Published online Nov 15, 2025. doi: 10.4239/wjd.v16.i11.109859
Revised: July 9, 2025
Accepted: October 10, 2025
Published online: November 15, 2025
Processing time: 167 Days and 8.1 Hours
Diabetic foot ulcers (DFUs) are a severe complication of diabetes and a leading cause of lower limb amputation due to impaired wound healing. Adipose-derived mesenchymal stem cells (ADSCs) have emerged as a promising therapeutic option for DFUs because of their angiogenic, immunomodulatory, and regenerative properties. However, studies on the molecular mechanisms and regulatory pathways of ADSCs in DFUs are limited.
To investigate the dose-response relationship, the optimal administration route, persistence, and molecular mechanisms of ADSCs in DFU healing.
In this study, human ADSCs were isolated and cultured, and their differentiation potential was characterized. A DFU mouse model was established to evaluate the dose-dependent effects and persistence of ADSCs administered subcutaneously or intramuscularly. Wound closure rate, angiogenesis, inflammation, and collagen deposition were assessed in the ADSC-treated and model groups. Additionally, in vitro experiments using human dermal fibroblasts and endothelial cells were conducted to elucidate the molecular mechanisms underlying ADSC-mediated wound healing.
ADSC treatment significantly enhanced wound closure, promoted angiogenesis, modulated inflammatory responses, and accelerated tissue regeneration in the DFU model. Notably, the therapeutic efficacy and retention of ADSCs were influenced by both dosage and administration route, with subcutaneous injection of 5 × 105 ADSCs yielding the most favorable outcomes, particularly when injected into the feet, which resulted in prolonged retention. In vitro experiments further revealed that ADSCs exert their therapeutic effects via multiple mechanisms, including phosphatidylinositol 3-kinase signaling pathway activation to enhance vascular endothelial growth factor secretion, thereby promoting angiogenesis and modulating the Notch signaling pathway in DFUs to suppress inflammation and facilitate tissue regeneration.
ADSCs effectively promote DFU healing and have clinical potential as a treatment for chronic non-healing diabetic wounds. These findings provide a foundation for optimizing ADSC-based therapies for treating DFUs.
Core Tip: This study systematically evaluated the dose-response relationship, administration route, persistence, and mechanisms of adipose-derived mesenchymal stem cells (ADSCs) in diabetic foot ulcer healing. Subcutaneous injection of 5 × 105 ADSCs showed optimal efficacy with prolonged retention. Mechanistically, ADSCs restore the phosphatidylinositol 3-kinase-protein kinase B-mammalian target of rapamycin-hypoxia-inducible factor-1α-vascular endothelial growth factor pathway to improve angiogenesis and inhibit Notch signaling pathway overactivation to reduce inflammation and enhance collagen deposition. These results provide critical insights into the optimization of ADSC-based therapies and offer a promising approach for treating chronic diabetic wounds.
- Citation: Cao J, Liu ZC, An WQ, Zhang S, Zhang X, Li LJ, Ji HL, Long X, Yang YM. Optimizing adipose-derived stem cell therapy for diabetic foot ulcers. World J Diabetes 2025; 16(11): 109859
- URL: https://www.wjgnet.com/1948-9358/full/v16/i11/109859.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i11.109859
Diabetes mellitus (DM) is a common disease affecting millions of people worldwide. The mechanism of DM pathogenesis is complex, multifactorial, and is affected by the environment, health, and lifestyle. Globally, approximately 537 million adults worldwide have diabetes[1]. Diabetic foot ulcers (DFUs) are a common complication of DM, frequently associated with neuropathy and/or peripheral arterial disease of the lower extremities[2,3]. This condition imposes a substantial burden on both patient well-being and the global healthcare system, affecting approximately 25% of patients with diabetes over their lifetime[4]. If left untreated, foot ulcers may progress to soft tissue infections, gangrene, and limb loss[3]. Epidemiological data show that approximately 50% of all patients with ulcers develop infections, of which up to 20% require hospitalization. Moreover, 15%-20% of moderate to severe infections eventually lead to lower-extremity amputation. The 5-year mortality rate of patients with DFUs is 30%, whereas that of patients with above-foot amputations exceeds 70%[5,6]. Being primary drivers of diabetes-related hospitalization and lower limb amputation, DFUs require effective treatment strategies[2].
Wound healing is a highly dynamic and complex biological process divided into four interrelated phases: Hemostasis, inflammation, proliferation, and remodeling[7]. It follows a well-coordinated sequence in healthy individuals without underlying pathophysiological defects[8]. However, in chronic wounds such as DFUs, this process is significantly impaired due to a combination of hyperglycemia, neuropathy, vascular insufficiency, and dysregulated inflammatory responses[9]. Persistent chronic inflammation, reduced angiogenesis, and diminished tissue regeneration contribute to poor wound-healing outcomes[7]. In particular, persistent inflammation within the DFU microenvironment has been identified as a cardinal feature of chronic wounds in patients with hyperglycemia, stemming from the stagnation of DFUs during the inflammatory phase without progression to resolution[10]. Uncontrolled inflammation and aberrant macrophage polarization act as critical barriers to wound repair, disrupting the sequential phases of tissue regeneration; this observation indicates the involvement of macrophage phenotypic imbalance[11,12]. Given the multifactorial pathogenesis of DFUs, conventional treatments, including debridement, infection control, and offloading, often fail to address the biological deficiencies underlying chronic wound formation[3].
