Published online Feb 15, 2026. doi: 10.4239/wjd.v17.i2.110701
Revised: August 1, 2025
Accepted: December 15, 2025
Published online: February 15, 2026
Processing time: 239 Days and 2.5 Hours
In recent years, diabetes has become a major global health concern, and India is one of the countries most affected by the growing number of cases. In this review, we explore the diabetes scenario in the Indian community in comparison with global patterns. The extensive literature on diabetes suggests that poor diet, sedentary lifestyles, and genetic predisposition have been risk factors for diabetes. We further discuss disease management and treatment strategies related to diabetes in India, highlighting the challenges in healthcare accessibility and affordability. In addition, particularly in Indian scenarios, the role of traditional medicine as an alternative medicine is also discussed. This overall highlights possible interventions, including the National Diabetes Control Program, tele
Core Tip: Diabetes mellitus is expected to become a global health emergency, with 643 million cases expected by 2030 and 792 million by 2045. India is among the most affected countries, where younger and less fortunate populations are increasingly being impacted by a complex interplay of genetic, environmental, behavioral, and socioeconomic factors. Despite government efforts, issues like poor glycemic control, delayed diagnosis, and limited access to healthcare, especially in rural areas, remain. Gene-environment interactions further raise risk, emphasizing the need for targeted prevention, early detection, and customized treatment regimens.
- Citation: Rana NS, Vishvakarma NK, Sonkar SC, Beg MMA. Diabetes: A comprehensive review of the Indian landscape in contrast with global trends. World J Diabetes 2026; 17(2): 110701
- URL: https://www.wjgnet.com/1948-9358/full/v17/i2/110701.htm
- DOI: https://dx.doi.org/10.4239/wjd.v17.i2.110701
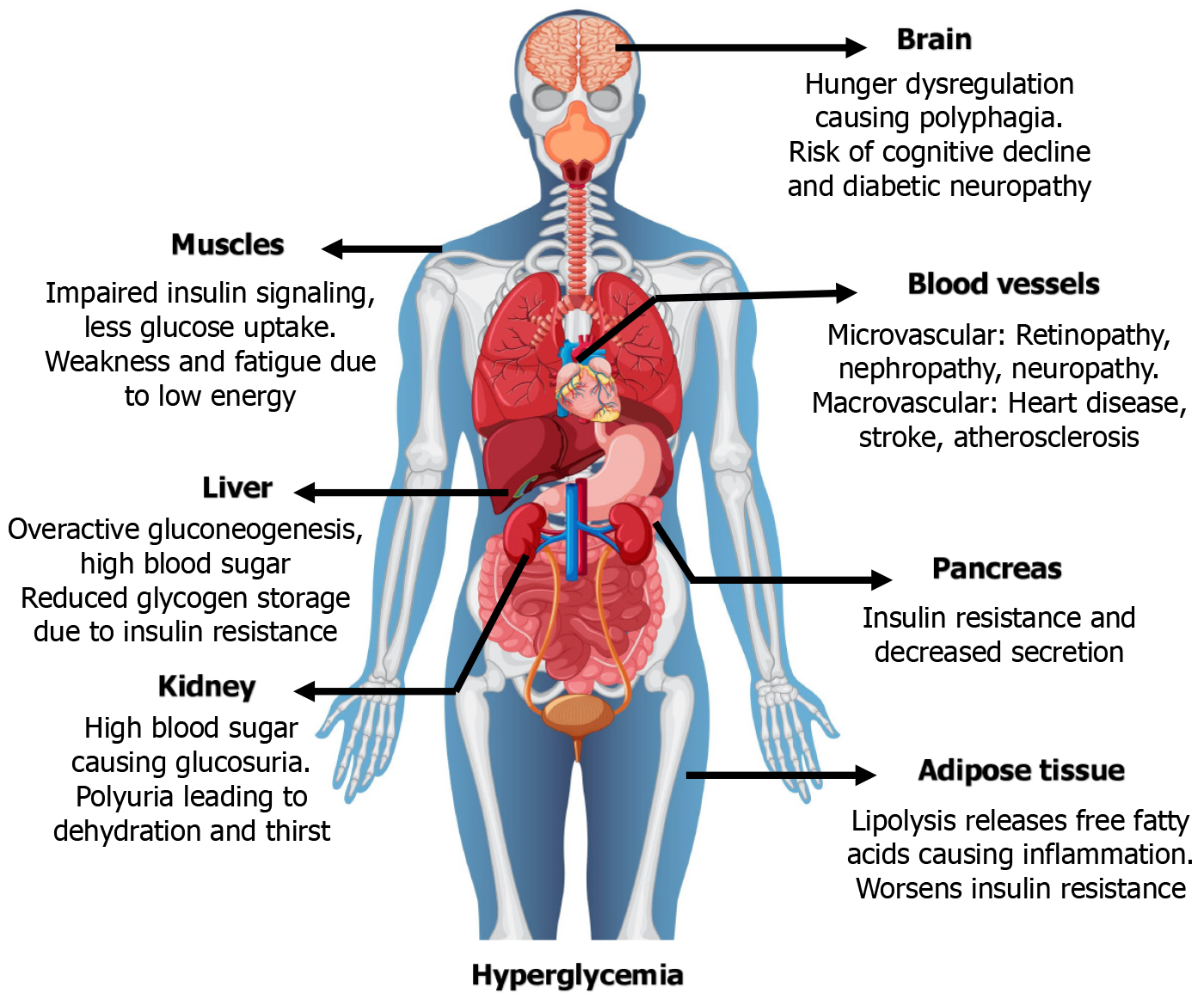
Diabetes, in general, is a chronic metabolic disorder characterized by sustained hyperglycemia resulting from impaired insulin secretion, action, or sometimes both, and affecting multiple organs (Figure 1). Its etiology includes autoimmune beta cell loss (type 1), hormonal changes during pregnancy [gestational diabetes (GD)], and insulin resistance, which is often linked to obesity and lifestyle choices (type 2). Typical clinical signs include increased thirst, frequent urination, unexplained weight loss, fatigue, and chronic problems with the kidneys, heart, eyes, and nerves[1]. In terms of disease burden, non-communicable diseases (NCDs), particularly type 2 diabetes mellitus (T2DM), are overtaking communicable diseases. Ironically, diabetes has become more prevalent as a result of the economic boom, which is marked by increased industry, urbanization, and income levels. The International Diabetes Federation (2024) reports that over 101 million adults in India currently have diabetes, ranking second only to China. By 2045, that number is expected to rise to 134 million[1]. India’s economic growth has led to urbanization, dietary changes, and a decrease in physical activity, especially among the wealthier classes. This “nutrition transition” has increased the risk of obesity and diabetes as a result of sedentary lifestyles and increased consumption of processed foods[2,3]. Interestingly, despite the fact that diabetes was once believed to be a disease of wealth, recent studies reveal that the prevalence of the disease has significantly increased among low- and middle-income groups in both rural and peri-urban areas. This is attributed to a number of factors, including low health literacy, limited access to nutrient-dense meals, ignorance, economic disparity, and delayed diagnosis and treatment[4]. Over the past 20 years, the prevalence of diabetes in rural India has doubled, indicating a narrowing of the urban-rural divide, according to the Indian Council of Medical Research-India Diabetes study[5]. Because of the increasing prevalence of diabetes in India, it is estimated that the total costs of healthcare, including direct medical expenses and indirect effects like employee absenteeism, will surpass $35 billion annually by 2030[6]. Out-of-pocket costs are still high because so few people have health insurance, especially those with lower incomes. This financial burden is made worse by the early onset of diabetes (usually < 40 years), which affects the most productive demographic[7].
The rising burden of diabetes is particularly evident in India, where its prevalence has surged dramatically over the past few decades. This trend is mirrored in many other developing nations, especially in the Southeast Asian region, where rapid economic growth, urbanization, and dietary shifts contribute to an escalating public health challenge[8]. With an estimated 77 million people with diabetes in 2021 and predictions indicating a continued upward trend in the years to come, India stands out as a particularly notable example in the global context[9]. The complex interplay of genetic predisposition, environmental factors, socioeconomic disparities, and cultural nuances contributes to the escalating diabetes epidemic in India[10-12]. Emerging genomic studies have identified several susceptibility loci associated with type 2 diabetes (T2D) in both Indian and global populations[10]. Beyond genetics, environmental factors like air pollution, endocrine disruptors, and heavy metals contribute to T2D by disrupting glucose metabolism, promoting inflammation, and interacting with genetic susceptibilities through epigenetic mechanisms[11]. Socioeconomic disparities significantly worsen diabetes outcomes in low- and middle-income countries (LMICs) like India. A national family health survey analysis revealed that wealthier, urban, and more educated individuals have substantially greater awareness, treatment access, and disease control compared to their poorer, rural counterparts[12]. Chronic hyperglycemia, the hallmark of uncontrolled diabetes, initiates a cascade of systemic pathophysiology leading to widespread organ failure. Key mechanisms include insulin resistance, oxidative stress, chronic inflammation, and the accumulation of advanced glycation end-products[2,13]. These processes manifest across vital organs: Impaired cerebral glucose regulation disrupts appetite and elevates the risk of cognitive decline and neuropathy[2,13]; skeletal muscle insulin resistance causes fatigue and energy deficits[3,14]; and hepatic insulin resistance promotes hyperglycemia via increased gluconeogenesis[15].
Concurrently, pancreatic β-cell failure diminishes insulin secretion[16], while adipose tissue inflammation and renal overload further exacerbate the metabolic dysregulation[17]. The final common pathway is microvascular and macrovascular damage, including atherosclerosis and coronary artery disease[18], underscoring the critical need for consistent glycemic control. This review examines the global diabetes landscape, focusing on its epidemiology, genetic underpinnings, and management strategies. It provides a comparative analysis of the specific challenges and opportunities within the Indian context, highlighting key disparities and strategic interventions for prevention and improved clinical outcomes.
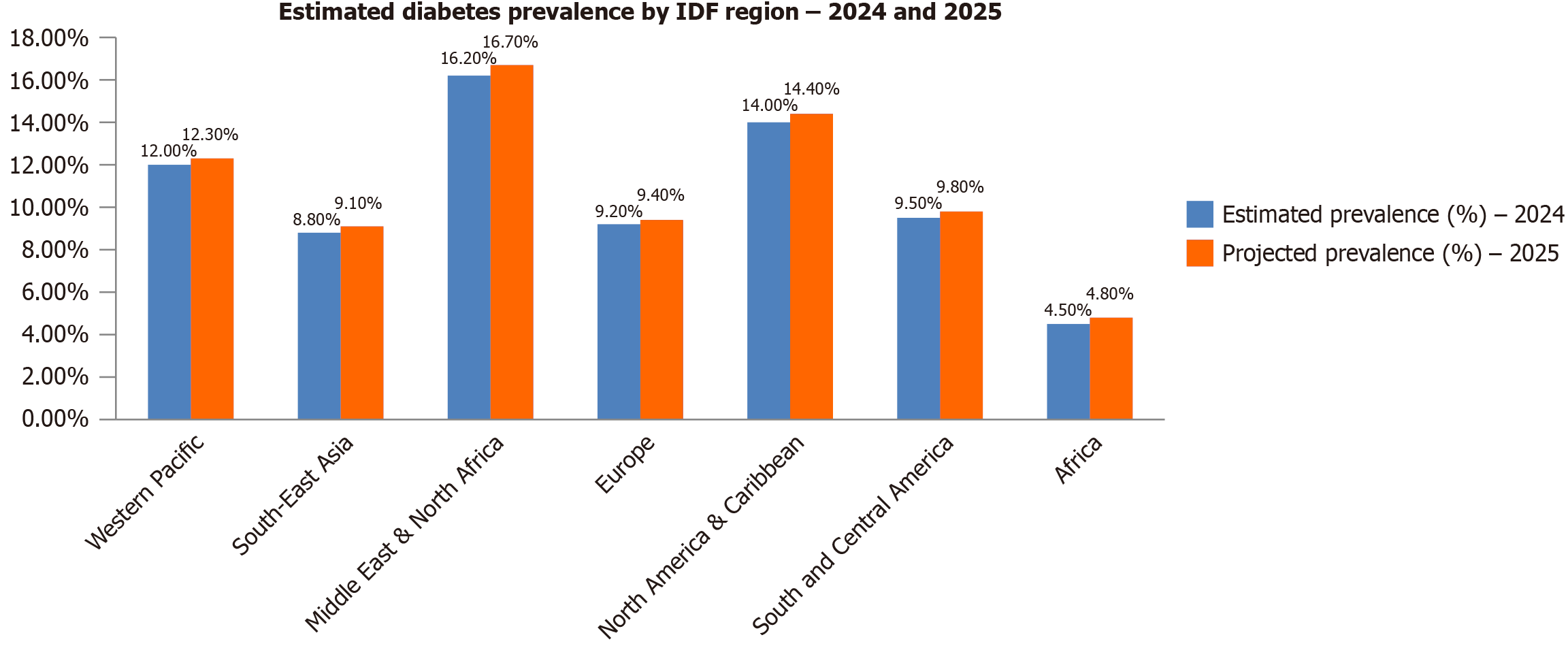
Diabetes remains a major global health concern, with 536.6 million adults affected in 2024, a prevalence of 10.5% projected to rise to 783.2 million by 2045[1]. The burden is unevenly distributed, with the highest concentrations in the Western Pacific (206 million), South-East Asia (101 million), and the Middle East and North Africa (73 million) regions[19]. China (141 million) and India (101 million) alone account for nearly 40% of global cases[1]. T2D constitutes over 90% of all cases and is strongly associated with modifiable risk factors, including obesity and unhealthy diets. A concerning trend is the rising incidence among younger adults under 40, which is associated with longer disease duration, earlier complications, and increased mortality[20]. Significant geographic and socioeconomic disparities characterize the global diabetes landscape. LMICs bear over 75% of the global diabetes burden, despite frequently lacking robust diagnostic and long-term management systems[19]. While urban areas typically show higher prevalence (12%-15%) than rural regions (6%-8%), epidemiological models now indicate a faster growth rate in rural areas of South Asia, Sub-Saharan Africa, and Latin America[21]. Concurrently, prediabetes affects over 470 million people globally, with up to 70% progressing to T2D without intervention[1,22]. Diabetes accounted for 6.7 million deaths in 2023 alone[1].
