Published online Jan 15, 2026. doi: 10.4239/wjd.v17.i1.111847
Revised: September 30, 2025
Accepted: November 19, 2025
Published online: January 15, 2026
Processing time: 161 Days and 0.3 Hours
Diabetic foot ulcers (DFUs), a common and severe long-term complication of diabetes, are associated with high rates of amputation and mortality. Transverse tibial bone transport (TTT) has recently been applied in DFU management to accelerate wound healing.
To explore the potential mechanism through which TTT aids in the treatment of DF, with a special focus on the role of postoperative distraction osteogenesis and angiogenesis in the affected limb.
Fifteen patients with DFUs (Wagner grades 2-5) treated with TTT were enrolled. Pain intensity was assessed using the Visual Analog Scale (VAS), and skin tem
Complete wound healing occurred in all patients, with a mean healing duration of 10.1 ± 3.7 weeks. The rege
In patients with DR, TTT appears to promote ulcer healing by enhancing limb blood circulation. This effect is associated with distraction osteogenesis at the surgical site, which supports collateral angiogenesis. These findings suggest a relationship among distraction osteogenesis, local angiogenesis, and improved tissue perfusion that may contribute to ulcer healing; however, the causal mechanisms underlying this relationship remain unclear.
Core Tip: Current treatment options for diabetic foot (DF) primarily involve glycemic control and surgical intervention; however, these approaches do not address the underlying pathological state of ischemia and hypoxia. Consequently, many patients with refractory DF ultimately require amputation. Transverse tibial bone transport (TTT), initially developed to correct limb deformities, has shown favorable outcomes in research studies. This technique has also been widely applied in managing chronic osteomyelitis due to its advantages, including minimal invasiveness, enhancement of microcirculation, and reconstruction of both osseous and soft tissue defects. This study explored the effects of TTT on treatment outcomes in patients with DF ulcers.
- Citation: Liao MM, Zhang F, Wang YK, Wang MW, Cao JR, Jin ZH, Ren YJ, Chen S. Transverse tibial bone transport promotes distraction osteogenesis and improves blood flow in the management of diabetic foot. World J Diabetes 2026; 17(1): 111847
- URL: https://www.wjgnet.com/1948-9358/full/v17/i1/111847.htm
- DOI: https://dx.doi.org/10.4239/wjd.v17.i1.111847
Diabetic foot (DF), a severe complication of diabetes mellitus (DM), is associated with a 5-year mortality rate approaching 50%[1]. Current management strategies are multifaceted, emphasizing blood sugar control and local wound care. In most cases classified as Wagner grades 1 and 2, wound healing can be achieved through measures such as blood glucose regulation, regular dressing changes, and vacuum-assisted wound closure. However, in cases classified above Wagner grade 3, the efficacy of conventional treatment methods remains suboptimal.
Following its use in lower extremity ischemic diseases, transverse tibial bone transport (TTT) was first applied for DF by Professor Hua Qikai’s team at the First Affiliated Hospital of Guangxi Medical University (Guangxi, China) in 2014, with encouraging results. Since then, the technique has expanded and is now widely implemented in hospitals across the country, benefiting many patients with DF[2,3]. To explore the mechanisms underlying TTT in treating DF, numerous scholars have conducted extensive research. Several reports have shown that, compared with the preoperative state, patients exhibited neovascularization and improved perfusion within 3 months following TTT surgery. This finding mirrors the well-documented expansion of granulation tissue during ulcer healing, suggesting that TTT facilitates neovascularization in ischemic limbs or lesions throughout the body, enhances perfusion, and thereby improves microcirculation. It has been hypothesized that this may constitute the pathological basis of TTT in DF management[4,5]. Furthermore, both animal and clinical studies have indicated that bone transport stimulates the formation of collateral circulations near the bone block and in ischemic limbs. The newly formed blood vessels are predominantly microvessels with lumen diameters smaller than 10 μm. It has also been speculated that inflammation resulting from local injury associated with bone transport may be a principal cause of neovascularization[1,6,7].
Since 2019, our institution has conducted clinical research on the use of TTT for DF, achieving favorable outcomes. During treatment, we observed that patients developed abundant collateral vessels in the affected limb, accompanied by a marked increase in blood flow volume, velocity, and peripheral circulation, which ultimately contributed to regenerative healing of ulcerated skin. Distinct distraction osteogenesis at the TTT site was also observed in all patients. The aim of this study was to explore the potential mechanisms underlying TTT in DF by assessing distraction osteogenesis and angiogenesis in the affected limb after surgery.
This study was a single-arm, retrospective study. Fifteen patients with DF who underwent TTT and completed a 6-month follow-up were included. Participants were recruited from the Department of Orthopedics at Renmin Hospital of Wuhan University (Wuhan, China) between January 2021 and August 2022. The study population consisted of 11 males and four females aged 51-78 (mean age, 63 ± 9.4) years. According to Wagner’s classification, 2 cases were grade 2, 3 cases were grade 3, 7 cases were grade 4, and 3 cases were grade 5.
Inclusion criteria: (1) Age 18-80 years; (2) Diagnosis of DF with Wagner grades 2-5; (3) Willingness to undergo TTT with high adherence to treatment; (4) Computed tomography angiography (CTA) confirmation of stenosis or occlusion of infrapopliteal arteries (anterior tibial, posterior tibial, or peroneal), resulting in foot ischemia, with severe cases exhibiting nonpalpable pulses; and (5) Ankle-brachial index (ABI) < 0.9 and transcutaneous oxygen pressure < 60 mmHg.