Adipose-derived mesenchymal stem cells (ADSCs) are mesenchymal stem cells (MSCs) isolated from adipose tissue that can differentiate into various cell lineages, including adipocytes, osteoblasts, chondrocytes, and myocytes[13]. In recent years, MSC-based therapies have gained attention as promising treatments for refractory wounds, owing to their potent angiogenic, immunomodulatory, and regenerative properties[14-17]. Angiogenesis is critical for wound healing because it ensures an adequate oxygen and nutrient supply for tissue repair. However, it is markedly impaired in DFUs due to endothelial dysfunction[7,14,17]. ADSCs secrete a wide range of angiogenic cytokines, including vascular endothelial growth factor (VEGF) and placental growth factors, which enhance vascularization[18-21]. Chronic inflammation, characterized by persistent infiltration of pro-inflammatory cells, is a hallmark of non-healing diabetic wounds[7,22]. The immunomodulatory properties of MSCs have been well-documented in various pathological conditions, including acute myocardial infarction and wound healing[15,23]. Additionally, ADSCs facilitate tissue regeneration by differentiating into skin cells and secreting growth factors that promote fibroblast and keratinocyte proliferation and migration, ultimately accelerating collagen deposition and wound closure[18,19]. Although previous studies have demonstrated the beneficial effects of ADSCs in DFU healing, several critical aspects remain unclear, including the dose-response relationship, the influence of administration routes on therapeutic efficacy, in vivo distribution and persistence of ADSCs, and the molecular mechanisms by which ADSCs promote wound healing[24].
In this study, we systematically investigated the dose-dependent effects of ADSCs, compared different administration methods, assessed their distribution and persistence after transplantation, and explored the molecular mechanisms underlying their therapeutic potential in DFU healing. Human ADSCs were administered via subcutaneous or intramuscular injection in a DFU mouse model. Wound healing rates were evaluated, and histological analyses were conducted to assess angiogenesis, growth factor levels, inflammatory cell infiltration, and collagen deposition in ulcerative tissues. The distribution of transplanted ADSCs was tracked using 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD) cell membrane dye, demonstrating that ADSCs predominantly localized around the injection site and persisted for several weeks. To further elucidate the mechanisms by which ADSCs promote DFU healing, we conducted in vitro experiments to examine their pro-angiogenic, immunomodulatory, and tissue repair functions. Our findings demonstrate that ADSCs primarily enhance angiogenesis through paracrine VEGF secretion, mitigate inflammation by inducing a phenotypic shift in macrophages toward the anti-inflammatory M2 phenotype, and reduce pro-inflammatory cytokine release. Furthermore, ADSCs accelerate tissue repair by promoting the proliferation and migration of human dermal fibroblasts (HDFs) and keratinocytes (HaCaT), processes regulated by the Notch signaling pathway. This study provides the first comprehensive evaluation of the optimal ADSC dosage and administration route for DFU treatment and offers novel insights into the molecular mechanisms underlying ADSC-mediated wound healing.
Adipose tissue was harvested from consenting adult donors undergoing liposuction following protocols approved by the institutional review board. In the lab, the tissue was washed with saline, minced, and digested with 0.1% collagenase type I at 37 °C for 30 minutes. The enzyme was neutralized, and the cell suspension was filtered through a 100 μm strainer and centrifuged at 300 × g for 5 minutes. The cell pellet was resuspended in complete culture medium and seeded in T75 flasks. Cells were incubated at 37 °C with 5% CO2, and the medium was changed every 48 hours. After reaching 80%-90% confluence, ADSCs were detached with TrypLE™ and passaged.
