Published online Nov 15, 2025. doi: 10.4239/wjd.v16.i11.111280
Revised: August 28, 2025
Accepted: October 23, 2025
Published online: November 15, 2025
Processing time: 140 Days and 22.4 Hours
Type 2 diabetes mellitus (T2DM), one of the most common chronic metabolic diseases, is also one of the most significant risk factors for cardiovascular disease (CVD) and chronic kidney disease (CKD).
To conduct a systematic review and network meta-analysis of cardiovascular (CV) and renal benefits of glucagon-like peptide-1 receptor agonists (GLP-1RA), sodium-glucose cotransporter-2 inhibitors (SGLT2i), and nonsteroidal mineralocorticoid receptor antagonists (nsMRA) in T2DM patients.
We searched four databases-PubMed, EMBASE, Cochrane Library, and Web of Science- for publications from inception to March 6, 2025. Total 500 participants were enrolled and had an intervention period of at least one year (or 52 weeks). Eligible studies included adult patients with T2DM and interventions with a placebo or another GLP-1RA, SGLT2i, or nsMRA. Data were standardized using Stata 17.0 software. The quality of evidence was assessed using the CINeMA and GRADE approaches.
Total 14970 articles were retrieved, of which 25 high-quality studies were included for the systematic review and network meta-analysis, covering 189797 patients and three drug classes (14 drugs). Network meta-analysis revealed low heterogeneity, thus ensuring reliable results. Meta-regression analysis indicated that baseline factors, such as comorbidities and blood glucose levels, did not affect our results. Overall, all included drugs demonstrated significant CV and renal benefits compared with the placebo. nsMRA showed the best efficacy in reducing the incidence of major adverse CV events and myocardial infarction. SGLT2i were most effective in reducing all-cause mortality, CV mortality, and the incidence of renal outcomes. GLP-1RA showed the greatest benefits in reducing the incidence of stroke. SC-semaglutide had the most significant effect on reducing major adverse CV events, oral semaglutide was most effective in reducing all-cause mortality and CV mortality, empagliflozin had the strongest effect in reducing composite renal outcomes and renal replacement therapy, canagliflozin was most effective in slowing the progression of proteinuria, and dapagliflozin showed the most significant reduction in end-stage renal disease.
T2DM, as one of the most common chronic metabolic diseases, is also one of the most significant risk factors for CVD and CKD. GLP-1RA, SGLT2i, and nsMRAs have emerged as novel therapeutic agents to comprehensively manage T2DM-related CVD and CKD. We conducted a network meta-analysis to compare the efficacy and safety of GLP-1RAs, SGLT2i, and nsMRA in patients with T2DM.
Core Tip: Type 2 diabetes mellitus (T2DM), as one of the most common chronic metabolic diseases, is also one of the most significant risk factors for cardiovascular disease (CVD) and chronic kidney disease (CKD). Glucagon-like peptide-1 receptor agonists (GLP-1RA), sodium-glucose cotransporter-2 inhibitors (SGLT2i), and nonsteroidal mineralocorticoid receptor antagonists (nsMRA) have emerged as novel therapeutic agents to comprehensively manage T2DM -related CVD and CKD. We conducted a network meta-analysis to compare the efficacy and safety of GLP-1RAs, SGLT2i, and nsMRA in patients with T2DM.
- Citation: An XD, Li XQ, Zhang H, Jia QY, Zhang YH, Yang GG. Comparison of three types of drugs for cardiovascular and renal benefits in type 2 diabetes mellitus. World J Diabetes 2025; 16(11): 111280
- URL: https://www.wjgnet.com/1948-9358/full/v16/i11/111280.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i11.111280
Type 2 diabetes mellitus (T2DM), one of the most common chronic metabolic diseases, is also one of the most significant risk factors for cardiovascular disease (CVD) and chronic kidney disease (CKD). Recent studies have revealed a closer relationship among T2DM, CVD, and CKD[1,2], collectively termed as cardiovascular (CV) -kidney-metabolic syndrome[3]. Research indicates that diabetic patients have a 2 to 4 fold higher risk of developing atherosclerotic CVD than non-diabetic individuals[4], and approximately 40% of diabetic patients may develop CKD[5]. A cross-sectional study involving 11607 participants from the National Health and Nutrition Examination Survey estimated that 25% of the participants had at least one CV, renal, or metabolic disease[6]. These diseases impose a substantial burden on morbidity and mortality[5]. The medical community is continuously exploring novel therapeutic strategies to comprehensively manage T2DM -related CVD and/or CKD.
Therefore, glucagon-like peptide-1 receptor agonists (GLP-1RA), sodium-glucose cotransporter-2 inhibitors (SGLT2i), and nonsteroidal mineralocorticoid receptor antagonists (nsMRA) have emerged as novel therapeutic agents of great interest. GLP-1RA and SGLT2i are currently recommended as first-line glucose-lowering agents for diabetes patients with established CVD or multiple CV and renal risk factors[7-9]. The novel nsMRA, finerenone has also been demonstrated to provide CV and renal protection in patients with diabetes and CKD[10].
GLP-1RA achieves effective glycemic control by stimulating insulin secretion, inhibiting glucagon release, and delaying gastric emptying. Additionally, it suppresses appetite and promotes weight loss[11], significantly reducing adverse CV and renal adverse events[12]. SGLT2i blocks the reabsorption of filtered glucose in the renal tubules, promoting glucose excretion in urine, thereby lowering blood glucose and body weight[13], and significantly reducing major adverse CV and renal events[14-16]. Finerenone is a highly selective and potent nsMRA[17], and multiple studies have confirmed its CV and renal benefits in T2DM patients[10,18,19].
These findings suggest that GLP-1RA, SGLT2i, and nsMRAs are promising treatment options for T2DM-related CVD and CKD. However, differences in efficacy and safety among these drug classes remain unclear. Although several previous systematic reviews and network meta-analyses have compared the effectiveness of these three drug classes[20-25], no study has systematically compared the efficacy and safety of SGLT2i, GLP-1RA, and nsMRA in preventing CV and renal events in T2DM patients based on long-term (≥ 1 year or 52 weeks), large-scale (≥ 500 participants) high-quality clinical studies.
Therefore, to address this gap, we conducted a systematic review and network meta-analysis to provide a more scientific and comprehensive evidence base for the optimal selection of GLP-1RA, SGLT2i, and nsMRA in the clinical management of T2DM-related CVD and CKD.
This study employed a systematic review and network meta-analysis summarizing the latest high-quality, large-sample clinical studies comparing the CV and renal benefits of GLP-1RA, SGLT2i, and nsMRAs in patients with T2DM. The study protocol was registered in PROSPERO (CRD420251003383); detailed information is available online (https://www.crd.york.ac.uk/prospero/).
We searched four databases-PubMed, Web of Science, the Cochrane Central Register of Controlled Trials, and EMBASE-for randomized controlled trials that evaluated the use of GLP-1RA, SGLT2i, or nsMRAs as monotherapy in T2DM patients. The search period was from the database inception to March 6, 2025. Additionally, we reviewed the reference lists of published articles and relevant systematic reviews to ensure the comprehensive inclusion of third-party-reviewed published literature. The language was restricted to English, with no limitations on the publication date or status. Search keywords included but were not limited to: “GLP-1RA”, “SGLT2i”, “nsMRA” and “type 2 diabetes mellitus” (Detailed search strategies for different databases are provided in the Supplementary material).
Participants: Adults (≥ 18 years old) with T2DM.
Interventions: The intervention group received GLP-1RA, SGLT2i, or nsMRA, whereas the control group received a placebo or another monotherapy with GLP-1RA, SGLT2i, or nsMRA.
Outcomes: Studies must provide sufficient data for a meta-analysis, including at least one efficacy outcome. CV events: Major adverse CV events, all-cause mortality, CV mortality, stroke, myocardial infarction, and hospitalization for heart failure. Renal events: Composite renal events, progression of proteinuria, end-stage renal disease, renal replacement therapy, and a sustained decrease in estimated glomerular filtration rate (eGFR) to < 15 mL/min/1.73 m².
Study design: Randomized controlled trials with an intervention duration of at least one year (or 52 weeks) and a minimum sample size of 500 participants.
Study type: Systematic reviews, narrative reviews, clinical trial protocols, basic research, conference abstracts, and other non-original randomized controlled trials.
Language: Non-English articles.
Duplicate references were screened using the ENDNOTE 20. We compared the references based on title and year. Two researchers independently screened titles and abstracts to identify potentially eligible studies. Full-text screening was conducted to finalize the studies included in the analysis.
After literature screening, data were extracted using Microsoft Excel (version 16.95.1) to collect the following information from eligible studies.
Study characteristics: Study name, first author, publication year, clinical trial registration number, study design, inter
CV events: Major adverse CV events, all-cause mortality, CV mortality, stroke, myocardial infarction, and hospitalization for heart failure. Renal outcomes: Composite renal events, progression of proteinuria, end-stage renal disease, renal replacement therapy, and a sustained decrease in eGFR to < 15 mL/min/1.73 m2.
Safety outcomes: Serious adverse events, diabetic ketoacidosis, pancreatitis, hypoglycemic events, acute kidney injury, urinary tract infection, leg amputation, foot amputation, any cancer, and thyroid cancer.
Discrepancies in data extraction were resolved through discussions with a third-party researcher (Zhang YH) until a consensus was reached.
When incomplete data were encountered, we first searched ClinicalTrials.gov for relevant information or contacted the authors directly to obtain the missing data.
A Bayesian random effects model was used to conduct a network meta-analysis of randomized controlled trials using Stata 17.0. The Bayesian random-effects model was implemented using Markov chain Monte Carlo methods with four chains, 50000 iterations, and a burn-in of 10000. The “Network” command set was used for data processing. In the evidence network, interventions are represented as nodes, with larger nodes indicating a greater number of patients receiving the intervention. Lines between nodes represent direct comparisons between interventions, with the line thickness reflecting the number of studies included.
For networks with closed loops, the node-splitting method was used to assess both local and global inconsistencies by direct and indirect comparisons. A P value > 0.05 indicated no statistically significant difference, suggesting consistency between direct and indirect comparisons and thereby justifying the use of a consistency model for analysis.
We visualized the indirect evidence network for different drugs and calculated the Surface Under the Cumulative Ranking Curve (SUCRA) scores. SUCRA values were calculated to rank treatments according to their probability of being the most effective, based on the cumulative ranking probabilities derived from posterior distributions. A higher SUCRA value indicated better intervention effectiveness, allowing for comparisons and ranking of different interventions. Forest plots were generated to compare each drug with the placebo.