Exclusion criteria: (1) History of cerebral infarction, myocardial infarction, heart failure, cancer, or renal failure within the previous 3 months; (2) Hematological or autoimmune dysfunction; (3) Other endocrinologist-diagnosed, uncontrolled DM-related comorbidities; and (4) Contraindications to anesthesia, or inability to tolerate anesthesia due to cardio
Ethical approval was obtained from the Ethics Committee of Renmin Hospital of Wuhan University (Approval No. WDRY2022-K200).
The surgical procedure began with an approximately 10 cm arc-shaped incision along the medial aspect of the mid-tibia following induction of anesthesia (nerve block or general anesthesia). A periosteal incision was then made along the medial tibia, followed by subperiosteal elevation to both sides. A bone transport window was created, measuring 5 cm in length and 1.5 cm in width. The bone block prepared for transport was secured using two 2-mm external fixation pins placed within the bone window. Using a drill and a pendulum saw, a mobile bone flap was fashioned from the transport block through an osteotomy, while preserving the integrity of the medullary cavity. Four additional 4-mm external fixation pins were inserted into the tibia, with two placed on each side of the bone window. The transverse tibial transport frame was installed, adjusted, and tightened, with the bone transport direction marked. Multilayer wound closure was then performed. During the procedure, thorough debridement of ulcerative lesions and drainage of abscess cavities were conducted. Necrotic and nonviable skin and tendons were excised, and devitalized tarsal bones and blackened toes were amputated. The wound was dressed under sterile conditions and, when indicated, was supplemented with vacuum sealing drainage to provide negative-pressure suction. Postoperatively, routine dressing changes were performed for both the external fixator and wound site. Strict bed rest was enforced to prevent weight bearing on the affected limb. Advancement of the external stent began on postoperative day 5. The incisions and foot wounds were managed with regular dressing changes, and wound debridement was performed when necessary. Additionally, prophylactic antibiotics and strict glycemic control were consistently maintained.
Comprehensive follow-up evaluations were conducted. At postoperative month 6, wound healing and residual limb retention rates were assessed. Clinical outcomes, including Visual Analog Scale (VAS) pain score, skin temperature, and ABI, were measured preoperatively and at 1 week, 1 month, and 6 months postoperatively.
A 64-slice spiral computed tomography (CT) scanner (LightSpeed VCT-XT; GE Healthcare, Chicago, IL, United States) was used to obtain CT scans of the tibias of the study participants. Four-dimensional image reconstruction was performed to generate sagittal and coronal views of the tibia. Scanning procedures were conducted by experienced CT technicians. The CT attenuation values of the medullary cavities corresponding to the TTT bone blocks were measured using dedicated imaging software. These measurements were performed independently by two radiologists with more than 3 years of professional experience. The measurement procedure was as follows: At the axial position corresponding to the transport bone block, three cross-sectional sites-upper, middle, and lower medullary cavities-were selected. Within each site, a region of interest of maximum feasible size was delineated, avoiding cortical bone margins, and the CT attenuation value was recorded. The independently obtained values from the two radiologists were compiled, outlier values were excluded, and the average was calculated. CT attenuation values were expressed as Hounsfield units.
Ultrasonic examination: Ultrasonography was performed using the Philips EPIQ5 color ultrasound diagnostic system equipped with a linear array probe (frequency, 5.0-12 MHz). Blood flow volume in the popliteal artery (POA) was measured before and 1 month postoperatively. Additionally, the inner diameters and peak flow velocities of the anterior tibial artery (ATA), posterior tibial artery, peroneal artery (PA), and dorsalis pedis artery (DPA). The number of collateral branches arising from the three major midcalf arteries was also recorded.
Detection of plantar microcirculation: Laser Doppler flowmetry detects the Doppler shift generated by the movement of blood cells in blood vessels located within approximately 1 mm beneath the skin and converts the signal into a current value, which is displayed as a color-coded image: Red indicates maximal blood flow perfusion, dark blue indicates minimal perfusion, and yellow indicates intermediate levels. Plantar skin blood flow perfusion was assessed before surgery and 1 month postoperatively using the PeriScan PIM3 Laser Doppler flowmetry system. Cutaneous perfusion mapping of the plantar surface was performed using a laser Doppler probe after the patient was positioned supine and the affected foot was immobilized. Forefoot perfusion images were obtained, and the corresponding regional perfusion values were recorded to quantify plantar microcirculation.
Data analyses were conducted using SPSS version 22.0 (SPSS Inc., Armonk, NY, United States). The Kolmogorov-Smirnov test was applied to assess normality. Continuous variables with a normal distribution are expressed as the mean ± SD, whereas nonparametric continuous and categorical variables are presented as percentages. For quantitative data, the Student’s t-test was used, and within-group differences were analyzed using the paired-sample t-test. Longitudinal comparisons of parameters were conducted using one-way repeated-measures analysis of variance, followed by Tukey’s post hoc test. Nonparametric data were analyzed using the Mann-Whitney test, and categorical variables were analyzed using the χ2 test. P < 0.05 was considered statistically significant.