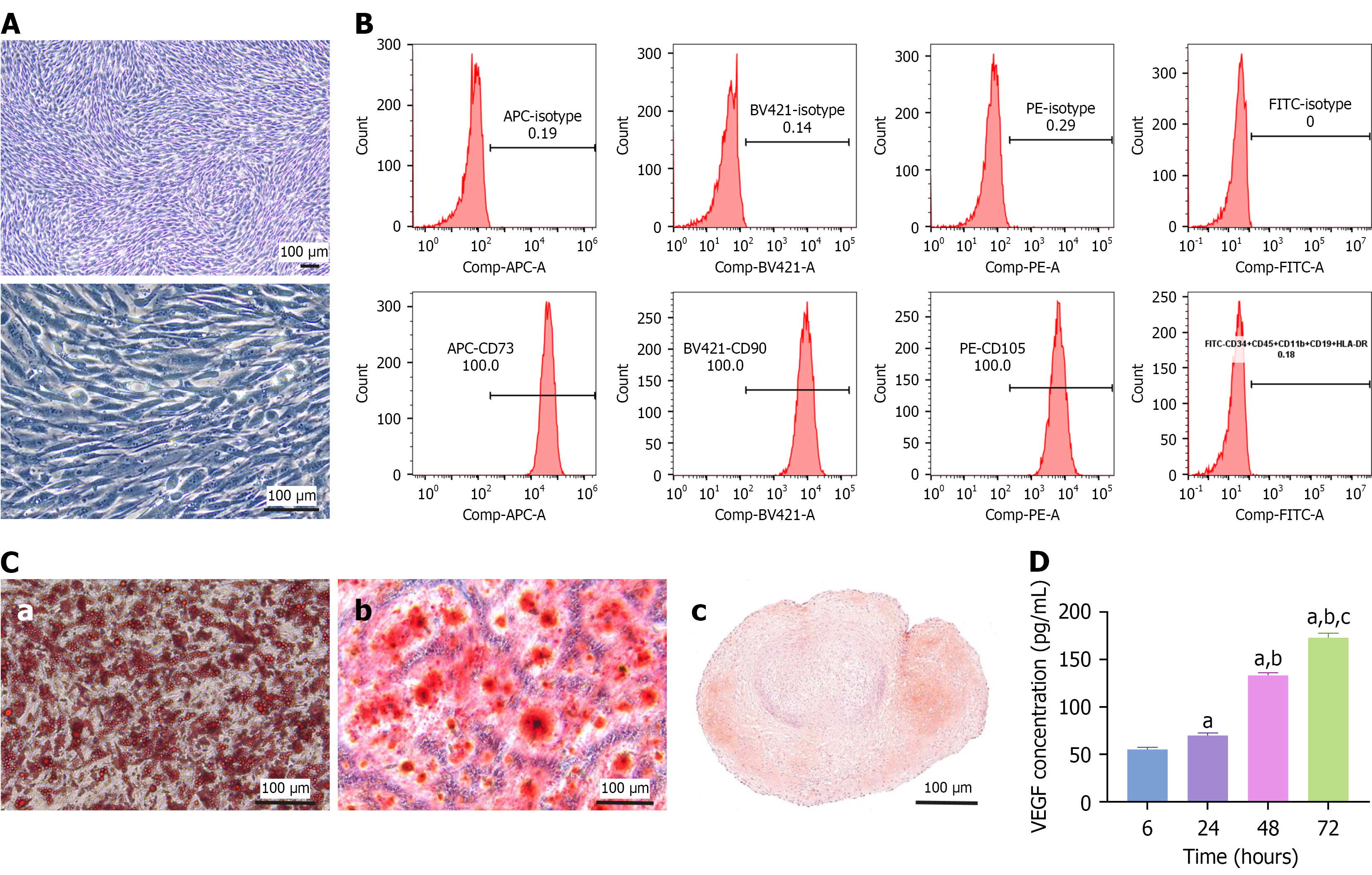
Adipogenic, chondrogenic, and osteogenic differentiation were conducted using the MesenCult™ Adipogenic Differentiation Kit (Human), MesenCult™ Osteogenic Differentiation Kit, and MesenCult™-ACF Chondrogenic Differentiation Kit, respectively, following the manufacturer’s protocols (Catalog #05412, Catalog #05465, Catalog #05455, STEMCELL Technologies, Shanghai, China). ADSCs at passage 5 were seeded in 6-well plates; upon reaching confluency, the culture medium was replaced with an induction medium. Adipogenic differentiation was assessed by staining intracellular lipid droplets with Oil Red O. Osteogenic differentiation was evaluated by alizarin red staining, which identifies calcium deposition indicative of mineralized matrix formation. Chondrogenic differentiation was confirmed by safranin O staining.
Specific ADSC surface markers were detected using flow cytometry. Approximately 1 × 106 cells were aliquoted into flow cytometry tubes for flow cytometric analysis. The cells were incubated with fluorescently labeled monoclonal antibodies against the MSC markers CD73, CD90, and CD105. CD34, CD45, CD11b, CD19, and human leukocyte antigen-DR antibodies were used to assess purity and stem cell phenotype. Appropriate isotype-matched control antibodies were used for fluorescent labels to control for nonspecific antibody binding. After incubation for 30 minutes at 4 °C in the dark, the cells were washed twice with phosphate buffered saline (PBS) to remove unbound antibodies. Subsequently, the cells were resuspended in 500 μL PBS and analyzed on a flow cytometer. The results were analyzed to determine the percentage of cells expressing each marker.
Male BALB/c mice, aged 6-8 weeks and weighing 18-20 g, were purchased from Sipeifu Biotechnology Co., Ltd. (Beijing, China) to establish a DFU mouse model. Diabetes was induced using streptozotocin (STZ). The mice fasted for 12 hours before induction but had free access to water. STZ (120 mg/kg) was administered intraperitoneally. Blood glucose levels were monitored daily for 7 days, and mice with fasting blood glucose levels exceeding 11.1 mmol/L were considered diabetic[25]. Diabetic mice were anesthetized and, under aseptic conditions, a single circular full-thickness skin wound (4 mm diameter) was created on the dorsal side of the hind leg using a skin biopsy punch to establish the DFU model. All mice were housed under a 12-hour light-dark cycle at the animal care facilities of the Chinese Academy of Medical Sciences. All animals were euthanized via barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
Male Sprague-Dawley rats aged 6-8 weeks and weighing 180-200 g were purchased from Sipeifu Biotechnology Co., Ltd. (Beijing, China). The STZ dose was 60 mg/kg and skin wounds were created on the right hind foot of the rats. The other processes used for model establishment were the same as those used for the mouse models. At the end of the experiment, blood was collected from the orbit, and the rats were euthanized via cervical dislocation, and skin and organ tissues were harvested. Rats were euthanized by intraperitoneal administration of sodium pentobarbital (60 mg/kg). Experiments involving animals strictly complied with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Ethics Committee of the Chinese Academy of Medical Sciences, Peking Union Medical College Hospital granted ethical approval for these experiments.
For subcutaneous injection, ADSCs in 100 μL PBS were carefully injected into the subcutaneous tissue surrounding the wound margin at four equidistant points (25 μL/site). For intramuscular injection, the same volume and concentration of ADSCs were injected into the muscle tissue adjacent to the wound bed to ensure minimal disturbance of the ulcerated area.