In high-income countries, diabetes prevalence remains elevated (11.1% in 2024) and continues to rise, driven by sedentary lifestyles, processed food consumption, and aging populations[1,23]. Although technological innovations in diabetes management are advancing, the increasing incidence of young-onset T2D underscores the urgent need for enhanced preventive strategies[24]. The complex interplay of behavioral, genetic, and environmental factors necessitates coordinated global action focused on prevention, early detection, and equitable healthcare access[25,26] (Figure 2 and Table 1). T2DM constitutes 90%-95% of all global diabetes cases and is strongly linked to modifiable lifestyle factors, making it largely preventable[27]. In contrast, type 1 diabetes (T1D) is an autoimmune disorder characterized by the destruction of pancreatic β-cells, leading to absolute insulin deficiency and accounting for 5%-10% of cases[28]. Additionally, GD mellitus (GDM), a hyperglycemic state first identified during pregnancy, contributes to the overall disease burden and is a significant risk factor for the subsequent development of T2DM[29,30] (Table 2)[10,30]. The global rise in diabetes is marked by a critical shift towards younger-onset T2D, largely driven by unhealthy diets, sedentary behavior, and rising obesity. A report by diabetes United Kingdom noted a nearly 40% increase in T2D cases among those under 40 within five years[31]. While prevalence increases with age and is complicated by comorbidities in older adults[32], early-onset T2D is linked to greater insulin resistance, faster β-cell function decline, and more severe complications, elevating long-term morbidity and mortality[31]. Socioeconomic and ethnic disparities intensify this burden, disproportionately affecting deprived and minority groups. Obesity, a leading global cause of malnutrition, remains a primary risk factor, with higher body mass index correlating to earlier T2D onset[33]. Epidemiologically, diabetes is more common in men, with 17.7 million more men affected than women worldwide[34]. Genetic predisposition also plays a key role, as population-specific genetic variations influence susceptibility, disease progression, and treatment response[35]. Understanding these genetic, environmental, and socioeconomic factors is essential for developing personalized and effective prevention strategies.
| Global diabetes epidemiology (2025) | |
| Ref. | International Diabetes Federation[25], 2023; World Health Organization[26], 2025 |
| Aspect | Details |
| Global prevalence | Approximately 589 million adults (20-79 years) are living with diabetes, representing 11.1% of the global adult population; projected to rise to 853 million by 2050 |
| Undiagnosed cases | An estimated 252 million adults are unaware they have diabetes, highlighting a substantial gap in diagnosis and awareness |
| Type distribution | Type 2 diabetes accounts for over 90% of all diabetes cases worldwide |
| Regional burden | Low- and middle-income countries (LMICs) bear the majority of the burden, with over 75% of people with diabetes residing in these regions |
| Mortality | Diabetes is responsible for over 3.4 million deaths annually, equating to one death every 9 seconds |
| Economic impact | Global health expenditure on diabetes has reached USD 1 trillion, marking a 338% increase over the last 17 years |
| Risk factors | Key risk factors include obesity, sedentary lifestyle, unhealthy diet, smoking, alcohol consumption, hypertension, dyslipidemia, age, genetic predisposition, and family history |
| COVID-19 impact | The COVID-19 pandemic has exacerbated diabetes risk factors due to increased sedentary behavior and disrupted healthcare services, leading to higher morbidity and mortality among diabetic patients |
| Prevention strategies | Primary prevention: Lifestyle interventions, public health education, and community-level initiatives; secondary prevention: Early diagnosis through screening, especially in high-risk populations; tertiary prevention: Comprehensive disease management to prevent complications |
| Classification of diabetes | ||
| Ref. | Joseph et al[10], 2022; International Diabetes Federation[30], 2025 | |
| Diabetes type | Etiology/pathophysiology | Clinical and diagnostic features |
| Type 1 diabetes mellitus | An autoimmune-mediated destruction of pancreatic β-cells, often involving islet cell autoantibodies (e.g., GAD65, IA-2); this leads to absolute insulin deficiency; affects both children and adults, including latent autoimmune diabetes in adults (LADA) | Acute onset with symptoms like polyuria, polydipsia, weight loss, and fatigue; ketoacidosis is common at presentation; diagnosed by low C-peptide levels, presence of autoantibodies, and fasting hyperglycemia |
| Type 2 diabetes mellitus | Characterized by insulin resistance and a progressive decline in β-cell function; influenced by obesity, sedentary lifestyle, age, and genetic predisposition; often preceded by prediabetes (impaired glucose tolerance or fasting glucose) | Insidious onset, often asymptomatic for years; commonly diagnosed via routine screening; associated with metabolic syndrome; HbA1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL, or 2-hour OGTT ≥ 200 mg/dL |
| Gestational diabetes mellitus (GDM) | Glucose intolerance is first recognized during pregnancy, typically in the 2nd or 3rd trimester; caused by hormonal changes leading to insulin resistance (e.g., placental lactogen, estrogen, cortisol) | Screened between 24-28 weeks of gestation using OGTT; usually asymptomatic but can lead to macrosomia, preeclampsia, and neonatal hypoglycemia; increases lifetime risk of T2DM |
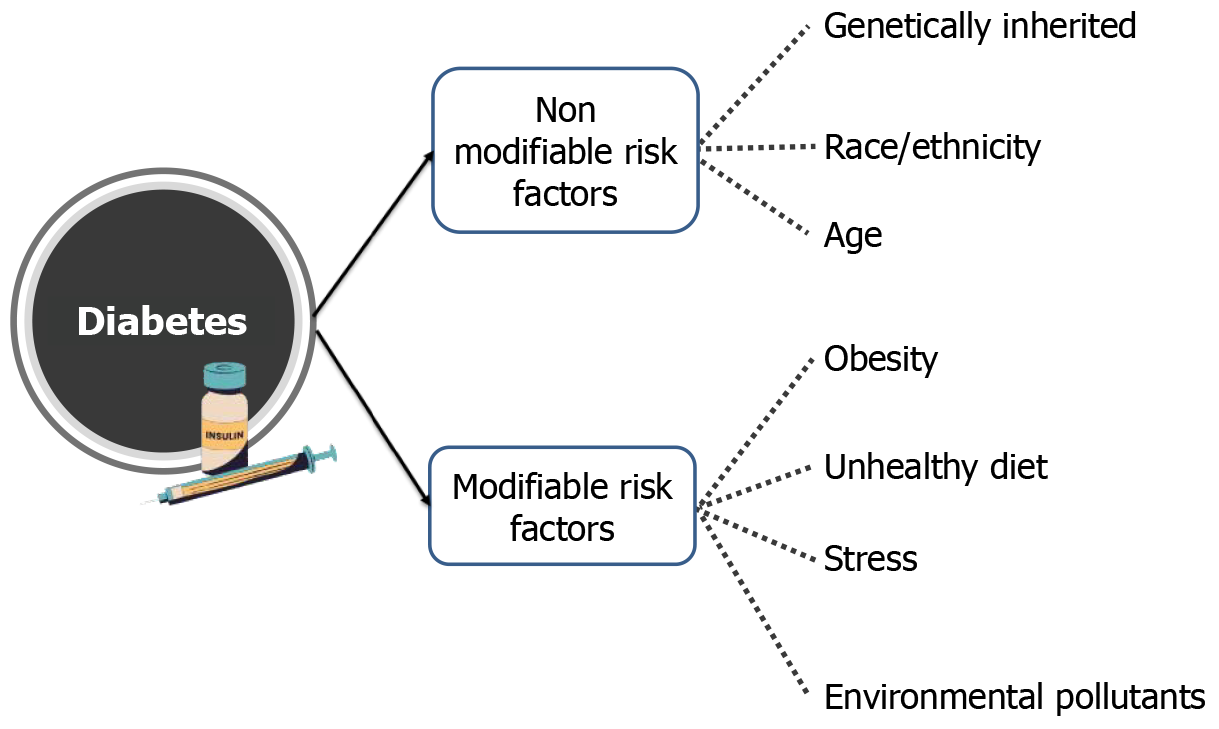
The escalating global burden of diabetes is driven by a complex interplay of genetic, environmental, lifestyle, and socioeconomic factors. While genetic predisposition plays a role, particularly in T1D and influencing susceptibility to T2D, the rapid increase in global prevalence is largely attributed to modifiable environmental and lifestyle factors.
Genetic susceptibility contributes to the risk of developing both T1D and T2D. In T1D, specific genetic variations, particularly within the human leukocyte antigen (HLA) complex, are strongly associated with the autoimmune destruction of beta cells[36]. In T2D, numerous genes have been identified that increase the risk of developing the disease, often influencing insulin secretion, insulin sensitivity, or both[37]. However, it’s crucial to understand that genetic predisposition alone does not guarantee the development of T2D; environmental and lifestyle factors play a crucial role in determining whether this genetic susceptibility is expressed. Polygenic risk scores, which combine the effects of multiple genetic variants, are increasingly being used to assess individual risk for T2D[38] (Table 3)[39-64].
| Genes associated with type 1 diabetes | ||||
| Ref. | Ke et al[39], 2022; Nejentsev et al[40], 2009 | |||
| Gene Symbol | Key function/mechanism | Variant types | Clinical significance | Primary population-specific data |
| HLA | Antigen presentation; strongest genetic risk factor | HLA Haplotypes | High-risk alleles | European, Scandinavian, Hispanic |
| INS | Thymic insulin expression; immune tolerance | VNTR | Risk alleles | European, Asian |
| IL2RA | Altered Treg function and immune tolerance | SNPs | Risk alleles | European, Asian |
| PTPN22 | Reduced inhibitory signaling in lymphocytes | SNPs | Risk alleles | European, Asian |
| IFIH1 | Viral RNA sensor; reduced antiviral response | Rare Variants | Protective alleles | European |
| BACH2 | Affects lymphocyte development; autoimmunity | SNPs | Risk alleles | European |
| TYK2 | Influences beta-cell survival and immune responses | SNPs | Risk alleles | European |
| CLEC16A | Impaired autophagy affects antigen presentation | SNPs | Risk alleles | European |
| CD226 | T-cell activation | SNPs | Risk alleles | European |
| CCR5 | Chemokine receptor; T-cell migration | Deletion (Δ32) | Protective allele | European |
| CTLA4 | Immune checkpoint regulation | SNPs | Risk alleles | Various populations |
| STAT4 | Signal transduction in inflammatory responses | SNPs | Risk alleles | Various populations |
| EPO | Linked to T1D complications (nephropathy) | SNPs | Risk alleles | Various populations |
| NOS3 | Associated with vascular complications | SNPs | Risk alleles | Various populations |
| MIR375 | MicroRNA regulating insulin secretion & beta-cell mass | MicroRNA | Regulatory role | Various populations |
| Genes associated with type 2 diabetes | ||||
| Ref. | Gloyn et al[41], 2003; Dornbos et al[42], 2022; Kumar et al[43], 2024; Flannick et al[44], 2019; Shi et al[45], 2025; Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research et al[46], 2007; Abu Aqel et al[47], 2024; Russ-Silsby et al[48], 2025; Gerber et al[49], 2017; Fuchsberger et al[50], 2016; Hwang et al[51], 2023; Udler et al[52], 2019; Morris et al[53], 2012 | |||
| TCF7 L2 | Wnt signaling regulates insulin secretion | SNPs | Strongest common risk allele | European, Hispanic, Asian |
| PPARG | Nuclear receptor; influences insulin sensitivity | Missense (Pro12Ala) | Risk and Protective alleles | Predominantly European |
| SLC30A8 | Zinc transporter in insulin granules | Loss-of-function | Protective allele | Multiethnic cohorts |
| KCNJ11 | Potassium channel regulates insulin secretion | Missense (E23K) | Confirmed risk allele | Global distribution |
| FTO | Regulates appetite and adiposity | Intronic SNPs | Risk allele via obesity | Worldwide (strongest in Europeans) |
| GCK | Glucose-sensing enzyme; modulates β-cell function | Missense, Nonsense | Risk and rare MODY alleles | European populations |
| MTNR1B | Melatonin receptor; impairs insulin secretion | SNPs | Risk alleles | European and Asian populations |
| IRS1 | Mediates insulin signaling; insulin resistance | SNPs | Risk allele | European and Asian populations |
| HHEX | Pancreas development; impaired insulin secretion | SNPs | Risk alleles | Asian and European populations |
| CDKAL1 | β-cell insulin secretion regulator | SNPs | Risk alleles | European populations |
| KCNQ1 | Potassium channel; affects β-cell electrical activity | SNPs | Risk alleles | East Asian populations |
| WFS1 | Linked to beta-cell survival and apoptosis | SNPs | Risk alleles | European populations |
| ANK1 | Linked to insulin secretion defects | SNPs | Risk alleles | European populations |
| GIPR | Influences insulin release and glucose tolerance | Missense, SNPs | Risk alleles | European populations |
| PDX1 | Influences beta-cell function and development | SNPs | Risk alleles | European populations |
| Genes associated with gestational diabetes | ||||
| Ref. | Lu et al[54], 2024; Keels et al[55], 2024; Liang et al[56], 2024; Mittal et al[57], 2025; Fan et al[58], 2021; Sladek et al[59], 2007; Gwenzi and Brenner[60], 2024; Li et al[61], 2020; Goyal et al[62], 2023; Saini[63], 2010; Sayyed Kassem et al[64], 2023 | |||
| TCF7 L2 | Wnt signaling regulates insulin secretion | SNPs | Higher susceptibility to GDM | Chinese Han population |
| MTNR1B | Melatonin receptor; influences circadian rhythm | SNPs | Elevated fasting glucose; GDM risk | Russian women |
| GCK | Glucose-sensing enzyme in β-cells | SNPs | Impaired glucose sensing and GDM | Multiple populations |
| IRS1 | Mediates insulin signaling; insulin resistance | SNPs | Increased insulin resistance; GDM | Multiple populations |
| KCNJ11 | Potassium channel; insulin release | SNPs | Altered secretion; GDM risk | Multiple populations |
| CDKAL1 | β-cell insulin secretion regulator | SNPs | Impaired secretion; GDM risk | Women < 30 years |
| HHEX | Pancreas development regulator | SNPs | Increased GDM risk | Multiple populations |
| SLC30A8 | Zinc transporter in insulin granules | SNPs | Defective insulin storage; GDM | North Indian population |
| CDKN2A/2B | Regulate β-cell cycle; impair proliferation | SNPs | Increased GDM risk | Multiple populations |
| KCNQ1 | Potassium channel; β-cell function | SNPs | Altered insulin secretion | Multiple populations |
| HNF1B | Transcription factor for pancreas development | SNPs | Reduced insulin secretion | Multiple populations |
| ABCC8 | Regulates insulin secretion via K⁺ channels | SNPs | Disrupted insulin control; GDM | Multiple populations |
| KIAA0825 | Potential oncogene; linked to high glucose | SNPs | Elevated glucose levels | Chinese Han population |
| FOXC2 | Adipocyte differentiation and insulin sensitivity | SNPs | Protective effect against GDM | Multiple populations |
| HKDC1 | Hexokinase is involved in glucose metabolism | SNPs | Impaired glucose metabolism; GDM risk | Multiple populations |
| TRA2A, NPM3, PHF5A, PLXNA3 | RNA splicing, cell cycle, signaling (biomarkers) | SNPs | Diagnostic utility for GDM | Multiple populations |
Environmental factors, particularly obesity and Western dietary patterns, significantly drive the onset and progression of T2DM. Diets high in processed foods, saturated fats, and refined carbohydrates promote weight gain and induce oxidative stress, insulin resistance, and systemic low-grade inflammation. These diets also cause gut dysbiosis, which further exacerbates metabolic dysfunction and inflammation[65]. Visceral obesity plays a central role by creating a pro-inflammatory state. Adipose tissue releases elevated levels of inflammatory cytokines (e.g., tumor necrosis factor-α, interleukin-6) and reduces protective adiponectin, impairing insulin signaling. Furthermore, ectopic fat deposition in the liver and pancreas leads to lipotoxicity and β-cell dysfunction, ultimately reducing insulin secretion[66]. These factors, combined with physical inactivity, sleep disorders, and exposure to environmental pollutants, underscore that T2DM is not solely genetic but is heavily influenced by modifiable lifestyle and environmental determinants. Public health strategies must therefore prioritize dietary improvement and obesity prevention to combat the diabetes epidemic.
Physical activity: Regular physical activity is crucial for maintaining insulin sensitivity and glucose homeostasis[67]. Sedentary lifestyles, characterized by prolonged sitting and lack of exercise, significantly increase the risk of T2D[68].
Obesity: Obesity, particularly abdominal obesity, is a major risk factor for insulin resistance and the development of T2D[69]. The relationship between obesity and diabetes is complex and bidirectional, with obesity contributing to diabetes risk and diabetes increasing the risk of further weight gain.