Fifteen patients were enrolled between January 2021 and August 2022, comprising 11 males and four females aged 51 to 78 years (63 ± 9.4 years). Wagner grades were distributed as follows: Grade 2 (n = 2), grade 3 (n = 3), grade 4 (n = 7), and grade 5 (n = 3). Complete clinical outcomes and radiographic data were obtained for all participants (Table 1).
| Characteristic | Number (n = 15) |
| Type 2 diabetes mellitus | 15 |
| Number of feet | 15 |
| Age | 63.5 ± 9.4 |
| Sex | |
| Male/female | 11/4 |
| Wagner’s grades | |
| 2 | 2 |
| 3 | 3 |
| 4 | 7 |
| 5 | 3 |
| Duration of diabetes (years) | 7.26 ± 1.21 |
| HbA1c (%) | 10.26 ± 1.52 |
| Coronary artery disease | 5 |
| Smoking history | 6 |
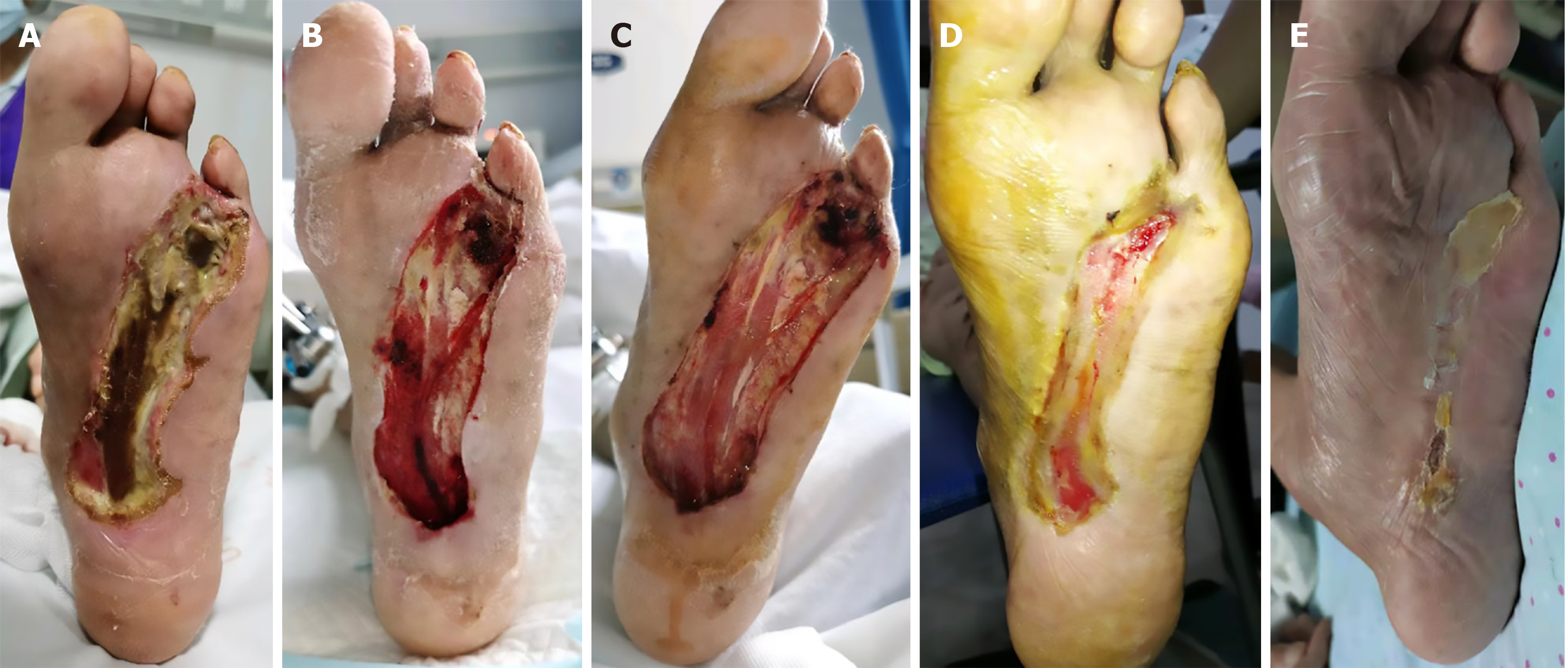
Bone transport was initiated 1 week postoperatively, and the external fixator was removed 4 weeks after transport. Within 1 week of transport, fresh granulation tissue appeared on the wound surface, and the skin advanced centripetally from the periphery toward the center. More minor wounds typically healed within approximately 4 weeks, while the longest healing duration was 17 weeks (mean, 10.1 ± 3.7 weeks) with a healing rate of 100%. The newly formed skin tissue had no distinct boundary with the surrounding tissue and showed intact dermatoglyphics (Figure 1). At both the 1- and 2-year follow-ups, the wound healing rate was 100% (15/15), with no amputations required.