ADSCs were traced after transplantation using the lipophilic tracer dye DiD. For labeling, DiD was added to the cell suspension at a final concentration of 2.5 μmol/L. The cells were then incubated with the dye at 37 °C for 20 minutes in the dark. Following incubation, the labeled cells were washed three times with PBS to remove excess dye and resuspended in a suitable volume of PBS for transplantation. For in vivo experiments, DiD-labeled ADSCs were injected into the dorsal side of the hind legs of DFU model mice and the right hind foot of DFU model rats. Animals were monitored weekly after transplantation.
The wound healing rate was calculated using Image-Pro Plus software as follows: Wound closure rate (%) = (wound area on the modeling day - area on day 7)/wound area on the modeling day × 100.
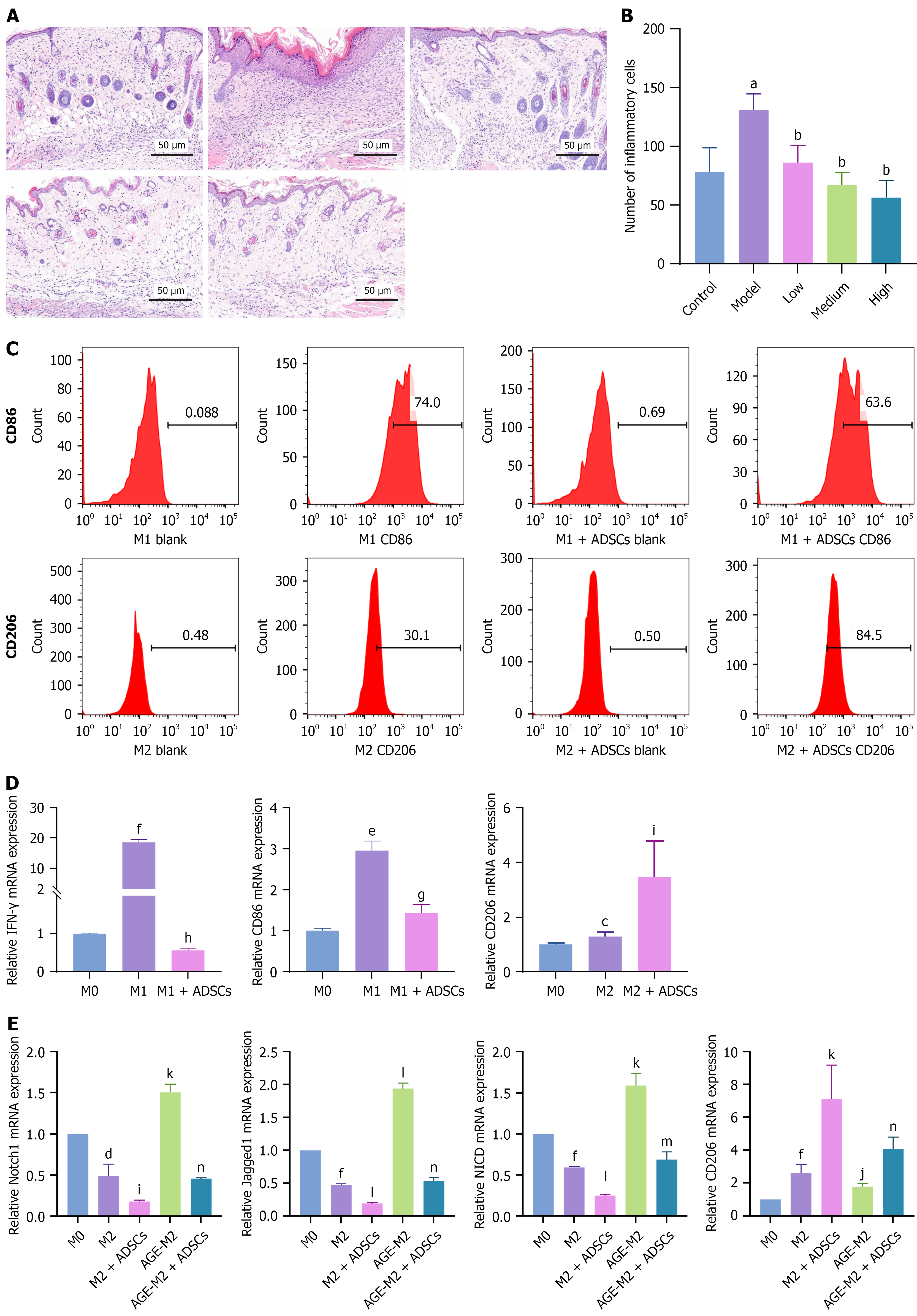
On days 7 and 14 after ADSC administration, the mice were euthanized, and the wound area and surrounding tissues were excised. Embedded tissues were sectioned at a thickness of 5 μm. Sections were stained with hematoxylin and eosin or Masson’s trichrome to visualize inflammatory cell infiltration and collagen fibers, respectively. The number of infiltrating inflammatory cells and collagen thickness were assessed.
Immunohistochemistry (IHC) was performed for CD31, VEGF, Ki67, and collagen I. Antigens were retrieved by heating the slides in citrate buffer (pH = 6.0) in a microwave. Diaminobenzidine was used as the chromogen, and the sections were counterstained with hematoxylin. The number of CD31+ vessels and Ki67+ cells in each field, and the optical densities of VEGF and collagen I were assessed.