The Cochrane Risk of Bias Tool was used to evaluate the methodological quality of the included studies, covering the following domains: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other sources of bias. Studies were categorized as having a low, high, or unclear risk of bias. Quality assessment was conducted by two reviewers (An XD and Li XQ), and discrepancies were resolved through discussion or consultation with a third reviewer (Zhang H).
Additionally, we used CINeMA to assess the certainty of evidence across six key domains: Within-study bias, reporting bias, indirectness, imprecision, heterogeneity, and inconsistency. We evaluated the uncertainty in CINeMA by comparing potential effect modifiers across studies, providing both direct and indirect evidence for each comparison. Additionally, we adopted the GRADE approach to evaluate the quality of evidence in each network, including assessments of the risk of bias, inconsistency, indirectness, imprecision, and publication bias[26].
To assess the robustness of the included study results, we conducted a sensitivity analysis by systematically removing one study at a time to examine its impact on the overall results. Funnel plots and statistical tests such as the Egger test were used to evaluate publication bias and detect asymmetry. If publication bias was identified, appropriate corrections and adjustments (e.g., the trim-and-fill method) were applied.
In this study, we performed a subgroup analysis based on the specific characteristics of the study population, particularly in participants with baseline HbA1c ≥ 7%, to obtain more precise efficacy comparisons.
We performed a meta-regression analysis using Stata software. Considering the study designs and population characteristics of the 25 clinical trials included, we analyzed potential influencing factors, such as comorbidities (presence or absence of CV or kidney disease) and baseline glycemic levels (baseline HbA1c was greater than 7). The outcomes of the meta-regression analysis were based on at least ten studies.
In this study, we conducted a systematic review and network meta-analysis to compare the CV and renal benefits of GLP-1RA, SGLT2i, and nsMRAs in patients with T2DM. After integrating and analyzing the data from the included studies, we ultimately incorporated 25 Large-scale randomized controlled trials, involving 189797 patients (Figure 1). These studies were conducted in various countries and regions between 2017 and 2024. The three classes of drugs included in the analysis comprised 14 specific drugs: GLP-1RA (9 drugs): Albiglutide, Dulaglutide, Efpeglenatide, long-acting Exenatide, short-acting Exenatide (SA-Exenatide), Liraglutide, Lixisenatide, oral Semaglutide (Oral-Semaglutide), and subcutaneous injection Semaglutide (SC-Semaglutide); SGLT2i (4 drugs): Canagliflozin, Dapagliflozin, Empagliflozin, and Ertugliflozin; nsMRA (1 drug): Finerenone (Supplementary material).
As detailed in the Supplementary material, we included long-term, high-quality, large-sample, randomized, blinded, placebo-controlled clinical trials and independently assessed the quality of each study. Consistency checks for various outcome measures indicated no significant inconsistencies (P > 0.05), allowing us to use a consistency model for the analysis. We evaluated the agreement between direct and indirect evidence. For indirect evidence, we observed significant heterogeneity, potentially because all existing studies compared target intervention drugs to placebo. However, this did not affect the stability or reliability of the results. In addition, we found no significant evidence of funnel plot asymmetry, suggesting a lack of publication bias.
According to the GRADE method, all included studies were randomized trials, and the evaluations for the risk of bias, inconsistency, indirectness, imprecision, and publication bias were rated as “not serious” or “none.” Our findings suggest that for both critical outcomes (major adverse CV events, composite renal events, serious adverse events) and important outcomes, the certainty of the evidence was rated as high (Supplementary material).
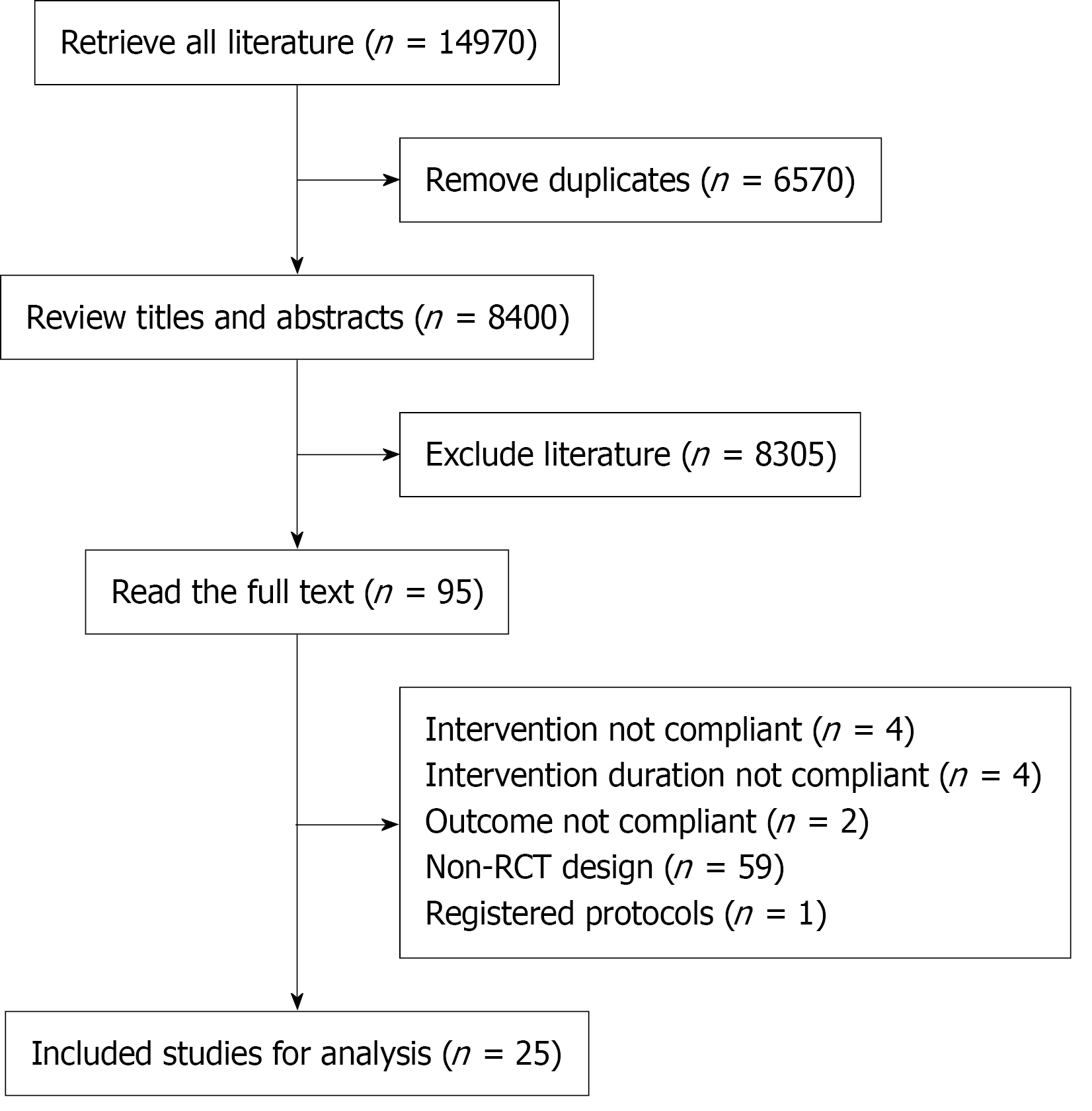
Major adverse CV events: Seventeen studies including 113358 patients were analyzed for major adverse CV events. The results showed that all three drug classes significantly reduced major adverse CV the incidence of major adverse cardiovascular events compared with the placebo, with nsMRA demonstrating the most significant effect [odds ratio (OR), -0.15; 95% confidence interval (CI): -0.26, -0.05; SUCRA, 77.3%]. Overall, the quality of the studies was high (Figure 2; Supplementary material).
Among the different drugs, SC-semaglutide showed the most significant efficacy (OR, -0.33; 95%CI: -0.59, -0.07; SUCRA, 86.5%). Among the SGLT2i, Canagliflozin had the highest efficacy (OR, -0.18; 95%CI: -0.42, 0.07; SUCRA, 72.4%) (Figure 2; Supplementary material).
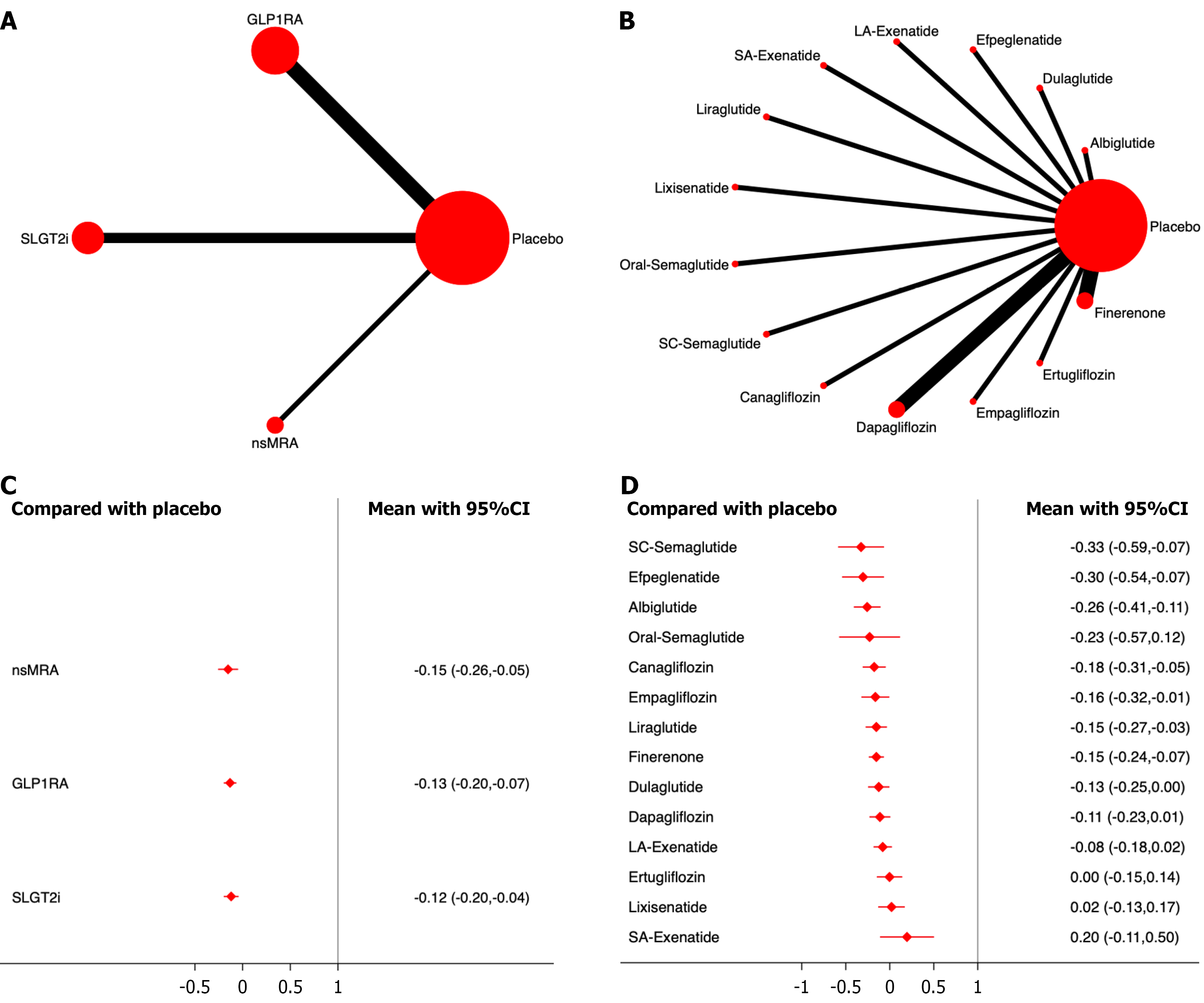
Eighteen studies, including 125449 patients, were analyzed for all-cause mortality. The results showed that all three drug classes significantly reduced all-cause mortality compared with the placebo, with SGLT2i demonstrating the most significant effect (OR, -0.17; 95%CI: -0.25, -0.09; SUCRA, 81.6%). Overall, the quality of the studies was high. (Figure 3; Supplementary material).
