as part of a comprehensive risk assessment rather than as standalone criteria. Considering these limitations, it is necessary to further explore the application value of these IR indexes in a strict prospective cohort study and future studies should incorporate advanced statistical measures such as net reclassification improvement or decision-curve analysis to better evaluate their clinical utility.
Taken together, IR indexes of TyG index, TyG-BMI and TyG-WC are all risk factors of the progression of diabetes in pre-diabetic subjects. The TyG-BMI was superior to the TyG index and TyG-WC in predicting the progression of diabetes in adult pre-diabetic subjects in Dalian.
The TyG index and its derived indicators were independent risk factors for diabetes progression in adults with pre-diabetes. The TyG-BMI demonstrated a modest advantage over TyG and TyG-WC in prediction, although its discriminative ability was limited. These indices may serve as simple, initial screening tools in primary care. Before clinical implementation, these findings should be validated in larger, multi-center cohorts with diverse populations and external validation.
| 1. | Tian Y, Qiu Z, Wang F, Deng S, Wang Y, Wang Z, Yin P, Huo Y, Zhou M, Liu G, Huang K. Associations of Diabetes and Prediabetes With Mortality and Life Expectancy in China: A National Study. Diabetes Care. 2024;47:1969-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 2. | Satman I. Prediabetes and diabetes: main characteristics. Pol Arch Intern Med. 2023;133:16469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Wang Z, Xiong H, Ren TYS. Repair of Damaged Pancreatic β Cells: New Hope for a Type 2 Diabetes Reversal? J Transl Int Med. 2021;9:150-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Song J, Jin C, Shan Z, Teng W, Li J. Prevalence and Risk Factors of Hyperuricemia and Gout: A Cross-sectional Survey from 31 Provinces in Mainland China. J Transl Int Med. 2022;10:134-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 76] [Reference Citation Analysis (0)] |
| 5. | Selvi NMK, Nandhini S, Sakthivadivel V, Lokesh S, Srinivasan AR, Sumathi S. Association of Triglyceride-Glucose Index (TyG index) with HbA1c and Insulin Resistance in Type 2 Diabetes Mellitus. Maedica (Bucur). 2021;16:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Minh HV, Tien HA, Sinh CT, Thang DC, Chen CH, Tay JC, Siddique S, Wang TD, Sogunuru GP, Chia YC, Kario K. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens (Greenwich). 2021;23:529-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 7. | Sun Y, Ji H, Sun W, An X, Lian F. Triglyceride glucose (TyG) index: A promising biomarker for diagnosis and treatment of different diseases. Eur J Intern Med. 2025;131:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 101] [Reference Citation Analysis (0)] |
| 8. | Qin Y, Qiao Y, Yan G, Wang D, Tang C. Relationship between indices of insulin resistance and incident type 2 diabetes mellitus in Chinese adults. Endocrine. 2024;85:1228-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 9. | Zeng ZY, Liu SX, Xu H, Xu X, Liu XZ, Zhao XX. Association of triglyceride glucose index and its combination of obesity indices with prehypertension in lean individuals: A cross-sectional study of Chinese adults. J Clin Hypertens (Greenwich). 2020;22:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, Shi B, Sun H, Ba J, Chen B, Du J, He L, Lai X, Li Y, Chi H, Liao E, Liu C, Liu L, Tang X, Tong N, Wang G, Zhang JA, Wang Y, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Ning G, Mu Y, Zhao J, Teng W, Shan Z. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1030] [Cited by in RCA: 1085] [Article Influence: 180.8] [Reference Citation Analysis (2)] |
| 11. | Braga T, Kraemer-Aguiar LG, Docherty NG, Le Roux CW. Treating prediabetes: why and how should we do it? Minerva Med. 2019;110:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Zhang Y, Shen T, Wang S. Progression from prediabetes to type 2 diabetes mellitus induced by overnutrition. Hormones (Athens). 2022;21:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Lian K, Feng H, Liu S, Wang K, Liu Q, Deng L, Wang G, Chen Y, Liu G. Insulin quantification towards early diagnosis of prediabetes/diabetes. Biosens Bioelectron. 2022;203:114029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Bansal SK, Bansal MB. Pathogenesis of MASLD and MASH - role of insulin resistance and lipotoxicity. Aliment Pharmacol Ther. 2024;59 Suppl 1:S10-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 15. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 25034] [Article Influence: 610.6] [Reference Citation Analysis (2)] |
