Published online Jan 15, 2026. doi: 10.4239/wjd.v17.i1.114618
Revised: November 8, 2025
Accepted: December 3, 2025
Published online: January 15, 2026
Processing time: 112 Days and 15.5 Hours
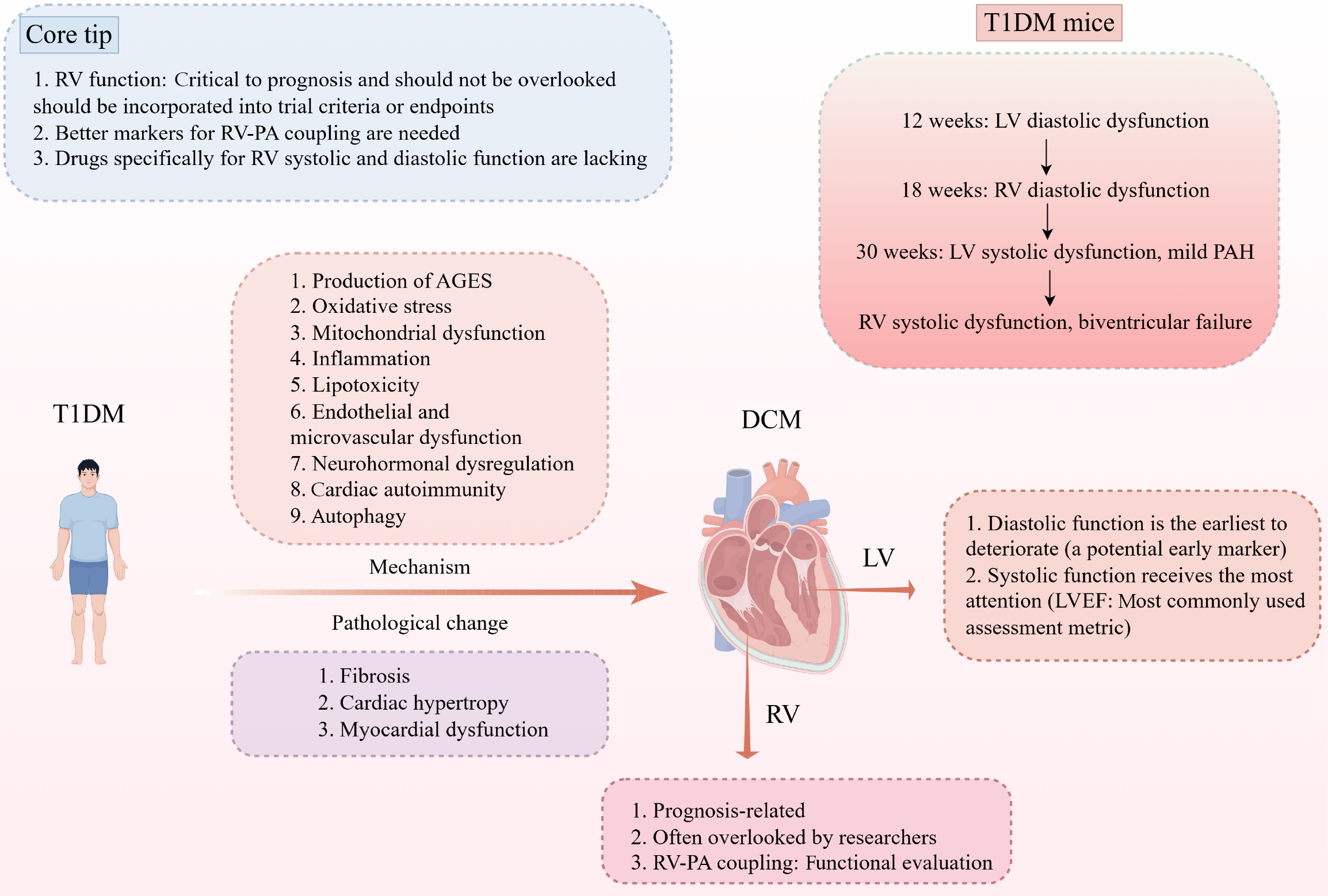
While left ventricular (LV) impairment in diabetic cardiomyopathy is well recognized, the contribution of right ventricular (RV) dysfunction has received far less attention. In their longitudinal investigation, Yu et al systematically examined RV and LV performance in a type 1 diabetic mouse model and demonstrated that RV diastolic dysfunction develops later than LV abnormalities, coinciding with structural remodeling marked by fibrosis, hypertrophy, and mild pulmonary hypertension. These observations underscore the progressive yet distinct tra
Core Tip: In type 1 diabetic cardiomyopathy, right ventricular (RV) function is a key determinant of patient prognosis and must not be neglected. RV performance should be incorporated into the inclusion criteria and endpoint selection of clinical trials. There is a critical need for more robust surrogate markers to evaluate RV-pulmonary arterial coupling. Additionally, the development of therapies that directly improve RV systolic and diastolic function is urgently required.