The serum levels of growth factors and inflammatory factors, including VEGF, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), in DFU model mice were detected using enzyme-linked immunosorbent assay (ELISA) kits. Blood samples were collected from DFU model mice on days 7 and 14 after ADSC treatment. The collected blood was allowed to clot at room temperature for 2 hours and then centrifuged at 1500 × g for 15 minutes at 4 °C. The supernatant was carefully transferred to a new tube and stored at -80 °C for later analysis. The serum concentrations of specific growth and inflammatory factors were quantified using ELISA kits.
ADSCs were seeded in T75 flasks at 10000 cells/cm2 and cultured in 10 mL of serum-free medium (without supplementation) for 72 hours. The ADSC-conditioned medium (CM) was collected at the end of the culture period, and the cell numbers were counted. The collected medium was centrifuged at 500 × g for 5 minutes to remove dead cells and debris. The CM was kept at -20 °C or -80 °C after harvest, and repeated freeze-thaw cycles were avoided to maintain intact protein composition.
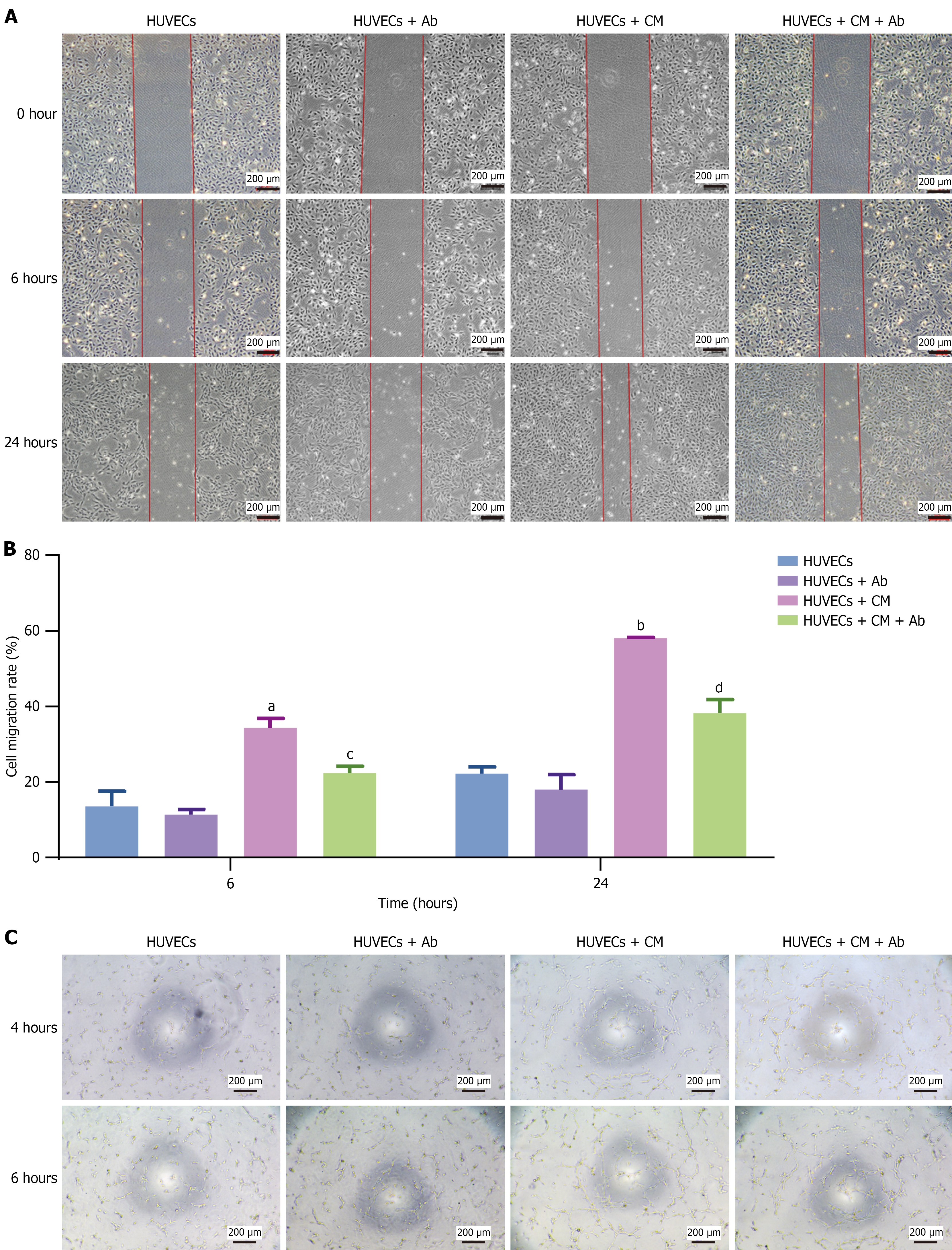
The angiogenic capacity of human umbilical vein endothelial cells (HUVECs) was assessed in vitro through a tube formation assay. Each well of a 96-well plate was coated with 60 μL of Matrigel (BD Biosciences, Shanghai, China) first, ensuring a uniform layer and avoiding bubbles. The gel was allowed to solidify into a firm gel layer by incubating the plate at 37 °C for 30 minutes to 1 hour. Then, 5000 HUVECs with or without 5000 ADSCs in 200 μL of complete endothelial cell medium were inoculated onto the pre-solidified gel. The cells were incubated at 37 °C in a 5% CO2 incubator for 6 hours. Tube formation was photographed via an inverted light microscope (× 100) at 4 and 6 hours after seeding.
