Published online Nov 15, 2025. doi: 10.4239/wjd.v16.i11.109919
Revised: July 31, 2025
Accepted: September 29, 2025
Published online: November 15, 2025
Processing time: 149 Days and 23.5 Hours
Diabetic nephropathy (DN) is one of the most serious microvascular complications of type 2 diabetes mellitus (T2DM), and its incidence increases with the global rise in diabetes prevalence. It is the leading cause of chronic kidney disease and end-stage kidney disease. Patients with DN often experience complex metabolic disorders and chronic inflammatory states, which not only accelerate the decline of renal function but are also closely related to complications such as cardiovascular events and osteoporosis (OP), seriously compromising quality of life. With the in-depth research on the gut microbiota and the emergence of concepts such as the "gut-kidney axis" and the "enteric-bone axis", the key roles of the gut microbiota and its metabolites in metabolic disorders, inflammatory responses, and target organ damage have been increasingly recognized. However, the specific role of gut microbiota in the pathogenesis of DN remains to be further explored. The results obtained may provide evidence to better understand the pathogenesis of DN and to identify high-risk populations at an early stage. This research direction is of strategic significance.
To assess the correlation of the gut microbiota metabolite trimethylamine N-oxide (TMAO) with inflammatory marker levels and OP in patients with DN.
A total of 115 patients diagnosed with type 2 DN and treated at the Department of Endocrinology, Second Affiliated Hospital of Shandong First Medical University from August 2022 to December 2024 were enrolled in the DN group, and 115 patients with T2DM without nephropathy were included in the T2DM group. The two groups were compared in terms of gastrointestinal microbiota abundance and relative abundance at the genus level; levels of TMAO, inflammatory markers [including C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α (TNF-α)], and bone metabolism markers [including procollagen type I N-terminal propeptide (PINP), β-CrossLaps (β-CTX), and alkaline phosphatase (ALP)]; and lumbar spine and hip bone mineral density (BMD). The correlation of TMAO level with inflammatory factor and bone metabolism indicator levels was further analyzed.
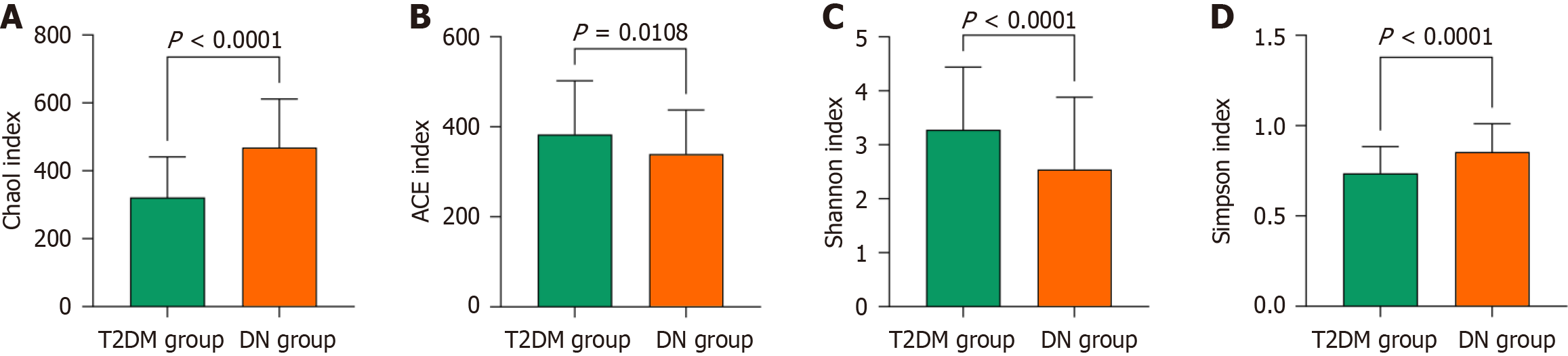
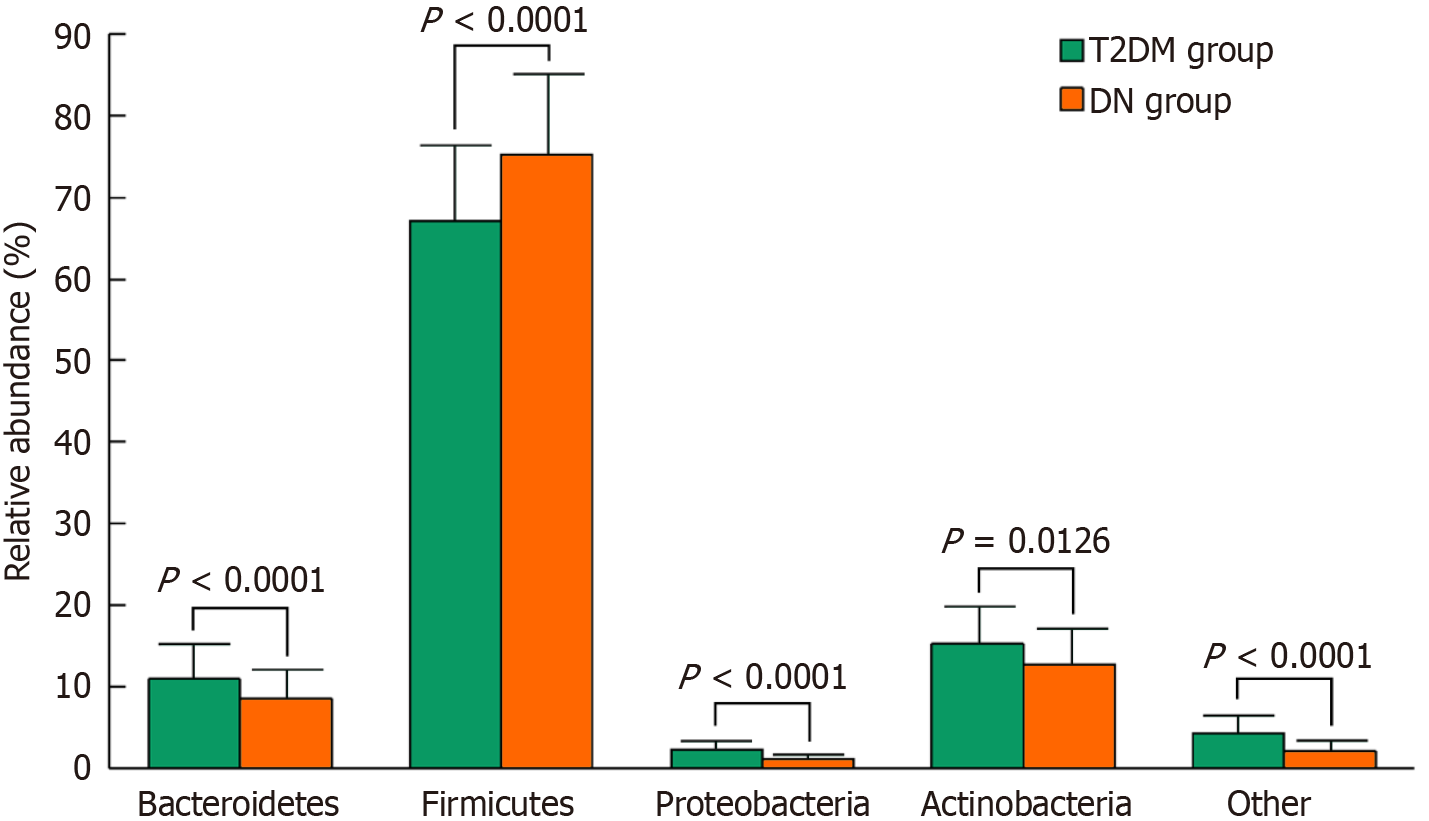
The DN group had higher Chao1 and Simpson indices of gastrointestinal microbiota diversity than the T2DM group, whereas the ACE and Shannon indices were lower (P < 0.05). The relative abundance of Firmicutes was higher, and the relative abundances of Bacteroidetes, Proteobacteria, and Actinobacteria were lower in the DN group than in the T2DM group (P < 0.05). CRP, IL-6, IL-8, TNF-α, and TMAO levels were considerably elevated in the DN group compared to the T2DM group (P < 0.05). Moreover, the DN group had higher levels of bone turnover markers-including PINP, β-CTX, and ALP-but lower lumbar spine and hip BMDs than the T2DM group (P < 0.05). TMAO level positively correlated with the Chao1 and Simpson indices and negatively correlated with the ACE and Shannon indices of gut microbiota diversity. TMAO level also negatively correlated with the relative abundances of Bacteroidetes, Proteobacteria, and Actinobacteria and positively correlated with the abundance of Firmicutes. Additionally, TMAO level positively correlated with the inflammatory markers CRP, IL-6, IL-8, and TNF-α, as well as with the bone turnover markers PINP, β-CTX, and ALP. It negatively correlated with lumbar spine and hip BMDs (P < 0.05).