Foot skin temperature was significantly higher at 1 week, 1 month, and 6 months postoperatively compared to preoperative levels (P < 0.05). VAS scores decreased markedly at all three postoperative intervals (P < 0.05). ABI values were significantly elevated at 1 and 6 months after surgery compared with the baseline (P < 0.05) (Table 2).
| Foot skin temperature (℃) | ABI index | VAS score | |
| Preoperative | 28.67 ± 0.57 | 0.51 ± 0.11 | 4.93 ± 0.70 |
| 1 week after surgery | 30.75 ± 0.55a | 0.56 ± 0.14 | 1.87 ± 0.52a |
| 1 month after surgery | 31.05 ± 0.45a | 0.72 ± 0.07a | 1.13 ± 0.35a |
| 6 months after surgery | 32.11 ± 0.66a | 0.87 ± 0.05a | 0.47 ± 0.52a |
| F value | 98.38 | 39.52 | 202.8 |
| P value | < 0.0001 | < 0.0001 | < 0.0001 |
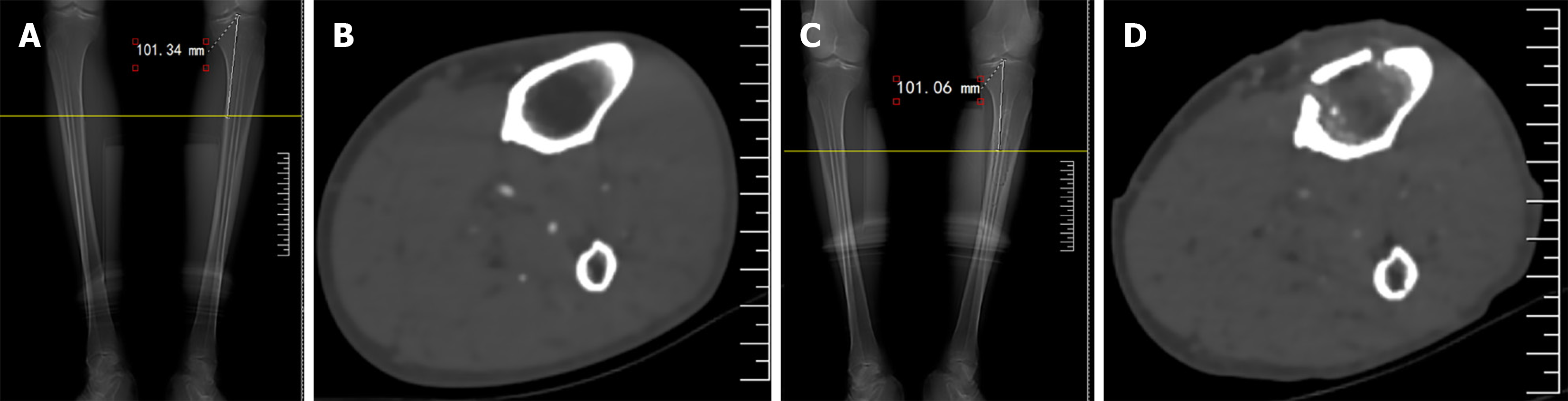
At 1 month postoperatively, CT imaging revealed significant bone formation beneath the distraction bone block. The orientation of the new bone was consistent with the direction of distraction, showing a transverse arrangement. No bone aggregation was observed in the corresponding region of the medullary cavity before surgery (Figure 2). Compared with baseline, the CT value of the corresponding part of the distraction bone block increased significantly 1 month after surgery (55.26 ± 10.32 vs -45.89 ± 15.60; P < 0.05), suggesting that TTT induces distraction osteogenesis within the medullary cavity corresponding to the bone transport block.
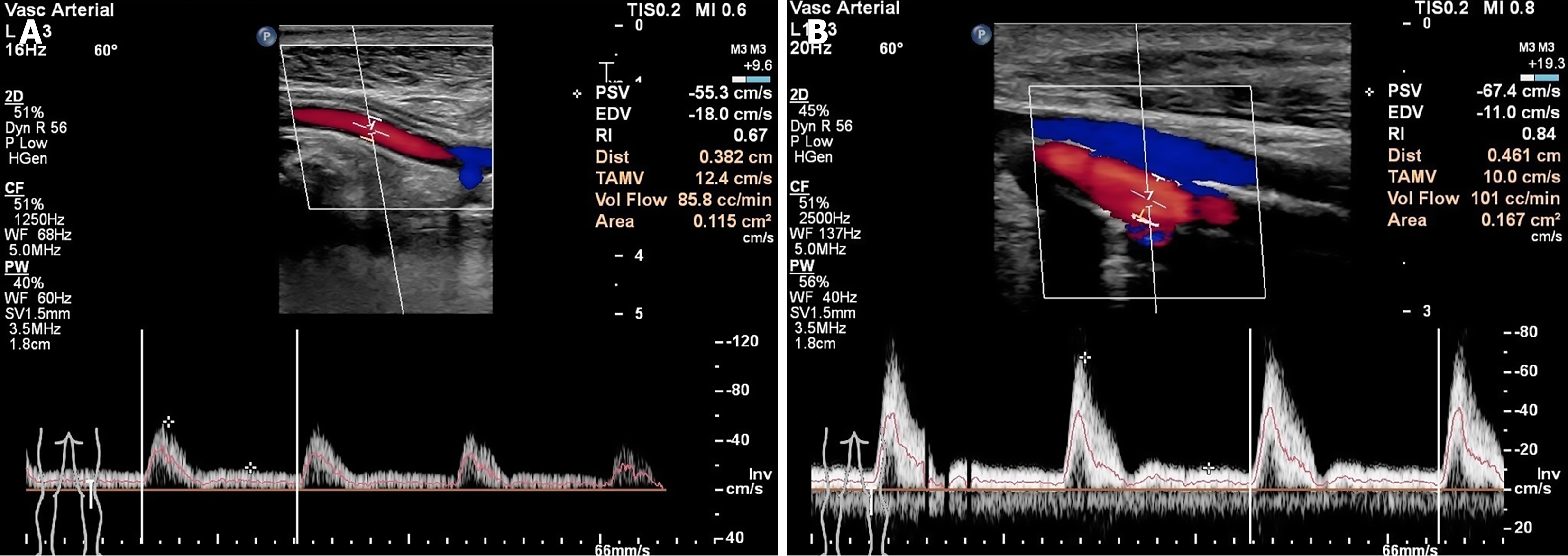
At 1 month postoperatively, POA blood flow increased significantly compared with preoperative levels (t = 2.306, P = 0.029). At the 1-month postoperative follow-up, the diameter of the ATA showed no significant difference from preoperatively (t = 0.322, P = 0.750), and blood flow velocity also showed no significant variation (t = 0.851, P = 0.402). The diameter of the PA increased significantly compared with the baseline (t = 2.148, P = 0.040), whereas blood flow velocity did not differ significantly (t = 0.441, P = 0.663). The diameter of the DPA increased (t = 2.148, P = 0.040), and blood flow velocity was significantly higher (t = 2.144, P = 0.041) 1 month postoperatively compared to the preoperative values (Figure 3; Table 3).