Among the different drugs, oral semaglutide showed the most significant efficacy (OR, -0.68; 95%CI: -1.21, -0.16; SUCRA, 96.2%). Among the SGLT2i, Empagliflozin had the highest efficacy ranking (OR, -0.40; 95%CI: -0.63, -0.17; SUCRA, 97.0%) (Figure 3; Supplementary material).
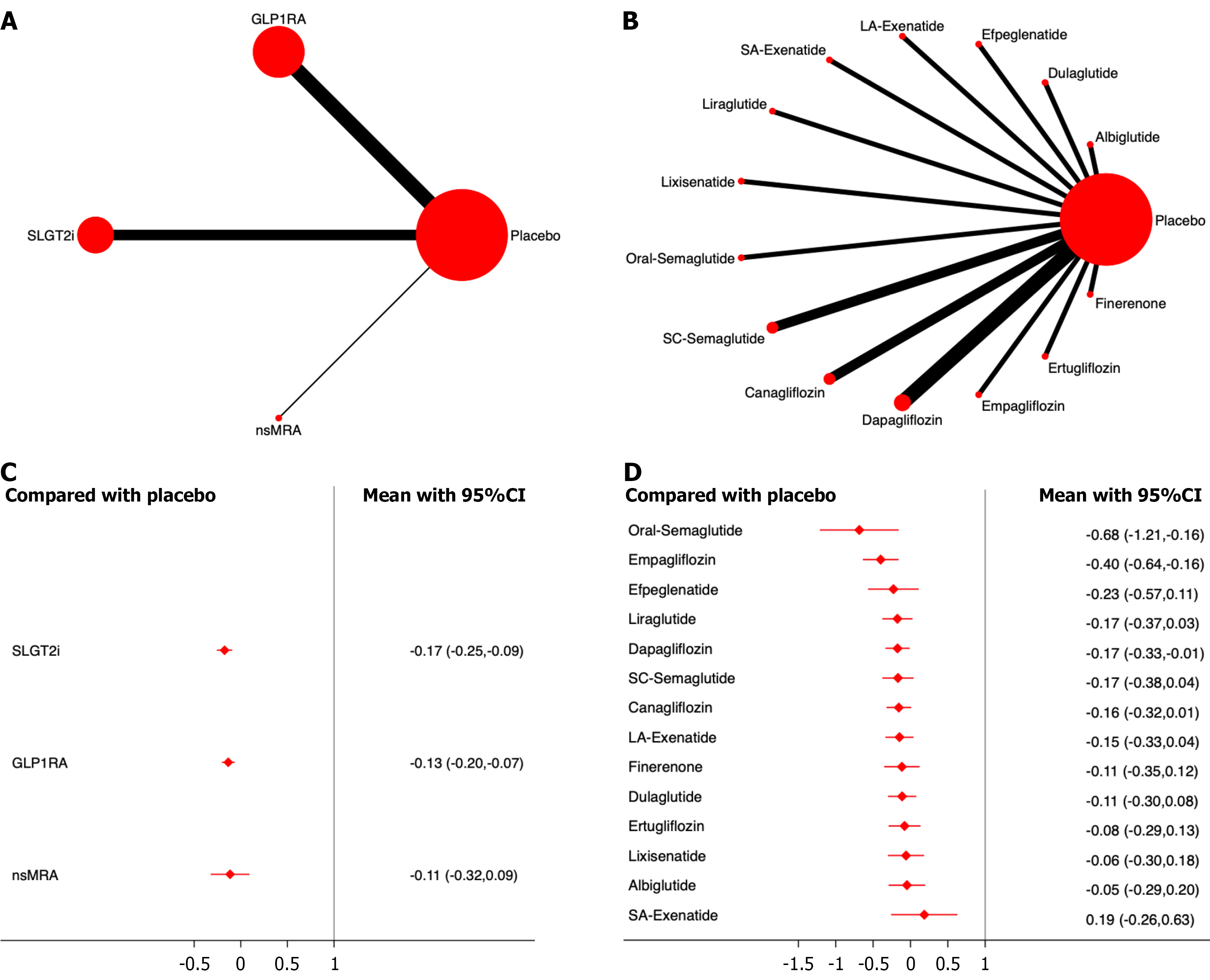
Twenty studies, including 137425 patients, were analyzed for CV mortality. The results showed that all three drug classes significantly reduced CV mortality compared with the placebo, with SGLT2i demonstrating the most significant effect (OR, -0.17; 95%CI: -0.27, -0.07; SUCRA, 73.3%). Overall, the quality of the studies was high (Figure 4; Supplementary material).
Among the different drugs, oral semaglutide showed the most significant efficacy (OR, -0.70; 95%CI: -1.35, -0.06; SUCRA, 94.0%). Among the SGLT2i, Empagliflozin had the highest efficacy ranking (OR, -0.31; 95%CI: -0.59, -0.03; SUCRA, 85.6%) (Figure 4; Supplementary material).
Stroke: Seventeen studies, including 129029 patients, were analyzed for stroke incidence. The results showed that all three drug classes significantly reduced stroke incidence compared with the placebo, with GLP-1RA demonstrating the most significant effect (OR, -0.15; 95%CI: -0.24, -0.05; SUCRA, 97.3%). Overall, the quality of the studies was high (Supplementary material).
Among the different drugs, dulaglutide showed the most significant efficacy (OR, -0.27; 95%CI: -0.53, -0.01; SUCRA, 79.7%). Among theSGLT2i, Canagliflozin had the highest efficacy (mean difference, -0.20; 95%CI: -0.42, 0.03; SUCRA, 95.0%) (Supplementary material).
Myocardial infarction: Seventeen studies, including 129029 patients, were analyzed for myocardial infarction. The results showed that all three drug classes significantly reduced the incidence of myocardial infarction compared to placebo, with nsMRA demonstrating the most significant effect (OR, -0.13; 95%CI: -0.32, 0.05; SUCRA, 74.8%). Overall, the quality of the studies was high (Supplementary material).
Among the different drugs, albiglutide showed the most significant efficacy (OR, -0.29; 95%CI: -0.49, -0.10; SUCRA, 91.1%). Among the SGLT2i, Empagliflozin had the highest efficacy (OR, -0.13; 95%CI: -0.36, 0.09; SUCRA, 71.0%) (Supplementary material).
Hospitalization for heart failure: Seventeen studies including 113358 patients were analyzed for hospitalization due to heart failure. The results showed that all three drug classes significantly reduced hospitalization rates compared with the placebo, with SGLT2i demonstrating the most significant effect (OR, -0.43; 95%CI: -0.52, -0.34; SUCRA, 99.9%). Overall, the quality of the studies was high (Supplementary material).
Among the different drugs, empagliflozin showed the most significant efficacy (OR, -0.54; 95%CI: -0.70, -0.38; SUCRA, 94.0%). Among the GLP-1RA drugs, liraglutide had the highest efficacy (OR, -0.13; 95%CI: -0.32, 0.05; SUCRA, 71.9%) (Supplementary material).