Inflammatory and bone metabolic levels in patients with DN were found to be associated with the gut microbiota–derived metabolite TMAO. Elevated TMAO levels may mediate inflammatory responses and bone metabolism disorders in patients with DN, thereby contributing to the progression of systemic inflammation and OP.
Core Tip: In recent years, with in-depth research on the gut microbiota and the emergence of concepts such as the "gut-kidney axis" and the "intestinal-bone axis", the key role of the gut microbiota and its metabolites in metabolic disorders, inflammatory responses, and target organ damage has been increasingly recognized. Trimethylamine N-oxide (TMAO) is the main metabolite produced by the gut microbiota from dietary nutrients such as phosphatidylcholine and choline, and has attracted attention due to its participation in the pathogenesis of diabetic nephropathy (DN). In this study, we aim to explore the correlation between TMAO levels and the levels of inflammatory markers and osteoporosis (OP) in patients with DN, providing a theoretical and practical basis for the early identification of high-risk patients and timely clinical intervention. The research results confirm that elevated TMAO levels may mediate the inflammatory response and bone metabolism disorder in patients with DN, thereby promoting the progression of systemic inflammation and OP.
- Citation: Pan ZL, Li MQ, Zhang J, Xue LY, Shi YP. Correlation of gut microbiota metabolite trimethylamine N-oxide with inflammatory levels and osteoporosis in patients with diabetic nephropathy. World J Diabetes 2025; 16(11): 109919
- URL: https://www.wjgnet.com/1948-9358/full/v16/i11/109919.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i11.109919
Diabetic nephropathy (DN) is one of the most serious microvascular complications of type 2 diabetes mellitus (T2DM). Its development is primarily caused by prolonged hyperglycemia, which damages the renal microvasculature and filtration system. Key pathogenic mechanisms underlying the progression from T2DM to DN include persistent hyperglycemia, glomerular hyperfiltration and hypertension, metabolic disturbances, glomerulosclerosis, and tubulointerstitial fibrosis[1]. The kidneys are the key organs responsible for synthesizing active vitamin D. In DN, declining renal function leads to reduced production of active vitamin D, which in turn decreases intestinal calcium absorption and lowers serum calcium levels. This hypocalcemia stimulates parathyroid hormone secretion, accelerating bone resorption and resulting in net bone loss. Consequently, it impairs osteoblast function, induces apoptosis of bone cells, and increases the risk of osteoporosis (OP) in affected patients[2]. Relevant studies have shown that gut microbiota imbalance plays a critical role in the development and progression of both DN and OP. For instance, short-chain fatty acids produced by the fermentation of dietary fiber by bacteria like Bifidobacterium are directly associated with bone resorption, bone formation, and calcium absorption[3]. Additionally, Bacteroides species are involved in the synthesis of vitamin K2, which is essential for promoting bone mineralization and inhibiting vascular calcification[4]. An increase in harmful bacteria can disrupt the Th17/Treg cell balance, thereby affecting the regulation of bone metabolism[5]. Gut microbiota dysbiosis can also compromise intestinal barrier integrity, allowing lipopolysaccharides (LPS) to enter the bloodstream and activate inflammatory pathways. Additionally, the accumulation of protein fermentation byproducts can be converted into uremic toxins, inducing nephrotoxicity. Furthermore, dysregulated gut microbiota can alter bile acid composition, leading to imbalanced activation of FXR and TGR5 receptors, which exacerbates renal inflammation and fibrosis[6]. However, the interaction mechanisms between gut microbiota and the development of DN and OP are highly complex. The involvement of numerous related indicators and the technical complexity of microbiota analysis have limited the clinical application of microbiota-targeted therapies for DN and OP. Trimethylamine N-oxide (TMAO), a key metabolite produced by gut microbiota from dietary components such as choline, phosphatidylcholine, and L-carnitine, has been found to correlate closely with gut microbiota composition. Existing studies indicate that TMAO can activate inflammatory signaling pathways, induce oxidative stress, upregulate profibrotic factors, and impair vascular endothelial function[7]. Nevertheless, the precise mechanisms linking TMAO to the pathogenesis of DN and OP remain incompletely understood. Based on this, the present study aims to investigate the correlation between TMAO levels, inflammatory markers, and bone metabolism indicators in patients with DN, in order to provide theoretical support and practical evidence for the precise identification and early intervention of high-risk patients with DN/OP.
In this study, TMAO was used as the primary observational indicator, and the required sample size was calculated using formula. In the formula, n represents the required sample size for each group; Z1-α/2 is the Z value corresponding to the significance level α, where α = 0.05 and Z1-α/2 = 1.96; Zβ is the Z value corresponding to the type II error probability β, where β = 0.1 and Zβ = 1.28; μ1 and μ2 are the means of the core observational indicator in the two groups, and σ is the standard deviation of the core indicator.
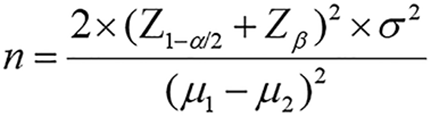
A pilot study was conducted by randomly selecting 30 patients with DN and 30 patients with T2DM without nephropathy. The TMAO level in patients with DN was 6.27 ± 2.18 ng/mL, while in patients with T2DM, it was 4.96 ± 1.25 μmol/L, with an overall standard deviation of 2.45 μmol/L. According to formula, the estimated required sample size per group was approximately 73 cases. Considering the multiple observational indicators in this study and the need for greater statistical power, 115 patients were planned for inclusion in each group. A total of 115 patients with confirmed type 2 DN admitted to the Department of Endocrinology, Second Affiliated Hospital of Shandong First Medical University between August 2022 and December 2024 were retrospectively enrolled as the DN group. Additionally, 115 patients with confirmed T2DM without nephropathy admitted during the same period were selected as the T2DM group. The study was reviewed and approved by the hospital’s ethics committee, and all patients and their families provided written informed consent.
Diagnostic criteria: (1) The diagnostic criteria for DN in this study were based on the Asian Pacific Society of Nephrology (APSN) Clinical Practice Guideline on Diabetic Kidney Disease (DKD)-2025 Update published by the APSN[8]; and (2) The diagnostic criteria for T2DM were based on the Standards of Care in Diabetes-2025 issued by the American Diabetes Association[9].
Inclusion criteria: (1) An age ≥ 60 years; (2) Duration of diabetes ≥ 5 years; (3) No use of antibiotics, metformin, SGLT2 inhibitors, or other medications known to affect gut microbiota and inflammation levels within the past month; (4) No use of medications influencing bone metabolism within the past 3 months; and (5) Receipt of a stable antidiabetic treatment regimen over the past 6 months.
Exclusion criteria (1) The presence of malignant tumors, hematological disorders, or autoimmune diseases; (2) Coexisting primary renal diseases such as glomerulonephritis or nephrotic syndrome; (3) Complications involving gastrointestinal infections, perforation, bleeding, or ulcers; (4) Underlying conditions that affect bone metabolism, such as thyroid dysfunction or rheumatoid arthritis; (5) Dysfunction of major organs or the presence of systemic diseases; (6) Presence of human immunodeficiency virus infection, urinary tract infection, or other acute infections, including various bacterial, viral, or fungal infections; and (7) Long-term bedridden status.