| Blood flow volume of the popliteal artery (cc/min) | Anterior Tibial artery | Peroneal artery | Dorsalis Pedis artery | ||||
| Diameter (mm) | Blood flow velocity (cm/s) | Diameter (mm) | Blood flow velocity (cc/min) | Diameter (mm) | Blood flow velocity (cm/s) | ||
| Preoperative | 76.20 ± 29.98 | 0.13 ± 0.08 | 34.47 ± 32.17 | 0.13 ± 0.03 | 40.73 ± 33.65 | 0.11 ± 0.03 | 22.13 ± 13.89 |
| 1 month after surgery | 98.07 ± 21.23 | 0.12 ± 0.09 | 43.80 ± 27.73 | 0.15 ± 0.02 | 45.53 ± 25.39 | 0.13 ± 0.02 | 33.13 ± 14.21 |
| t | 2.306 | 0.322 | 0.851 | 2.148 | 0.441 | 2.148 | 2.144 |
| P value | 0.029 | 0.750 | 0.402 | 0.040 | 0.663 | 0.040 | 0.041 |
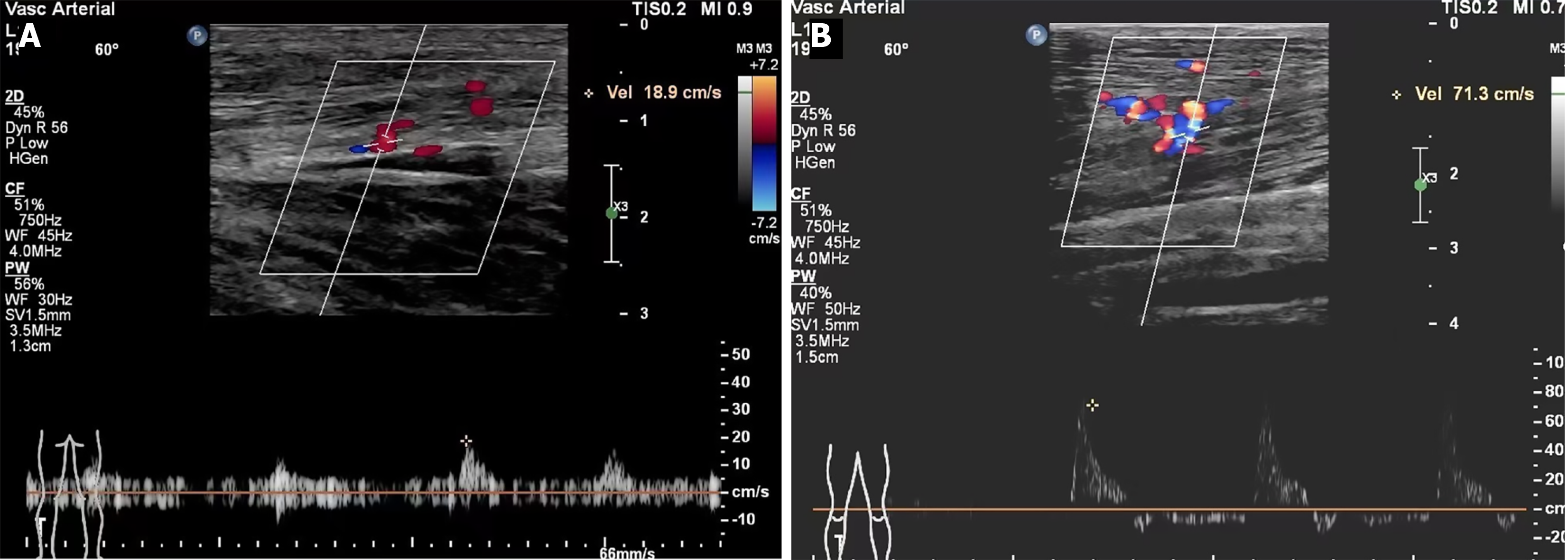
B-ultrasound revealed that collateral branches around the three major middle arteries of the lower leg were sparse before surgery. One month postoperatively, the number of collateral vessels increased significantly (t = 9.676, P < 0.0001), the vessel diameter showed no significant change (t = 0.939, P = 0.356), and blood flow velocity increased significantly (t = 9.816, P < 0.0001) (Figure 4; Table 4).