A wound healing assay was performed to evaluate the migration of HUVECs, HDFs, and HaCaT cells, a keratinocyte cell line. Briefly, cells were seeded in 6-well plates at a density of 5 × 105 cells/well and cultured in a complete medium for 48 hours. The complete medium was replaced with a serum-free medium for the next 12 hours. Then, the cell monolayer was scratched with a sterile 0.2 mL pipette tip to form a wound. The cells were then maintained in 50% CM + 50% endothelial cell medium in a transwell system (top chambers: ADSCs; bottom chambers: HDFs or HaCaT cells) for 24 hours. Next, the distance between the wound edges was measured at different time points. The percentage of cell migration was calculated using the following formula: Cell migration rate (%) = (initial wound width - width at specific time points)/initial wound width × 100.
All statistical analyses were performed using GraphPad Prism 9, with data presented as the mean ± SD from at least three independent experiments. A Student’s t-test was used for comparisons between two groups. For multiple-group comparisons, a one-way ANOVA was performed, followed by Tukey’s honest significant difference test for all pairwise comparisons or Dunnett’s test when comparing multiple treatments against the control group. An adjusted P-value < 0.05 was considered statistically significant.
Morphologically, ADSCs exhibited a fibroblast-like adherent shape (Figure 1A). Flow cytometric analysis confirmed that ADSCs expressed the MSC markers CD73, CD90, and CD105 but not the hematopoietic and immune cell markers CD34, CD45, CD11b, CD19, and human leukocyte antigen-DR (Figure 1B). Under specific induction conditions, ADSCs successfully differentiated into adipocytes, osteoblasts, and chondrocytes, as confirmed by histological staining (Figure 1C). To assess their secretory capacity, we cultured ADSCs in a serum-free medium for 72 hours and quantified VEGF levels in the CM using ELISA. VEGF concentration progressively increased from 55 pg/mL at 6 hours to 172 pg/mL at 72 hours, demonstrating a significant time-dependent secretion pattern (Figure 1D).
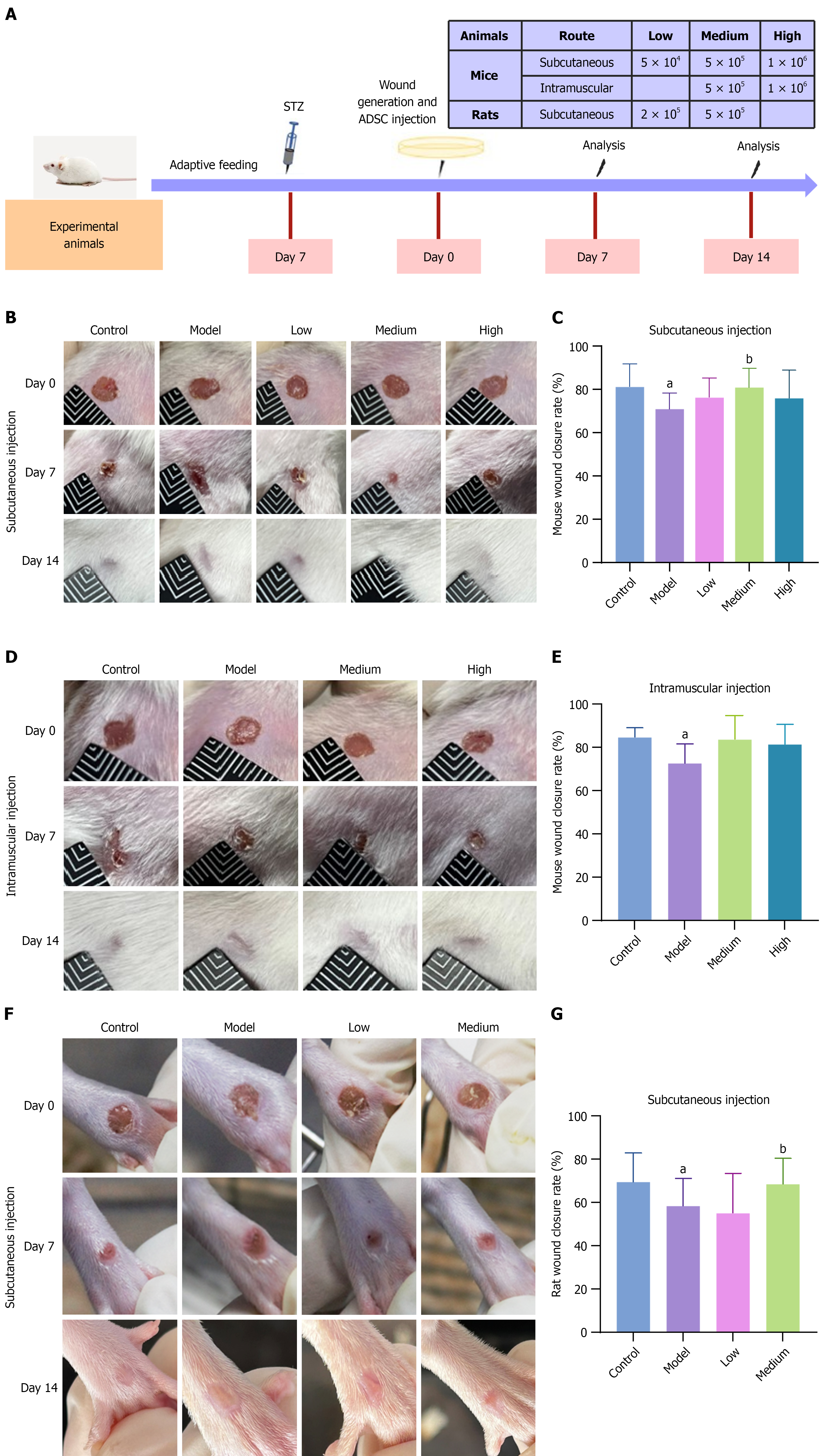
To investigate the dose-response relationship between ADSC quantity and therapeutic efficacy, as well as the impact of different administration routes, we established DFU models in mice and rats (Figure 2A, Tables 1 and 2). Mice were randomly assigned to groups using the Excel RAND function. After STZ injection, we monitored the fasting blood glucose levels of mice to confirm the successful establishment of diabetes. We made full-thickness excisional skin wounds and administered different ADSC doses subcutaneously or intramuscularly. We continuously monitored body weights of the experimental animals during the experiment. ADSC treatment did not significantly change the body weight of diabetic mice (Supplementary Figure 1). Compared to non-diabetic mice, the wound healing rate in diabetic model mice was significantly lower (Figure 2B-E). However, after treatment with 