Smoking: Cigarette smoking is associated with an increased risk of T2D and impairs insulin sensitivity and glucose metabolism[70] (Table 4)[71-81].
| Ref. | Kleinberger et al[71], 2015; Cerf et al[72], 2013; Pervjakova et al[73], 2022; Crudele et al[74], 2023; Guidotti et al[75], 2013; Andersen et al[76], 2012; Landin-Olsson[77], 2002; Garcia-Gutierrez[78], 2024; Schulz et al[79], 2021; Kota et al[80], 2012; Pilla et al[81], 2022 | ||
| Environmental factor | Type of diabetes affected | Gene(s) involved | Mechanism of interaction |
| Obesity/high-fat diet | T2D | TCF7 L2, FTO, PPARG | Alters gene expression involved in insulin sensitivity and metabolism through epigenetic changes |
| Physical inactivity | T2D | PPARG, IRS1 | Reduces insulin sensitivity via modulation of glucose metabolism genes |
| Maternal nutrition | GDM, T2D | IGF2, H19, PDX1 | Epigenetic modifications affecting pancreatic beta-cell development and fetal metabolic programming |
| Endocrine disruptors (e.g., BPA) | T2D | PPARG, GLUT4 | Mimics or blocks hormonal action, disrupting insulin signaling and glucose transport |
| Chronic stress/cortisol | T2D | NR3C1, FKBP5 | Alters glucocorticoid receptor expression and function, affecting glucose metabolism |
| Smoking | T2D, GDM | CYP1A1, GSTM1 | Increases oxidative stress and induces insulin resistance |
| Air pollution (PM2.5, NO2) | T2D | GSTP1, NFE2 L2 | Induces oxidative stress and systemic inflammation, impairing insulin signaling |
| Vitamin D deficiency | T1D, T2D | VDR | Modulates immune response and beta-cell function, increasing susceptibility |
| Viral infections | T1D | HLA-DR, INS | Triggers autoimmune destruction of pancreatic beta cells in genetically susceptible individuals |
| Gut microbiome dysbiosis | T1D, T2D, GDM | NOD2, TLR4 | Alters immune homeostasis and promotes systemic inflammation, impacting insulin sensitivity |
| Chemical EXPosure (e.g., pesticides, phthalates) | T2D | PPARG, IRS1 | Acts as an endocrine disruptor, interfering with insulin signaling pathways |
| Sleep/circadian disruption | T2D | CLOCK, BMAL1 | Alters circadian regulation of metabolic gene expression, leading to impaired glucose metabolism |
| Socioeconomic status (SES) | All types | Multiple genes | Influences access to healthcare, nutrition, and stress levels, leading to epigenetic modifications |
Socio-economic factors, such as poverty, limited access to education and healthcare, and food insecurity, also contribute to the global diabetes burden[82]. Individuals from lower socioeconomic backgrounds may have limited access to healthy food choices, safe environments for physical activity, and quality healthcare, all of which can increase their risk of developing diabetes. Furthermore, stress related to poverty and social disadvantage may also play a role (Figure 3).
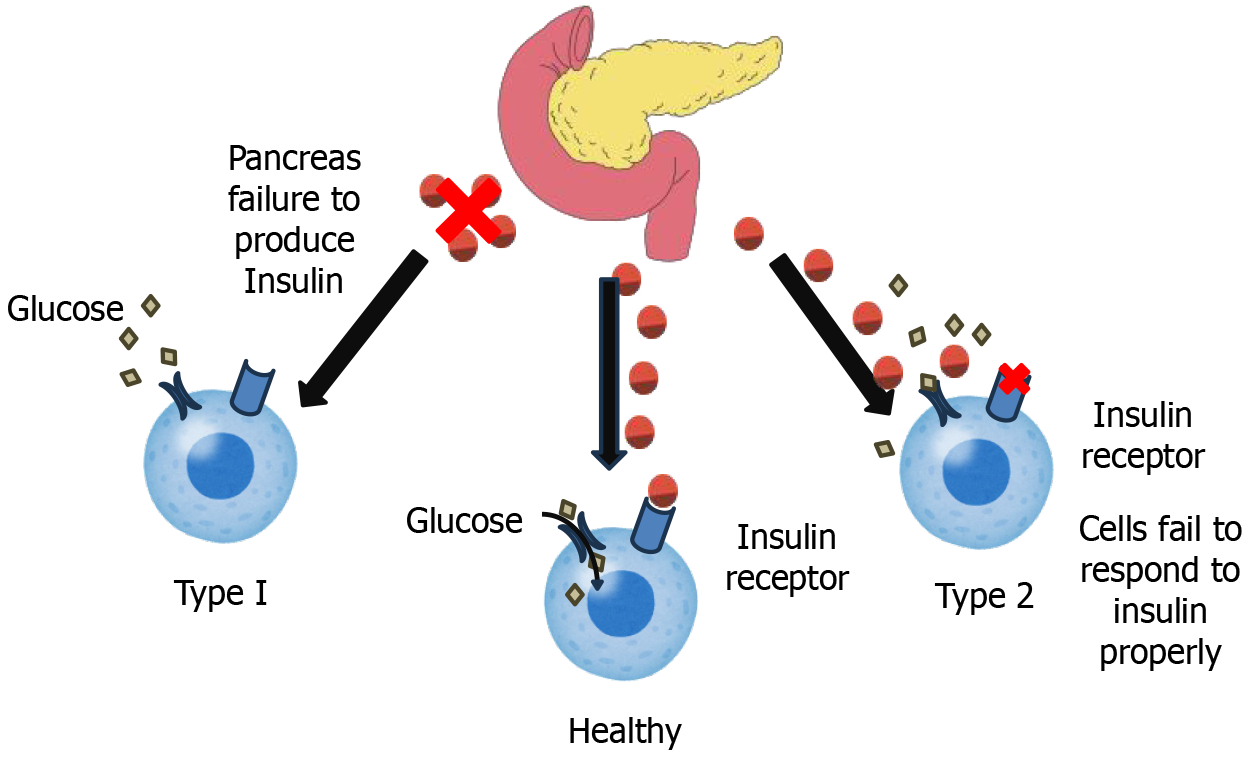
Diabetes mellitus is a complex metabolic disorder characterized by persistent hyperglycemia, or high blood glucose levels. This hyperglycemia arises from defects in either the secretion of insulin, the action of insulin, or sometimes a mixture of both. Understanding the intricate pathophysiological mechanisms underlying these defects is crucial for developing effective prevention and treatment strategies. Here, we further explore the distinct Pathophysiology of both T1D, T2D, and GD (Figure 4). T1D is an autoimmune disorder characterized by the immune-mediated destruction of pancreatic β-cells, the exclusive producers of insulin, a hormone critical for glucose homeostasis. This autoimmune response is primarily driven by autoreactive CD4+ and CD8+ T lymphocytes, which infiltrate the pancreatic islets and initiate β-cell apoptosis[83]. Although the precise etiology remains unclear, a multifactorial interplay of genetic predisposition and environmental triggers is strongly implicated. The HLA complex on chromosome 6p21 is the strongest genetic determinant of T1D susceptibility. Specifically, HLA-DR and HLA-DQ alleles play a key role, with haplotypes such as HLA-DR3-DQ2 (DRB103:01-DQB102:01) and HLA-DR4-DQ8 (DRB104:01-DQB103:02) increasing risk, while HLA-DR2-DQ6 (DRB115:01-DQB106:02) provides protection. These variants influence antigen presentation, leading to improper immune recognition of β-cell antigens like insulin and glutamic acid decarboxylase 65, triggering autoimmunity. The peptide-binding properties of HLA-DQ2 and HLA-DQ8 enhance autoreactive T-cell activation, promoting β-cell destruction. Interactions with non-HLA genes, such as PTPN22 and INS, further modulate risk, highlighting the complex genetic basis of the disease[84]. Environmental factors, including viral infections and dietary influences, have been associated with disease onset, though definitive causal links remain elusive[85]. β-cell destruction occurs through mechanisms such as molecular mimicry, wherein exogenous antigens resemble β-cell epitopes, eliciting an immune response; the generation of autoantibodies against β-cell antigens, such as glutamic acid decarboxylase 65; and the release of pro-inflammatory cytokines by activated T cells[86]. This progressive decline in β-cell mass ultimately leads to a severe insulin deficiency, manifesting as overt hyperglycemia once approximately 80%-90% of β-cells are lost.
T2D, typically, is a more intricate and multifactorial condition defined by two primary defects: Insulin resistance and impaired insulin secretion. Insulin resistance describes a state where cells, primarily in muscle, fat, and liver tissues, fail to respond appropriately to insulin[87]. Insulin, crucial for glucose uptake by cells, encounters resistance, requiring the pancreas to produce increasingly higher levels of the hormone to achieve the same glucose-lowering effect[88]. Obesity, especially visceral fat accumulation, is a major driver of insulin resistance. Excess adipose tissue releases hormones and inflammatory cytokines that interfere with insulin signaling pathways. Physical inactivity further exacerbates insulin resistance. Dietary patterns high in saturated fats and refined carbohydrates also contribute. Genetic factors also play a role in influencing individual insulin sensitivity[89]. Alongside insulin resistance, impaired insulin secretion develops. Initially, the pancreas compensates for insulin resistance by producing more insulin, leading to hyperinsulinemia. However, over time, the beta cells become overwhelmed and are no longer able to sustain this increased insulin output. This leads to a decline in insulin secretion, a progressive rise in blood glucose levels, and ultimately, the clinical manifestation of T2D[90]. Chronic hyperglycemia itself can further damage beta cells, creating a vicious cycle of worsening insulin deficiency. Furthermore, elevated free fatty acids (lipotoxicity) and chronic low-grade inflammation, often associated with obesity, can also impair beta cell function[91] (Table 5)[77,82,92-118].
| Pathophysiology of T1D with genetic network | |||
| Ref. | Landin-Olsson[77], 2002; Liu et al[82], 2023; Noble and Valdes[92], 2011; Bacchetta and Roncarolo[93], 2024; James et al[94], 2023; Yang et al[95], 2024; Herold and Krischer JP[96], 2024; Mancuso et al[97], 2023; Wang et al[98], 2024; De Franco[99], 2020; Abdul-Ghani and DeFronzo[100], 2008 | ||
| Pathophysiological process | Description | Key genes | |
| Autoimmune beta-cell destruction | Insulin insufficiency results from CD4+ and CD8+ T-cell-mediated immune destruction of pancreatic β-cells | HLA-DR, HLA-DQ, INS, PTPN22 | |
| Antigen presentation and immune activation | β-cell antigens are presented by MHC class II molecules to autoreactive T-cells, initiating an immune response | HLA-DR3, HLA-DR4, HLA-DQ8 | |
| T-cell receptor signaling and immune regulation | Defective regulatory T-cell function and abnormal activation of T-cells contribute to loss of immune tolerance | PTPN22, CTLA4, IL2RA, FOXP3 | |
| β-cell stress and apoptosis | Endoplasmic reticulum stress and exposure to proinflammatory cytokines lead to apoptosis of β-cells | INS, EIF2AK3, TXNIP | |
| Cytokine-mediated inflammation | Inflammatory cytokines like IFN-γ, TNF-α, and IL-1β induce β-cell dysfunction and promote cell death | IFIH1, IL2RA, STAT4, IL-10 | |
| Genetic susceptibility and environmental triggers interaction | Viral infections and other environmental factors interact with genetic predispositions to initiate autoimmunity | HLA, IFIH1, PTPN22 | |
| Defective central and peripheral tolerance | Autoreactive T-cells escape elimination in the thymus or are not suppressed in peripheral tissues | AIRE, FOXP3, CTLA4 | |
| Innate immune response dysregulation | Abnormal innate immune activity enhances proinflammatory responses and autoimmunity | IFIH1, TLR7, NOD2 | |
| Pancreatic islet inflammation (Insulitis) | Persistent infiltration of immune cells into pancreatic islets leads to chronic inflammation and β-cell damage | CXCL10, CCR5 | |
| Beta-cell regeneration failure | Impaired β-cell regenerative capacity limits the replacement of destroyed insulin-producing cells | PDX1, MAFA | |
| Autoantibody production | Production of autoantibodies against β-cell proteins marks autoimmune activity and precedes clinical diagnosis | INS, GAD65, IA-2, PTPRN | |
| Pathophysiology of T2D with a genetic network | |||
| Ref. | Liu et al[101], 2021; Febbraio and Karin[102], 2021;Donath[103], 2014; Zhu et al[104], 2025; Dhatariya[105], 2022; Zhang et al[106], 2016; Wu et al[107], 2023 | ||
| Pathophysiological process | Description | Key genes | |
| Insulin resistance | Decreased insulin sensitivity of peripheral tissues (liver, muscle, and fat) | IRS1, PPARG, TCF7 L2, INSR, AKT2 | |
| Impaired insulin secretion | Pancreatic β-cells’ inability to detect glucose and release insulin | KCNJ11, ABCC8, HNF1A, TCF7 L2, GLIS3 | |
| Lipotoxicity and ectopic fat accumulation | Fatty acid buildup in the liver and muscles disrupts insulin transmission. | PNPLA3, SREBF1, FABP4 | |
| Mitochondrial dysfunction | The metabolism of glucose is impacted by decreased oxidative phosphorylation and ATP generation | NDUFS4, UCP2, SIRT1 | |
| Inflammation and immune activation | Insulin resistance is facilitated by persistent low-grade inflammation | TNF, IL-6, NLRP3, TLR4 | |
| Adipokine dysregulation | Metabolic homeostasis is disturbed by an imbalance in adipokines, such as leptin and adiponectin | LEP, ADIPOQ, RETN | |
| Glucose transport dysfunction | Lower glucose uptake is caused by decreased GLUT4 translocation in muscle and fat | SLC2A4, AS160 | |
| Hepatic gluconeogenesis overactivity | Overproduction of glucose in the liver in spite of hyperglycemia | G6PC, PCK1, FOXO1, CREB | |
| Β-cell dedifferentiation and apoptosis | β-cell failure is a result of both increased apoptosis and loss of β-cell identity | PDX1, MAFA, NKX6-1, FOXO1 | |
| Gut microbiota and metabolic endotoxemia | Changes in the microbiota impact insulin sensitivity and inflammation | NOD2, TLR5, FFAR2 | |
| Pathophysiology of GDM with genetic network | |||
| Ref. | Damm et al[108], 2016; Kwak et al[109], 2012; Godfrey[110], 2002; Wicklow and Retnakaran[111], 2023; Dias et al[112], 2023; Franzago et al[113], 2019; Ruchat et al[114], 2013; Neven et al[115], 2022; Ibrahim et al[116], 2022; Zhang et al[117], 2022; Niu et al[118], 2023 | ||
| Pathophysiological process | Description | Key genes | |
| Progressive insulin resistance in pregnancy | Later in pregnancy, maternal insulin resistance is increased by placental hormones (such as hPL, estrogen, and progesterone) | IRS1, PPARG, INSR, SOCS3 | |
| Inadequate β-cell adaptation | Hyperglycemia results from the inability of pancreatic β-cells to compensate for the increased demand for insulin | TCF7 L2, HNF1A, GCK, CDKAL1 | |
| Placental hormonal dysregulation | Systemic insulin resistance and disturbed glucose metabolism are caused by altered placental hormone production | LEP, TNF, PAPP-A, PSGs | |
| Adipokine imbalance and metabolic stress | Insulin signaling and energy homeostasis are hampered by decreased adiponectin and elevated leptin/resistin | ADIPOQ, LEP, RETN, NAMPT | |
| Inflammation and oxidative stress | Insulin resistance is brought on by cytokine-mediated inflammation (IL-6, TNF-α) through interference with signaling | IL-6, TNF, CRP, NLRP3 | |
| Epigenetic modifications and fetal programming | Changes in miRNA and DNA methylation impact long-term results and maternal-fetal metabolism | DNMT3B, miR-29a, miR-103, MEG3 | |
| Obesity-associated insulin resistance | Insulin resistance and the risk of GDM are increased by maternal obesity via inflammatory and hormonal mechanisms | FTO, MC4R, SLC30A8, IL-1β | |
| Gut microbiota alterations and endotoxemia | Endotoxemia and chronic inflammation brought on by microbial imbalance exacerbate insulin resistance | TLR4, NOD2, FFAR2, LBP | |
| Mitochondrial dysfunction | β-cell dysfunction and reduced ATP generation are caused by impaired mitochondrial oxidative capability | UCP2, SIRT3, MFN2 | |
| Impaired insulin signaling pathway | The absorption and use of glucose are impacted by disruptions in the insulin receptor and downstream signaling. | INSR, IRS2, AKT2 | |
| Endocrine disruptor exposure and GDM risk | Through epigenetic modifications, EDCs like BPA and phthalates may affect β-cell activity and insulin sensitivity | ESR1, NR3C1, PPARG | |
As of 2024, India has approximately 89.8 million adults (aged 20-79 years) living with diabetes, and this number is projected to rise to 101 million by 2025[119]. Additionally, an estimated 136 million individuals in the country are living with prediabetes, indicating a significant population at risk[120]. There is a marked urban–rural disparity, with urban areas showing a prevalence of 15%-20%, while rural areas report a lower prevalence of 8%-12%[119]. When looking at gender distribution, males have a slightly higher prevalence of diabetes compared to females[119]. The most affected age group comprises individuals aged 45-59 years, though there is a growing trend among younger adults aged 30-45 years, reflecting a concerning shift in disease onset[121]. Among the states and union territories, Goa (26.4%), Puducherry (26.3%), and Kerala (25.5%) report the highest diabetes prevalence rates. Other regions with notably high rates include Lakshadweep (23.2%), Chandigarh (20.4%), and Delhi (17.8%)[119]. Conversely, Bihar (4.3%), Mizoram (6%), and Nagaland (approximately 6%) are among the least affected[119]. Key risk factors contributing to the rise in diabetes cases include obesity, physical inactivity, diets high in sugar and fat, family history, smoking, and alcohol consumption[119]. The disease is associated with several serious complications, such as retinopathy, nephropathy, neuropathy, cardiovascular disease, and foot ulcers[119]. The economic burden of diabetes in India is substantial, with an estimated $30 billion spent annually on both direct medical costs and indirect costs[122]. To combat the diabetes epidemic, the Indian government has implemented several initiatives, including the national program for prevention and control of cancer, diabetes, cardiovascular diseases, and stroke[123], Ayushman Bharat, and the E-Sanjeevani teleconsultation services[124]. Recent trends show that diabetes is increasing in rural and lower socio-economic groups, and there is also a rise in T2D among children, which was previously rare[120]. Despite efforts, India continues to face significant challenges, such as late diagnosis, poor glycemic control, and an inadequate healthcare infrastructure, particularly in rural and tribal regions[121]. The Ayushman Bharat program was started in 2018 with the intention of transforming India’s primary healthcare system by establishing Health and Wellness Centers (HWCs), also referred to as Ayushman Arogya Mandirs. These clinics are essential centers for the early detection, prevention, and treatment of NCDs, including T2DM, especially among disadvantaged populations[123]. HWCs conduct population-based screening for diabetes in individuals aged 30 and older using established protocols. Auxiliary nurse midwives and accredited social health activists typically assist community health officers in conducting screenings. For tracking and follow-up, they make use of glucometers and digital tools such as the Clinical and Public Health Committee-NCD portal and Ayushman Bharat Health Account IDs[124]. The three essential antidiabetic medications - glibenclamide, gliclazide, and metformin - are administered to patients with diabetes by HWCs in accordance with the Standard Treatment Guidelines. Monthly follow-ups are provided to patients for glucose monitoring and medication adjustments. eSanjeevani telemedicine services are being used for virtual consultations with medical officers and specialists to support clinical decision-making at the periphery[125,126]. In the treatment of diabetes, HWCs have demonstrated promising outcomes. A multi-state implementation evaluation found that most centers had > 85% of the required diabetes medications on hand, and over 70% of patients with diabetes were initiated on treatment at the HWC level[125,126] (Tables 6, 7, and 8)[119-122,124,127-143].