| Number of collateral vessels | Inner diameter of the collateral branches | Blood flow velocity of the collateral branches | |
| Preoperative | 2.7 ± 1.6 | 0.12 ± 0.04 | 25.33 ± 8.41 |
| 1 month after surgery | 9.7 ± 2.3 | 0.13 ± 0.01 | 54.73 ± 7.99 |
| t | 9.676 | 0.939 | 9.816 |
| P value | < 0.0001 | 0.356 | < 0.0001 |
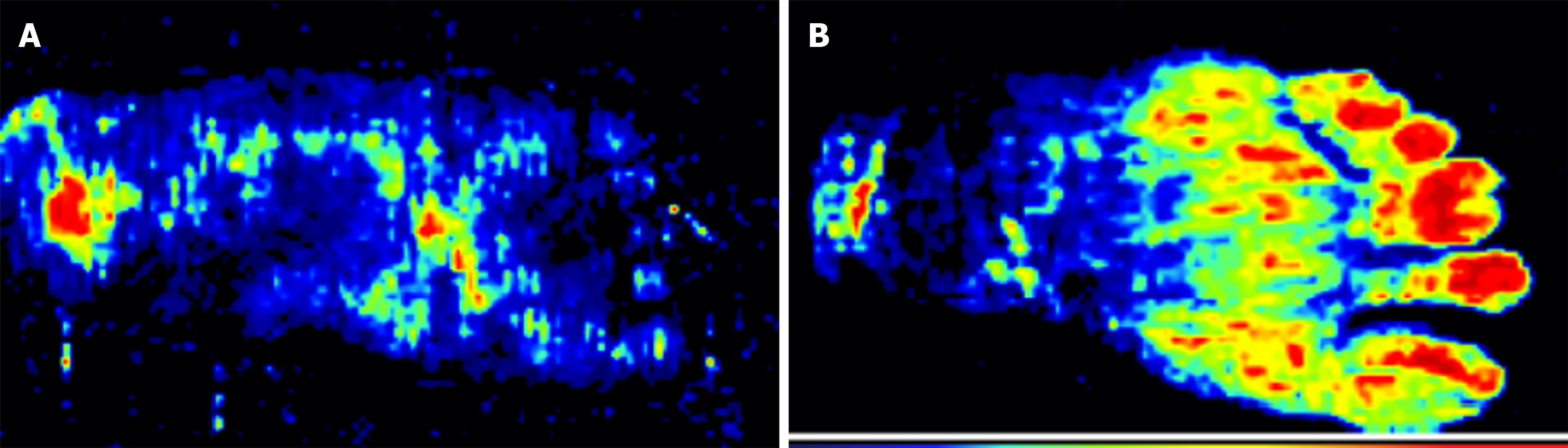
Preoperative laser Doppler imaging showed sparse blood flow signals and poor microcirculatory perfusion in the plantar skin. At 1 month postoperatively, blood flow in the plantar region-particularly in the forefoot and midfoot-was abundant, with marked improved microcirculatory perfusion compared with the preoperative levels (52.02 ± 7.45 vs 33.72 ± 7.50, P < 0.05) (Figure 5).
Management of DF ulcers (DFUs) typically requires a multidisciplinary approach, including glycemic control, improvement of blood circulation, local debridement, dressing changes, anti-infective therapy, and neurotrophic support[8,9]. Several treatment protocols are available, such as endovascular stenting, percutaneous transluminal angioplasty, and lower limb bypass grafting. However, their effect is limited to enhancing local blood circulation, which results in suboptimal outcomes. Sustained slow traction stimulation has been indicated to facilitate local tissue regeneration[10,11]. Currently, TTT is widely used in the treatment of infectious osteomyelitis; however, its use in DFU therapy remains rarely reported.
In this study, TTT improved blood circulation in the affected limb, accelerated wound healing in DFUs, and prevented amputations. During the first 24-48 hours following a fracture, hematoma formation, inflammatory responses, and the recruitment of mesenchymal stem cells are the predominant pathological features at the fracture site. Cartilage callus growth peaks 7-9 days postfracture, while periosteal reactions give rise to early intramembranous osteogenesis, involving cell proliferation, early intravascular growth, and neovascularization. In clinical practice, bone distraction typically commences 7 days after osteotomy[12]. Instead of a soft callus, an osteoepiphysis fibrovascular bridge forms in the distraction gap during bone distraction, termed the fibrous interzone (FIZ)[13]. The FIZ is rich in chondroid cells, fibroblasts, and oval cells that morphologically lie between fibroblasts and chondrocytes. Throughout distraction, the FIZ maintains a thickness of approximately 4 mm, which is also the last ossified region of the distraction gap. On both sides of the FIZ are primary mineralization zones populated by densely proliferating osteoblasts. These osteoblasts undergo primary mineralization around the newly formed capillary and vascular sinus, leading to the formation of bone columns resembling stalagmites and stalactites, known as the microcolumn formation zone. At the end of distraction, the primary mineralized zone advances from both ends toward the center, eventually connecting the FIZ[14]. In this study, CT imaging confirmed distinct osteogenesis in the medullary cavity at the level corresponding to the bone transport block, while the corresponding preoperative region was filled with yellow bone marrow tissue. According to Professor Ilizarov, bone transport technology adheres to the tension-stress law of tissue regeneration, whereby the continuous, stable, and slow stretching of living tissue generates a certain tension, which stimulates the regeneration and vigorous growth of the tissue[15,16]. Distraction osteogenesis is the primary pathophysiological process occurring at the bone transport site and should also be the initiating factor for biological effects such as angiogenesis and skin regeneration after bone transport. During the practical application of the bone transport technique, scholars observed a marked improvement in distal limb circulation in postsurgical patients[17], prompting them to apply TTT to lower extremity ischemic disorders.