5 × 104, 5 × 105, and 1 × 106 ADSCs, the wound healing rate improved in all treated groups compared to the model group, with a statistically significant difference in the group receiving a subcutaneous medium dose (Figure 2C). To further determine the dose-response relationship, we generated full-thickness skin wounds on the hind feet of rats, and subcutaneously administered 2 × 105 and 5 × 105 ADSCs. Our results showed that the medium dose group significantly improved wound closure rate (Figure 2F and G). Therefore, 5 × 105 (medium dose) was identified as the optimal treatment dose for experimental animals.
| No. | Groups | Injections | Number of mice | |
| 1 | Subcutaneous injection | Control | Vehicle | 14 |
| 2 | Model | Vehicle | 14 | |
| 3 | Low | 5 × 104 ADSCs | 14 | |
| 4 | Medium | 5 × 105 ADSCs | 14 | |
| 5 | High | 1 × 106 ADSCs | 14 | |
| 6 | Intramuscular injection | Control | Vehicle | 7 |
| 7 | Model | Vehicle | 7 | |
| 8 | Medium | 5 × 105 ADSCs | 7 | |
| 9 | High | 1 × 106 ADSCs | 7 | |
| No. | Groups | Injections | Number of rats | |
| 1 | Subcutaneous injection | Control | Vehicle | 14 |
| 2 | Model | Vehicle | 14 | |
| 3 | Low | 2 × 105 ADSCs | 14 | |
| 4 | Medium | 5 × 105 ADSCs | 14 | |
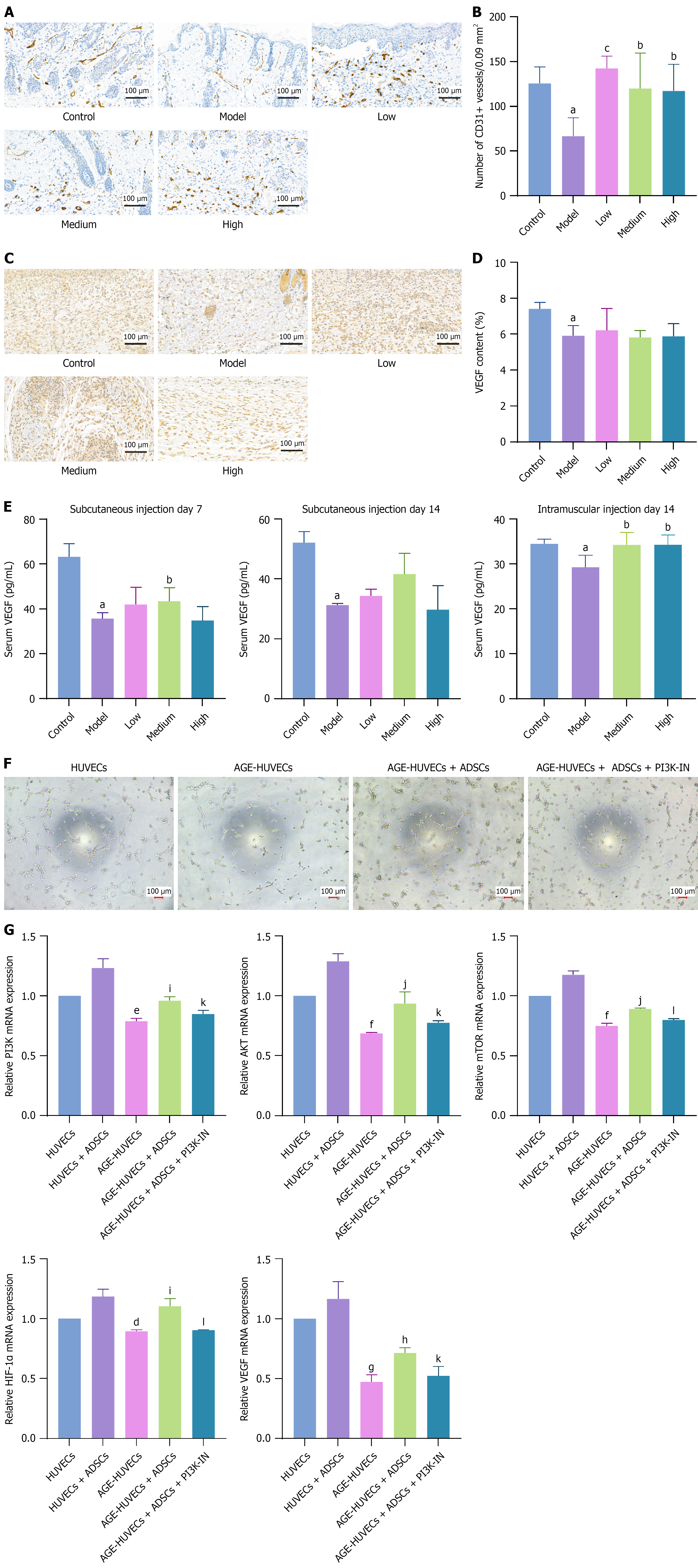
To explore the mechanisms by which ADSCs promote DFU healing, we examined the number of blood vessels and VEGF levels in the wound beds of the mouse models using IHC analysis. The results indicated that the number of blood vessels and the density of VEGF in the wound beds of DFU model mice were significantly lower than those in non-diabetic mice. On day 7 after subcutaneous ADSC injection, the number of blood vessels significantly increased (Figure 3A and B), but VEGF levels did not change significantly (Figure 3C and D). Fourteen days after subcutaneous or intramuscular ADSC treatment, the number of blood vessels significantly increased. VEGF levels increased, although not significantly (Supplementary Figure 2A-D). However, serum VEGF levels significantly increased 7 days after subcutaneous injection and 14 days after intramuscular injection (Figure 3E). In in vitro cell experiments, we treated HUVECs with advanced glycation end products (AGE) to mimic hyperglycemic conditions in patients with diabetes. Our results showed that AGE impaired vascular formation in HUVECs, whereas ADSCs counteracted this suppression and promoted angiogenesis (Figure 3F). We found that ADSCs lost their proangiogenic capability in an AGE environment when a phosphatidylinositol 3-kinase (PI3K) inhibitor was introduced. Further analysis revealed that AGE reduced PI3K, protein kinase B (AKT), mammalian target of rapamycin (mTOR), and hypoxia-inducible factor-1α (HIF-1α) levels in HUVECs, and then decreased VEGF expression and ultimately impaired vascular formation in DFUs. These changes were reversed in the ADSC-treated group (Figure 3G). To verify PI3K signaling pathway inhibition in DFU mice, we assessed AKT expression in mouse skin tissue using reverse transcription-quantitative polymerase chain reaction. AKT expression was suppressed in DFU mice but was significantly restored after ADSC treatment (Supplementary Figure 2E). Therefore, ADSCs may facilitate vascular formation in DFUs by restoring the PI3K-AKT-mTOR-HIF-1α-VEGF signaling pathway under long-term high-glucose conditions.