Data regarding patient demographics and clinical characteristics were sourced from the hospital’s electronic medical record system and obtained using a standardized questionnaire. The collected information included age, sex, body mass index (BMI), smoking history, alcohol consumption history, the presence of hypertension and hyperlipidemia, and the duration of diabetes.
Metabolic indicator detection method: After a 12-hour overnight fast, 5 mL of early morning fasting venous blood was collected from each participant and divided into three tubes. The first tube was allowed to stand for 30 minutes, then centrifuged at 3000 rpm for 10 minutes with a centrifugal radius of 12 cm. The supernatant was carefully collected, aliquoted into EP tubes, and stored at -80 °C for further analysis.
Fasting blood glucose, fasting insulin, and serum uric acid: Serum samples were used for the detection. Fasting blood glucose, serum uric acid, blood urea nitrogen, and serum creatinine were measured using the URIT-8020A fully automatic biochemical analyzer (URIT Medical Electronic Co., Ltd., Guilin, China; registration number: 20172220142). The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula based on the serum creatinine level. Fasting insulin was measured using the YnY2020 fully automated electrochemiluminescence immunoassay analyzer (Ansei Diagnostic Technology Co., Ltd., Shenzhen, China; registration number: 20222220387).
On the day of enrollment, approximately 5 g of fresh fecal samples were collected from each participant and placed in sterile cryogenic tubes. Samples were immediately stored in a -80 °C portable freezer box and transferred to the laboratory for long-term storage within 24 hours. Genomic DNA was extracted using the QIAamp Fast DNA Stool Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. Specifically, a 200 mg fecal sample was processed using bead-beating to lyse the bacterial cell walls, followed by silica membrane column-based purification. DNA concentration and purity were assessed using a UV spectrophotometer, with an acceptable A260/A280 ratio ranging from 1.8 to 2.0. The V3-V4 region of the bacterial 16S rRNA gene was amplified using the primers 341F (5’-CCTACGGGNGGCWGCAG-3’) and 806R (5’-GGACTACHVGGGTATCTAAT-3’), with sample-specific barcodes incorporated to distinguish individual samples. The polymerase chain reaction (PCR) system had a total volume of 25 μL: 12.5 μL of 2 × Taq Master Mix, 0.5 μL of forward primer (341F), 0.5 μL of reverse primer (806R), 1.0 μL of DNA template, and 10.5 μL of ddH2O. PCR was carried out under the following conditions: Initial denaturation at 95 °C for 3 minutes, followed by 30 cycles of denaturation at 95 °C for 30 seconds, annealing at 55 °C for 30 seconds, and extension at 72 °C for 45 seconds, with a final extension at 72 °C for 10 minutes. High-throughput sequencing was performed using the Illumina MiSeq platform (Illumina, United States) with paired-end sequencing (2 × 300 bp). Sequencing depth was ≥ 10000 reads per sample, with rarefaction curve coverage > 99%. The human DNA contamination rate was < 0.1%, the chimeric sequence ratio was < 3%, and the Q30 score was 95.4%. The PERMANOVA test yielded a P-value < 0.01. Sequencing was conducted by Shanghai Sangon Biotech Co., Ltd. Data processing: Low-quality sequences were filtered and chimeras removed using QIIME 2 (v2023.8). Operational taxonomic units were clustered at 97% sequence similarity. Alpha diversity metrics, including Chao1 index, ACE index, Shannon index, and Simpson index, were calculated. Taxonomic annotation was performed based on the Greengenes database (v13.8), and the relative abundance of major bacterial phyla-including Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria-was determined as the ratio of the number of sequences assigned to each phylum to the total number of valid sequences in the sample.
Inflammatory markers: Serum samples were used to measure C-reactive protein (CRP) level using the colloidal gold method, with a reagent kit provided by Wuhan Jinghong Biotechnology Co., Ltd. Interleukin-6 (IL-6) levels were measured using the magnetic microparticle chemiluminescence method and a reagent kit from Leadman Biochemistry Co., Ltd. (Beijing, China). Interleukin-8 (IL-8) was detected using a chemiluminescence immunoassay and a reagent kit from Tongxin Biotechnology Co., Ltd. (Beijing, China). Tumor necrosis factor-α (TNF-α) was measured using the magnetic microparticle chemiluminescence method and a reagent kit from Shandong Zhonghong Tejian Biotechnology Co., Ltd.
Serum samples were used for gut microbiota metabolite TMAO detection. Plasma was subjected to protein precipitation using a methanol/acetonitrile mixture (1:1, v/v) followed by centrifugation at 13000 rpm for 15 minutes. The resulting supernatant was filtered through a 0.22 μm membrane prior to analysis. Chromatographic separation was performed on a C18 reversed-phase column (2.1 mm × 100 mm, 1.8 μm) using a mobile phase consisting of 0.1% formic acid aqueous solution (A) and 0.1% formic acid acetonitrile solution (B) with a gradient elution program as follows: 0-1 minutes, 5% B; 1-4 minutes, 5%-95% B; 4-5 minutes, 95% B; 5-5.1 minutes, 95%-5% B; 5.1-7 minutes, 5% B. The flow rate was set to 0.3 mL/min, and the column temperature was maintained at 40 °C. Mass spectrometry was performed using an electrospray ionization source in positive mode (ESI+) under multiple reaction monitoring. The qualitative ion transition was m/z 76.1 → 58.1, and the quantitative ion transition was m/z 76.1 → 46.1. The data acquisition time was 50 ms, and the collision energy was set to 15 eV. All analyses were conducted by BGI clinical laboratories (Beijing, China).
Serum samples were used to measure procollagen type I N-terminal propeptide (PINP) with a YnY 2030 fully automated electrochemiluminescence immunoassay analyzer and a reagent kit from Ansei Diagnostic Technology Co., Ltd. (Shenzhen, China). β-CrossLaps (β-CTX) were measured using the magnetic microparticle chemiluminescence method and a reagent kit from Hunan Kangqing Biotechnology Co., Ltd. (registration number: Hunan Medical Device Approval 20242400879). Alkaline phosphatase (ALP) was measured using the NPP substrate-AMP buffer method and a reagent kit from Wuhan Baideruikang Biotechnology Co., Ltd.
Lumbar spine and hip bone mineral densities (BMD) were measured using an LM-LUX dual-energy X-ray absorptiometry scanner manufactured by Lefu Medical Equipment Co., Ltd.
Statistical analyses were performed using R (version 4.4.2; R Foundation for Statistical Computing, Vienna, Austria). The measurement data collected in this study were normally distributed, as assessed using the Shapiro-Wilk test, and are expressed as mean ± SD. The t-test was used for comparison between groups. Count data are expressed as frequencies (percentages) [n (%)]. The χ2 test was used for comparisons between groups, and the Mann-Whitney U test was applied to ordinal categorical variables. Correlations among multiple indicators were analyzed using the Pearson method, with partial correlation analysis performed to adjust for potential confounding effects of diabetes duration and eGFR. Correlation scatter plots were generated, and statistical significance was set at P < 0.05.