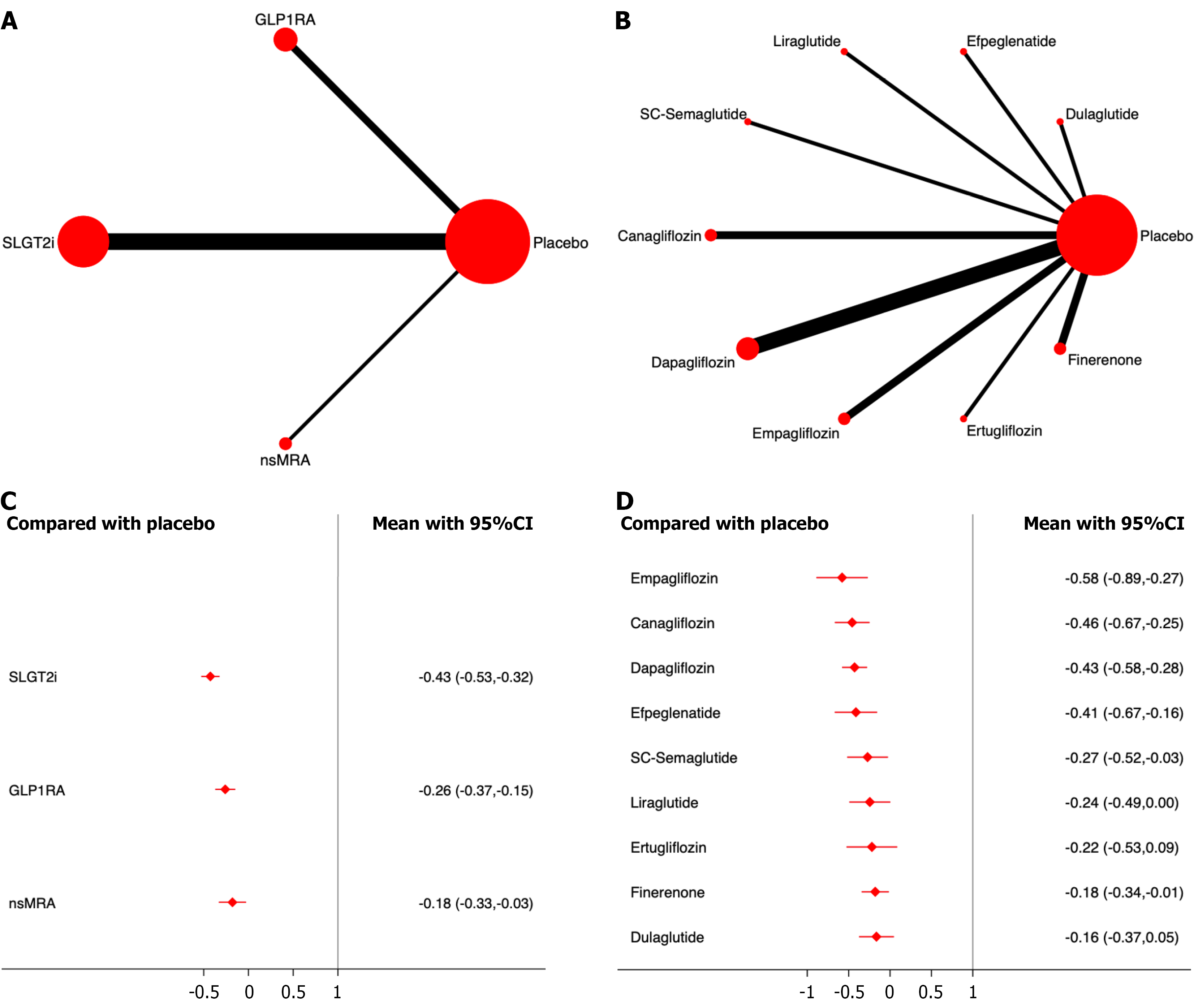
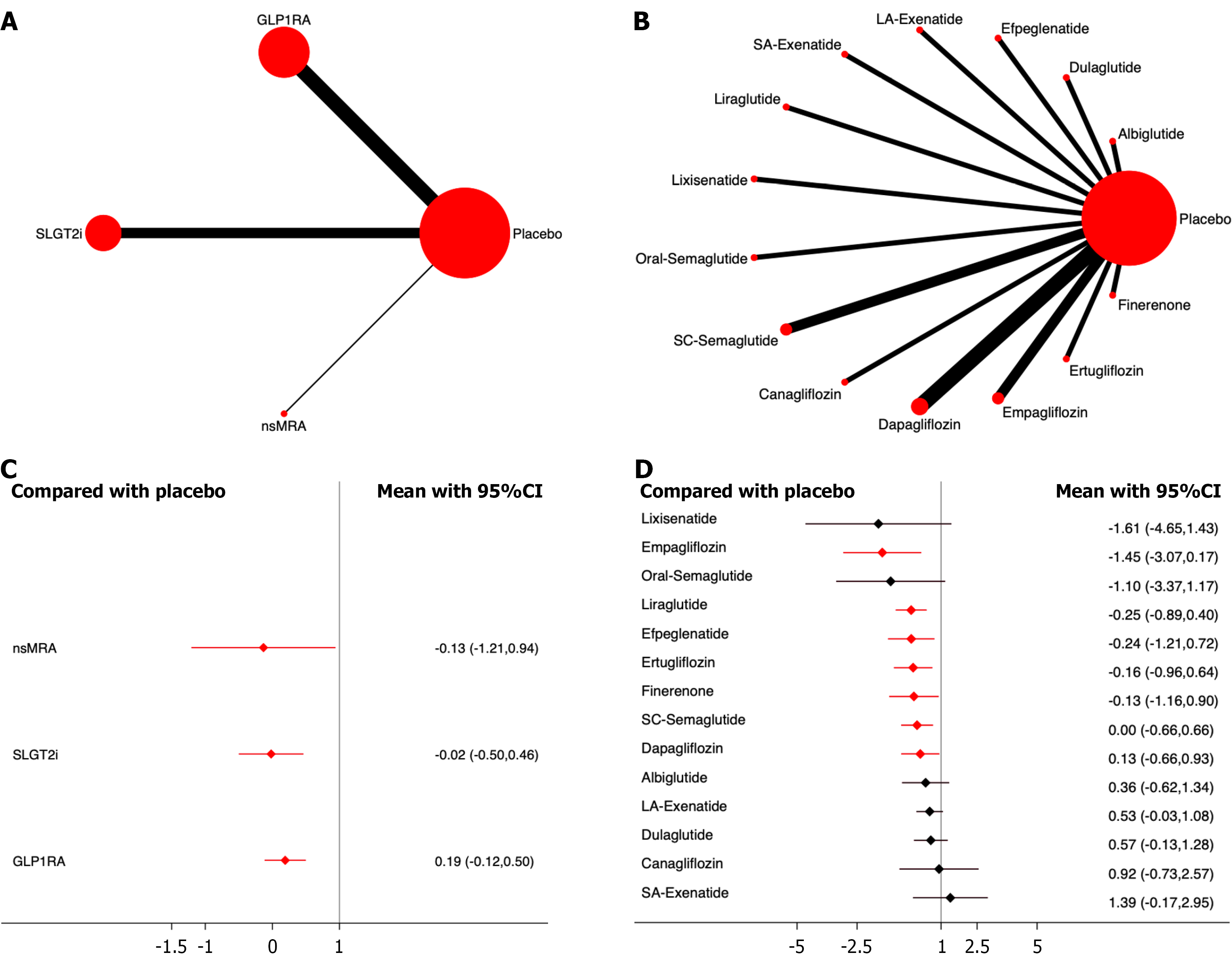
Composite renal events: Fifteen studies, including 110854 patients, were included in the analysis of composite renal events. The results showed that compared to placebo, all three drug classes significantly reduced the incidence of composite renal events. Among them, SGLT2i demonstrated the most significant effect on drug efficacy ranking (OR,
Among the different drugs, empagliflozin showed the most significant effect on drug efficacy (OR, -0.58 ;95%CI: -0.89,
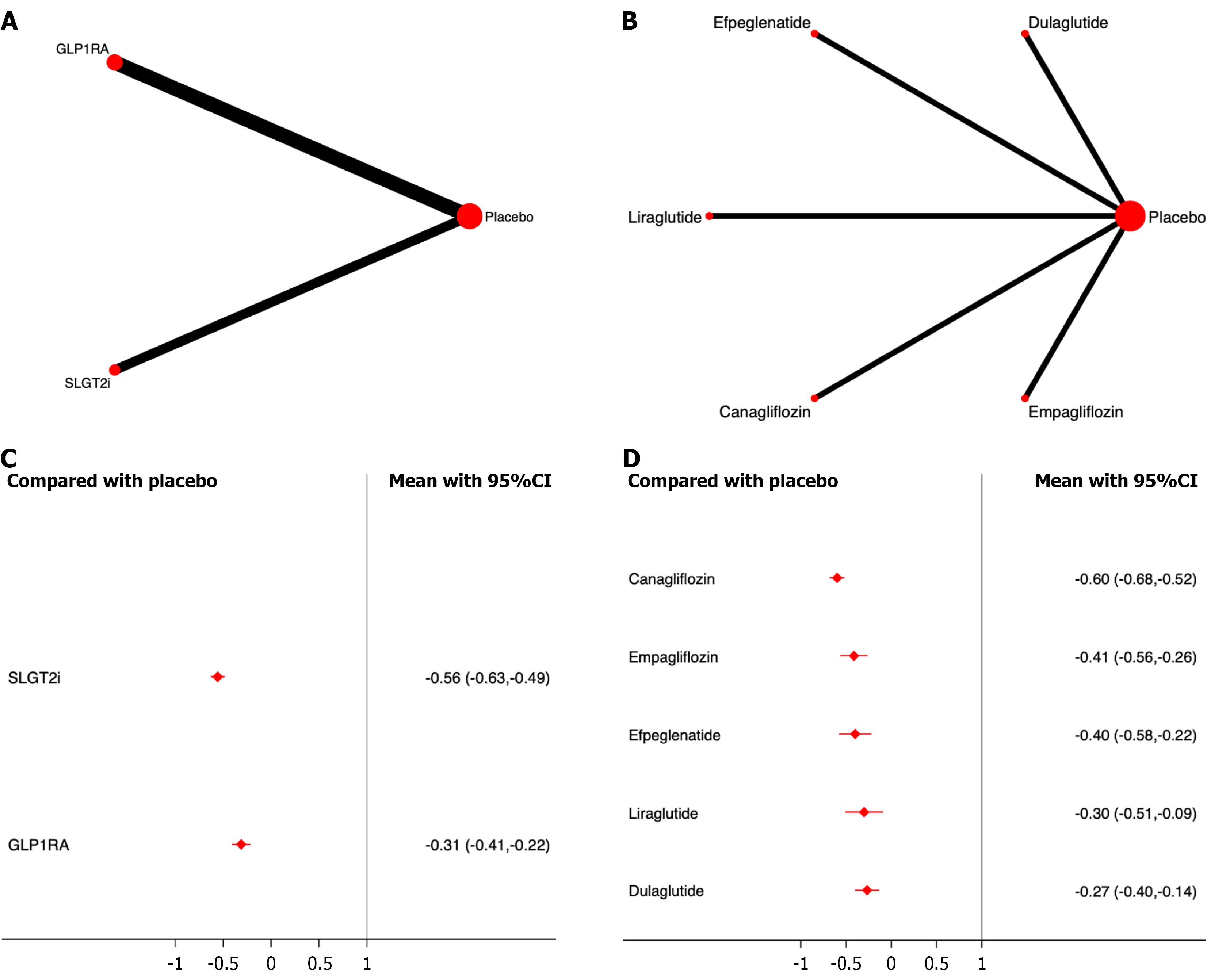
Progression of proteinuria: Five studies, including 40427 patients, were included in the analysis of proteinuria progression. The results showed that, compared to placebo, both SGLT2i and GLP-1RA significantly reduced the incidence of proteinuria progression, with SGLT2i demonstrating the most significant effect on drug efficacy ranking (OR, -0.56; 95%CI: -0.63, -0.49; SUCRA, 100.0%). The overall quality of the studies was high. (Figure 6; Supplementary material).