| Epidemiology of diabetes (2024-2025) | |
| Parameter | Details |
| Ref. | Duncan et al[119], 2025; Indian Council of Medical Research[120], 2023; The Times of India[121], 2025; MedBound Times[122]; India Today NE[124] |
| Total diabetics (20-79 years) | As of 2024, approximately 89.8 million individuals in India have diabetes, projected to rise to 101 million by 2025 |
| Prediabetes prevalence | An estimated 136 million Indians have prediabetes, highlighting a critical population requiring preventive interventions |
| Urban vs rural prevalence | Urban areas report a higher prevalence (15%-20%) compared to rural areas (8%-12%), though rural rates are rising steadily |
| Gender distribution | Males show a slightly higher prevalence, though women, especially those with a history of gestational diabetes, are significantly affected |
| Age group most affected | Adults aged 45-59 years are most affected, with a concerning increase in cases among those aged 30-45 |
| Top states/UTs by prevalence (%) | Goa (26.4%), Puducherry (26.3%), and Kerala (25.5%) report the highest prevalence rates |
| Least affected states | Bihar (4.3%), Mizoram (6%), and Nagaland (approximately 6%) have the lowest reported prevalence |
| Key risk factors | Obesity, physical inactivity, unhealthy diets, family history, and tobacco/alcohol use are major contributors |
| Complications | Includes retinopathy, nephropathy, neuropathy, cardiovascular disease, and diabetic foot ulcers |
| Economic burden | Diabetes costs India an estimated $30 billion annually in direct and indirect costs |
| Government initiatives | Key programs include the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases, and Stroke (NPCDCS), Ayushman Bharat, and eSanjeevani teleconsultation services |
| Recent trends | The disease burden is shifting towards rural areas and lower socio-economic groups, with a rise in type 2 diabetes among children and adolescents |
| Challenges | Key hurdles include late diagnosis, poor glycemic control, inadequate healthcare infrastructure in rural regions, and a lack of public awareness |
| Comparison of diabetes prevalence across all Indian states and Union Territories (2023 and 2024) | |||||||
| Ref. | Anjana et al[127], 2023; International Diabetes Federation[128], 2023; Ministry of Electronics and Information Technology[129]; Anjana et al[130], 2023; Anjana et al[131], 2024; Anjana et al[132], 2011; Mahajan et al[133], 2025; National Family Health Survey[134] | ||||||
| State/Union Territory | Prevalence | Urban | Rural | Key risk factors | |||
| Southern States | 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | |
| Andhra Pradesh | 12.5 | 13.0 | 14.7 | 15.3 | 10.6 | 11.0 | Rice-heavy diet |
| Karnataka | 11.9 | 12.4 | 14.2 | 14.7 | 9.8 | 10.3 | IT sector sedentary jobs |
| Kerala | 19.4 | 20.1 | 23.1 | 23.8 | 16.2 | 16.9 | Aging population |
| Tamil Nadu | 15.7 | 16.3 | 18.9 | 19.5 | 12.8 | 13.3 | Genetic predisposition |
| Telangana | 13.1 | 13.7 | 15.8 | 16.4 | 10.9 | 11.4 | Processed food consumption |
| Northern States | |||||||
| Delhi (National Capital Territory) | 15.3 | 15.9 | 16.8 | 17.4 | 8.2 | 8.6 | Urban stress, pollution |
| Haryana | 9.2 | 9.7 | 12.1 | 12.7 | 7.3 | 7.7 | High body mass index (> 25) prevalence |
| Himachal Pradesh | 7.8 | 8.3 | 9.5 | 10.0 | 6.7 | 7.2 | Alcohol consumption |
| Jammu and Kashmir | 6.9 | 7.4 | 8.7 | 9.3 | 5.8 | 6.2 | Low screening rates |
| Punjab | 14.2 | 14.8 | 16.5 | 17.1 | 12.1 | 12.7 | Wheat-heavy diet |
| Rajasthan | 7.1 | 7.6 | 9.3 | 9.8 | 6.2 | 6.6 | Limited healthcare access |
| Uttarakhand | 8.3 | 8.8 | 10.6 | 11.2 | 7.1 | 7.6 | Tourism-related dietary shifts |
| Western States | |||||||
| Goa | 12.3 | 12.9 | 14.9 | 15.5 | 9.8 | 10.3 | Alcohol, seafood diet |
| Gujarat | 10.8 | 11.4 | 13.1 | 13.8 | 8.9 | 9.4 | Trans-fat consumption |
| Maharashtra | 12.8 | 13.3 | 15.3 | 15.9 | 10.4 | 10.8 | Stress, fast-food culture |
| Eastern States | |||||||
| Bihar | 5.7 | 6.1 | 8.4 | 8.9 | 4.9 | 5.3 | Low awareness |
| Jharkhand | 6.2 | 6.7 | 8.9 | 9.5 | 5.3 | 5.7 | Tribal health disparities |
| Odisha | 7.5 | 8.0 | 10.1 | 10.8 | 6.4 | 6.9 | Rice-based malnutrition |
| West Bengal | 9.7 | 10.3 | 12.4 | 13.1 | 7.6 | 8.1 | Sweetened food culture |
| North-Eastern States | |||||||
| Assam | 6.8 | 7.3 | 9.2 | 9.7 | 5.9 | 6.3 | Betel nut consumption |
| Manipur | 7.8 | 8.3 | 10.5 | 11.0 | 6.7 | 7.1 | Rapid urbanization |
| Meghalaya | 6.5 | 6.9 | 8.3 | 8.7 | 5.8 | 6.1 | Indigenous dietary patterns |
| Mizoram | 7.1 | 7.6 | 9.7 | 10.3 | 6.2 | 6.7 | Smoking prevalence |
| Nagaland | 6.3 | 6.7 | 8.6 | 9.1 | 5.5 | 5.8 | Low health infrastructure |
| Sikkim | 8.9 | 9.5 | 11.2 | 11.8 | 7.6 | 8.0 | Alcohol use |
| Tripura | 7.4 | 7.9 | 9.8 | 10.4 | 6.5 | 7.0 | Rapid lifestyle changes |
| Union Territories | |||||||
| Chandigarh | 13.5 | 15.2 | 14.1 | 18.0 | 9.3 | 12.4 | Affluence-linked obesity |
| Puducherry | 14.6 | 14.1 | 17.3 | 14.7 | 11.9 | 9.7 | French-influenced diet |
| Diabetes prevalence across Indian States and Union Territories (2025 Projections) | ||||
| Ref. | Ministry of Health and Family Welfare[135], 2024; International Diabetes Federation[136], 2025; Government of Goa[137], 2025; Makkar et al[138], 2025; International Diabetes Federation[139], 2025; Ministry of Health and Family Welfare[140], 2024; Imai et al[141], 1988; International Diabetes Federation[142]; Ministry of Health and Family Welfare Government of India[143] | |||
| State/Union Territory | 2025 prevalence | Urban | Rural | Key risk factors |
| Andhra Pradesh | 13.1 | 15.6 | 11.2 | Rice-heavy diet, low activity |
| Bihar | 6.4 | 9.0 | 5.5 | Low awareness, processed food uptake |
| Delhi (National Capital Territory) | 16.5 | 17.8 | 9.1 | Pollution, stress, and obesity |
| Goa | 14.0 | 16.3 | 11.2 | Alcohol, seafood, tourism diet |
| Gujarat | 11.5 | 14.0 | 9.4 | High trans-fat intake |
| Karnataka | 12.7 | 15.1 | 10.6 | IT sector inactivity |
| Kerala | 20.8 | 24.3 | 17.5 | Aging, sedentary jobs |
| Maharashtra | 14.1 | 16.7 | 11.9 | Fast-food culture, stress |
| Punjab | 15.1 | 17.6 | 13.2 | Wheat-based diet, low exercise |
| Tamil Nadu | 17.2 | 20.1 | 14.0 | Genetic risk + urban lifestyle |
| Telangana | 14.3 | 17.0 | 12.1 | IT corridor stress |
| Uttar Pradesh | 7.2 | 10.0 | 6.0 | Low screening rates |
| West Bengal | 10.5 | 13.2 | 8.4 | Sweetened food habits |
Diabetes management and treatment in India present a complex picture, shaped by a mix of healthcare system structure, access to resources, cultural beliefs, and economic realities. India’s healthcare system is a multi-tiered structure, comprising public and private sectors. Public healthcare facilities, including primary health centers in rural areas and government hospitals in urban centers, provide basic diabetes care. However, access to specialist care and advanced diagnostic facilities can be limited, particularly in rural areas[144]. The private sector plays a significant role, with private clinics and hospitals offering a wider range of services, but often at a higher cost. This mixed system creates disparities in access to quality diabetes care, with those in urban areas and with higher socioeconomic status generally having better access[145]. Many countries face similar challenges regarding healthcare access, but the scale and complexity of India’s population make these challenges particularly acute (Table 9)[146-151].
| India’s approach to healthcare: Treatment and management[146-151] | |
| Ref. | World Health Organization[146], 2022; Muralidharan[147], 2024; Mohan et al[148], 2007; International Diabetes Federation[149], 2023; Anjana et al[150], 2017; Ranasinghe et al[151], 2024 |
| Component | Details |
| National program | NPCDCS (2010) - screening, lifestyle advice, free medication/diagnostics at PHCs; implemented in 600+ districts |
| Primary care infrastructure | Health and Wellness Centers (HWCs) - diabetes screening, lifestyle education, digital health records via ABHA ID |
| Private sector role | Handles approximately 70% of diabetes cases; offers specialist care, advanced diagnostics, but with higher out-of-pocket expenses |
| Affordable medicines | Jan Aushadhi Kendras supply low-cost generics (Metformin, glimepiride, basic insulin) |
| Diagnostic access | Public labs provide subsidized HbA1c, glucose, and lipid profile tests; mobile units support rural outreach |
| Insulin availability | Cold chain limitations in rural areas; limited access to analogs and newer injectables (GLP-1, SGLT2i) in the public sector |
| Digital health tools | eSanjeevani: Govt. teleconsultation platform - private apps (BeatO, 1 mg, HealthifyMe), sugar tracking, online consults, lifestyle advice |
| Challenges | Poor awareness and treatment adherence - high cost in the private sector - rural supply chain gaps - fragmented care and follow-up |
| Policy recommendations | Universal screening - subsidized diagnostics and newer drugs - better referral system - rural insulin supply - integrate nutrition and mental health |
The treatment and management of diabetes in India are hindered by significant challenges, including disparities in medicine accessibility, affordability, and healthcare infrastructure. Despite government initiatives such as Jan Aushadhi and state-level subsidized pharmacies, essential anti-diabetic medicines remain inadequately available in public healthcare facilities, forcing patients to depend on private pharmacies where prices are often prohibitive. The high out-of-pocket expenditure on diabetes treatment exacerbates financial strain, particularly among low-income populations, contributing to poor adherence and suboptimal disease management[152]. Fixed-dose combinations offer a promising approach to improving compliance and treatment outcomes, but their integration into the healthcare system remains limited. Additionally, prescribing patterns favor costlier branded medicines over generic alternatives, further increasing the financial burden on patients. To enhance diabetes disease management, policymakers must ensure a steady supply of essential medicines in public healthcare facilities, enforce generic prescribing, implement transparent medicine price monitoring, and expand insurance coverage for diabetes care. Strengthening these aspects is essential for improving access to affordable and effective diabetes treatment across India[153] (Table 10)[126-128,154].
| Diabetes prevention, treatment options, and management stratigies | |
| Ref. | Government of India[126], 2023; Anjana et al[127], 2023; International Diabetes Federation[128], 2023; Prasanna Kumar et al[154], 2024 |
| Aspect | Details |
| Essential drugs | Metformin, glimepiride, insulin (human), and pioglitazone are included in the National List of Essential Medicines (NLEM) |
| Generic drug access | Available via Jan Aushadhi Kendras (government-run generic medicine outlets) at 50%-90% lower cost than branded versions |
| Insulin access | Human insulin is widely available; analogs (e.g., glargine, lispro) are expensive and less accessible in rural Primary Health Centers (PHCs) |
| Cost burden | Monthly cost [branded insulin + oral antidiabetic drugs (OADs)]: Approximately 1500-3000; generics: 300-800 |
| Public sector availability | State-run hospitals provide free/basic medications; stockouts and geographic variation are common |
| Private sector access | Full range of medications available, but high out-of-pocket (OOP) expenses; patients often switch to cheaper or irregular treatment |
| Innovative treatments | Newer classes like sodium-glucose cotransporter 2 inhibitors (SGLT2i), glucagon-like peptide-1 receptor agonists (GLP-1 RA), and dipeptidyl peptidase-4 inhibitors (DPP-4i) are limited to metropolitan areas and private hospitals due to cost and awareness gaps |
| Insurance coverage | Partial under Ayushman Bharat - Pradhan Mantri Jan Arogya Yojana (AB-PMJAY); many private plans do not cover chronic outpatient department (OPD) care |
| Policy recommendations | Expand NLEM to include newer drugs - ensure insulin cold chain in rural areas- Subsidize analog insulins |
Traditional medicine systems like Ayurveda, Siddha, and Unani are commonly used in India for diabetes management, employing herbal formulations such as Gymnema sylvestre (Gurmar), Momordica charantia (Bitter melon), and Trigonella foenum-graecum (Fenugreek), which demonstrate anti-diabetic properties through enhanced insulin secretion and improved glucose uptake[155-157]. Clinical evidence suggests traditional formulations can help regulate blood glucose and manage complications through antioxidant and anti-inflammatory properties[158]. However, significant gaps remain in large-scale validation and standardization. While integration with modern medicine could improve outcomes in resource-limited settings, combining treatments requires medical supervision to avoid adverse effects. Government initiatives like Ayurveda, Yoga, and Naturopathy, Unani, Siddha, and Homeopathy support research and standardization efforts[159].
