Published online Jan 15, 2026. doi: 10.4239/wjd.v17.i1.115685
Revised: November 12, 2025
Accepted: November 28, 2025
Published online: January 15, 2026
Processing time: 84 Days and 3.4 Hours
Recent real-world evidence suggests that improved regulation of metabolic and inflammatory pathways can substantially lower the risk of periodontal compli
Core Tip: Periodontitis is a frequent but often overlooked complication of type 2 dia
- Citation: Tang HW, Zhang N. Improving metabolic and inflammatory balance prevents periodontal complications in diabetes. World J Diabetes 2026; 17(1): 115685
- URL: https://www.wjgnet.com/1948-9358/full/v17/i1/115685.htm
- DOI: https://dx.doi.org/10.4239/wjd.v17.i1.115685
Persistent hyperglycaemia, insulin resistance, and chronic low-grade inflammation in type 2 diabetes (T2D) create a systemic environment that renders periodontal tissues particularly vulnerable[1]. Periodontitis has long been recognised as one of the most frequent yet underestimated complications of T2D[2]. Epidemiological evidence shows that patients with diabetes have a substantially higher risk of developing periodontitis and often present with more severe periodontal destruction than non-diabetic individuals[2].
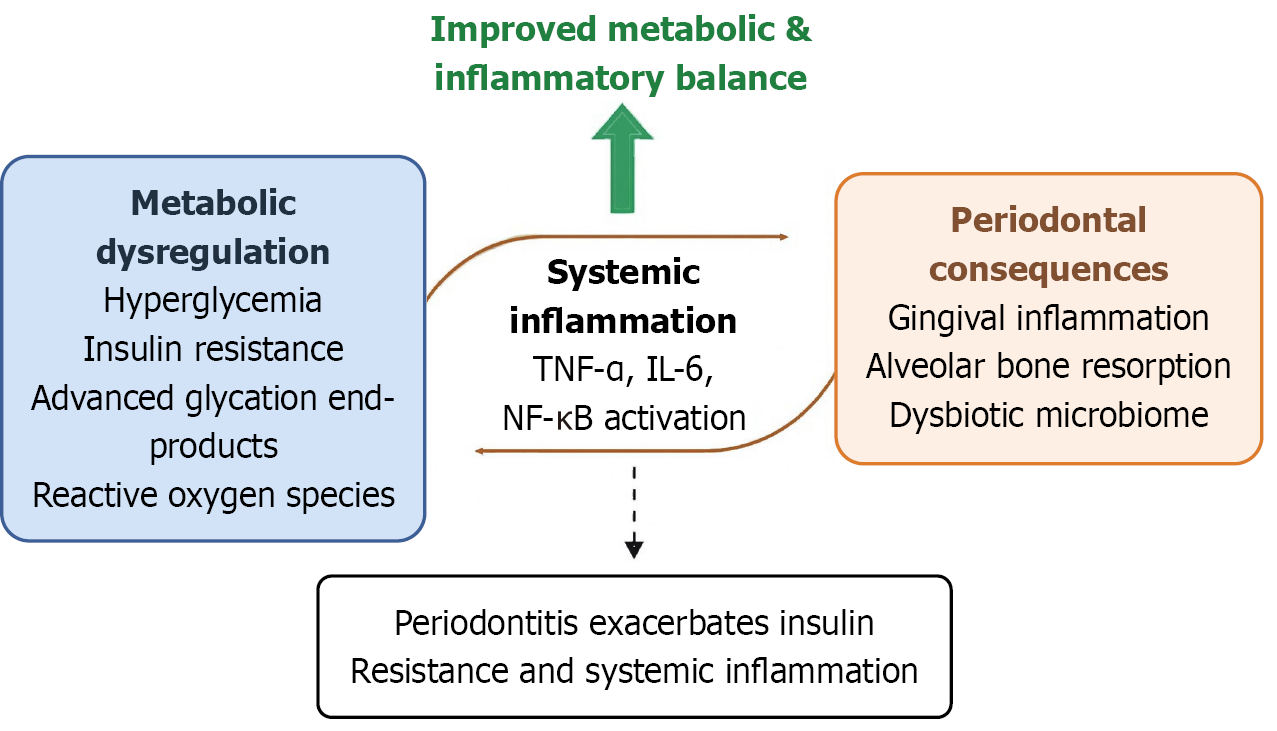
In diabetes, the accumulation of advanced glycation end-products, oxidative stress, endothelial dysfunction, and immune dysregulation collectively impair host-microbiome homeostasis in periodontal tissues[3]. Conversely, chronic periodontal inflammation can aggravate systemic metabolic dysfunction through the release of pro-inflammatory cytokines (interleukin-1 beta, tumor necrosis factor-alpha, interleukin-6), activation of nuclear factor kappa B, and increased insulin resistance[4]. This bidirectional link between diabetes and periodontal disease underscores the need for integrated management approaches that address both metabolic and inflammatory components of the disease process.