Among the different drugs, canagliflozin showed the most significant effect on drug efficacy (OR, -0.60; 95%CI: -0.68,
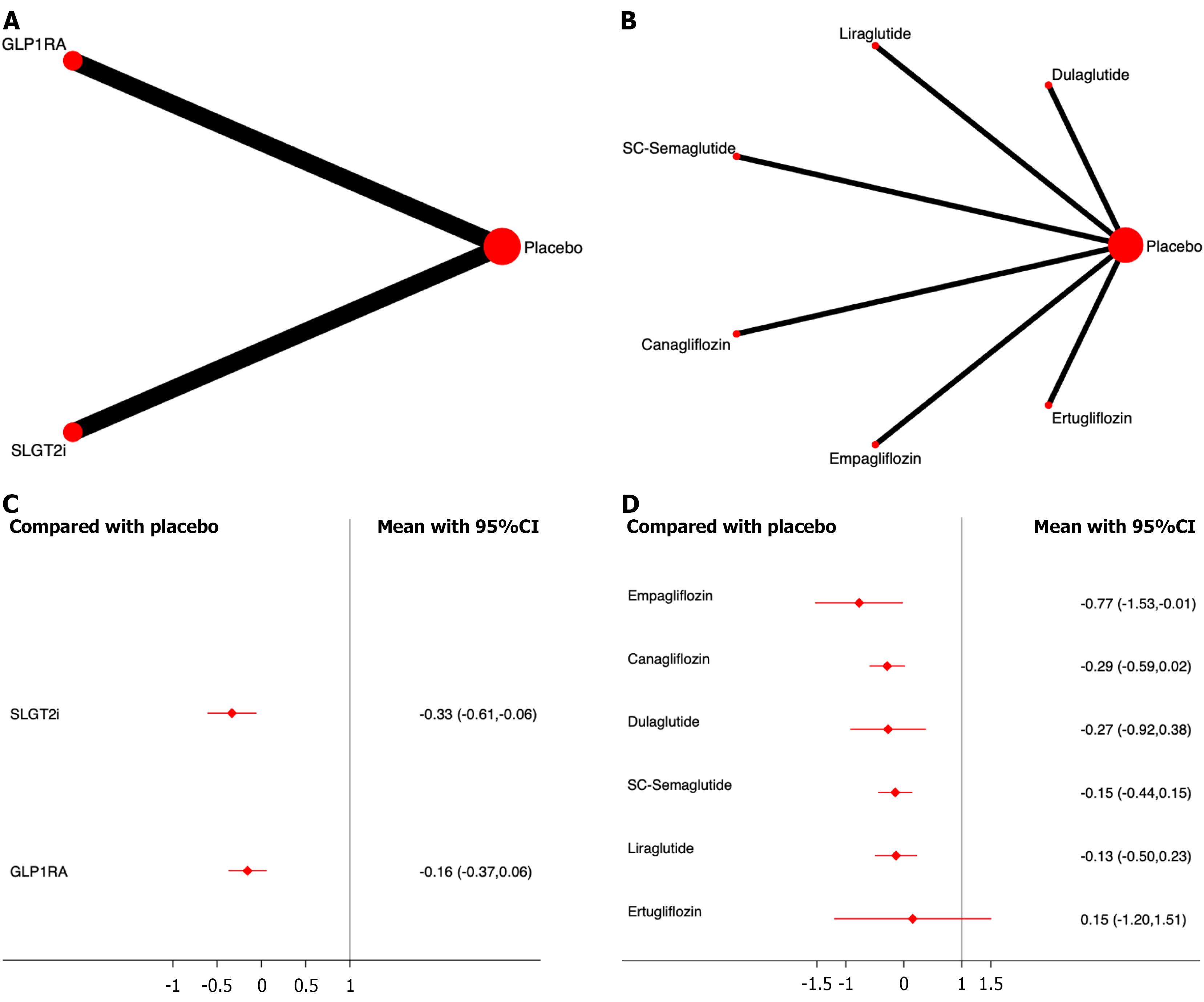
Renal replacement therapy: Six studies including 42389 patients were included in the analysis of renal replacement therapy. The results showed that, compared to placebo, both SGLT2i and GLP-1RA significantly reduced the incidence of renal replacement therapy, with SGLT2i demonstrating the most significant effect on drug efficacy ranking (OR, -0.33; 95%CI: -0.61, -0.06; SUCRA, 91.6%). The overall quality of the studies was high (Figure 7; Supplementary material).
Among the different drugs, empagliflozin showed the most significant effect on drug efficacy (OR, -0.77; 95%CI: -1.53,
End-stage renal disease: Five studies including 37493 patients were included in the analysis of end-stage renal disease. The results showed that, compared to placebo, both SGLT2i and nsMRA significantly reduced the incidence of end-stage renal disease, with SGLT2i demonstrating the most significant effect on drug efficacy ranking (OR, -0.41; 95%CI: -0.59,
Among different drugs, dapagliflozin showed the most significant effect in drug efficacy ranking (OR, -0.52; 95%CI:
Four studies, including 20960 patients, were included in the analysis of a sustained decrease in eGFR to < 15 mL/min/1.73 m2. The results showed that, compared to placebo, all three drug classes significantly reduced the incidence of sustained decrease in eGFR to < 15 mL/min/1.73 m2, with SGLT2i demonstrating the most significant effect in drug efficacy ranking (OR, -0.50; 95%CI: -0.78, -0.21; SUCRA, 95.5%). The overall quality of the studies was high.
Among the different drugs, canagliflozin showed the most significant effect on drug efficacy (OR, -0.50; 95%CI: -0.78,
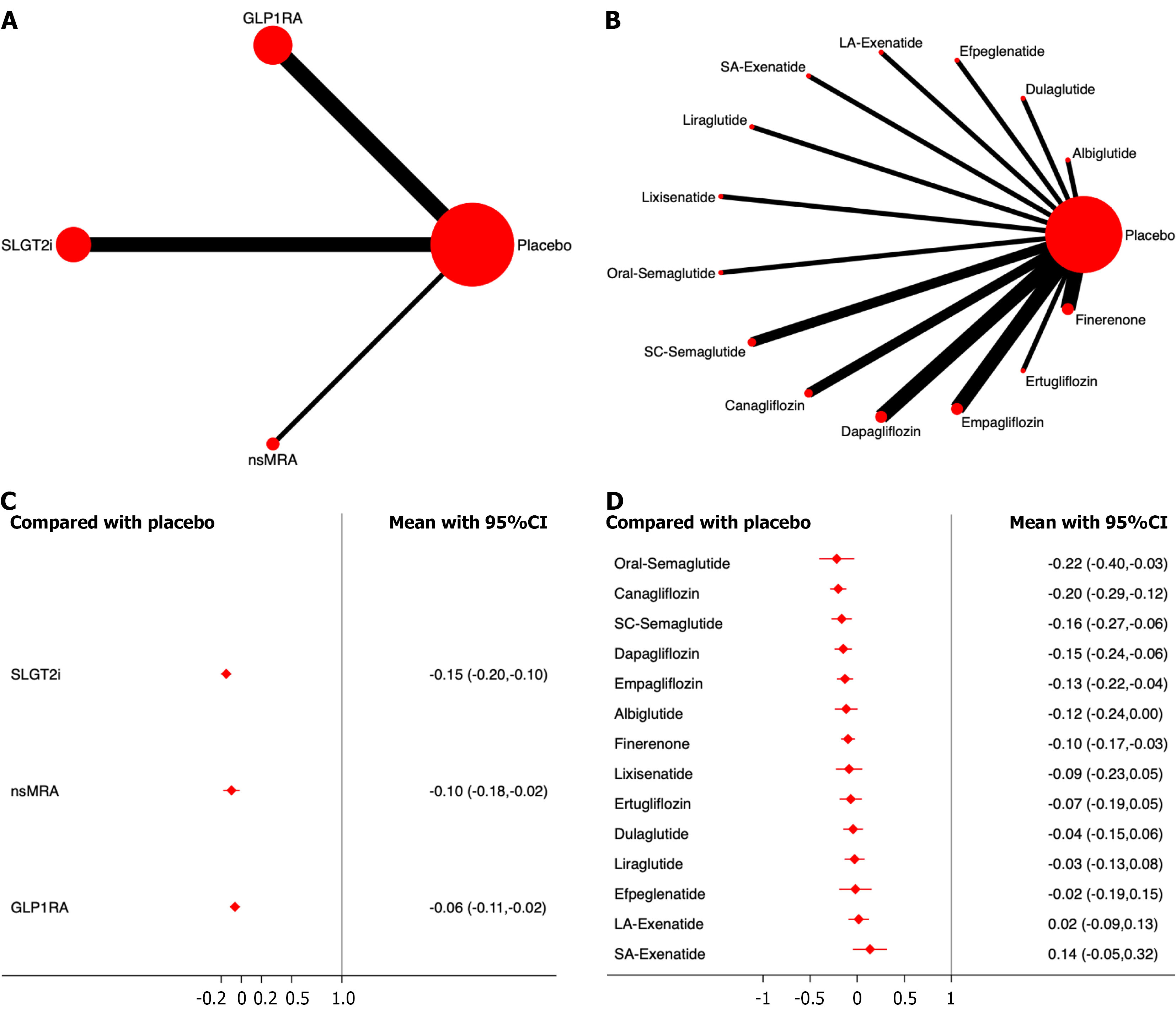
Serious adverse events: A total of 22 studies, including 147165 patients were included in the analysis of serious adverse events. The results showed that, compared with the placebo, none of the three drug classes significantly increased the incidence of serious adverse events. Among them, SGLT2i demonstrated the most significant effect on drug safety ranking (OR, -0.15; 95%CI: -0.20, -0.10; SUCRA, 95.0%). The overall quality of the studies was high.
None of the individual drugs significantly increased the incidence of serious adverse events. Among them, canagliflozin showed the most significant effect on the drug safety ranking (OR, -0.22; 95%CI: -0.40, -0.03; SUCRA, 89.0%). Among the different GLP-1RAs, oral semaglutide showed the most significant effect (OR, -0.22; 95%CI: -0.39, -0.05; SUCRA, 92.3%) (Figure 8; Supplementary material).