Traditional and alternative medicine practices are gaining popularity due to their cultural relevance, affordability, and holistic approach, and are increasingly being integrated into conventional diabetes care[160]. For example, Momordica charantia (bitter melon) demonstrates insulin-mimetic and insulin-releasing properties, leading to reductions in glycated hemoglobin A1c (HbA1c) and fasting blood glucose[161]. Similarly, Trigonella foenum-graecum (fenugreek) seeds, rich in soluble fiber, improve glycemic control by slowing carbohydrate digestion and enhancing insulin sensitivity[162]. In Ayurvedic practice, Gymnema sylvestre (“gurmar”) helps manage dietary habits by reducing sugar cravings and supporting pancreatic β-cell regeneration[163].
An Indian randomized controlled trial demonstrated that a standardized polyherbal formulation containing Syzygium cumini, Emblica officinalis, and Tinospora cordifolia significantly reduced HbA1c, fasting plasma glucose, and postprandial glucose levels after 12 weeks of treatment[164]. Complementing herbal interventions, traditional practices like yoga and meditation show empirical support for diabetes management. A meta-analysis of 23 randomized trials confirmed that regular yoga practice significantly reduces body mass index, fasting glucose, and HbA1c in type 2 diabetes patients[165]. These benefits are attributed to improved glucose utilization, enhanced parasympathetic activity, and reduced cortisol levels. Similarly, mindfulness-based interventions indirectly improve glycemic control by alleviating psychological stress and enhancing dietary adherence[166].
Diabetes management employs distinct allopathic and Ayurvedic approaches. Allopathic treatment targets glucose metabolism, using metformin to improve insulin sensitivity[167], sulfonylureas like glibenclamide to stimulate insulin secretion (with hypoglycemia risk)[168], and dipeptidyl peptidase-4 inhibitors such as sitagliptin to enhance incretin effects[169]. Conversely, Ayurveda utilizes polyherbal formulations to restore metabolic balance, employing Gymnema sylvestre to reduce sugar cravings and support β-cell function[170], Momordica charantia with its insulin-mimetic compounds[161], Trigonella foenum-graecum to slow glucose absorption[162], and Emblica officinalis for antioxidant protection of β-cells[171]. Guduchi, or Tinospora cordifolia, has anti-inflammatory, insulin-sensitive, and immune-regulating qualities[164]. Ayurvedic treatments address systemic dysfunctions like Agni (digestion), Ama (toxins), and dosha imbalance to promote metabolic stability. While both systems offer glycemic benefits, combining them requires caution to prevent adverse herb-drug interactions. The World Health Organization Traditional Medicine Strategy (2014-2023) emphasizes the need for evidence-based integration, advocating for research and regulation to ensure safe, standardized use of traditional medicine in managing diabetes[172]. The global report also highlighted the need to standardize regulatory frameworks across countries and the increasing use of digital platforms to sell conventional drugs[173]. Future efforts should prioritize rigorous scientific validation, quality control, and formulation standardization to ensure safety and efficacy, ultimately enhancing diabetes management strategies in India (Table 11)[126,154,174,175].
| Indian perspective on the function of traditional and alternative medicine in the treatment of diabetes | |
| Ref. | Government of India[126], 2023; Prasanna Kumar KM et al[154], 2024; Central Council for Research in Ayurvedic Sciences (CCRAS)[174], 2023; Council of Scientific and Industrial Research (CSIR), Ministry of AYUSH[175], 2023 |
| Aspect | Details |
| Systems involved | India’s pluralistic healthcare system includes AYUSH: Ayurveda, Yoga, Unani, Siddha, and Homeopathy; these systems emphasize holistic approaches focusing on mind-body balance and lifestyle regulation for diabetes care |
| Popular herbs used | Gymnema sylvestre (Gurmar): Glucose-lowering effect, β-cell regeneration; Momordica charantia (Bitter gourd): Insulin-like compounds; Trigonella foenum-graecum (Fenugreek): Improves insulin sensitivity |
| Ayurvedic formulations | Common preparations include Chandraprabha Vati, Nishamalaki Churna, Dhanvantari Kashayam, and proprietary formulations like Diabecon and BGR-34, used as adjunct therapies for glycemic control |
| Yoga and lifestyle therapy | Yoga practices such as Surya Namaskar, Pranayama, and meditation have shown benefits in improving glycemic control, insulin sensitivity, and stress reduction in clinical and observational studies |
| Usage statistics | An estimated 20%-25% of Indian diabetes patients utilize some form of AYUSH therapy, commonly in conjunction with allopathic treatments |
| Evidence and Limitations | Preliminary studies and small-scale clinical trials indicate the efficacy of several AYUSH therapies; however, there is a lack of large-scale RCTs, standardization, and systematic safety evaluations |
| Government support | NMITLI project on herbal anti-diabeticsAYUSH research portal for data consolidation, government funding for clinical trials, and establishment of integrative healthcare centers |
| Challenges | Key barriers include quality control of herbal products, unregulated markets, potential herb-drug interactions, and poor disclosure by patients to conventional healthcare providers |
| Policy recommendations | Promote large-scale RCTs and meta-analyses to validate efficacy, develop standardized, quality-controlled formulations, establish integrative diabetes care clinics, and enhance patient education |
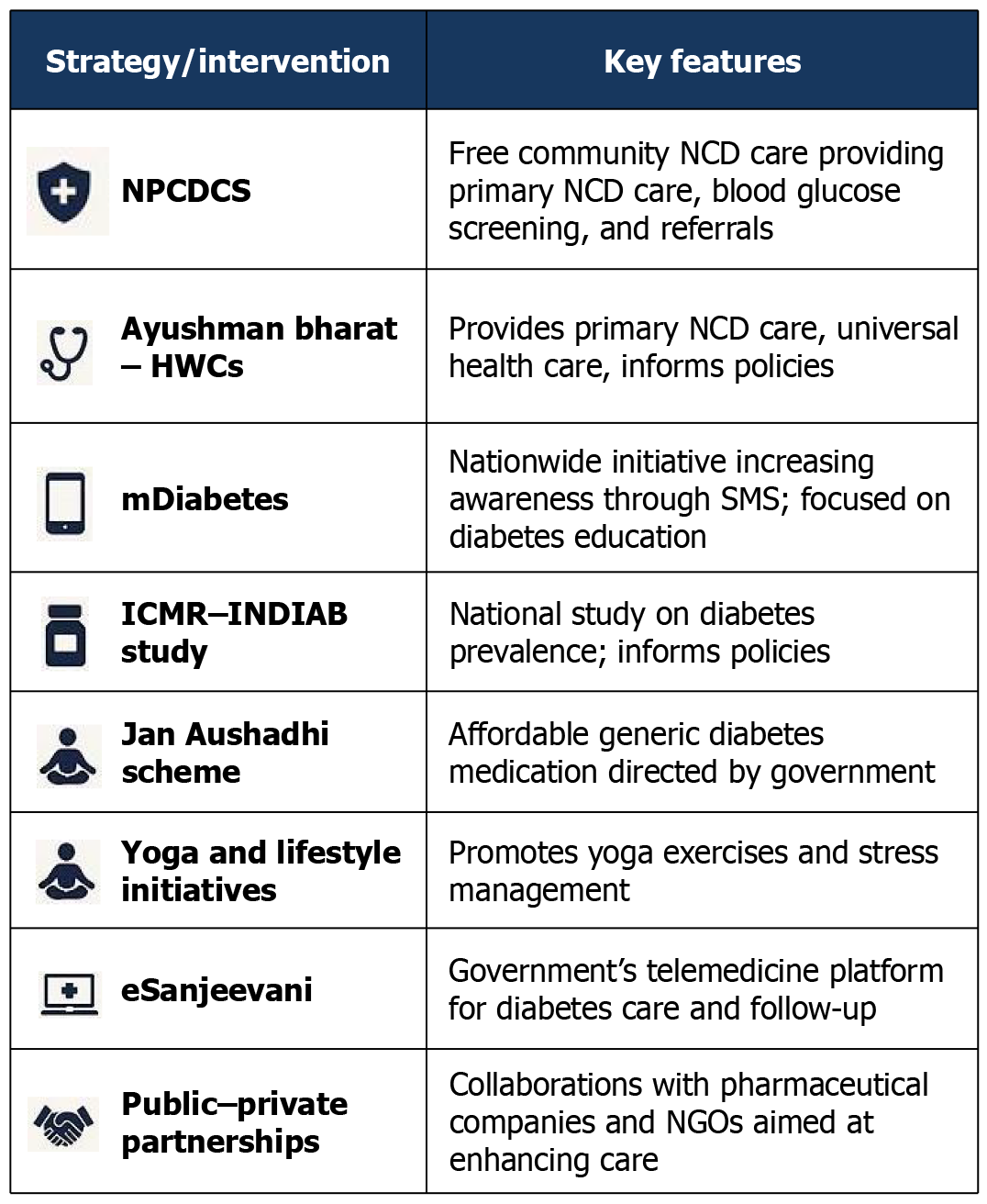
Several strategies and interventions have shown promise in addressing the growing diabetes burden in India, offering valuable lessons for other LMICs grappling with similar challenges. The National Diabetes Control Program in India, with its focus on early detection, health education, and affordable treatment, provides a potential model for large-scale diabetes management in resource-constrained settings[176]. Community-based initiatives, often driven by nongovernmental organizations and local organizations, have proven effective in raising awareness and promoting early detection, especially among vulnerable populations in both rural and urban settings. These campaigns can be particularly impactful in reaching communities with limited access to formal healthcare[177]. Establishing strong referral systems and integrating community-based initiatives with existing healthcare infrastructure are crucial for ensuring long-term care, a challenge shared by many LMICs, which can be a critical consideration for policymakers as well. The increasing use of telehealth in India offers promising solutions for diabetes management, especially in remote areas[178]. These technologies can improve access to specialist care, remote monitoring, and personalized support. Telehealth can bridge geographical barriers and provide remote monitoring and support, particularly beneficial in LMICs with dispersed populations and limited healthcare infrastructure. While further scientific evaluation and validation are needed, exploring the potential role of traditional practices alongside conventional care is relevant for many LMICs. Collaborative research and knowledge sharing across LMICs can facilitate the development of evidence-based approaches to integrating traditional medicine into diabetes care, not only at the local level but globally too[179] (Figure 5 and Table 12)[122,125,126,133,139,179].
| Diabetes prevention and management in India | ||
| Ref. | MedBound Times[122]; Ministry of Health and Family Welfare[125], 2022; Government of India[126], 2023; Mahajan et al[133], 2025; International Diabetes Federation[139], 2025; Mohan et al[179], 2024 | |
| Strategy/intervention | Key features | Impact/remarks |
| NPCDCS | National program for NCD screening, health promotion, and free medication at primary health centers | Screened 150+ million people; improved early detection in low-income groups |
| Ayushman Bharat HWCs | Network of health centers providing primary care, diabetes screening, and counseling | Expanded preventive care in rural/underserved areas; a pillar of Universal Health Coverage |
| mDiabetes Initiative | WHO-MoHFW SMS program delivering lifestyle advice in 12 languages | Reached 1+ million people; cost-effective digital health model |
| Jan Aushadhi Scheme | Government pharmacies provide low-cost generic diabetes medicines | Reduced out-of-pocket expenses; improved drug access in rural areas |
| eSanjeevani Telemedicine | Government teleconsultation platform for diabetes follow-up and specialist access | 100+ million consultations; improved care continuity in remote areas |
| Yoga and Lifestyle Initiatives | AYUSH-led programs promoting yoga and stress management for diabetes | Evidence shows reduced HbA1c; culturally accepted prevention strategy |
| ICMR-INDIAB study | National study on diabetes prevalence and risk factors | Data revealed 100+ million diabetics; informed national policy |
| Public-Private Partnerships | Collaborations with pharma/NGOs for insulin access and diabetes education | Enhanced care in underserved communities through targeted programs |
Diabetes mellitus presents a critical and growing health challenge, particularly in LMICs. Driven by genetic, environmental, and lifestyle factors, its rising prevalence increasingly affects younger and more vulnerable populations. In India, management is hindered by issues of access, affordability, and infrastructure. The national response includes programs like the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases, and Stroke, and Ayushman Bharat, digital health platforms like eSanjeevani, and the integration of Ayurveda, Yoga, and Naturopathy, Unani, Siddha, and Homeopathy systems. Efforts such as the Jan Aushadhi scheme for affordable medicines, yoga-based interventions, and public-private partnerships enhance care. Growing public awareness and digital tools are improving education and monitoring, yet further scientific validation and infrastructure strengthening are needed to effectively address the escalating burden.
India’s diabetes care system leverages a robust network of community health workers for rural outreach, yet it contends with critical challenges, including shortages of medical personnel, essential drugs, and diagnostic tools. These limitations contribute to low screening rates, delayed diagnosis, and fragmented care, exacerbating poor glycemic control and imposing significant financial strain on families. Emerging opportunities through digital health technologies, public-private partnerships, and community-based lifestyle programs offer promising avenues for improved tracking, affordable medication access, and preventive strategies. The growing burden of diabetes-related complications necessitates a strengthened primary healthcare system focused on early detection and personalized prevention. A comprehensive, integrated approach is crucial to mitigate India's substantial diabetes burden, though current assessments remain limited by reliance on secondary data and lack of direct program evaluation.