- Citation: Luo LY, Liu ZX, Yang TS, Liang W, Luo XL. Right ventricular dysfunction in type 1 diabetic cardiomyopathy: An overlooked component? World J Diabetes 2026; 17(1): 114618
- URL: https://www.wjgnet.com/1948-9358/full/v17/i1/114618.htm
- DOI: https://dx.doi.org/10.4239/wjd.v17.i1.114618
Diabetes is a multifaceted metabolic disease that is clinically defined by elevated circulating glucose levels[1]. In type 1 diabetes mellitus (T1DM), disturbances in glucose regulation arise primarily from impaired insulin secretion and dysregulated immune responses[2]. Diabetic cardiomyopathy (DCM), a major chronic complication of diabetes, is characterized by cardiac hypertrophy, interstitial fibrosis, cardiomyocyte apoptosis, and ensuing diastolic and/or systolic dysfunction that can ultimately lead to heart failure (HF)[3]. Although most previous research has centered on left ventricular (LV) abnormalities, Yu et al[4] innovatively delineated the landscape of biventricular injury in a T1DM mouse model. Building on these findings, this letter underscores the importance of recognizing right ventricular (RV) involvement as an integral component of T1DM-induced cardiomyopathy, rather than a secondary or incidental feature. A deeper understanding of RV pathophysiology in diabetes is crucial for refining diagnostic strategies, improving risk stratification, and ultimately developing targeted interventions that address the full spectrum of biventricular dysfunction in diabetic patients.
During embryogenesis, the RV originates from the secondary heart field and develops as a crescent-shaped, thin-walled chamber[5]. Anatomically and functionally, it remains tightly integrated with the left ventricle via the interventricular septum[6]. In 1943, studies by Starr et al[7] suggested that the RV contributed minimally to the clinical picture of HF. However, accumulating evidence over subsequent decades has firmly established the RV as a key determinant of cardiovascular pathophysiology and patient outcomes across a wide range of conditions. For instance, Obokata et al[8] reported that among 271 patients with HF with preserved ejection fraction (HFpEF), those who developed new-onset RV dysfunction during a median 4-year follow-up nearly doubled their risk of mortality (adjusted hazard ratio = 1.89, 95% confidence interval: 1.01-3.44; P = 0.04). Frea et al[9] further demonstrated that estimated right atrial pressure and the RV contractile pressure index serve as strong predictors of in-hospital and short-term outcomes in individuals with advanced acute decompensated chronic HF. Complementing these findings, a meta-analysis of 108 studies by Kitano et al[10] showed that RV ejection fraction remains significantly associated with prognosis in both dilated and hypertrophic cardiomyopathy even after correcting for bias. Similarly, Sayour et al[11], through a meta-analysis of 10 studies, found that RV ejection fraction exhibits a stronger prognostic association than other commonly used RV parameters, including tricuspid annular plane systolic excursion, fractional area change, and free-wall longitudinal strain in cardiopulmonary disorders.