| 16. | Balkau B, Charles MA, Drivsholm T, Borch-Johnsen K, Wareham N, Yudkin JS, Morris R, Zavaroni I, van Dam R, Feskins E, Gabriel R, Diet M, Nilsson P, Hedblad B; European Group For The Study Of Insulin Resistance (EGIR). Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28:364-376. [PubMed] |
| 17. | Yeh WC, Tsao YC, Li WC, Tzeng IS, Chen LS, Chen JY. Elevated triglyceride-to-HDL cholesterol ratio is an indicator for insulin resistance in middle-aged and elderly Taiwanese population: a cross-sectional study. Lipids Health Dis. 2019;18:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Bickerton AS, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJ, Gilbert M, Karpe F, Frayn KN. Preferential uptake of dietary Fatty acids in adipose tissue and muscle in the postprandial period. Diabetes. 2007;56:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Li X, Xue Y, Dang Y, Liu W, Wang Q, Zhao Y, Zhang Y. Association of Non-Insulin-Based Insulin Resistance Indices with Risk of Incident Prediabetes and Diabetes in a Chinese Rural Population: A 12-Year Prospective Study. Diabetes Metab Syndr Obes. 2022;15:3809-3819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 20. | Luo P, Cao Y, Li P, Li W, Song Z, Fu Z, Zhou H, Yi X, Zhu L, Zhu S. TyG Index Performs Better Than HOMA-IR in Chinese Type 2 Diabetes Mellitus with a BMI < 35 kg/m2: A Hyperglycemic Clamp Validated Study. Medicina (Kaunas). 2022;58:876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, Álvarez-Villalobos NA, González-González JG. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int J Endocrinol. 2020;2020:4678526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 293] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 22. | Ramdas Nayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride Glucose (TyG) Index: A surrogate biomarker of insulin resistance. J Pak Med Assoc. 2022;72:986-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 220] [Reference Citation Analysis (0)] |
| 23. | Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 1498] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 24. | Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatr Diabetes. 2016;17:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347-3351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 1395] [Article Influence: 87.2] [Reference Citation Analysis (8)] |
| 26. | Vasques AC, Novaes FS, de Oliveira Mda S, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98-e100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 518] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 27. | Locateli JC, Lopes WA, Simões CF, de Oliveira GH, Oltramari K, Bim RH, de Souza Mendes VH, Remor JM, Lopera CA, Nardo Junior N. Triglyceride/glucose index is a reliable alternative marker for insulin resistance in South American overweight and obese children and adolescents. J Pediatr Endocrinol Metab. 2019;32:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Lee JW, Lim NK, Park HY. The product of fasting plasma glucose and triglycerides improves risk prediction of type 2 diabetes in middle-aged Koreans. BMC Endocr Disord. 2018;18:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, Lee JH, Yim HW, Kang MI, Lee WC, Son HY, Yoon KH. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS One. 2014;9:e90430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 30. | Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol. 2010;9:402-409. [PubMed] |
| 31. | Pulipati VP, Brinton EA, Hatipoglu B. Management of Mild-to-Moderate Hypertriglyceridemia. Endocr Pract. 2022;28:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Er LK, Wu S, Chou HH, Hsu LA, Teng MS, Sun YC, Ko YL. Triglyceride Glucose-Body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS One. 2016;11:e0149731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 426] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 33. | Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PLoS One. 2019;14:e0212963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 290] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 34. | Xuan W, Liu D, Zhong J, Luo H, Zhang X. Impacts of Triglyceride Glucose-Waist to Height Ratio on Diabetes Incidence: A Secondary Analysis of A Population-Based Longitudinal Data. Front Endocrinol (Lausanne). 2022;13:949831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Zheng S, Shi S, Ren X, Han T, Li Y, Chen Y, Liu W, Hou PC, Hu Y. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: cross-sectional and prospective cohort study. J Transl Med. 2016;14:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 36. | Ramdas Nayak VK, Nayak KR, Vidyasagar S, P R. Predictive performance of traditional and novel lipid combined anthropometric indices to identify prediabetes. Diabetes Metab Syndr. 2020;14:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/