We are grateful to the Multidisciplinary Research Unit at Maulana Azad Medical College and Associated Hospitals, Department of Biotechnology, Guru Ghasidas Vishwavidyalaya, Delhi, and Faculty of Medicine, Alatoo International University, Bishkek. We would like to thank Raj Rajeshwar Malinda for their input in language correction.
| 1. | Magliano DJ, Boyko EJ; IDF Diabetes Atlas 10th edition scientific committee. 10th edition. Brussels: International Diabetes Federation; 2021. [PubMed] |
| 2. | Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr. 2006;84:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 360] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Mohan V, Mathur P, Deepa R, Deepa M, Shukla DK, Menon GR, Anand K, Desai NG, Joshi PP, Mahanta J, Thankappan KR, Shah B. Urban rural differences in prevalence of self-reported diabetes in India--the WHO-ICMR Indian NCD risk factor surveillance. Diabetes Res Clin Pract. 2008;80:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, Bhansali A, Joshi SR, Joshi PP, Yajnik CS, Dhandhania VK, Nath LM, Das AK, Rao PV, Madhu SV, Shukla DK, Kaur T, Priya M, Nirmal E, Parvathi SJ, Subhashini S, Subashini R, Ali MK, Mohan V; ICMR–INDIAB Collaborative Study Group. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022-3027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 5. | Verma M, Kalra S, Deepa M, Venkatesan U, Sharma N, Pradeepa R, Chauhan K, Singh O, Elangovan N, Aggarwal S, Kakkar R, Dhaliwal RS, Kaur T, Mohan V, Anjana RM. Understanding Epidemiology of Physical Activity and Sedentary Behaviour Among Adults in Haryana: Insights from the ICMR-INDIAB Study [ICMR-INDIAB-19]. Adv Ther. 2025;42:3265-3284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. Global report on diabetes. Geneva: WHO. 2016. [cited 2023 Nov 10]. Available from: https://www.who.int/publications/i/item/9789241565257. |
| 7. | Lal P, Mishra D, Singh R. A “Bottom-up approach” to introduce ban on tobacco products to prevent spitting during COVID-19: An early review of progress made and challenges. Int J Non-Commun Dis. 2020;5:138. [DOI] [Full Text] |
| 8. | Mohan V, Sudha V, Shobana S, Gayathri R, Krishnaswamy K. Are Unhealthy Diets Contributing to the Rapid Rise of Type 2 Diabetes in India? J Nutr. 2023;153:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 9. | Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69:2932-2938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 10. | Joseph A, Thirupathamma M, Mathews E, Alagu M. Genetics of type 2 diabetes mellitus in Indian and Global Population: A Review. Egypt J Med Hum Genet. 2022;23:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Beulens JWJ, Pinho MGM, Abreu TC, den Braver NR, Lam TM, Huss A, Vlaanderen J, Sonnenschein T, Siddiqui NZ, Yuan Z, Kerckhoffs J, Zhernakova A, Brandao Gois MF, Vermeulen RCH. Environmental risk factors of type 2 diabetes-an exposome approach. Diabetologia. 2022;65:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (2)] |
| 12. | Maiti S, Akhtar S, Upadhyay AK, Mohanty SK. Socioeconomic inequality in awareness, treatment and control of diabetes among adults in India: Evidence from National Family Health Survey of India (NFHS), 2019-2021. Sci Rep. 2023;13:2971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 13. | Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14:591-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 943] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 14. | Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17:20-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 622] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 15. | Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 764] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 16. | Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, Basson AK, Pheiffer C, Kengne AP. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J Diabetes. 2023;14:130-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 152] [Article Influence: 50.7] [Reference Citation Analysis (12)] |
| 17. | Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12:2032-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1955] [Article Influence: 217.2] [Reference Citation Analysis (0)] |
| 18. | Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int J Mol Sci. 2020;21:1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 725] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 19. | World Health Organization. Compendium of WHO and other UN guidance in health and environment, 2024 update. World Health Organization, 2024. [cited 3 August 2025]. Available from: https://www.who.int/publications/i/item/9789240095380. |
| 20. | Pappachan JM, Fernandez CJ, Ashraf AP. Rising tide: The global surge of type 2 diabetes in children and adolescents demands action now. World J Diabetes. 2024;15:797-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (3)] |
| 21. | Kalra S, Anjana RM, Verma M, Pradeepa R, Sharma N, Deepa M, Singh O, Venkatesan U, Elangovan N, Aggarwal S, Kakkar R, Mohan V. Urban-Rural Differences in the Prevalence of Diabetes Among Adults in Haryana, India: The ICMR-INDIAB Study (ICMR-INDIAB-18). Diabetes Ther. 2024;15:1597-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2049] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 23. | The World Bank. World Bank country classifications by income level (2024). Aug 6, 2024. [cited 3 May 2025]. Available from: https://datahelpdesk.worldbank.org. |
| 24. | Zhuo X, Zhang P, Barker L, Albright A, Thompson TJ, Gregg E. The lifetime cost of diabetes and its implications for diabetes prevention. Diabetes Care. 2014;37:2557-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | International Diabetes Federation. The Diabetes Atlas. 11th edition. Brussels: IDF; 2023. Available from: https://diabetesatlas.org. |
| 26. | World Health Organization. Diabetes [Internet]. Geneva: WHO; 2025 [cited 9 Jun 2025]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes. |
| 27. | GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402:203-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2437] [Cited by in RCA: 2574] [Article Influence: 858.0] [Reference Citation Analysis (18)] |
| 28. | Popoviciu MS, Kaka N, Sethi Y, Patel N, Chopra H, Cavalu S. Type 1 Diabetes Mellitus and Autoimmune Diseases: A Critical Review of the Association and the Application of Personalized Medicine. J Pers Med. 2023;13:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 75] [Reference Citation Analysis (9)] |
| 29. | Sweeting A, Wong J, Murphy HR, Ross GP. A Clinical Update on Gestational Diabetes Mellitus. Endocr Rev. 2022;43:763-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 515] [Article Influence: 128.8] [Reference Citation Analysis (1)] |
| 30. | International Diabetes Federation. Facts and figures [Internet]. Brussels: IDF; 2025 [cited 9 Jun 2025]. Available from: https://idf.org/about-diabetes/diabetes-facts-figures/. |
| 31. | The Lancet Diabetes Endocrinology. Alarming rise in young-onset type 2 diabetes. Lancet Diabetes Endocrinol. 2024;12:433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 32. | Corriere M, Rooparinesingh N, Kalyani RR. Epidemiology of diabetes and diabetes complications in the elderly: an emerging public health burden. Curr Diab Rep. 2013;13:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 33. | Strati M, Moustaki M, Psaltopoulou T, Vryonidou A, Paschou SA. Early onset type 2 diabetes mellitus: an update. Endocrine. 2024;85:965-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 34. | Kautzky-Willer A, Leutner M, Harreiter J. Sex differences in type 2 diabetes. Diabetologia. 2023;66:986-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 325] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 35. | Golden SH, Yajnik C, Phatak S, Hanson RL, Knowler WC. Racial/ethnic differences in the burden of type 2 diabetes over the life course: a focus on the USA and India. Diabetologia. 2019;62:1751-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Tait BD. A review of the genetics of type I diabetes. Explor Immunol. 2024;4:568-576. [DOI] [Full Text] |
| 37. | Himanshu D, Ali W, Wamique M. Type 2 diabetes mellitus: pathogenesis and genetic diagnosis. J Diabetes Metab Disord. 2020;19:1959-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Laakso M, Fernandes Silva L. Genetics of Type 2 Diabetes: Past, Present, and Future. Nutrients. 2022;14:3201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 39. | Ke C, Narayan KMV, Chan JCN, Jha P, Shah BR. Pathophysiology, phenotypes and management of type 2 diabetes mellitus in Indian and Chinese populations. Nat Rev Endocrinol. 2022;18:413-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 40. | Nejentsev S, Walker N, Riches D, Egholm M, Todd JA. Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science. 2009;324:387-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 792] [Cited by in RCA: 748] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 41. | Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, Walker M, Levy JC, Sampson M, Halford S, McCarthy MI, Hattersley AT, Frayling TM. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 515] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 42. | Dornbos P, Koesterer R, Ruttenburg A, Nguyen T, Cole JB; AMP-T2D-GENES Consortium, Leong A, Meigs JB, Florez JC, Rotter JI, Udler MS, Flannick J. A combined polygenic score of 21,293 rare and 22 common variants improves diabetes diagnosis based on hemoglobin A1C levels. Nat Genet. 2022;54:1609-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 43. | Kumar KMP, Unnikrishnan AG, Jariwala P, Mehta A, Chaturvedi R, Panchal S, Lakhani P, Acharya R, Dixit J. SGLT2 Inhibitors: Paradigm Shift from Diabetes Care to Metabolic Care-An Indian Perspective. Indian J Endocrinol Metab. 2024;28:11-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 44. | Flannick J, Mercader JM, Fuchsberger C, Udler MS, Mahajan A, Wessel J, Teslovich TM, Caulkins L, Koesterer R, Barajas-Olmos F, Blackwell TW, Boerwinkle E, Brody JA, Centeno-Cruz F, Chen L, Chen S, Contreras-Cubas C, Córdova E, Correa A, Cortes M, DeFronzo RA, Dolan L, Drews KL, Elliott A, Floyd JS, Gabriel S, Garay-Sevilla ME, García-Ortiz H, Gross M, Han S, Heard-Costa NL, Jackson AU, Jørgensen ME, Kang HM, Kelsey M, Kim BJ, Koistinen HA, Kuusisto J, Leader JB, Linneberg A, Liu CT, Liu J, Lyssenko V, Manning AK, Marcketta A, Malacara-Hernandez JM, Martínez-Hernández A, Matsuo K, Mayer-Davis E, Mendoza-Caamal E, Mohlke KL, Morrison AC, Ndungu A, Ng MCY, O'Dushlaine C, Payne AJ, Pihoker C; Broad Genomics Platform, Post WS, Preuss M, Psaty BM, Vasan RS, Rayner NW, Reiner AP, Revilla-Monsalve C, Robertson NR, Santoro N, Schurmann C, So WY, Soberón X, Stringham HM, Strom TM, Tam CHT, Thameem F, Tomlinson B, Torres JM, Tracy RP, van Dam RM, Vujkovic M, Wang S, Welch RP, Witte DR, Wong TY, Atzmon G, Barzilai N, Blangero J, Bonnycastle LL, Bowden DW, Chambers JC, Chan E, Cheng CY, Cho YS, Collins FS, de Vries PS, Duggirala R, Glaser B, Gonzalez C, Gonzalez ME, Groop L, Kooner JS, Kwak SH, Laakso M, Lehman DM, Nilsson P, Spector TD, Tai ES, Tuomi T, Tuomilehto J, Wilson JG, Aguilar-Salinas CA, Bottinger E, Burke B, Carey DJ, Chan JCN, Dupuis J, Frossard P, Heckbert SR, Hwang MY, Kim YJ, Kirchner HL, Lee JY, Lee J, Loos RJF, Ma RCW, Morris AD, O'Donnell CJ, Palmer CNA, Pankow J, Park KS, Rasheed A, Saleheen D, Sim X, Small KS, Teo YY, Haiman C, Hanis CL, Henderson BE, Orozco L, Tusié-Luna T, Dewey FE, Baras A, Gieger C, Meitinger T, Strauch K, Lange L, Grarup N, Hansen T, Pedersen O, Zeitler P, Dabelea D, Abecasis G, Bell GI, Cox NJ, Seielstad M, Sladek R, Meigs JB, Rich SS, Rotter JI; DiscovEHR Collaboration; CHARGE; LuCamp; ProDiGY; GoT2D; ESP; SIGMA-T2D; T2D-GENES; AMP-T2D-GENES, Altshuler D, Burtt NP, Scott LJ, Morris AP, Florez JC, McCarthy MI, Boehnke M. Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature. 2019;570:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 229] [Article Influence: 32.7] [Reference Citation Analysis (1)] |
| 45. | Shi S, Li X, Chen Y, Li J, Dai Y. Cardiovascular Therapy Benefits of Novel Antidiabetic Drugs in Patients With Type 2 Diabetes Mellitus Complicated With Cardiovascular Disease: A Network Meta-Analysis. J Diabetes. 2025;17:e70044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 46. | Diabetes Genetics Initiative of Broad Institute of Harvard and MIT; Lund University, and Novartis Institutes of BioMedical Research, Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Boström K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Råstam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjögren M, Sterner M, Surti A, Svensson M, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2243] [Cited by in RCA: 2139] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 47. | Abu Aqel Y, Alnesf A, Aigha II, Islam Z, Kolatkar PR, Teo A, Abdelalim EM. Glucokinase (GCK) in diabetes: from molecular mechanisms to disease pathogenesis. Cell Mol Biol Lett. 2024;29:120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 48. | Russ-Silsby J, Teles M, Hassan SS, Elbarbary NS, Ngọc CTB, De Franco E. Global perspectives on monogenic forms of diabetes. Diabetologia. 2025;68:2362-2373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Gerber PA, Rutter GA. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid Redox Signal. 2017;26:501-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 472] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 50. | Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, Ma C, Fontanillas P, Moutsianas L, McCarthy DJ, Rivas MA, Perry JRB, Sim X, Blackwell TW, Robertson NR, Rayner NW, Cingolani P, Locke AE, Tajes JF, Highland HM, Dupuis J, Chines PS, Lindgren CM, Hartl C, Jackson AU, Chen H, Huyghe JR, van de Bunt M, Pearson RD, Kumar A, Müller-Nurasyid M, Grarup N, Stringham HM, Gamazon ER, Lee J, Chen Y, Scott RA, Below JE, Chen P, Huang J, Go MJ, Stitzel ML, Pasko D, Parker SCJ, Varga TV, Green T, Beer NL, Day-Williams AG, Ferreira T, Fingerlin T, Horikoshi M, Hu C, Huh I, Ikram MK, Kim BJ, Kim Y, Kim YJ, Kwon MS, Lee J, Lee S, Lin KH, Maxwell TJ, Nagai Y, Wang X, Welch RP, Yoon J, Zhang W, Barzilai N, Voight BF, Han BG, Jenkinson CP, Kuulasmaa T, Kuusisto J, Manning A, Ng MCY, Palmer ND, Balkau B, Stančáková A, Abboud HE, Boeing H, Giedraitis V, Prabhakaran D, Gottesman O, Scott J, Carey J, Kwan P, Grant G, Smith JD, Neale BM, Purcell S, Butterworth AS, Howson JMM, Lee HM, Lu Y, Kwak SH, Zhao W, Danesh J, Lam VKL, Park KS, Saleheen D, So WY, Tam CHT, Afzal U, Aguilar D, Arya R, Aung T, Chan E, Navarro C, Cheng CY, Palli D, Correa A, Curran JE, Rybin D, Farook VS, Fowler SP, Freedman BI, Griswold M, Hale DE, Hicks PJ, Khor CC, Kumar S, Lehne B, Thuillier D, Lim WY, Liu J, van der Schouw YT, Loh M, Musani SK, Puppala S, Scott WR, Yengo L, Tan ST, Taylor HA Jr, Thameem F, Wilson G Sr, Wong TY, Njølstad PR, Levy JC, Mangino M, Bonnycastle LL, Schwarzmayr T, Fadista J, Surdulescu GL, Herder C, Groves CJ, Wieland T, Bork-Jensen J, Brandslund I, Christensen C, Koistinen HA, Doney ASF, Kinnunen L, Esko T, Farmer AJ, Hakaste L, Hodgkiss D, Kravic J, Lyssenko V, Hollensted M, Jørgensen ME, Jørgensen T, Ladenvall C, Justesen JM, Käräjämäki A, Kriebel J, Rathmann W, Lannfelt L, Lauritzen T, Narisu N, Linneberg A, Melander O, Milani L, Neville M, Orho-Melander M, Qi L, Qi Q, Roden M, Rolandsson O, Swift A, Rosengren AH, Stirrups K, Wood AR, Mihailov E, Blancher C, Carneiro MO, Maguire J, Poplin R, Shakir K, Fennell T, DePristo M, de Angelis MH, Deloukas P, Gjesing AP, Jun G, Nilsson P, Murphy J, Onofrio R, Thorand B, Hansen T, Meisinger C, Hu FB, Isomaa B, Karpe F, Liang L, Peters A, Huth C, O'Rahilly SP, Palmer CNA, Pedersen O, Rauramaa R, Tuomilehto J, Salomaa V, Watanabe RM, Syvänen AC, Bergman RN, Bharadwaj D, Bottinger EP, Cho YS, Chandak GR, Chan JCN, Chia KS, Daly MJ, Ebrahim SB, Langenberg C, Elliott P, Jablonski KA, Lehman DM, Jia W, Ma RCW, Pollin TI, Sandhu M, Tandon N, Froguel P, Barroso I, Teo YY, Zeggini E, Loos RJF, Small KS, Ried JS, DeFronzo RA, Grallert H, Glaser B, Metspalu A, Wareham NJ, Walker M, Banks E, Gieger C, Ingelsson E, Im HK, Illig T, Franks PW, Buck G, Trakalo J, Buck D, Prokopenko I, Mägi R, Lind L, Farjoun Y, Owen KR, Gloyn AL, Strauch K, Tuomi T, Kooner JS, Lee JY, Park T, Donnelly P, Morris AD, Hattersley AT, Bowden DW, Collins FS, Atzmon G, Chambers JC, Spector TD, Laakso M, Strom TM, Bell GI, Blangero J, Duggirala R, Tai ES, McVean G, Hanis CL, Wilson JG, Seielstad M, Frayling TM, Meigs JB, Cox NJ, Sladek R, Lander ES, Gabriel S, Burtt NP, Mohlke KL, Meitinger T, Groop L, Abecasis G, Florez JC, Scott LJ, Morris AP, Kang HM, Boehnke M, Altshuler D, McCarthy MI. The genetic architecture of type 2 diabetes. Nature. 