Although much of the existing research on DCM focuses on LV systolic and diastolic impairment, emerging evidence indicates that RV remodeling can also develop in patients with diabetes or even prediabetes, independent of other comor
The mechanisms linking T1DM to HF are multifactorial and remain incompletely understood. Julián et al[20] outlined several major pathogenic pathways through which T1DM contributes to HF development, including chronic hyper
Regarding RV involvement in T1DM, early evidence dates back to 2007 when Karamitsos et al[22] evaluated echocardiograms from 66 patients with T1DM and 66 age- and sex-matched healthy controls, showing that biventricular diastolic dysfunction, particularly impaired myocardial relaxation appears before overt systolic impairment develops in T1DM. These changes were attributed to ventricular interdependence and the global impact of diabetes on myocardial per
Currently, there are no pharmacological agents specifically designed to improve RV systolic or diastolic function. Current clinical approaches to managing RV remodeling rely on three main strategies. The first is reducing RV load, which includes preload reduction with diuretics and afterload reduction using calcium channel blockers, inhaled vasodilators, endothelin receptor antagonists, prostacyclin analogs, phosphodiesterase-5 inhibitors, and soluble guanylate cyclase stimulators[26,27]. The second strategy focuses on augmenting RV contractility, employing β1-adrenergic agonists, phosphodiesterase-3 inhibitors, vasopressors, or mechanical circulatory support when necessary[28,29]. The third cornerstone of therapy is addressing the underlying disease process to halt or reverse continued RV deterioration.
The cardiovascular benefits of sodium-glucose co-transporter 2 inhibitors (SGLT2i) and GLP-1 receptor agonists (GLP1-RA) are increasingly recognized. The STEP-HFpEF trial showed that semaglutide, a GLP1-RA, reduced RV enlargement in patients with obesity- or diabetes-related HFpEF[30]. In contrast, the EMPA-HEART CardioLink-6 study found no effect of empagliflozin, an SGLT2i, on RV structure, including RV mass, in individuals with diabetes and coronary artery disease[31]. A meta-analysis of 23 studies similarly concluded that SGLT2i do not meaningfully improve RV structure or function[32]. Collectively, these findings suggest that GLP1-RA may offer greater promise than SGLT2i for modulating RV remodeling in DCM.
In summary, RV diastolic dysfunction can emerge early during T1DM-induced DCM, underscoring the importance of recognizing RV involvement rather than focusing solely on LV abnormalities. RV structural and functional remodeling has strong prognostic implications in DCM, yet the current tools and biomarkers used to evaluate RV performance remain limited in accuracy and sensitivity. This highlights an urgent need for more refined and reliable surrogate markers, particularly those capable of assessing RV-PA coupling in both clinical practice and experimental research. Therefore, advancing these assessment methods will not only improve risk stratification but may also uncover specific mechanistic pathways driving RV vulnerability in diabetes. Furthermore, equally important is the development of therapeutic strategies that directly target RV systolic and diastolic dysfunction. Overall, such interventions could meaningfully alter disease progression and ultimately enhance outcomes for patients with DCM.