The DN and T2DM groups were similar in terms of age, sex, BMI, and the proportion of patients with a history of smoking, alcohol consumption, hypertension, and hyperlipidemia (P > 0.05). However, the DN group exhibited significantly higher levels of fasting blood glucose, fasting insulin, serum uric acid, and blood urea nitrogen than the T2DM group (P < 0.05) (Table 1).
| DN group (n = 115) | T2DM group (n = 115) | t/χ2 | P value | |
| Age (years old) | 70.32 ± 7.41 | 68.88 ± 8.29 | 1.468 | 0.144 |
| Sex | 1.430 | 0.232 | ||
| Male (cases) | 69 (60.00) | 60 (52.17) | ||
| Female (cases) | 46 (40.00) | 55 (47.83) | ||
| BMI (kg/m2) | 24.40 ± 2.15 | 24.21 ± 2.43 | 0.630 | 0.529 |
| Smoking (cases) | 43 (37.39) | 37 (32.17) | 0.690 | 0.406 |
| Drinking (cases) | 39 (33.91) | 47 (40.87) | 1.189 | 0.276 |
| Hypertension (cases) | 65 (56.52) | 73 (63.48) | 1.159 | 0.282 |
| Hyperlipidemia (cases) | 79 (68.70) | 84 (73.04) | 0.527 | 0.468 |
| Duration of diabetes (years) | 12.64 ± 3.91 | 9.57 ± 2.85 | 6.896 | 0.000 |
| eGFR (mL/min/1.73 m2) | 48.52 ± 9.77 | 78.92 ± 14.65 | -23.227 | 0.000 |
| Fasting blood glucose (mmol/L) | 7.59 ± 1.85 | 6.97 ± 1.88 | 2.507 | 0.013 |
| Fasting insulin level (mIU/L) | 9.53 ± 1.72 | 8.39 ± 1.19 | 5.847 | 0.000 |
| Blood uric acid (μmol/L) | 377.65 ± 65.23 | 358.07 ± 33.29 | 4.333 | 0.000 |
| Urea nitrogen (mmol/L) | 11.84 ± 2.03 | 8.67 ± 1.67 | 12.999 | 0.000 |
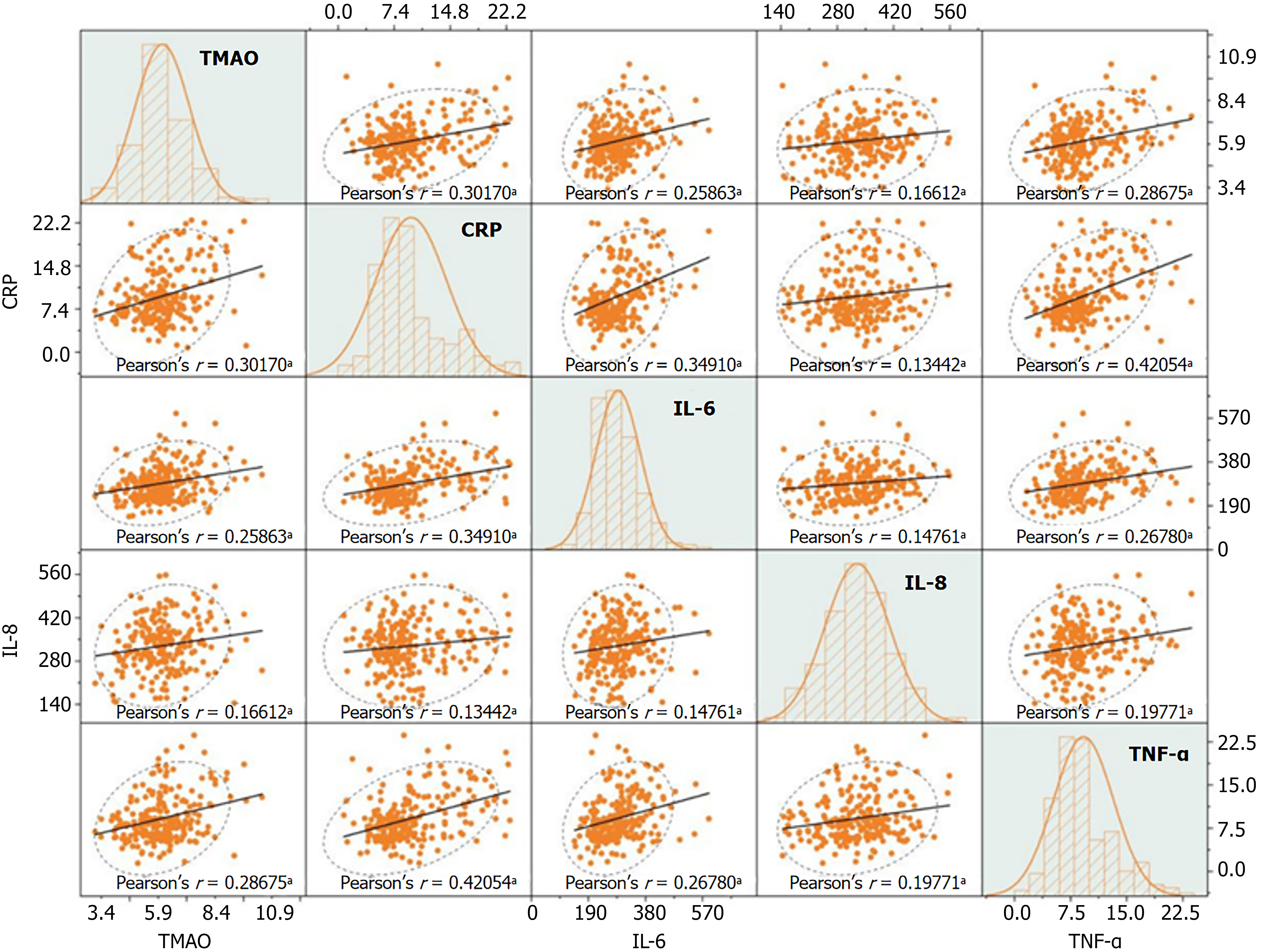
The DN group exhibited markedly higher levels of CRP, IL-6, IL-8, TNF-α, and TMAO compared to the T2DM group (P < 0.05) (Table 2).
| DN group (n = 115) | T2DM group (n = 115) | t | P value | |
| CRP (mg/L) | 12.16 ± 5.44 | 6.95 ± 1.77 | 5.760 | 0.000 |
| IL-6 (ng/L) | 331.14 ± 72.13 | 245.57 ± 42.04 | 9.749 | 0.000 |
| IL-8 (ng/L) | 345.04 ± 86.16 | 314.12 ± 75.13 | 10.659 | 0.000 |
| TNF-α (ng/L) | 11.28 ± 4.55 | 7.15 ± 1.80 | 2.972 | 0.003 |
| TMAO (ng/mL) | 123.47 ± 22.65 | 108.06 ± 17.62 | 9.041 | 0.000 |
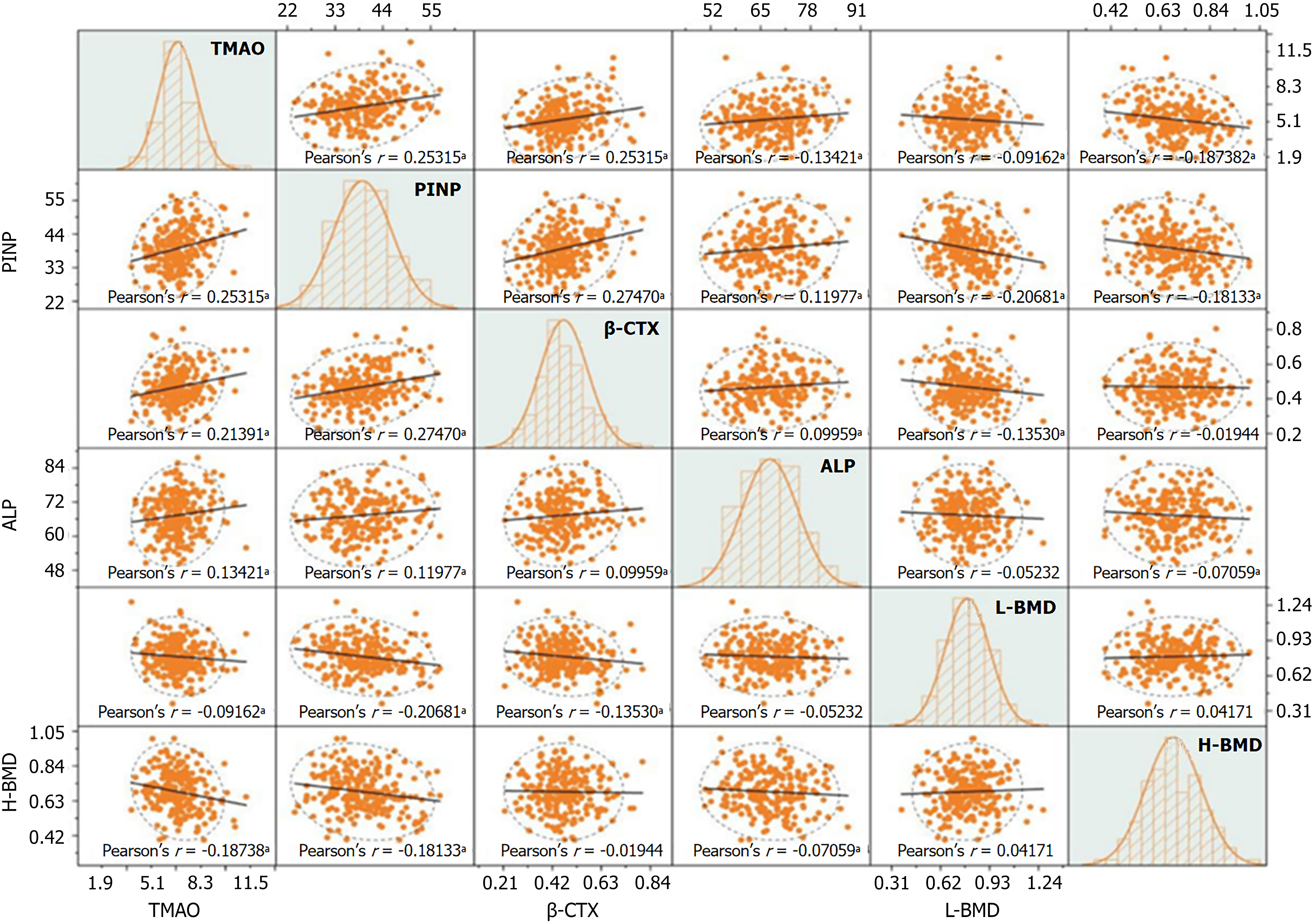
The DN group showed significantly higher levels of PINP, β-CTX, and ALP compared to the T2DM group, while lumbar spine BMD and hip BMD were significantly lower (P < 0.05) (Table 3).