2016;536:41-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 809] [Cited by in RCA: 795] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 51. | Hwang YC, Ahn HY, Jun JE, Jeong IK, Ahn KJ, Chung HY. Subtypes of type 2 diabetes and their association with outcomes in Korean adults - A cluster analysis of community-based prospective cohort. Metabolism. 2023;141:155514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 52. | Udler MS, McCarthy MI, Florez JC, Mahajan A. Genetic Risk Scores for Diabetes Diagnosis and Precision Medicine. Endocr Rev. 2019;40:1500-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 53. | Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, Prokopenko I, Kang HM, Dina C, Esko T, Fraser RM, Kanoni S, Kumar A, Lagou V, Langenberg C, Luan J, Lindgren CM, Müller-Nurasyid M, Pechlivanis S, Rayner NW, Scott LJ, Wiltshire S, Yengo L, Kinnunen L, Rossin EJ, Raychaudhuri S, Johnson AD, Dimas AS, Loos RJ, Vedantam S, Chen H, Florez JC, Fox C, Liu CT, Rybin D, Couper DJ, Kao WH, Li M, Cornelis MC, Kraft P, Sun Q, van Dam RM, Stringham HM, Chines PS, Fischer K, Fontanillas P, Holmen OL, Hunt SE, Jackson AU, Kong A, Lawrence R, Meyer J, Perry JR, Platou CG, Potter S, Rehnberg E, Robertson N, Sivapalaratnam S, Stančáková A, Stirrups K, Thorleifsson G, Tikkanen E, Wood AR, Almgren P, Atalay M, Benediktsson R, Bonnycastle LL, Burtt N, Carey J, Charpentier G, Crenshaw AT, Doney AS, Dorkhan M, Edkins S, Emilsson V, Eury E, Forsen T, Gertow K, Gigante B, Grant GB, Groves CJ, Guiducci C, Herder C, Hreidarsson AB, Hui J, James A, Jonsson A, Rathmann W, Klopp N, Kravic J, Krjutškov K, Langford C, Leander K, Lindholm E, Lobbens S, Männistö S, Mirza G, Mühleisen TW, Musk B, Parkin M, Rallidis L, Saramies J, Sennblad B, Shah S, Sigurðsson G, Silveira A, Steinbach G, Thorand B, Trakalo J, Veglia F, Wennauer R, Winckler W, Zabaneh D, Campbell H, van Duijn C, Uitterlinden AG, Hofman A, Sijbrands E, Abecasis GR, Owen KR, Zeggini E, Trip MD, Forouhi NG, Syvänen AC, Eriksson JG, Peltonen L, Nöthen MM, Balkau B, Palmer CN, Lyssenko V, Tuomi T, Isomaa B, Hunter DJ, Qi L; Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium, Shuldiner AR, Roden M, Barroso I, Wilsgaard T, Beilby J, Hovingh K, Price JF, Wilson JF, Rauramaa R, Lakka TA, Lind L, Dedoussis G, Njølstad I, Pedersen NL, Khaw KT, Wareham NJ, Keinanen-Kiukaanniemi SM, Saaristo TE, Korpi-Hyövälti E, Saltevo J, Laakso M, Kuusisto J, Metspalu A, Collins FS, Mohlke KL, Bergman RN, Tuomilehto J, Boehm BO, Gieger C, Hveem K, Cauchi S, Froguel P, Baldassarre D, Tremoli E, Humphries SE, Saleheen D, Danesh J, Ingelsson E, Ripatti S, Salomaa V, Erbel R, Jöckel KH, Moebus S, Peters A, Illig T, de Faire U, Hamsten A, Morris AD, Donnelly PJ, Frayling TM, Hattersley AT, Boerwinkle E, Melander O, Kathiresan S, Nilsson PM, Deloukas P, Thorsteinsdottir U, Groop LC, Stefansson K, Hu F, Pankow JS, Dupuis J, Meigs JB, Altshuler D, Boehnke M, McCarthy MI; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1715] [Cited by in RCA: 1502] [Article Influence: 107.3] [Reference Citation Analysis (1)] |
| 54. | Lu X, Xie Q, Pan X, Zhang R, Zhang X, Peng G, Zhang Y, Shen S, Tong N. Type 2 diabetes mellitus in adults: pathogenesis, prevention and therapy. Signal Transduct Target Ther. 2024;9:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 225] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 55. | Keels JN, McDonald IR, Lee CS, Dwyer AA. Antidiabetic agent use and clinical outcomes in patients with diabetes hospitalized for COVID-19: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2024;15:1482853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 56. | Liang Q, Sun Y, Li M, Li R, Nie L, Lin L, Yu X. Association and function analysis of genetic variants and the risk of gestational diabetes mellitus in a southern Chinese population. Front Endocrinol (Lausanne). 2024;15:1476222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Mittal R, Prasad K, Lemos JRN, Arevalo G, Hirani K. Unveiling Gestational Diabetes: An Overview of Pathophysiology and Management. Int J Mol Sci. 2025;26:2320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 58. | Fan Y, Long E, Cai L, Cao Q, Wu X, Tong R. Machine Learning Approaches to Predict Risks of Diabetic Complications and Poor Glycemic Control in Nonadherent Type 2 Diabetes. Front Pharmacol. 2021;12:665951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2125] [Article Influence: 111.8] [Reference Citation Analysis (1)] |
| 60. | Gwenzi T, Brenner H. Reply - Letter to the Editor - Patients with cancer and precancerous lesions: Systematic review and meta-analysis of randomized trials. Clin Nutr. 2024;43:1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Li WZ, Stirling K, Yang JJ, Zhang L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J Diabetes. 2020;11:293-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (5)] |
| 62. | Goyal S, Rani J, Bhat MA, Vanita V. Genetics of diabetes. World J Diabetes. 2023;14:656-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (3)] |
| 63. | Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J Diabetes. 2010;1:68-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (6)] |
| 64. | Sayyed Kassem L, Rajpal A, Barreiro MV, Ismail-Beigi F. Beta-cell function in type 2 diabetes (T2DM): Can it be preserved or enhanced? J Diabetes. 2023;15:817-837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 65. | Clemente-Suárez VJ, Beltrán-Velasco AI, Redondo-Flórez L, Martín-Rodríguez A, Tornero-Aguilera JF. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients. 2023;15:2749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 399] [Article Influence: 133.0] [Reference Citation Analysis (1)] |
| 66. | Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE. Why does obesity cause diabetes? Cell Metab. 2022;34:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 575] [Article Influence: 143.8] [Reference Citation Analysis (0)] |
| 67. | Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2016;2:e000143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 400] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 68. | Hamilton MT, Hamilton DG, Zderic TW. Sedentary behavior as a mediator of type 2 diabetes. Med Sport Sci. 2014;60:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 69. | Ruze R, Liu T, Zou X, Song J, Chen Y, Xu R, Yin X, Xu Q. Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front Endocrinol (Lausanne). 2023;14:1161521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 417] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 70. | Maddatu J, Anderson-Baucum E, Evans-Molina C. Smoking and the risk of type 2 diabetes. Transl Res. 2017;184:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 71. | Kleinberger JW, Pollin TI. Personalized medicine in diabetes mellitus: current opportunities and future prospects. Ann N Y Acad Sci. 2015;1346:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 72. | Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne). 2013;4:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 581] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 73. | Pervjakova N, Moen GH, Borges MC, Ferreira T, Cook JP, Allard C, Beaumont RN, Canouil M, Hatem G, Heiskala A, Joensuu A, Karhunen V, Kwak SH, Lin FTJ, Liu J, Rifas-Shiman S, Tam CH, Tam WH, Thorleifsson G, Andrew T, Auvinen J, Bhowmik B, Bonnefond A, Delahaye F, Demirkan A, Froguel P, Haller-Kikkatalo K, Hardardottir H, Hummel S, Hussain A, Kajantie E, Keikkala E, Khamis A, Lahti J, Lekva T, Mustaniemi S, Sommer C, Tagoma A, Tzala E, Uibo R, Vääräsmäki M, Villa PM, Birkeland KI, Bouchard L, Duijn CM, Finer S, Groop L, Hämäläinen E, Hayes GM, Hitman GA, Jang HC, Järvelin MR, Jenum AK, Laivuori H, Ma RC, Melander O, Oken E, Park KS, Perron P, Prasad RB, Qvigstad E, Sebert S, Stefansson K, Steinthorsdottir V, Tuomi T, Hivert MF, Franks PW, McCarthy MI, Lindgren CM, Freathy RM, Lawlor DA, Morris AP, Mägi R. Multi-ancestry genome-wide association study of gestational diabetes mellitus highlights genetic links with type 2 diabetes. Hum Mol Genet. 2022;31:3377-3391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 74. | Crudele L, Gadaleta RM, Cariello M, Moschetta A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBioMedicine. 2023;97:104821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 146] [Reference Citation Analysis (1)] |
| 75. | Guidotti G, Calabrese F, Anacker C, Racagni G, Pariante CM, Riva MA. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment. Neuropsychopharmacology. 2013;38:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 76. | Andersen ZJ, Raaschou-Nielsen O, Ketzel M, Jensen SS, Hvidberg M, Loft S, Tjønneland A, Overvad K, Sørensen M. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care. 2012;35:92-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 77. | Landin-Olsson M. Latent autoimmune diabetes in adults. Ann N Y Acad Sci. 2002;958:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Garcia-Gutierrez E, O'Mahony AK, Dos Santos RS, Marroquí L, Cotter PD. Gut microbial metabolic signatures in diabetes mellitus and potential preventive and therapeutic applications. Gut Microbes. 2024;16:2401654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 79. | Schulz MC, Sargis RM. Inappropriately sweet: Environmental endocrine-disrupting chemicals and the diabetes pandemic. Adv Pharmacol. 2021;92:419-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Kota SK, Meher LK, Jammula S, Krishna SV, Kota SK, Modi KD. Neuropsychiatric screening in type 2 diabetes mellitus. Indian J Endocrinol Metab. 2012;16 Suppl 1:S37-S40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 81. | Pilla SJ, Shahidzadeh Yazdi Z, Taylor SI. Individualized Glycemic Goals for Older Adults Are a Moving Target. Diabetes Care. 2022;45:1029-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Liu C, He L, Li Y, Yang A, Zhang K, Luo B. Diabetes risk among US adults with different socioeconomic status and behavioral lifestyles: evidence from the National Health and Nutrition Examination Survey. Front Public Health. 2023;11:1197947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 83. | Toren E, Burnette KS, Banerjee RR, Hunter CS, Tse HM. Partners in Crime: Beta-Cells and Autoimmune Responses Complicit in Type 1 Diabetes Pathogenesis. Front Immunol. 2021;12:756548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 84. | Sticht J, Álvaro-Benito M, Konigorski S. Type 1 Diabetes and the HLA Region: Genetic Association Besides Classical HLA Class II Genes. Front Genet. 2021;12:683946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 85. | Alves Abrantes JJP, Veríssimo de Azevedo JC, Fernandes FL, Duarte Almeida V, Custódio De Oliveira LA, Ferreira de Oliveira MT, Galvão De Araújo JM, Lanza DCF, Bezerra FL, Andrade VS, Araújo de Medeiros Fernandes TA, Fernandes JV. Viruses as a potential environmental trigger of type 1 diabetes mellitus (Review). Biomed Rep. 2024;20:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 86. | Suliman BA. Potential clinical implications of molecular mimicry-induced autoimmunity. Immun Inflamm Dis. 2024;12:e1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 87. | da Silva Rosa SC, Nayak N, Caymo AM, Gordon JW. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol Rep. 2020;8:e14607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 88. | Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 491] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 89. | Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 1240] [Article Influence: 310.0] [Reference Citation Analysis (0)] |
| 90. | Park SY, Gautier JF, Chon S. Assessment of Insulin Secretion and Insulin Resistance in Human. Diabetes Metab J. 2021;45:641-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 91. | Ye R, Onodera T, Scherer PE. Lipotoxicity and β Cell Maintenance in Obesity and Type 2 Diabetes. J Endocr Soc. 2019;3:617-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 92. | Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011;11:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 327] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 93. | Bacchetta R, Roncarolo MG. IPEX syndrome from diagnosis to cure, learning along the way. J Allergy Clin Immunol. 2024;153:595-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 94. | James EA, Joglekar AV, Linnemann AK, Russ HA, Kent SC. The beta cell-immune cell interface in type 1 diabetes (T1D). Mol Metab. 2023;78:101809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 95. | Yang Z, Zhang Z, Li L, Jing Z, Ma Y, Lan T, Li Y, Lin Z, Fang W, Zhang J, Zhang J, Liang X, Wu B, Zheng Y, Zhang X. Bioengineered Artificial Extracellular Vesicles Presenting PD-L1 and Gal-9 Ameliorate New-Onset Type 1 Diabetes. Diabetes. 2024;73:1325-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 96. | Herold KC, Krischer JP. The Pathogenesis of Type 1 Diabetes. Cold Spring Harb Perspect Med. 2025;15:a041623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 97. | Mancuso G, Bechi Genzano C, Fierabracci A, Fousteri G. Type 1 diabetes and inborn errors of immunity: Complete strangers or 2 sides of the same coin? J Allergy Clin Immunol. 2023;151:1429-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Wang X, Yuan W, Yang C, Wang Z, Zhang J, Xu D, Sun X, Sun W. Emerging role of gut microbiota in autoimmune diseases. Front Immunol. 2024;15:1365554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 99. | De Franco E. From Biology to Genes and Back Again: Gene Discovery for Monogenic Forms of Beta-Cell Dysfunction in Diabetes. J Mol Biol. 2020;432:1535-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Abdul-Ghani MA, DeFronzo RA. Mitochondrial dysfunction, insulin resistance, and type 2 diabetes mellitus. Curr Diab Rep. 2008;8:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 101. | Liu M, Huang Y, Xu X, Li X, Alam M, Arunagiri A, Haataja L, Ding L, Wang S, Itkin-Ansari P, Kaufman RJ, Tsai B, Qi L, Arvan P. Normal and defective pathways in biogenesis and maintenance of the insulin storage pool. J Clin Invest. 2021;131:e142240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 102. | Febbraio MA, Karin M. "Sweet death": Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021;33:2316-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 103. | Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13:465-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 104. | Zhu Q, Jia X, Li S, Feng J. Role of adiponectin and its receptors AdipoR1/2 in inflammatory bowel disease. Cell Commun Signal. 2025;23:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 105. | Dhatariya K. Initiation and Continuation of Sodium-Glucose Cotransporter 2 Inhibitors in Hospital Inpatients: Ready for Prime Time? Diabetes Care. 2022;45:2806-2807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 106. | Zhang T, Kim DH, Xiao X, Lee S, Gong Z, Muzumdar R, Calabuig-Navarro V, Yamauchi J, Harashima H, Wang R, Bottino R, Alvarez-Perez JC, Garcia-Ocaña A, Gittes G, Dong HH. FoxO1 Plays an Important Role in Regulating β-Cell Compensation for Insulin Resistance in Male Mice. Endocrinology. 2016;157:1055-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 107. | Wu J, Yang K, Fan H, Wei M, Xiong Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2023;14:1114424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 79] [Reference Citation Analysis (0)] |
| 108. | Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59:1396-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 454] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 109. | Kwak SH, Kim SH, Cho YM, Go MJ, Cho YS, Choi SH, Moon MK, Jung HS, Shin HD, Kang HM, Cho NH, Lee IK, Kim SY, Han BG, Jang HC, Park KS. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes. 2012;61:531-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 110. | Godfrey KM. The role of the placenta in fetal programming-a review. Placenta. 2002;23 Suppl A:S20-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 207] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 111. | Wicklow B, Retnakaran R. Gestational Diabetes Mellitus and Its Implications across the Life Span. Diabetes Metab J. 2023;47:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (1)] |