| 1. | Ziegler AG. The countdown to type 1 diabetes: when, how and why does the clock start? Diabetologia. 2023;66:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 2. | Atkinson MA, Mirmira RG. The pathogenic "symphony" in type 1 diabetes: A disorder of the immune system, β cells, and exocrine pancreas. Cell Metab. 2023;35:1500-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 3. | Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res. 2018;122:624-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1363] [Article Influence: 194.7] [Reference Citation Analysis (0)] |
| 4. | Yu JJ, Han JG, Tan Y, Xu JX, LeBlanc A, Keller BB, Huang J, Cai L. Right ventricular dysfunctions in type 1 diabetic mice: A longitudinal study. World J Diabetes. 2025;16:109526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:1463-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 528] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 6. | Naeije R, Badagliacca R. The overloaded right heart and ventricular interdependence. Cardiovasc Res. 2017;113:1474-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 7. | Starr I, Jeffers WA, Meade RH. The absence of conspicuous increments of venous pressure after severe damage to the right ventricle of the dog, with a discussion of the relation between clinical congestive failure and heart disease. Am Heart J. 1943;26:291-301. [RCA] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 325] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J. 2019;40:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 242] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 9. | Frea S, Pidello S, Bovolo V, Iacovino C, Franco E, Pinneri F, Galluzzo A, Volpe A, Visconti M, Peirone A, Morello M, Bergerone S, Gaita F. Prognostic incremental role of right ventricular function in acute decompensation of advanced chronic heart failure. Eur J Heart Fail. 2016;18:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Kitano T, Bartoš F, Nabeshima Y, Sayour AA, Kovács A, Takeuchi M. Impact of cardiovascular magnetic resonance-derived right ventricular ejection fraction on adverse outcomes: A robust Bayesian model-averaged meta-analysis. J Cardiovasc Magn Reson. 2024;26:101118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Sayour AA, Tokodi M, Celeng C, Takx RAP, Fábián A, Lakatos BK, Friebel R, Surkova E, Merkely B, Kovács A. Association of Right Ventricular Functional Parameters With Adverse Cardiopulmonary Outcomes: A Meta-analysis. J Am Soc Echocardiogr. 2023;36:624-633.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Linssen PBC, Veugen MGJ, Henry RMA, van der Kallen CJH, Kroon AA, Schram MT, Brunner-La Rocca HP, Stehouwer CDA. Associations of (pre)diabetes with right ventricular and atrial structure and function: the Maastricht Study. Cardiovasc Diabetol. 2020;19:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Widya RL, van der Meer RW, Smit JW, Rijzewijk LJ, Diamant M, Bax JJ, de Roos A, Lamb HJ. Right ventricular involvement in diabetic cardiomyopathy. Diabetes Care. 2013;36:457-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Houston BA, Brittain EL, Tedford RJ. Right Ventricular Failure. N Engl J Med. 2023;388:1111-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 171] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 15. | Tampakakis E, Shah SJ, Borlaug BA, Leary PJ, Patel HH, Miller WL, Kelemen BW, Houston BA, Kolb TM, Damico R, Mathai SC, Kasper EK, Hassoun PM, Kass DA, Tedford RJ. Pulmonary Effective Arterial Elastance as a Measure of Right Ventricular Afterload and Its Prognostic Value in Pulmonary Hypertension Due to Left Heart Disease. Circ Heart Fail. 2018;11:e004436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Tello K, Wan J, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Mohajerani E, Seeger W, Herberg U, Sommer N, Gall H, Richter MJ. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ Cardiovasc Imaging. 2019;12:e009047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 17. | Tello K, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Seeger W, Wilhelm J, Gall H, Richter MJ. Cardiac Magnetic Resonance Imaging-Based Right Ventricular Strain Analysis for Assessment of Coupling and Diastolic Function in Pulmonary Hypertension. JACC Cardiovasc Imaging. 