| DN group (n = 115) | T2DM group (n = 115) | t | P value | |
| PINP (μg/L) | 43.13 ± 6.11 | 35.51 ± 4.87 | 10.461 | 0.000 |
| β-CTX (ng/mL) | 0.51 ± 0.11 | 0.43 ± 0.08 | 5.996 | 0.000 |
| ALP (U/L) | 69.16 ± 7.12 | 65.61 ± 7.09 | 3.788 | 0.000 |
| Lumbar spine BMD (g/cm2) | 0.77 ± 0.14 | 0.81 ± 0.14 | -2.552 | 0.011 |
| Hip BMD (g/cm2) | 0.66 ± 0.11 | 0.71 ± 0.12 | -3.624 | 0.000 |
The DN group had higher Chao1 and Simpson indices of gut microbiota compared to the T2DM group, while the ACE and Shannon indices were lower (P < 0.05) (Figure 1).
Both groups exhibited relatively high abundances of Bacteroidetes, Firmicutes, and Actinobacteria, with Firmicutes being the most dominant phylum. However, the DN group showed a significantly higher relative abundance of Firmicutes and significantly lower relative abundances of Bacteroidetes, Proteobacteria, and Actinobacteria and other phyla compared to the T2DM group (t = 6.025, -7.722, 13.683, 3.884, 5.497 respectively; P < 0.05) (Figure 2).
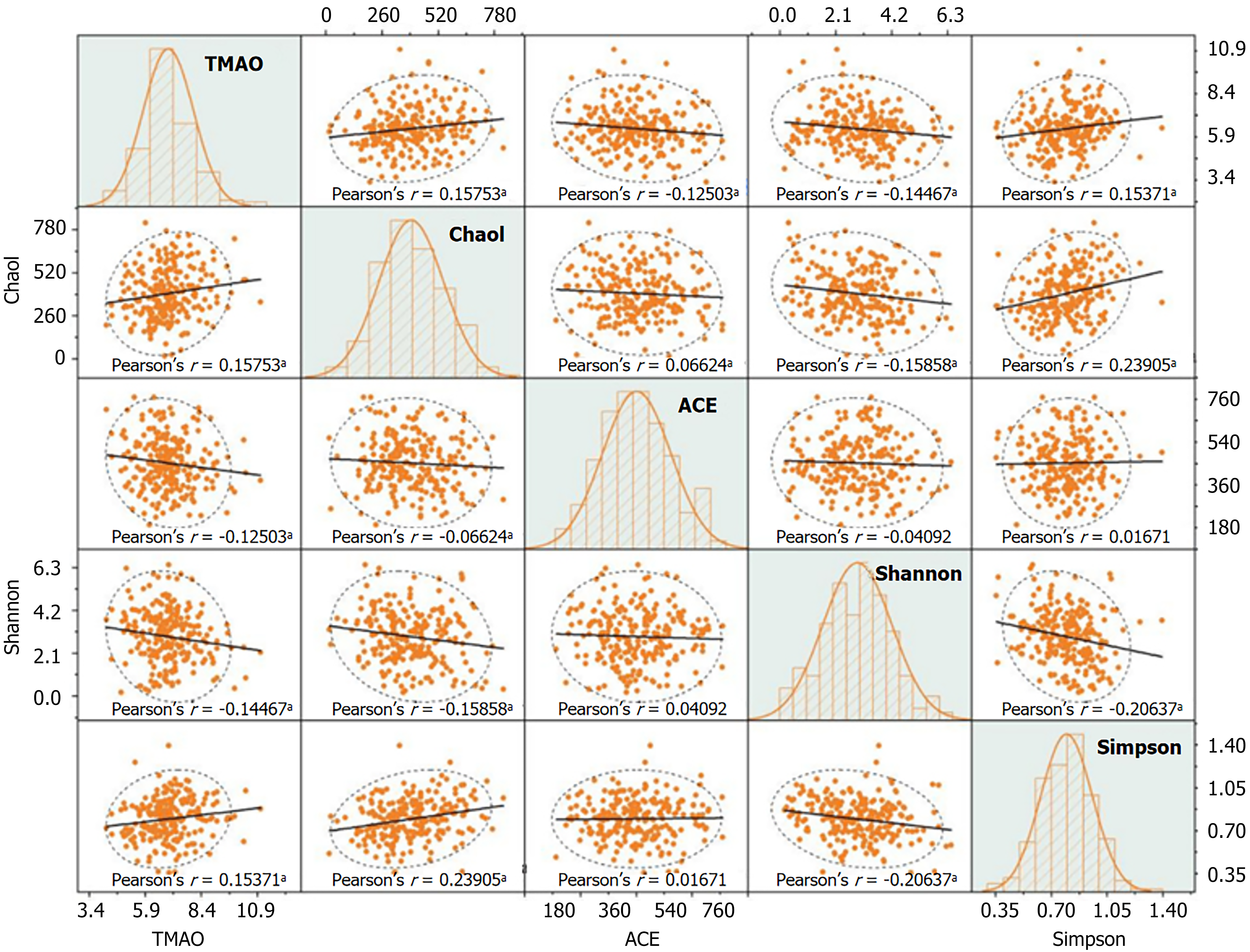
In patients with DN, TMAO was positively correlated with the Chao1 (r = 0.158) and Simpson (r = 0.154) indices of gut microbiota, and negatively correlated with the ACE (r = -0.125) and Shannon (r = -0.145) indices (P < 0.05) (Figure 3).