This linkage has been supported by multiple types of data, including real-world clinical cohort studies, experimental models, and molecular mechanistic analyses. A recent large-scale real-world cohort study by Lin et al[5] reported that long-term adjunctive therapy in T2D was associated with approximately a 52% lower incidence of periodontitis and reduced periodontitis-related ambulatory visits. These findings highlight the under-appreciated interface between metabolic regulation and oral inflammatory disease, suggesting that sustained metabolic-inflammatory balance may provide tangible benefits beyond glycaemic control alone.
Evidence from animal experiments, cellular studies, and multi-omics analyses further illustrates the biological mechanisms underlying this linkage. Improving metabolic and inflammatory balance may protect periodontal tissues through several complementary biological mechanisms (Figure 1). First, tighter glycaemic control reduces advanced glycation end-product accumulation, inhibits nuclear factor kappa B signaling, and limits cytokine overexpression within gingival tissues. Second, alleviating oxidative stress enhances endothelial microcirculation and neutrophil function, restoring immune homeostasis and reducing tissue breakdown[6]. Third, stabilising systemic inflammatory status may support a more balanced subgingival microbiome, mitigating dysbiosis and alveolar bone loss[7]. Together, these mechanisms provide a biological rationale for the observed reduction in periodontal complications with improved metabolic control.
The results from Lin et al[5] and related studies support the integration of oral health into standard diabetes care frameworks. Endocrinologists and diabetes-care teams should routinely screen for oral symptoms and encourage preventive dental evaluations, while dental professionals should consider systemic metabolic and inflammatory status when planning periodontal therapy. This interdisciplinary collaboration can promote early detection, optimise treatment outcomes, and reduce healthcare utilisation and costs[8]. In addition, health-system planners should recognise that preventive metabolic-inflammatory interventions may decrease the burden of both metabolic and oral disease, sup
Although compelling, current evidence remains primarily observational. Future studies should aim to: (1) Establish causality through prospective interventional trials comparing standard and integrative diabetes care models; (2) Utilise multi-omics and systems-biology approaches to identify key immunometabolism biomarkers that predict oral complications; and (3) Evaluate implementation and cost-effectiveness of interdisciplinary management strategies in different healthcare contexts[9]. Such research will help define precise clinical targets and further elucidate the shared molecular pathways linking metabolism, inflammation, and oral health.
Growing evidence links metabolic-inflammatory balance with periodontal health in T2D, underscoring the need for a holistic approach to management. Periodontitis should be viewed as a systemic consequence of chronic metabolic and inflammatory dysregulation. Integrative interventions that target both glucose and inflammation may help prevent oral complications and improve overall patient outcomes.
| 1. | Jansson H, Lindholm E, Lindh C, Groop L, Bratthall G. Type 2 diabetes and risk for periodontal disease: a role for dental health awareness. J Clin Periodontol. 2006;33:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Turkkahraman H, Flanagan S, Zhu T, Akel N, Marino S, Ortega-Gonzalez D, Yuan X, Bellido T. Sclerostin antibody corrects periodontal disease in type 2 diabetic mice. JCI Insight. 2024;9:e181940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Tavares BS, Tsosura TVS, Mattera MSLC, Santelli JO, Belardi BE, Chiba FY, Cintra LTA, Silva CC, Matsushita DH. Effects of melatonin on insulin signaling and inflammatory pathways of rats with apical periodontitis. Int Endod J. 2021;54:926-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Sanz M, Ceriello A, Buysschaert M, Chapple I, Demmer RT, Graziani F, Herrera D, Jepsen S, Lione L, Madianos P, Mathur M, Montanya E, Shapira L, Tonetti M, Vegh D. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45:138-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 427] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 5. | Lin WW, Yen CT, Livneh H, Huang HL, Lu MC, Chen WJ, Tsai TY. Real-world evidence for herbal medicine benefit in 9728 type 2 diabetes patients-peridonotitis risk and ambulatory care utilization. World J Diabetes. 2025;16:112171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Plachokova AS, Andreu-Sánchez S, Noz MP, Fu J, Riksen NP. Oral Microbiome in Relation to Periodontitis Severity and Systemic Inflammation. Int J Mol Sci. 2021;22:5876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Kawamoto D, Borges R, Ribeiro RA, de Souza RF, Amado PPP, Saraiva L, Horliana ACRT, Faveri M, Mayer MPA. Oral Dysbiosis in Severe Forms of Periodontitis Is Associated With Gut Dysbiosis and Correlated With Salivary Inflammatory Mediators: A Preliminary Study. Front Oral Health. 2021;2:722495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Adedokun SD, Sarwar M, Hwang K, Hans A, Baskaran J, Anantha Narayanan M. Outcomes of lower extremity peripheral arterial interventions in patients with and without chronic kidney disease or end-stage renal disease. J Cardiovasc Surg (Torino). 2023;64:624-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Giger OF, Fleisch E, Jovanova M, Kowatsch T. Barriers and facilitators of implementing value-based care: The case of SwissDiabeter. Digit Health. 2025;11:20552076251336322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/