2019;12:2155-2164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | Kovács A, Magunia H, Nicoara A, Oxborough D, Keller M, Augustine DX, Thijssen D, van Dijk A, Denault A, Haddad F, Surkova E. Challenges and opportunities in assessing right ventricular structure and function: a Roadmap for standardization, clinical implementation and research. Nat Rev Cardiol. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Narang A, Bae R, Hong H, Thomas Y, Surette S, Cadieu C, Chaudhry A, Martin RP, McCarthy PM, Rubenson DS, Goldstein S, Little SH, Lang RM, Weissman NJ, Thomas JD. Utility of a Deep-Learning Algorithm to Guide Novices to Acquire Echocardiograms for Limited Diagnostic Use. JAMA Cardiol. 2021;6:624-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (4)] |
| 20. | Julián MT, Pérez-Montes de Oca A, Julve J, Alonso N. The double burden: type 1 diabetes and heart failure-a comprehensive review. Cardiovasc Diabetol. 2024;23:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 21. | Luo L, Zuo Y, Dai L. Metabolic rewiring and inter-organ crosstalk in diabetic HFpEF. Cardiovasc Diabetol. 2025;24:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | Karamitsos TD, Karvounis HI, Dalamanga EG, Papadopoulos CE, Didangellos TP, Karamitsos DT, Parharidis GE, Louridas GE. Early diastolic impairment of diabetic heart: the significance of right ventricle. Int J Cardiol. 2007;114:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Khokhlova A, Myachina T, Volzhaninov D, Butova X, Kochurova A, Berg V, Gette I, Moroz G, Klinova S, Minigalieva I, Solovyova O, Danilova I, Sokolova K, Kopylova G, Shchepkin D. Type 1 Diabetes Impairs Cardiomyocyte Contractility in the Left and Right Ventricular Free Walls but Preserves It in the Interventricular Septum. Int J Mol Sci. 2022;23:1719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Polson S, Thornburg J, McNair B, Cook C, Straight E, Fontana K, Hoopes C, Nair S, Bruns DR. Right ventricular dysfunction in preclinical models of type I and type II diabetes. Can J Physiol Pharmacol. 2025;103:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Hao PP, Yang JM, Zhang MX, Zhang K, Chen YG, Zhang C, Zhang Y. Angiotensin-(1-7) treatment mitigates right ventricular fibrosis as a distinctive feature of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;308:H1007-H1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Hassoun PM. Pulmonary Arterial Hypertension. N Engl J Med. 2021;385:2361-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 445] [Article Influence: 89.0] [Reference Citation Analysis (2)] |
| 27. | Corrigendum to: 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur Heart J. 2023;44:1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 28. | Kapur NK, Esposito ML, Bader Y, Morine KJ, Kiernan MS, Pham DT, Burkhoff D. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation. 2017;136:314-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 263] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 29. | O'Rourke RA, Dell'Italia LJ. Diagnosis and management of right ventricular myocardial infarction. Curr Probl Cardiol. 2004;29:6-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Solomon SD, Ostrominski JW, Wang X, Shah SJ, Borlaug BA, Butler J, Davies MJ, Kitzman DW, Verma S, Abildstrøm SZ, Nygaard Einfeldt M, Rasmussen S, Abhayaratna WP, Ahmed FZ, Ben-Gal T, Chopra V, Ito H, Merkely B, Núñez J, Senni M, van der Meer P, Wolf D, Petrie MC, Kosiborod MN; STEP-HFpEF Trial Committees and Investigators. Effect of Semaglutide on Cardiac Structure and Function in Patients With Obesity-Related Heart Failure. J Am Coll Cardiol. 2024;84:1587-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 74] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 31. | Sarak B, Verma S, David Mazer C, Teoh H, Quan A, Gilbert RE, Goodman SG, Bami K, Coelho-Filho OR, Ahooja V, Deva DP, Garg V, Gandhi S, Connelly KA, Yan AT. Impact of empagliflozin on right ventricular parameters and function among patients with type 2 diabetes. Cardiovasc Diabetol. 2021;20:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 32. | Leo I, Salerno N, Figliozzi S, Cersosimo A, Ielapi J, Stankowski K, Bisaccia G, Dellegrottaglie S, Canino G, De Rosa S, Sorrentino S, Bucciarelli-Ducci C, Torella D. Effect of SGLT2 inhibitors on cardiac structure and function assessed by cardiac magnetic resonance: a systematic review and meta-analysis. Cardiovasc Diabetol. 2025;24:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/