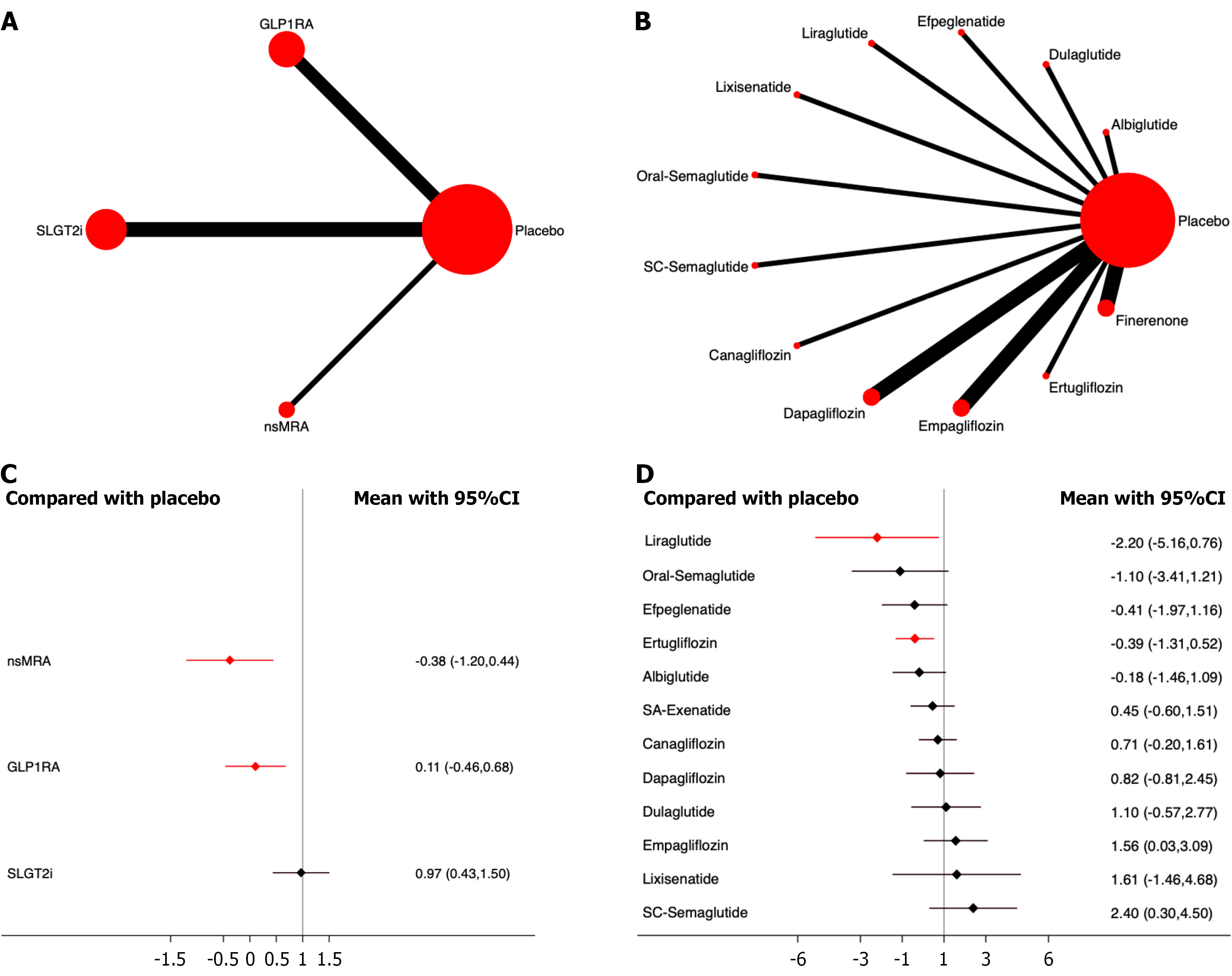
Diabetic ketoacidosis: Eighteen studies, including 114630 patients, were included in the analysis of diabetic ketoacidosis. The results showed that nsMRA had the most significant effect on the drug safety ranking (OR, -0.38; 95%CI: -1.20, 0.44; SUCRA 87.8%). The overall quality of the studies was high (Figure 9; Supplementary material).
None of the individual drugs significantly increased the incidence of diabetic ketoacidosis. Among them, Liraglutide showed the most significant effect in drug safety ranking (OR, -2.20; 95%CI: -5.16, 0.76; SUCRA, 92.3%) (Figure 9; Supplementary material).
Pancreatitis: Eighteen studies, including 116988 patients, were included in the analysis of pancreatitis. The results showed that, compared to placebo, nsMRA demonstrated the most significant effect on drug safety ranking (OR, -0.13; 95%CI: -1.21, 0.94; SUCRA, 62.4%). The overall quality of the studies was high.
None of the individual drugs significantly increased the incidence of diabetic ketoacidosis. Empagliflozin showed the most significant effect on drug safety ranking (OR, -1.45; 95%CI: -3.07, 0.17; SUCRA, 88.6%) (Figure 10; Supplementary material).
None of the included drugs significantly increased the incidence of the other adverse events of interest, including hypoglycemia, acute kidney injury, urinary tract infection, leg amputation, foot amputation, any cancer, and thyroid cancer (Supplementary material).
No drug class was associated with an increased incidence of any prespecified adverse event, supporting their overall clinical safety.
For the subgroup analysis, we conducted a separate analysis of subjects with baseline HbA1c ≥ 7%. The results showed that semaglutide significantly reduces the incidence of major serious CV events, stroke, and myocardial infarction. Efpeglenatide significantly reduced all-cause and CV mortality. Canagliflozin significantly reduced the incidence of hospitalization for heart failure, composite renal outcomes, progression of proteinuria, and sustained the decrease in eGFR to < 15 mL/min/1.73 m2. Dapagliflozin significantly reduced the incidence of end-stage renal disease, while liraglutide significantly reduces the incidence of renal replacement therapy (Supplementary material). These findings from subgroup analysis should be interpreted with caution due to limited sample sizes and post-hoc nature (Table 1).
| Intervention of studies | Albiglutide | Efpeglenatide | Long-acting Exenatide | Liraglutide | SC Semaglutide | Canagliflozin | Dapagliflozin | Ertugliflozin | Finerenone |
| Cardiovascular events | |||||||||
| Major adverse cardiovascular events | -0.26 (-0.41, -0.11) | -0.30 (-0.54, -0.07) | - | -0.15 (-0.27, -0.04) | -0.33 (-0.58, -0.07) | -0.18 (-0.30, -0.05) | - | 0.00 (-0.14, 0.14) | - |
| All-cause mortality | -0.05 (-0.25, 0.15) | -0.23 (-0.54, 0.08) | - | -0.17 (-0.32, -0.03) | 0.03 (-0.33, 0.40) | -0.13 (-0.29, 0.03) | - | -0.08 (-0.24, 0.08) | - |
| Cardiovascular mortality | -0.07 (-0.32, 0.19) | -0.30 (-0.66, 0.07) | - | -0.25 (-0.43, -0.07) | -0.05 (-0.46, 0.37) | -0.10 (-0.29, 0.09) | - | -0.08 (-0.27, 0.10) | - |
| Stroke | -0.14 (-0.42, 0.14) | -0.28 (-0.74, 0.18) | - | -0.14 (-0.35, 0.06) | -0.50 (-0.98, -0.01) | -0.20 (-0.42, 0.03) | - | 0.06 (-0.20, 0.32) | - |
| Myocardial infarction | -0.29 (-0.49, -0.10) | - | -0.25 (-0.59, 0.08) | -0.16 (-0.32, 0.00) | -0.32 (-0.70, 0.06) | -0.12 (-0.31, 0.07) | - | 0.05 (-0.15, 0.24) | - |
| Hospitalization for heart failure | - | - | - | -0.13 (-0.32, 0.05) | 0.09 (-0.28, 0.47) | -0.47 (-0.72, -0.22) | - | -0.37 (-0.63, -0.10) | - |
| Renal events | |||||||||
| Composite renal events | - | -0.41 (-0.59, -0.23) | - | -0.24 (-0.41, -0.08) | - | -0.50 (-0.75, -0.25) | - | -0.22 (-0.46, 0.03) | - |
| Progression of proteinuria | - | -0.40 (-0.58, -0.22) | - | -0.30 (-0.51, -0.09) | - | -0.60 (-0.68, -0.52) | - | - | - |
| End-stage renal disease | - | - | - | - | - | -0.38 (-0.81, 0.05) | -0.52 (-1.06, 0.03) | - | -0.26 (-0.63, 0.10) |
| Renal replacement therapy | - | - | - | -0.13 (-0.50, 0.23) | - | - | 0.15 (-1.20, 1.51) | ||
| Sustained decrease in eGFR to < 15 mL/min/1.73 m2 | - | - | - | - | -0.19 (-0.48, 0.10) | -0.50 (-0.78, -0.21) | - | - | -0.20 (-0.40, -0.01) |
We conducted meta-regression analyses on 12 outcomes, including the three most critical outcomes: Major adverse CV events, composite renal events, and serious adverse events. Meta-regression analysis showed that baseline comorbidities (presence of CVD/CKD) and HbA1c level (≥ 7% vs < 7%) were not significantly associated with the effect size for major CV or renal outcomes (P > 0.05) (Table 2).
| Intervention of studies | OR | 95%CI | t | P value |
| Major adverse cardiovascular events | ||||
| Comorbidities | 1.02 | 0.96-1.09 | 0.78 | 0.45 |
| HbA1c | 0.92 | 0.81-1.03 | -1.54 | 0.15 |
| All-cause mortality | ||||
| Comorbidities | 1.08 | 0.97-1.21 | 1.57 | 0.14 |
| HbA1c | 1.03 | 0.92-1.15 | 0.59 | 0.57 |
| Cardiovascular mortality | ||||
| Comorbidities | 1.05 | 0.96-1.15 | 1.24 | 0.23 |
| HbA1c | 1.01 | 0.86-1.18 | 0.08 | 0.93 |
| Stroke | ||||
| Comorbidities | 1.00 | 0.90-1.12 | -0.00 | 1.00 |
| HbA1c | 0.91 | 0.77-1.09 | -1.12 | 0.28 |
| Myocardial infarction | ||||
| Comorbidities | 1.00 | 0.91-1.08 | -0.14 | 0.89 |
| HbA1c | 0.91 | 0.79-1.04 | -1.51 | 0.16 |
| Hospitalization for heart failure | ||||
| Comorbidities | 1.06 | 0.94-1.20 | 1.05 | 0.31 |
| HbA1c | 0.94 | 0.73-1.21 | -0.50 | 0.62 |
| Composite renal events | ||||
| Comorbidities | 1.12 | 0.98-1.28 | 1.80 | 0.10 |
| HbA1c | 0.98 | 0.79-1.22 | -0.22 | 0.83 |
| Serious adverse events | ||||
| Comorbidities | 1.02 | 0.97-1.07 | 0.80 | 0.43 |
| HbA1c | 0.97 | 0.88-1.06 | -0.70 | 0.49 |
| Diabetic ketoacidosis | ||||
| Comorbidities | 0.93 | 0.32-2.68 | -0.15 | 0.88 |
| HbA1c | 0.70 | 0.11-4.28 | -0.43 | 0.67 |
| Pancreatitis | ||||
| Comorbidities | 0.94 | 0.67-1.33 | -0.37 | 0.72 |
| HbA1c | 0.62 | 0.34-1.13 | -1.71 | 0.11 |
| Hypoglycemia | ||||
| Comorbidities | 1.48 | 0.60-3.67 | 0.93 | 0.37 |
| HbA1c | 0.76 | 0.30-1.92 | -0.63 | 0.54 |
| Acute kidney injury | ||||
| Comorbidities | 1.14 | 0.99-1.31 | 1.94 | 0.07 |
| HbA1c | 1.07 | 0.84-1.38 | 0.62 | 0.55 |
To the best of our knowledge, this is the first network meta-analyses comparing the CV and renal benefits among GLP-1RA, SGLT2i, and nsMRA across high-quality (randomized controlled trials), long-term follow-up (1 year), large-sample RCTs (minimum sample size of 500 participants), including nsMRAs as a separate class in T2DM. Meta-regression analysis indicated that baseline factors such as comorbidities and blood glucose levels did not influence our results. The results indicated that most, but not all, agents demonstrated statistically significant CV and renal benefits compared with placebo. Regarding drug safety, the results showed that all drugs demonstrated good safety profiles, with no significant increase in adverse events compared to placebo. Overall, these agents demonstrated favorable tolerability and an acceptable risk-benefit profile.