| 112. | Dias S, Pheiffer C, Adam S. The Maternal Microbiome and Gestational Diabetes Mellitus: Cause and Effect. Microorganisms. 2023;11:2217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 113. | Franzago M, Fraticelli F, Stuppia L, Vitacolonna E. Nutrigenetics, epigenetics and gestational diabetes: consequences in mother and child. Epigenetics. 2019;14:215-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 114. | Ruchat SM, Hivert MF, Bouchard L. Epigenetic programming of obesity and diabetes by in utero exposure to gestational diabetes mellitus. Nutr Rev. 2013;71 Suppl 1:S88-S94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (8)] |
| 115. | Neven ACH, Mousa A, Boyle JA, Teede HJ. Endocrine and metabolic interactions in healthy pregnancies and hyperinsulinemic pregnancies affected by polycystic ovary syndrome, diabetes and obesity. Front Endocrinol (Lausanne). 2022;13:993619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 116. | Ibrahim I, Bashir M, Singh P, Al Khodor S, Abdullahi H. The Impact of Nutritional Supplementation During Pregnancy on the Incidence of Gestational Diabetes and Glycaemia Control. Front Nutr. 2022;9:867099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Zhang H, Wang S, Tuo L, Zhai Q, Cui J, Chen D, Xu D. Relationship between Maternal Vitamin D Levels and Adverse Outcomes. Nutrients. 2022;14:4230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 118. | Niu Z, Habre R, Yang T, Grubbs BH, Eckel SP, Toledo-Corral CM, Johnston J, Dunton GF, Lurvey N, Al-Marayati L, Lurmann F, Pavlovic N, Bastain TM, Breton CV, Farzan SF. Preconceptional and prenatal exposure to air pollutants and risk of gestational diabetes in the MADRES prospective pregnancy cohort study. Lancet Reg Health Am. 2023;25:100575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 119. | Duncan BB, Magliano DJ, Boyko EJ. IDF diabetes atlas 11th edition 2025: global prevalence and projections for 2050. Nephrol Dial Transplant. 2025;gfaf177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 120. | Over 100 million Indians have diabetes, estimates ICMR-supported study. Health Report [Internet]. 2023 Jun 9 [cited 2025 Jun 9]. Available from: https://scroll.in/latest/1050659/over-100-million-indians-have-diabetes-estimates-icmr-supported-study. |
| 121. | Diabetes, hypertension strongly linked: study warns of twin epidemics. The Times of India [Internet]. 2025 May 17 [cited 2025 Jun 9]. Available from: https://article.wn.com/view-steel/2025/05/17/Diabetes_hypertension_strongly_linked_Study_warns_of_twin_ep/. |
| 122. | MedBound Times. India Home to a Quarter of the World’s Diabetics: Lancet Study Showing Rising Global Diabetes Rates. [cited 3 August 2025]. Available from: https://www.medboundtimes.com/medbound-blog/india-home-to-a-quarter-of-the-worlds-diabetics-lancet-study-showing-rising-global-diabetes-rates. |
| 123. | Venugopal V, Richa R, Singh D, Gautam A, Jahnavi G. National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases, and Stroke: A Scoping Review in the Context of Hypertension Prevention and Control in India. Indian J Public Health. 2023;67:S50-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 124. | India Today NE. WHO chief lauds India’s Ayushman Bharat Scheme. [cited 3 August 2025]. Available from: https://www.indiatodayne.in/national/story/who-chief-lauds-indias-ayushman-bharat-scheme-637412-2023-08-20?utm_source=itneweb_story_share. |
| 125. | Ministry of Health and Family Welfare. National List of Essential Medicines (NLEM), 2022. New Delhi: Government of India. 2022. Available from: https://www.mohfw.gov.in/?q=en/newshighlights-104. |
| 126. | Government of India. Pradhan Mantri Bhartiya Janaushadhi Pariyojana. New Delhi: Government of India. 2023. Available from: https://janaushadhi.gov.in. |
| 127. | Anjana RM, Deepa M, Pradeepa R. The ICMR-INDIAB Study: Results from the National Study on Diabetes in India. J Indian Inst Sci. 2023;103:21-32. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 128. | International Diabetes Federation. The Diabetes Atlas. 10th edition. Brussels: IDF; 2023. Available from: https://diabetesatlas.org. |
| 129. | Ministry of Electronics and Information Technology. National Family Health Survey (NFHS-5). [cited 3 August 2025]. Available from: https://www.data.gov.in/catalog/national-family-health-survey-nfhs-5. |
| 130. | Anjana RM, Unnikrishnan R, Deepa M, Pradeepa R, Tandon N, Das AK, Joshi S, Bajaj S, Jabbar PK, Das HK, Kumar A, Dhandhania VK, Bhansali A, Rao PV, Desai A, Kalra S, Gupta A, Lakshmy R, Madhu SV, Elangovan N. ICMR-INDIAB Collaborative Study Group (2023). Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol 2023; 11: 474–489. [cited 3 August 2025]. Available from: https://www.thelancet.com/journals/landia/article/PIIS2213-8587(23)00119-5/fulltext. |
| 131. | Anjana RM, Hannah W, Deepa M, Pradeepa R. Burden of non-communicable diseases in India: Findings from the ICMR-INDIAB study. Int J Diabetes Dev Ctries. 2024;44:635-643. [DOI] [Full Text] |
| 132. | Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, Nath LM, Das AK, Madhu SV, Rao PV, Shukla DK, Kaur T, Ali MK, Mohan V. The Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) study: methodological details. J Diabetes Sci Technol. 2011;5:906-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 133. | Mahajan A, Deshmane A, Muley A. A Comparative Study on the Consumption Patterns of Processed Food Among Individuals With and Without Type 2 Diabetes. Int J Public Health. 2025;70:1607931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 134. | National Family Health Survey. National Family Health Survey (NFHS-6)- 2023-24. [cited 3 August 2025]. Available from: http://www.nfhsiips.in/nfhsnew/nfhsuser/nfhs6.php. |
| 135. | Ministry of Health and Family Welfare. 2023-24 Annual Report. New Delhi: Government of India; 2024. [cited 3 August 2025]. Available from: https://www.mohfw.gov.in/sites/default/files/Annual%20Report%202023%2024%20DoHFW%20English_0.pdf.. |
| 136. | International Diabetes Federation. IDF Diabetes Atlas 11th edition, 2025. Brussels: IDF; 2025. [cited 3 August 2025]. Available from: https://diabetesatlas.org/media/uploads/sites/3/2025/04/IDF_Atlas_11th_Edition_2025.pdf.. |
| 137. | Government of Goa. Annual NCD surveillance report. Goa: Government of Goa, 2025. [cited 3 August 2025]. Available from: https://dhs.goa.gov.in/cms-gov-be/public/uploads/cms_admin_files/Citizen-charter-2024.pdf.. |
| 138. | Makkar B, Agarwal S, Vishwanathan V, Sahay R, Maheshwari A, Sharma JK, Jethwani P, Ghosh S, J. A, Sreenivasamurthy L, Chawla M, Singh NK, Shunmugavelu M, Gupta A, Kesavadev J, Parikh R, Chawla R, Seshadri K, Saboo B, Virmani A, Deshpande N, Gupta S, Kapoor N. RSSDI white paper on diabetes: current status, challenges, and future vision. Int J Diabetes Dev Ctries. 2025;45:256-260. [DOI] [Full Text] |
| 139. | International Diabetes Federation. IDF Diabetes Atlas 11th edition 2025. Brussels: IDF; 2025. [cited 3 August 2025]. Available from: https://idf.org/about-diabetes/diabetes-facts-figures/. |
| 140. | Ministry of Health and Family Welfare. NFHS-6 Punjab state report 2024. New Delhi: Government of India; 2024. Available from: https://www.nfhsiips.in/nfhsuser/nfhs6.php. |
| 141. | Imai T, Funahashi H, Sato Y, Nozaki H, Asano M, Ueda M, Takagi H. Multiple functioning paraganglioma associated with polycythemia. J Surg Oncol. 1988;39:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 142. | International Diabetes Federation. The Diabetes Atlas. [cited 3 August 2025]. Available from: https://diabetesatlas.org/#:~:text=to%20tackle%20it.-,589%20million%20adults,3. |
| 143. | Ministry of Health and Family Welfare Government of India. National Programme for Prevention and Control of Non-Communicable Diseases. [cited 3 August 2025]. Available from: https://www.mohfw.gov.in/sites/default/files/NP-NCD%20Operational%20Guidelines_0.pdf.. |
| 144. | Kumar A. The Transformation of The Indian Healthcare System. Cureus. 2023;15:e39079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 145. | Ghammari F, Khodayari-Zarnaq R, Jalilian H, Gholizadeh M. Barriers to health care utilization among patients with type 2 diabetes living in slums: a qualitative study from providers' perspective. Glob Health Res Policy. 2023;8:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 146. | World Health Organization. Diabetes Country Profile: India. New Delhi: WHO; 2022. Available from: https://www.who.int/india. |
| 147. | Muralidharan S. Diabetes and current Indian scenario: A narrative review. JODB. 2024;15:12-17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 148. | Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217-230. [PubMed] |
| 149. | International Diabetes Federation. IDF South-East Asia. Brussels: IDF; 2023. Available from: https://idf.org/our-network/regions-and-members/south-east-asia/. |
| 150. | Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, Adhikari P, Rao PV, Saboo B, Kumar A, Bhansali A, John M, Luaia R, Reang T, Ningombam S, Jampa L, Budnah RO, Elangovan N, Subashini R, Venkatesan U, Unnikrishnan R, Das AK, Madhu SV, Ali MK, Pandey A, Dhaliwal RS, Kaur T, Swaminathan S, Mohan V; ICMR–INDIAB Collaborative Study Group. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 485] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 151. | Ranasinghe P, Rathnayake N, Wijayawardhana S, Jeyapragasam H, Meegoda VJ, Jayawardena R, Misra A. Rising trends of diabetes in South Asia: A systematic review and meta-analysis. Diabetes Metab Syndr. 2024;18:103160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 152. | Swain SP, Samal S, Sahu KS, Rout SK. Out-of-pocket expenditure and drug adherence of patients with diabetes in Odisha. J Family Med Prim Care. 2018;7:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Goswami P, Anand A. Impact of diabetes on healthcare utilization and expenditure among older adults in India. J Diabetes Metab Disord. 2024;23:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 154. | Prasanna Kumar KM, Chowdhury S, Bantwal G, Unnikrishnan AG, Kalra S, Aggarwal S, Singh AK, Pandit K, Shukla R, Vishwanathan V, Khobragade K, Sarda PS. Insulin Access Enhancement in India: Expert Views on Integrating Interchangeable Biosimilar Insulin Glargine. Cureus. 2024;16:e60983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 155. | Kim B, Lee HS, Kim HJ, Lee H, Lee IY, Ock S, Kwon S, Kang SS, Choi Y. Momordica charantia (bitter melon) efficacy and safety on glucose metabolism in Korean prediabetes participants: a 12-week, randomized clinical study. Food Sci Biotechnol. 2023;32:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 156. | Shabil M, Bushi G, Bodige PK, Maradi PS, Patra BP, Padhi BK, Khubchandani J. Effect of Fenugreek on Hyperglycemia: A Systematic Review and Meta-Analysis. Medicina (Kaunas). 2023;59:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 157. | Patil SM, Shirahatti PS, Ramu R. Azadirachta indica A. Juss (neem) against diabetes mellitus: a critical review on its phytochemistry, pharmacology, and toxicology. J Pharm Pharmacol. 2022;74:681-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 158. | Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, Ern LY, Ashraf NA, Kit SW, Yee TS, Pichika MR, Gorain B, Kesharwani P. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J Tradit Complement Med. 2018;8:361-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 159. | Mohanty P, Kishore J, Acharya GC, Mohanty I, Patnaik L, Bhowmik B, Sahoo M, Satpathy N, Sahoo PK, Jena PK. Utilization of Ayurveda, Yoga, Naturopathy, Unani, Siddha, and Homoeopathy (AYUSH) Practitioners' Services Among Older Adults: Results From the Longitudinal Aging Study in India. Cureus. 2024;16:e62192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 160. | American Diabetes Association Professional Practice Committee. 1. Improving Care and Promoting Health in Populations: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S11-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 98] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 161. | Leung L, Birtwhistle R, Kotecha J, Hannah S, Cuthbertson S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): a mini review. Br J Nutr. 2009;102:1703-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 162. | Haxhiraj M, White K, Terry C. The Role of Fenugreek in the Management of Type 2 Diabetes. Int J Mol Sci. 2024;25:6987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 163. | Devangan S, Varghese B, Johny E, Gurram S, Adela R. The effect of Gymnema sylvestre supplementation on glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Phytother Res. 2021;35:6802-6812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 164. | Suvarna R, Shenoy RP, Hadapad BS, Nayak AV. Effectiveness of polyherbal formulations for the treatment of type 2 Diabetes mellitus - A systematic review and meta-analysis. J Ayurveda Integr Med. 2021;12:213-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 165. | Saito K, Inoue T, Ariyasu H, Shimada T, Itoh H, Tanaka I, Terao C. Usefulness of subclassification of adult diabetes mellitus among inpatients in Japan. J Diabetes Investig. 2022;13:706-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 166. | Ee CC, Al-Kanini I, Armour M, Piya MK, McMorrow R, Rao VS, Naidoo D, Metzendorf MI, Kroeger CM, Sabag A. Mindfulness-based interventions for adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Integr Med Res. 2025;14:101138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 167. | Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1320] [Cited by in RCA: 1548] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 168. | Vidal J. Updated review on the benefits of weight loss. Int J Obes Relat Metab Disord. 2002;26 Suppl 4:S25-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 169. | Gallwitz B. Clinical Use of DPP-4 Inhibitors. Front Endocrinol (Lausanne). 2019;10:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 170. | Turner S, Diako C, Kruger R, Wong M, Wood W, Rutherfurd-Markwick K, Ali A. Consuming Gymnema sylvestre Reduces the Desire for High-Sugar Sweet Foods. Nutrients. 2020;12:1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 171. | Nain P, Saini V, Sharma S, Nain J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J Ethnopharmacol. 2012;142:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 172. | Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs. 2009;69:1777-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 370] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 173. | World Health Organization. WHO global report on traditional and complementary medicine 2019. [cited 3 August 2025]. Available from: https://www.who.int/publications/i/item/978924151536. |
| 174. | Central Council for Research in Ayurvedic Sciences (CCRAS). Annual Report 2022–2023. New Delhi: Ministry of AYUSH, Government of India; 2023. [cited 3 August 2025]. Available from: https://ccras.nic.in/annual-report-2022-2023/. |
| 175. | Council of Scientific and Industrial Research (CSIR), Ministry of AYUSH. Integrative Health Research and Innovation Report. New Delhi: Government of India; 2023. [cited 3 August 2025]. Available from: https://www.csir.res.in/. |
| 176. | Verma R, Khanna P, Mehta B. National programme on prevention and control of diabetes in India: Need to focus. Australas Med J. 2012;5:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 177. | Fatima A, Agrawal P, Singh PP. Herbal option for diabetes: an overview. Asian Pac J. Trop Dis. 2012;2:S536-S544. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 178. | Kesavadev J, Krishnan G, Mohan V. Digital health and diabetes: experience from India. Ther Adv Endocrinol Metab. 2021;12:20420188211054676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 179. | Mohan RR, Vadiraja HS, Moirangthem G, Sandhu S, Rao V, Rao M, Gupta R, Anburani S, Bendore P. Impact assessment of International Day of Yoga: results from a cross-sectional survey. CCRYN Indian Journal of Yoga & Naturopathy. 2024;1:24-29. [DOI] [Full Text] |