Angiographic and histological analyses from both animal and clinical studies of TTT have confirmed that bone transport induces abundant neovascularization around the transported bone segment, including the development of collateral circulation at the extremities. In a canine TTT experiment conducted by Professor Ilizarov[15,16], he found that, like longitudinal bone transport, capillary formation was evident 7 days after bone transport, with continuous and sinusoid capillaries as the major types. By 21 days, vascular growth outpaced the rate of transport, leading to the formation of meandering vessels. Angiographic assessment indicated a well-developed capillary network around the bone transport block, with a marked increase in vascular density observed at its distal end. Using CTA and perfusion imaging, Zeng et al[4] and Chen et al[5] observed angiogenesis and increased blood perfusion 3 months after TTT, compared to the preoperative status in patients with DF, a characteristic of the granulation tissue phase in ulcer healing[5]. In this study, the B-ultrasound examination results indicated that one month after the operation, POA blood flow of the affected limb increased obviously, the inner diameter of the DPA increased, the DPA blood flow velocity accelerated obviously, and the number of collateral vessels in the three middle arteries of the calf increased obviously. The plantar laser Doppler results revealed that the plantar microcirculation improved significantly 1 month postoperatively. Collectively, these results showed that TTT enhances blood circulation in the affected limb by mechanically stretching the tibial cross-section, thereby stimulating angiogenesis and accelerating wound healing in the lower extremities.
Following TTT treatment, DF wounds exhibited restoration of normal skin coverage rather than scar formation, suggesting that TTT may promote skin regeneration by activating intrinsic tissue regeneration mechanisms[18]. One study reported that patients with severe DF exhibited a marked reduction in numbness and sensory impairment 2 to 3 months after TTT, with sensory recovery observed at 6 months, suggesting the potential of TTT to facilitate nerve fiber regeneration in the affected limb[19]. Hua Qikai’s team further revealed that the local distraction effect of TTT activates stem cells through the upregulation of peripheral blood vascular endothelial growth factor (VEGF) or stromal cell–derived factor-1 (SDF-1), thereby modulating and mobilizing these cells. Additionally, TTT has been shown to favorably modulate macrophage polarization in distal wound tissues, thereby attenuating inflammatory responses by reducing the M1/M2 ratio and regulating immune cell dynamics. This modulation enhances the wound-healing microenvironment and promotes the contribution of skin stem cells to tissue regeneration and ulcer repair[5,20]. Research also indicates the activation of multiple pathways during bone distraction, including Fos/Jun-related genes, FAK, and the Wnt and Hippo pathways, all of which collectively stimulate tissue regenerative potential. Given that these pathways are closely linked to embryonic skeletal development and cell differentiation, their reactivation may represent the core principle underlying Ilizarov technology[21]. Therefore, the bone regeneration process initiated by TTT through distraction osteogenesis may serve as the linchpin for stimulating a multifaceted regenerative program in the affected limb, potentially involving vascular, neural, and skin regeneration. Bone tissue regeneration may therefore constitute a critical trigger for systemic regenerative activity, although the underlying mechanisms require further exploration.
However, this study has several limitations. As a single-center, single-arm retrospective analysis, it included a small sample size and lacked a control group, making it difficult to exclude interference from spontaneous healing or other confounding factors. Consequently, both the statistical power and the generalizability of the findings are limited. Due to the retrospective design, it is also impossible to establish a definitive temporal relationship between exposure and outcome, thereby restricting causal inference. Vascular assessment was constrained because ultrasound measurements focused solely on the number, internal diameter, and flow velocity of major arteries and collateral vessels in the lower limbs, without distinguishing between microvasculature (e.g., capillary density) and macrovasculature. Additionally, regional differences within the foot (e.g., heel, toes) were not quantified because of limited data collection. Another limitation is the lack of robust evidence directly linking distraction osteogenesis to angiogenesis; objective mechanistic markers such as VEGF and SDF-1 were not assessed. Future studies should therefore include prospective, multicenter controlled studies with larger sample sizes to validate and extend these findings.