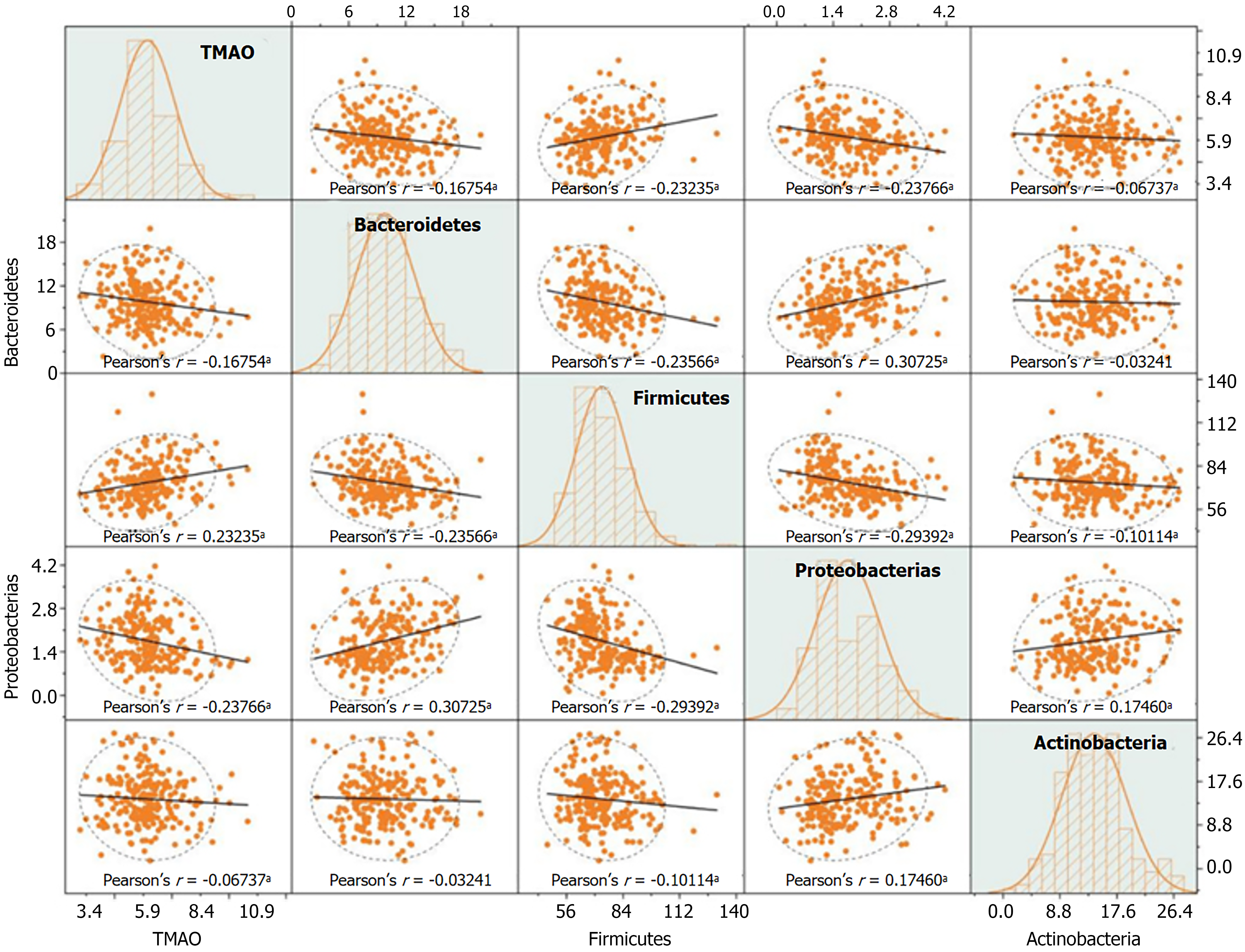
In patients with DN, TMAO levels were negatively correlated with the relative abundances of Bacteroidetes (r = -0.168), Proteobacteria (r = -0.238), and Actinobacteria (r = -0.067), and positively correlated with the relative abundance of Firmicutes (r = 0.232) (P < 0.05) (Figure 4).
TMAO levels in patients with DN were positively correlated with CRP, IL-6, IL-8, and TNF-α (r = 0.302, 0.259, 0.166, 0.287, respectively; P < 0.05) (Figure 5).
In patients with DN, TMAO was positively correlated with PINP, β-CTX, and ALP levels (r = 0.253, 0.214, 0.134, respectively; P < 0.05), and negatively correlated with lumbar BMD and hip BMD (r = -0.187; P < 0.05) (Figure 6).
DN is one of the most severe microvascular complications of diabetes and is often accompanied by systemic metabolic abnormalities such as chronic inflammation, gastrointestinal dysfunction, and OP[10]. Relevant studies have shown that, among patients with T2DM, those with DN exhibit higher levels of fasting blood glucose, fasting insulin, serum uric acid, blood urea nitrogen, CRP, IL-6, IL-8, and TNF-α[11-13]. The findings of the present study are consistent with these reports, indicating that patients with DN experience more severe metabolic disturbances and systemic chronic inflammatory responses. Persistent hyperglycemia induces oxidative stress, the formation of AGEs, and microvascular injury, while also activating inflammatory signaling pathways[14]. Moreover, insulin resistance not only exacerbates adipose tissue dysfunction and further promotes inflammation, but also directly damages the kidneys by activating the renin-angiotensin system and pro-inflammatory pathways[15]. Inflammatory cytokines, in turn, disrupt insulin signaling by activating pathways such as IKKβ/NF-κB and JNK/c-Jun, thereby aggravating insulin resistance. This establishes a bidirectional vicious cycle that contributes to renal interstitial inflammation and fibrosis progression[16]. With the decline in renal excretory function due to kidney injury, elevated uric acid levels further accelerate renal damage by activating the NLRP3 inflammasome[17]. Under the combined influence and interaction of metabolic disturbances, inflammatory responses, oxidative stress, and excessive activation of multiple signaling pathways, the renal structure undergoes progressive and self-perpetuating pathological damage.
Multiple studies have demonstrated that bone metabolism is impaired in patients with diabetes[18,19]. Zhu et al[20] found a negative correlation between eGFR and the levels of PINP and β-CTX in patients with diabetes, suggesting that bone turnover may be accelerated in those with DN. Further research by Chen et al[21] revealed that patients with DN have lower urinary calcium excretion and BMD compared to patients with T2DM without DN, along with elevated circulating phosphate levels and reduced urinary phosphate excretion, indicating a more pronounced disturbance in bone mineral metabolism. The present study yielded findings consistent with the aforementioned research: Patients in the DN group exhibited higher levels of PINP, β-CTX, and ALP compared to patients with T2DM without DN, along with lower BMD in the lumbar spine and hip. These results reflect a profile of high bone turnover and low bone density. This pattern is primarily attributed to impaired renal function in patients with DN, which reduces phosphate excretion and leads to hyperphosphatemia. Simultaneously, decreased synthesis of 1,25-(OH)2D3 results in reduced intestinal calcium absorption and hypocalcemia[22]. The combined effects of hyperphosphatemia and hypocalcemia stimulate increased secretion of PTH, resulting in SHPT. Persistently elevated PTH promotes both bone resorption and formation, contributing to increased levels of PINP, β-CTX, and ALP[23]. Under sustained SHPT, bone resorption exceeds bone formation, leading to net bone loss and skeletal structural deterioration, which is manifested as reduced BMD[24].
The gut microbiota plays a critical role in metabolic functions and immune regulation. This study found that, compared to patients with T2DM without DN, those with DN had higher Chao1 and Simpson indices, but lower ACE and Shannon indices. These findings suggest reduced microbial diversity and dominance of a limited number of species in patients with DN, indicating an imbalance in the gut microbial ecosystem. This is consistent with the findings reported by Zhang et al[25]. Further analysis of phylum-level microbial composition revealed that patients with DN had a higher relative abundance of Firmicutes, while the relative abundances of Bacteroidetes, Proteobacteria, and Actinobacteria were lower. These results suggest a reduction in beneficial bacteria such as Bacteroidetes and Actinobacteria, along with an enrichment of potentially harmful Firmicutes in these patients. The underlying reasons may be attributed to the combined effects of uremic toxins, systemic inflammation, dietary restrictions, chronic hyperglycemia, and metabolic disturbances in patients with DN. These factors collectively create a gut environment that favors the proliferation of certain Firmicutes species with strong stress tolerance and the ability to utilize specific substrates. These dominant bacteria are more involved in energy extraction and pro-inflammatory pathways, while simultaneously suppressing the growth of beneficial, protective microbiota[26]. Previous studies have confirmed that Firmicutes are closely associated with increased LPS production and enhanced intestinal permeability, making them one of the key drivers of metabolic inflammation[27]. Bacteroides, as a key genus involved in the production of short-chain fatty acids, play an essential role in maintaining the mucosal immune barrier. A reduction in Bacteroides may compromise this barrier function, thereby exacerbating systemic inflammation[28].