Several basic studies support the conclusion that GLP-1RA, SGLT2i, and nsMRAs provide CV and renal benefits. The protective effects of GLP-1RA on the heart and kidneys are primarily attributed to the comprehensive regulation of risk factors such as blood glucose, lipids, and blood pressure[27,28]. The glucose-lowering effect of GLP-1RA mainly occurs through targeting α- and β-cells, suppressing glucagon release while stimulating insulin secretion, delaying gastric emptying, and promoting weight loss[29]. GLP-1RA can reduce lipid synthesis, enhance β-oxidation of free fatty acids, and promote autophagy of adipocytes to exert lipid-lowering effects[30], and can also lower blood pressure via natriuretic mechanisms[31]. Direct CV protective actions involve vasodilation, natriuresis, inhibition of smooth muscle cell proliferation, anti-inflammatory effects on macrophages, delayed cardiomyocyte pyroptosis, and inhibition of platelet activity, all of which prevent the onset and progression of atherosclerosis[32-34]. GLP-1RA may influence the renal function by increasing diuresis and natriuresis and maintaining renal function by reducing intraglomerular pressure, alleviating inflammation, and attenuating oxidative stress, thereby limiting fibrosis[35-38].
For SGLT2i, they can reduce blood glucose independent of insulin levels, correct dyslipidemia by lowering serum cholesterol, promote weight loss and uric acid excretion, and normalize blood pressure, thereby improving multiple CV risk factors[39]. In addition, SGLT2i has demonstrated pleiotropic effects in animal models such as reducing inflammation, promoting vascular remodeling, delaying vascular aging, and providing systemic cardiometabolic benefits[40]. SGLT2 inhibition increases urinary sodium excretion and delivery to the distal nephron, which is a key mechanism of renal protection by normalizing the tubuloglomerular feedback that drives hyperfiltration[41,42].
As a mineralocorticoid receptor antagonist, finerenone neutralizes aldosterone-induced sodium retention, potassium excretion, fibrosis, and inflammation, thereby conferring renal and CV protection[43]. For instance, finerenone may improve glucose tolerance by activating the AMPK-ATGL-UCP-1 signaling pathway[44]. Its CV benefits include antifibrotic effects on the cardiac tissue, reduction of sodium retention, and alleviation of volume overload[45-47]. The renoprotective effects of finerenone may be attributed to its anti-inflammatory, antioxidant, and antifibrotic properties, which delay the progression of diabetic kidney disease[48,49].
Our findings were based on high-quality, long-term follow-up, and large-sample clinical studies, all of which employed a randomized, placebo-controlled study design. The quality of the included studies was high, and we addressed missing data. However, we must acknowledge that all the included studies were placebo-controlled, which introduces a higher risk of bias in obtaining indirect evidence. Finally, no significant evidence of funnel plot asymmetry was found, supporting the stability and reliability of the findings.
However, we must also acknowledge that since our study excluded small trials and there were certain differences in study designs and population characteristics across the included trials, the results may still be affected by potential confounding factors. Although we validated the findings using subgroup analysis and meta-regression and the results were not influenced, the interpretation of our findings should be approached with caution.
Our findings provide insights into the clinical application of GLP-1RA, SGLT2i, and nsMRAs for the treatment of T2DM with CV and renal complications. All the included drugs exhibited favorable CV and renal benefits, and nsMRA demonstrated greater efficacy in reducing composite CV events and myocardial infarction. Furthermore, these results may only be applicable to patients with CKD, as all evidence comes from this population. SGLT2i were more effective in reducing all-cause mortality, CV mortality, and renal outcomes. GLP-1RA was more effective at reducing stroke. Among the 14 drugs analyzed, the following exhibited superior effects in specific outcomes: SC-Semaglutide: Best for reducing major adverse CV events; Oral semaglutide: Best for reducing all-cause and CV mortality; dulaglutide: Best for reducing stroke incidence; Albiglutide: Best for reducing myocardial infarction incidence; Empagliflozin: Best for reducing hospitalization for heart failure, composite renal outcomes, and renal replacement therapy; Canagliflozin: Best for reducing proteinuria progression and sustained decrease in eGFR to < 15 mL/min/1.73 m²; Dapagliflozin: Best for reducing end-stage renal disease. Additionally, all included drugs exhibited good safety profiles. Our findings provide high-quality evidence to support precise drug selection for treating T2DM patients with CV and renal complications.
Although previous meta-analyses have compared GLP-1RAs and SGLT2is, few have simultaneously evaluated nsMRAs using a unified methodological framework. This inclusion distinguishes our study from prior reports. Several studies have conducted network meta-analyses to compare the CV and renal benefits of novel antidiabetic drugs[20-23,25,50-55]. Some results align with our findings, indicating that GLP-1RA are more likely to reduce CV events, while SGLT2i are more effective in improving renal outcomes. However, when categorizing different drugs, some variations emerged as follows: NsMRA was more effective in reducing composite CV events and myocardial infarction; SGLT2i demonstrated superior efficacy in reducing all-cause mortality, CV mortality, and renal outcomes; GLP-1RA exhibited greater efficacy in reducing the risk of stroke. These differences may be attributable to variations in the included studies, clinical characteristics of the patients, concomitant treatments, and drug-specific effects. For example, SA-exenatide weakened the overall effect of GLP-1RA. Therefore, we compared different drugs within each class to provide high-quality evidence for clinical decision-making in the treatment of T2DM with CV and renal complications.
Although our study presents important findings, several issues require further investigation: Potential confounding factors and biases. Because of the heterogeneity of the included studies (due to heterogeneity in study design methods, enrolled populations, and other potential confounding factors) and data limitations, we cannot entirely rule out potential confounders and biases. Although we minimized this risk by searching multiple databases and conducting sensitivity analyses, there is a risk of publication bias as our study was primarily based on published literature.
In summary, GLP-1RAs, SGLT2is, and nsMRAs each confer distinct cardiovascular and renal benefits in T2DM. The comparative ranking from this network meta-analysis supports individualized treatment selection based on specific clinical priorities and comorbidities. Given the limitations in the number and quality of the included studies and the potential heterogeneity of the different studies, our conclusions should be interpreted with caution. Large-scale high-quality clinical trials are needed to validate our findings and further optimize the comprehensive management of CV and renal complications in patients with T2DM.
| 1. | Lovre D, Shah S, Sihota A, Fonseca VA. Managing Diabetes and Cardiovascular Risk in Chronic Kidney Disease Patients. Endocrinol Metab Clin North Am. 2018;47:237-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143:1157-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 1293] [Article Influence: 258.6] [Reference Citation Analysis (2)] |
| 3. | Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS, Coresh J, Mathew RO, Baker-Smith CM, Carnethon MR, Despres JP, Ho JE, Joseph JJ, Kernan WN, Khera A, Kosiborod MN, Lekavich CL, Lewis EF, Lo KB, Ozkan B, Palaniappan LP, Patel SS, Pencina MJ, Powell-Wiley TM, Sperling LS, Virani SS, Wright JT, Rajgopal Singh R, Elkind MSV; American Heart Association. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation. 2023;148:1606-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 904] [Article Influence: 301.3] [Reference Citation Analysis (0)] |
| 4. | Wong ND, Sattar N. Cardiovascular risk in diabetes mellitus: epidemiology, assessment and prevention. Nat Rev Cardiol. 2023;20:685-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 249] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 5. | de Boer IH, Khunti K, Sadusky T, Tuttle KR, Neumiller JJ, Rhee CM, Rosas SE, Rossing P, Bakris G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care. 2022;45:3075-3090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 477] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 6. | Ostrominski JW, Arnold SV, Butler J, Fonarow GC, Hirsch JS, Palli SR, Donato BMK, Parrinello CM, O'Connell T, Collins EB, Woolley JJ, Kosiborod MN, Vaduganathan M. Prevalence and Overlap of Cardiac, Renal, and Metabolic Conditions in US Adults, 1999-2020. JAMA Cardiol. 2023;8:1050-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 155] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 7. | Davies MJ, Aroda VR, Collins BS, Gabbay RA, Green J, Maruthur NM, Rosas SE, Del Prato S, Mathieu C, Mingrone G, Rossing P, Tankova T, Tsapas A, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45:2753-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 1062] [Article Influence: 265.5] [Reference Citation Analysis (1)] |
| 8. | ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA; on behalf of the American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46:S140-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 587] [Article Influence: 195.7] [Reference Citation Analysis (1)] |
| 9. | Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, Christodorescu RM, Crawford C, Di Angelantonio E, Eliasson B, Espinola-Klein C, Fauchier L, Halle M, Herrington WG, Kautzky-Willer A, Lambrinou E, Lesiak M, Lettino M, McGuire DK, Mullens W, Rocca B, Sattar N; ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44:4043-4140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 871] [Article Influence: 290.3] [Reference Citation Analysis (0)] |
| 10. | Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, Kolkhof P, Nowack C, Gebel M, Ruilope LM, Bakris GL; FIDELIO-DKD and FIGARO-DKD investigators. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43:474-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 722] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 11. | Seufert J, Gallwitz B. The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes Metab. 2014;16:673-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, Lam CSP, Lopes RD, McMurray JJV, Pratley RE, Rosenstock J, Gerstein HC. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 1034] [Article Influence: 206.8] [Reference Citation Analysis (0)] |
| 13. | DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17:319-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 399] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 14. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 2033] [Article Influence: 290.4] [Reference Citation Analysis (0)] |
| 15. | Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, McMurray JJV, Solomon SD. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400:757-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 583] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 16. | Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788-1801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 627] [Article Influence: 156.8] [Reference Citation Analysis (0)] |