In summary, this study found that TTT promotes ulcer healing in patients with DF by enhancing limb blood circulation. Its efficacy is associated with tension osteogenesis at the surgical site and supportive collateral vascularization, though deeper mechanisms warrant further investigation.
| 1. | Chen L, Sun S, Gao Y, Ran X. Global mortality of diabetic foot ulcer: A systematic review and meta-analysis of observational studies. Diabetes Obes Metab. 2023;25:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 138] [Article Influence: 46.0] [Reference Citation Analysis (1)] |
| 2. | Wen R, Cheng X, Cao H, Zhang L, Luo F, Shang W. Transverse Tibial Bone Transfer in the Treatment of Diabetes Foot Ulcer: A Pilot Study. Diabetes Metab Syndr Obes. 2023;16:2005-2012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Ou S, Xu C, Yang Y, Chen Y, Li W, Lu H, Li G, Sun H, Qi Y. Transverse Tibial Bone Transport Enhances Distraction Osteogenesis and Vascularization in the Treatment of Diabetic Foot. Orthop Surg. 2022;14:2170-2179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 4. | Zeng Z, Dong Y, Hua Q, Kuang X, Li K, Deng X, Qiu S. Computed tomography perfusion study evaluating the curative effect of tibial transverse transport in patients with severe diabetic foot. J Orthop Translat. 2019;19:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Chen Y, Kuang X, Zhou J, Zhen P, Zeng Z, Lin Z, Gao W, He L, Ding Y, Liu G, Qiu S, Qin A, Lu W, Lao S, Zhao J, Hua Q. Proximal Tibial Cortex Transverse Distraction Facilitating Healing and Limb Salvage in Severe and Recalcitrant Diabetic Foot Ulcers. Clin Orthop Relat Res. 2020;478:836-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Goodman SB, Maruyama M. Inflammation, Bone Healing and Osteonecrosis: From Bedside to Bench. J Inflamm Res. 2020;13:913-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Shu LZ, Zhang XL, Ding YD, Lin H. From inflammation to bone formation: the intricate role of neutrophils in skeletal muscle injury and traumatic heterotopic ossification. Exp Mol Med. 2024;56:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 8. | Macfarlane SM, Zhao SX, Lafrenz JO, Nagaratnam MV, Tchen A, Linton CE, Yuen L. Effect of a multidisciplinary team approach on the management of diabetic foot ulcers on the Central Coast: A review of the Gosford Hospital High-Risk Foot Clinic. Int Wound J. 2024;21:e14570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (4)] |
| 9. | Riemer KL, Giurini JM. The Evolution of the Multidisciplinary Approach to the Diabetic Foot. Clin Podiatr Med Surg. 2025;42:585-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Roh J, Schellhardt L, Keane GC, Hunter DA, Moore AM, Snyder-Warwick AK, Mackinnon SE, Wood MD. Short-Duration, Pulsatile, Electrical Stimulation Therapy Accelerates Axon Regeneration and Recovery following Tibial Nerve Injury and Repair in Rats. Plast Reconstr Surg. 2022;149:681e-690e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Zhang X, Dong T, Yao S, Lu S, Li W. Application of transverse tibial bone transport and microcirculation reconstruction in the treatment of diabetic foot ulcer: a case report. Ann Palliat Med. 2021;10:8358-8364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Adejuyigbe B, Gharpure M, Wahle CF, Kallini JR. Distraction Osteogenesis: A Comprehensive Review. Appl Biosci. 2024;3:503-516. [DOI] [Full Text] |
| 13. | Kende PP, Sarda AS, Desai H, Landge J, Nimma V, Varte V. Complications of distraction osteogenesis: Narrative review. Oral Surg. 2021;14:412-422. [DOI] [Full Text] |
| 14. | Bafor A, Iobst C, Samchukov M, Cherkashin A, Singh S, Aguilar L, Glatt V. Reverse Dynamization Accelerates Regenerate Bone Formation and Remodeling in a Goat Distraction Osteogenesis Model. J Bone Joint Surg Am. 2023;105:1937-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;263-285. [PubMed] |
| 16. | Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin Orthop Relat Res. 1989;249-281. [PubMed] |
| 17. | Xie J, Liu X, Wu B, Chen B, Song Q, Guan Y, Gong Y, Yang C, Lin J, Huang M, Tan X, Lai R, Lin X, Zhang S, Xie X, Chen X, Zhang C, Yang M, Nong H, Zhao X, Xia L, Zhou W, Xiao G, Jiang Q, Zou W, Chen D, Lu D, Liu J, Bai X. Bone transport induces the release of factors with multi-tissue regenerative potential for diabetic wound healing in rats and patients. Cell Rep Med. 2024;5:101588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 18. | Yang Y, Li Y, Pan Q, Bai S, Wang H, Pan XH, Ling KK, Li G. Tibial cortex transverse transport accelerates wound healing via enhanced angiogenesis and immunomodulation. Bone Joint Res. 2022;11:189-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Qin W, Liu K, Su H, Hou J, Yang S, Pan K, Yang S, Liu J, Zhou P, Lin Z, Zhen P, Mo Y, Fan B, Li Z, Kuang X, Nie X, Hua Q. Tibial cortex transverse transport promotes ischemic diabetic foot ulcer healing via enhanced angiogenesis and inflammation modulation in a novel rat model. Eur J Med Res. 2024;29:155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Chen Y, Ding X, Zhu Y, Jia Z, Qi Y, Chen M, Lu J, Kuang X, Zhou J, Su Y, Zhao Y, Lu W, Zhao J, Hua Q. Effect of tibial cortex transverse transport in patients with recalcitrant diabetic foot ulcers: A prospective multicenter cohort study. J Orthop Translat. 2022;36:194-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Zhu YL, Guo BF, Zang JC, Pan Q, Zhang DW, Peng Y, Qin SH. Ilizarov technology in China: a historic review of thirty-one years. Int Orthop. 2022;46:661-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/