TMAO is a small organic compound produced through the metabolic activity of gut microbiota. A study by Zixin et al[29] demonstrated elevated TMAO levels in patients with DN, suggesting that TMAO may serve as a potential biomarker for DKD. This finding was further supported by research from Huo et al[30], which confirmed that TMAO levels were significantly higher in patients with DN compared to those with T2DM, and increased progressively with disease severity. The results of the present study are consistent with these findings, showing significantly higher TMAO levels in the DN group than in the T2DM group. However, Yu et al[31] noted that the kidneys are the primary organs responsible for TMAO excretion. In patients with DN, impaired renal function and reduced glomerular filtration rate contribute to the accumulation and elevation of TMAO levels. After adjusting for confounding factors such as diabetes duration and eGFR, this study found that in patients with DN, TMAO levels were positively correlated with the Chao1 and Simpson indices of the gut microbiota, and negatively correlated with the ACE and Shannon indices. Additionally, TMAO levels were positively associated with the relative abundance of Firmicutes and negatively associated with the relative abundances of Bacteroidetes, Proteobacteria, and Actinobacteria. These findings suggest that elevated TMAO levels in patients with DN may result from both increased TMAO production capacity and decreased degradation potential due to gut microbiota dysbiosis, as well as reduced renal clearance of TMAO owing to impaired kidney function.
After adjusting for the confounding effects of diabetes duration and eGFR, this study found that TMAO levels remained positively correlated with the levels of CRP, IL-6, IL-8, and TNF-α in patients with DN, suggesting that TMAO may play an important role in the activation and maintenance of chronic inflammatory responses. As a molecule derived from gut microbiota metabolism, TMAO has been shown to activate inflammatory signaling pathways through multiple mechanisms, including enhancing NF-κB transcriptional activity, promoting NLRP3 inflammasome assembly, and stimulating monocytes and macrophages to release pro-inflammatory mediators[32]. In patients with DN, persistent accumulation of TMAO due to gut microbiota dysbiosis and impaired renal function contributes to a sustained state of systemic immune activation[33]. Fang et al[34] demonstrated in DKD rat models that exogenous TMAO activates the NLRP3 inflammasome and promotes the release of pro-inflammatory cytokines such as IL-1β and IL-18, thereby exacerbating renal inflammation and fibrosis. Other studies have further confirmed that TMAO promotes inflammatory responses and activates and proliferates renal fibroblasts via multiple signaling pathways, including the PERK/Akt/mTOR axis, NLRP3 inflammasome activation, and caspase-1 signaling, ultimately leading to endothelial injury and renal fibrosis[35]. A study by Bai et al[36] also confirmed that the ZBP1-NLRP3 inflammasome pathway is involved in TMAO-induced chronic kidney injury. These findings suggest that TMAO plays a key role in the onset and progression of DN. It is possible that elevated TMAO levels, driven by gut microbiota dysbiosis, contribute to kidney injury, which in turn leads to reduced TMAO clearance and further TMAO accumulation, forming a vicious cycle of ‘’gut dysbiosis-elevated TMAO-renal damage-impaired excretion-further TMAO elevation.’’ However, as this study is retrospective and based on cross-sectional data, the proposed mechanism requires further validation through longitudinal time-series studies.
In patients with DN, impaired renal function leads to reduced calcium absorption and increased bone resorption, while excretory dysfunction further aggravates disturbances in bone metabolism[37]. After adjusting for diabetes duration and eGFR, this study found that TMAO levels in patients with DN remained positively correlated with PINP, β-CTX, and ALP levels, and negatively correlated with lumbar spine and hip BMD. These findings suggest that elevated TMAO levels may be one of the contributing factors to impaired bone metabolic function in patients with DN. The underlying mechanisms may involve TMAO-mediated activation of multiple inflammatory pathways, leading to the release of large quantities of pro-inflammatory cytokines. Cytokines such as IL-6 and TNF-α stimulate the differentiation and maturation of osteoclast precursors, thereby enhancing bone resorption, while simultaneously inhibiting osteoblast differentiation and promoting apoptosis of osteoblasts and osteocytes[38]. In addition, TMAO itself and its metabolic processes contribute to the generation of reactive oxygen species, and under conditions of heightened oxidative stress, osteoblasts and osteocytes are damaged, osteoclasts are activated, and bone matrix mineralization is suppressed[39]. Moreover, a study by Lin et al[40] using a mouse model demonstrated that TMAO activates PERK, leading to ATF4-induced disruption of endoplasmic reticulum autophagy, while suppressing ATF5 folding and impairing the mitochondrial unfolded protein response. This cascade hinders bone mineral acquisition and osteogenic differentiation, ultimately contributing to increased bone loss. These findings suggest that in patients with diabetes, elevated TMAO levels resulting from gut microbiota dysbiosis may contribute to bone metabolic disorders that occur in parallel with renal impairment.
However, this study is retrospective in nature and did not employ metagenomic or metabolomic analyses to verify the association between TMAO production and specific microbial functional pathways. Moreover, longitudinal or interventional trial data were not collected. Since TMAO is derived from dietary precursors, variations in dietary habits may directly influence both TMAO levels and gut microbiota composition, potentially introducing bias. This study did not control for the confounding effects of diet. Future research should aim to elucidate the mechanistic links between TMAO and specific gut microbial metabolic pathways, and validate the proposed "gut microbiota-TMAO-inflammatory response-renal function-bone metabolism" interaction through longitudinal datasets and interventional trials, while accounting for dietary influences.
Patients with T2DM and concurrent DN exhibited significant differences in gut microbiota diversity, phylum/genus-level relative abundance, TMAO levels, inflammatory markers, and bone metabolism indicators compared to those without DN. TMAO levels were significantly correlated with both gut microbiota diversity and relative abundance at the phylum/genus level. Higher TMAO levels were associated with elevated levels of CRP, IL-6, IL-8, and TNF-α, increased levels of bone turnover markers (PINP, β-CTX, and ALP), and reduced BMD at the lumbar spine and hip. These findings suggest that gut microbiota dysbiosis may contribute to inflammation and bone metabolic disorders in patients with DN through elevated TMAO, thereby promoting the onset and progression of systemic inflammation and OP.