| 17. | Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, Zannad F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 402] [Article Influence: 80.4] [Reference Citation Analysis (8)] |
| 18. | Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, Kolkhof P, Nowack C, Schloemer P, Joseph A, Filippatos G; FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020;383:2219-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1696] [Cited by in RCA: 1756] [Article Influence: 292.7] [Reference Citation Analysis (1)] |
| 19. | Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, Joseph A, Kolkhof P, Nowack C, Schloemer P, Ruilope LM; FIGARO-DKD Investigators. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med. 2021;385:2252-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 1037] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 20. | Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, Tunnicliffe D, Ruospo M, Natale P, Saglimbene V, Nicolucci A, Johnson DW, Tonelli M, Rossi MC, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque LI, Lloyd A, Ahmad N, Liu Y, Tiv S, Millard T, Gagliardi L, Kolanu N, Barmanray RD, McMorrow R, Raygoza Cortez AK, White H, Chen X, Zhou X, Liu J, Rodríguez AF, González-Colmenero AD, Wang Y, Li L, Sutanto S, Solis RC, Díaz González-Colmenero F, Rodriguez-Gutierrez R, Walsh M, Guyatt G, Strippoli GFM. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 442] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 21. | Zhang Y, Jiang L, Wang J, Wang T, Chien C, Huang W, Fu X, Xiao Y, Fu Q, Wang S, Zhao J. Network meta-analysis on the effects of finerenone versus SGLT2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus and chronic kidney disease. Cardiovasc Diabetol. 2022;21:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 22. | Yang Q, Lang Y, Yang W, Yang F, Yang J, Wu Y, Xiao X, Qin C, Zou Y, Zhao Y, Kang D, Liu F. Efficacy and safety of drugs for people with type 2 diabetes mellitus and chronic kidney disease on kidney and cardiovascular outcomes: A systematic review and network meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2023;198:110592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 23. | Neuen BL, Fletcher RA, Heath L, Perkovic A, Vaduganathan M, Badve SV, Tuttle KR, Pratley R, Gerstein HC, Perkovic V, Heerspink HJL. Cardiovascular, Kidney, and Safety Outcomes With GLP-1 Receptor Agonists Alone and in Combination With SGLT2 Inhibitors in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Circulation. 2024;150:1781-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 24. | Tsukamoto S, Morita R, Yamada T, Urate S, Azushima K, Uneda K, Kobayashi R, Kanaoka T, Wakui H, Tamura K. Cardiovascular and kidney outcomes of combination therapy with sodium-glucose cotransporter-2 inhibitors and mineralocorticoid receptor antagonists in patients with type 2 diabetes and chronic kidney disease: A systematic review and network meta-analysis. Diabetes Res Clin Pract. 2022;194:110161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Cao H, Liu T, Wang L, Ji Q. Comparative efficacy of novel antidiabetic drugs on cardiovascular and renal outcomes in patients with diabetic kidney disease: A systematic review and network meta-analysis. Diabetes Obes Metab. 2022;24:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9:e99682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 676] [Cited by in RCA: 1099] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 27. | An X, Sun W, Wen Z, Duan L, Zhang Y, Kang X, Ji H, Sun Y, Jiang L, Zhao X, Gao Q, Lian F. Comparison of the efficacy and safety of GLP-1 receptor agonists on cardiovascular events and risk factors: A review and network meta-analysis. Diabetes Obes Metab. 2025;27:1735-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Wen Z, Sun W, Wang H, Chang R, Wang J, Song C, Zhang S, Ni Q, An X. Comparison of the effectiveness and safety of GLP-1 receptor agonists for type 2 diabetes mellitus patients with overweight/obesity: A systematic review and network meta-analysis. Diabetes Res Clin Pract. 2025;222:111999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Yamagishi S, Matsui T. Pleiotropic effects of glucagon-like peptide-1 (GLP-1)-based therapies on vascular complications in diabetes. Curr Pharm Des. 2011;17:4379-4385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Rochoń J, Kalinowski P, Szymanek-Majchrzak K, Grąt M. Role of gut-liver axis and glucagon-like peptide-1 receptor agonists in the treatment of metabolic dysfunction-associated fatty liver disease. World J Gastroenterol. 2024;30:2964-2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (4)] |
| 31. | Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, Low PA. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R874-R880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Ferhatbegović L, Mršić D, Macić-Džanković A. The benefits of GLP1 receptors in cardiovascular diseases. Front Clin Diabetes Healthc. 2023;4:1293926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 33. | Pandey S, Mangmool S, Parichatikanond W. Multifaceted Roles of GLP-1 and Its Analogs: A Review on Molecular Mechanisms with a Cardiotherapeutic Perspective. Pharmaceuticals (Basel). 2023;16:836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 34. | Ma X, Liu Z, Ilyas I, Little PJ, Kamato D, Sahebka A, Chen Z, Luo S, Zheng X, Weng J, Xu S. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci. 2021;17:2050-2068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 35. | Wu Q, Zhang J, Zhang F, Li D. SGLT2 inhibitors as metabolic modulators: beyond glycemic control in type 2 diabetes. Front Endocrinol (Lausanne). 2025;16:1601633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 36. | Andrikou E, Tsioufis C, Andrikou I, Leontsinis I, Tousoulis D, Papanas N. GLP-1 receptor agonists and cardiovascular outcome trials: An update. Hellenic J Cardiol. 2019;60:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Alicic RZ, Cox EJ, Neumiller JJ, Tuttle KR. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol. 2021;17:227-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 38. | Sawami K, Tanaka A, Node K. Updated evidence on cardiovascular and renal effects of GLP-1 receptor agonists and combination therapy with SGLT2 inhibitors and finerenone: a narrative review and perspectives. Cardiovasc Diabetol. 2024;23:410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 39. | Xu J, Hirai T, Koya D, Kitada M. Effects of SGLT2 Inhibitors on Atherosclerosis: Lessons from Cardiovascular Clinical Outcomes in Type 2 Diabetic Patients and Basic Researches. J Clin Med. 2021;11:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 40. | Liu Z, Ma X, Ilyas I, Zheng X, Luo S, Little PJ, Kamato D, Sahebkar A, Wu W, Weng J, Xu S. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: from pharmacology to pre-clinical and clinical therapeutics. Theranostics. 2021;11:4502-4515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 41. | Aristizábal-Colorado D, Ocampo-Posada M, Rivera-Martínez WA, Corredor-Rengifo D, Rico-Fontalvo J, Gómez-Mesa JE, Duque-Ossman JJ, Abreu-Lomba A. SGLT2 Inhibitors and How They Work Beyond the Glucosuric Effect. State of the Art. Am J Cardiovasc Drugs. 2024;24:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 42. | Fonseca-Correa JI, Correa-Rotter R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front Med (Lausanne). 2021;8:777861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 43. | Pitt B, Filippatos G, Gheorghiade M, Kober L, Krum H, Ponikowski P, Nowack C, Kolkhof P, Kim SY, Zannad F. Rationale and design of ARTS: a randomized, double-blind study of BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease. Eur J Heart Fail. 2012;14:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Marzolla V, Feraco A, Gorini S, Mammi C, Marrese C, Mularoni V, Boitani C, Lombès M, Kolkhof P, Ciriolo MR, Armani A, Caprio M. The novel non-steroidal MR antagonist finerenone improves metabolic parameters in high-fat diet-fed mice and activates brown adipose tissue via AMPK-ATGL pathway. FASEB J. 2020;34:12450-12465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | Zhai S, Ma B, Chen W, Zhao Q. A comprehensive review of finerenone-a third-generation non-steroidal mineralocorticoid receptor antagonist. Front Cardiovasc Med. 2024;11:1476029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 46. | Dutzmann J, Musmann RJ, Haertlé M, Daniel JM, Sonnenschein K, Schäfer A, Kolkhof P, Bauersachs J, Sedding DG. The novel mineralocorticoid receptor antagonist finerenone attenuates neointima formation after vascular injury. PLoS One. 2017;12:e0184888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Grune J, Beyhoff N, Smeir E, Chudek R, Blumrich A, Ban Z, Brix S, Betz IR, Schupp M, Foryst-Ludwig A, Klopfleisch R, Stawowy P, Houtman R, Kolkhof P, Kintscher U. Selective Mineralocorticoid Receptor Cofactor Modulation as Molecular Basis for Finerenone's Antifibrotic Activity. Hypertension. 2018;71:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 48. | Kim DL, Lee SE, Kim NH. Renal Protection of Mineralocorticoid Receptor Antagonist, Finerenone, in Diabetic Kidney Disease. Endocrinol Metab (Seoul). 2023;38:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 49. | Yao L, Liang X, Liu Y, Li B, Hong M, Wang X, Chen B, Liu Z, Wang P. Non-steroidal mineralocorticoid receptor antagonist finerenone ameliorates mitochondrial dysfunction via PI3K/Akt/eNOS signaling pathway in diabetic tubulopathy. Redox Biol. 2023;68:102946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 50. | Drake T, Landsteiner A, Langsetmo L, MacDonald R, Anthony M, Kalinowski C, Ullman K, Billington CJ, Kaka A, Sultan S, Wilt TJ. Newer Pharmacologic Treatments in Adults With Type 2 Diabetes: A Systematic Review and Network Meta-analysis for the American College of Physicians. Ann Intern Med. 2024;177:618-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 51. | Kanie T, Mizuno A, Takaoka Y, Suzuki T, Yoneoka D, Nishikawa Y, Tam WWS, Morze J, Rynkiewicz A, Xin Y, Wu O, Providencia R, Kwong JS. Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis. Cochrane Database Syst Rev. 2021;10:CD013650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 52. | Lin DS, Lee JK, Hung CS, Chen WJ. The efficacy and safety of novel classes of glucose-lowering drugs for cardiovascular outcomes: a network meta-analysis of randomised clinical trials. Diabetologia. 2021;64:2676-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 53. | Yamada T, Wakabayashi M, Bhalla A, Chopra N, Miyashita H, Mikami T, Ueyama H, Fujisaki T, Saigusa Y, Yamaji T, Azushima K, Urate S, Suzuki T, Abe E, Wakui H, Tamura K. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol. 2021;20:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 54. | D'Andrea E, Kesselheim AS, Franklin JM, Jung EH, Hey SP, Patorno E. Heterogeneity of antidiabetic treatment effect on the risk of major adverse cardiovascular events in type 2 diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2020;19:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Fei Y, Tsoi MF, Cheung BMY. Cardiovascular outcomes in trials of new antidiabetic drug classes: a network meta-analysis. Cardiovasc Diabetol. 2019;18:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/