| 1. | Gupta S, Dominguez M, Golestaneh L. Diabetic Kidney Disease: An Update. Med Clin North Am. 2023;107:689-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 204] [Reference Citation Analysis (0)] |
| 2. | Chen P, Yan P, Wan Q, Zhang Z, Xu Y, Miao Y, Yang J. Association of circulating B-type natriuretic peptide with osteoporosis in a Chinese type 2 diabetic population. BMC Musculoskelet Disord. 2021;22:261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Indrio F, Salatto A. Gut Microbiota-Bone Axis. Ann Nutr Metab. 2025;81:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Lin X, Xiao HM, Liu HM, Lv WQ, Greenbaum J, Gong R, Zhang Q, Chen YC, Peng C, Xu XJ, Pan DY, Chen Z, Li ZF, Zhou R, Wang XF, Lu JM, Ao ZX, Song YQ, Zhang YH, Su KJ, Meng XH, Ge CL, Lv FY, Luo Z, Shi XM, Zhao Q, Guo BY, Yi NJ, Shen H, Papasian CJ, Shen J, Deng HW. Gut microbiota impacts bone via Bacteroides vulgatus-valeric acid-related pathways. Nat Commun. 2023;14:6853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Lu L, Chen X, Liu Y, Yu X. Gut microbiota and bone metabolism. FASEB J. 2021;35:e21740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2024;19:275-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 597] [Article Influence: 298.5] [Reference Citation Analysis (0)] |
| 7. | Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 837] [Article Influence: 167.4] [Reference Citation Analysis (0)] |
| 8. | Liew A, Bavanandan S, Hao CM, Lim SK, Prasad N, Sahay M, Susantitaphong P, Roberts V, Wijewickrama E, Wong MG, Tang SCW. Asian Pacific Society of Nephrology Clinical Practice Guideline on Diabetic Kidney Disease-2025 Update. Nephrology (Carlton). 2025;30 Suppl 2:3-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S27-S49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 440] [Article Influence: 440.0] [Reference Citation Analysis (0)] |
| 10. | Guo W, Song Y, Sun Y, Du H, Cai Y, You Q, Fu H, Shao L. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011-2018. Front Endocrinol (Lausanne). 2022;13:1071465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 11. | Shi L, Xue Y, Yu X, Wang Y, Hong T, Li X, Ma J, Zhu D, Mu Y. Prevalence and Risk Factors of Chronic Kidney Disease in Patients With Type 2 Diabetes in China: Cross-Sectional Study. JMIR Public Health Surveill. 2024;10:e54429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Vareldzis R, Perez A, Reisin E. Hyperuricemia: An Intriguing Connection to Metabolic Syndrome, Diabetes, Kidney Disease, and Hypertension. Curr Hypertens Rep. 2024;26:237-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 13. | Dwivedi S, Sikarwar MS. Diabetic Nephropathy: Pathogenesis, Mechanisms, and Therapeutic Strategies. Horm Metab Res. 2025;57:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Khalid M, Petroianu G, Adem A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules. 2022;12:542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 513] [Article Influence: 128.3] [Reference Citation Analysis (0)] |
| 15. | Masenga SK, Kabwe LS, Chakulya M, Kirabo A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int J Mol Sci. 2023;24:7898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 353] [Article Influence: 117.7] [Reference Citation Analysis (0)] |
| 16. | Szukiewicz D. Molecular Mechanisms for the Vicious Cycle between Insulin Resistance and the Inflammatory Response in Obesity. Int J Mol Sci. 2023;24:9818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 17. | Wang M, Lin X, Yang X, Yang Y. Research progress on related mechanisms of uric acid activating NLRP3 inflammasome in chronic kidney disease. Ren Fail. 2022;44:615-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Hofbauer LC, Busse B, Eastell R, Ferrari S, Frost M, Müller R, Burden AM, Rivadeneira F, Napoli N, Rauner M. Bone fragility in diabetes: novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022;10:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 267] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 19. | Sheu A, White CP, Center JR. Bone metabolism in diabetes: a clinician's guide to understanding the bone-glucose interplay. Diabetologia. 2024;67:1493-1506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Zhu X, Zhou Y, Hong S, Xue Y, Cui Y. Correlation between Serum Bone Turnover Markers and Estimated Glomerular Filtration Rate in Chinese Patients with Diabetes. Dis Markers. 2021;2021:6731218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Chen H, Li X, Yue R, Ren X, Zhang X, Ni A. The effects of diabetes mellitus and diabetic nephropathy on bone and mineral metabolism in T2DM patients. Diabetes Res Clin Pract. 2013;100:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Favero C, Carriazo S, Cuarental L, Fernandez-Prado R, Gomá-Garcés E, Perez-Gomez MV, Ortiz A, Fernandez-Fernandez B, Sanchez-Niño MD. Phosphate, Microbiota and CKD. Nutrients. 2021;13:1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Das L, Bhadada SK, Arvindbhai SM, Dahiya D, Behera A, Dutta P, Bhansali A, Sood A, Singh P, Prakash M, Kumari P, Rao SD. Baseline renal dysfunction determines mortality following parathyroidectomy in primary hyperparathyroidism: analysis of Indian PHPT registry. J Bone Miner Metab. 2022;40:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Pazianas M, Miller PD. Osteoporosis and Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD): Back to Basics. Am J Kidney Dis. 2021;78:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 25. | Zhang L, Wang Z, Zhang X, Zhao L, Chu J, Li H, Sun W, Yang C, Wang H, Dai W, Yan S, Chen X, Xu D. Alterations of the Gut Microbiota in Patients with Diabetic Nephropathy. Microbiol Spectr. 2022;10:e0032422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 26. | Zaky A, Glastras SJ, Wong MYW, Pollock CA, Saad S. The Role of the Gut Microbiome in Diabetes and Obesity-Related Kidney Disease. Int J Mol Sci. 2021;22:9641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 27. | Tanase DM, Gosav EM, Neculae E, Costea CF, Ciocoiu M, Hurjui LL, Tarniceriu CC, Maranduca MA, Lacatusu CM, Floria M, Serban IL. Role of Gut Microbiota on Onset and Progression of Microvascular Complications of Type 2 Diabetes (T2DM). Nutrients. 2020;12:3719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 28. | Zali F, Absalan A, Bahramali G, Mousavi Nasab SD, Esmaeili F, Ejtahed HS, Nasli-Esfahani E, Siadat SD, Pasalar P, Emamgholipour S, Razi F. Alterations of the gut microbiota in patients with diabetic nephropathy and its association with the renin-angiotensin system. J Diabetes Metab Disord. 2025;24:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Zixin Y, Lulu C, Xiangchang Z, Qing F, Binjie Z, Chunyang L, Tai R, Dongsheng O. TMAO as a potential biomarker and therapeutic target for chronic kidney disease: A review. Front Pharmacol. 2022;13:929262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 30. | Huo L, Li H, Zhu M, Liu Y, Ren L, Hu J, Wang X. Enhanced trimethylamine metabolism and gut dysbiosis in type 2 diabetes mellitus with microalbumin. Front Endocrinol (Lausanne). 2023;14:1257457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 31. | Yu PS, Wu PH, Hung WW, Lin MY, Zhen YY, Hung WC, Chang JM, Tsai JR, Chiu YW, Hwang SJ, Tsai YC. Association Between Trimethylamine N-oxide and Adverse Kidney Outcomes and Overall Mortality in Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2024;109:2097-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Fang X, Miao R, Wei J, Wu H, Tian J. Advances in multi-omics study of biomarkers of glycolipid metabolism disorder. Comput Struct Biotechnol J. 2022;20:5935-5951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 77] [Reference Citation Analysis (0)] |
| 33. | Gungor O, Hasbal NB, Alaygut D. Trimethylamine N-oxide and kidney diseases: what do we know? J Bras Nefrol. 2024;46:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Fang Q, Zheng B, Liu N, Liu J, Liu W, Huang X, Zeng X, Chen L, Li Z, Ouyang D. Trimethylamine N-Oxide Exacerbates Renal Inflammation and Fibrosis in Rats With Diabetic Kidney Disease. Front Physiol. 2021;12:682482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 35. | Kapetanaki S, Kumawat AK, Persson K, Demirel I. The Fibrotic Effects of TMAO on Human Renal Fibroblasts Is Mediated by NLRP3, Caspase-1 and the PERK/Akt/mTOR Pathway. Int J Mol Sci. 2021;22:11864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 36. | Bai L, Chen Q, Li Y, Wu F, Jin M, Chen Y, Teng X, Jin S, Fan H, Wu Y. Trimethylamine Induced Chronic Kidney Injury by Activating the ZBP1-NLRP3 Inflammasome Pathway. Physiol Res. 2024;73:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 37. | Vavanikunnel J, Sewing L, Triantafyllidou M, Steighardt A, Baumann S, Egger A, Grize L, Felix B, Kraenzlin M, Henzen C, Meier C. Determinants of Low Bone Turnover in Type 2 Diabetes-the Role of PTH. Calcif Tissue Int. 2022;111:587-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Li L, An J, Bai J, Zhang Y, Li X, Lv H. Association between systemic immune-inflammation index and trimethylamine N-oxide levels in peripheral blood and osteoporosis in overweight and obese patients. Front Endocrinol (Lausanne). 2025;16:1539594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Zhao Y, Wang C, Qiu F, Liu J, Xie Y, Lin Z, He J, Chen J. Trimethylamine-N-oxide promotes osteoclast differentiation and oxidative stress by activating NF-κB pathway. Aging (Albany NY). 2024;16:9251-9263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 40. | Lin YH, Lian WS, Wu RW, Chen YS, Wu SL, Ko JY, Wang SY, Jahr H, Wang FS. Trimethylamine-N-oxide accelerates osteoporosis by PERK activation of ATF5 unfolding. Cell Mol Life Sci. 2024;82:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/