Previous studies have shown that ADSCs secrete multiple factors via paracrine signaling[20]. In this study, we investigated whether these secretory factors promote angiogenesis. Our results showed that the ADSC culture supernatant promoted HUVEC migration; however, this migratory ability was significantly reduced after incubation with a VEGF-neutralizing antibody (Figure 4A and B). Similarly, this phenomenon was observed in the tube formation experiments (Figure 4C). These results demonstrated that ADSCs promote HUVEC proliferation and migration by secreting VEGF, thereby enhancing angiogenesis.
In contrast to acute wound healing, DFUs frequently remain in the inflammatory phase, with chronic inflammation worsening the ulcers. To investigate the regulatory effects of ADSCs on inflammation in DFUs, we obtained skin tissue sections from mice treated with or without ADSCs and evaluated the infiltration of inflammatory cells. The group treated with ADSCs exhibited a significant reduction in inflammatory cell infiltration on days 7 (Figure 5A and B) and 14 (Supplementary Figure 3A) after subcutaneous ADSC injection. Additionally, the serum inflammatory cytokines TNF-α, IL-1β, and IL-6 were significantly reduced after ADSC treatment (Supplementary Figure 3B).
The shift from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype in macrophages is critical for successful healing[7]. To investigate the effects of ADSCs on macrophage polarization, we added M1 or M2 polarization-stimulating factors to induce THP-1-derived or peripheral blood-derived macrophage polarization. While adding the polarization factors, we established a coculture group with ADSCs. CD86 and CD206 were used as surface markers of M1 and M2 macrophages, respectively. The results indicated that ADSCs inhibited M1 polarization and promoted M2 polarization (Figure 5C, Supplementary Figure 3C). Additionally, interferon-γ and CD86 expression in M1 macrophages decreased, while CD206 expression in M2 macrophages increased after co-culturing with ADSCs (Figure 5D). Further research revealed that AGE treatment activated the Notch signaling pathway and inhibited CD206 expression by overexpressing Notch1, Jagged1, and Notch intracellular domain (NICD) in macrophages. ADSCs reversed the effects of AGE (Figure 5E). To verify Notch signaling pathway overactivation in DFU mice, we assessed NICD expression in mouse skin tissue using reverse transcription-quantitative polymerase chain reaction. NICD was overexpressed in DFU mice, whereas its expression was significantly suppressed after ADSC treatment (Supplementary Figure 3D). Thus, ADSCs may promote macrophage polarization to the M2 phenotype by inhibiting the Notch signaling pathway.
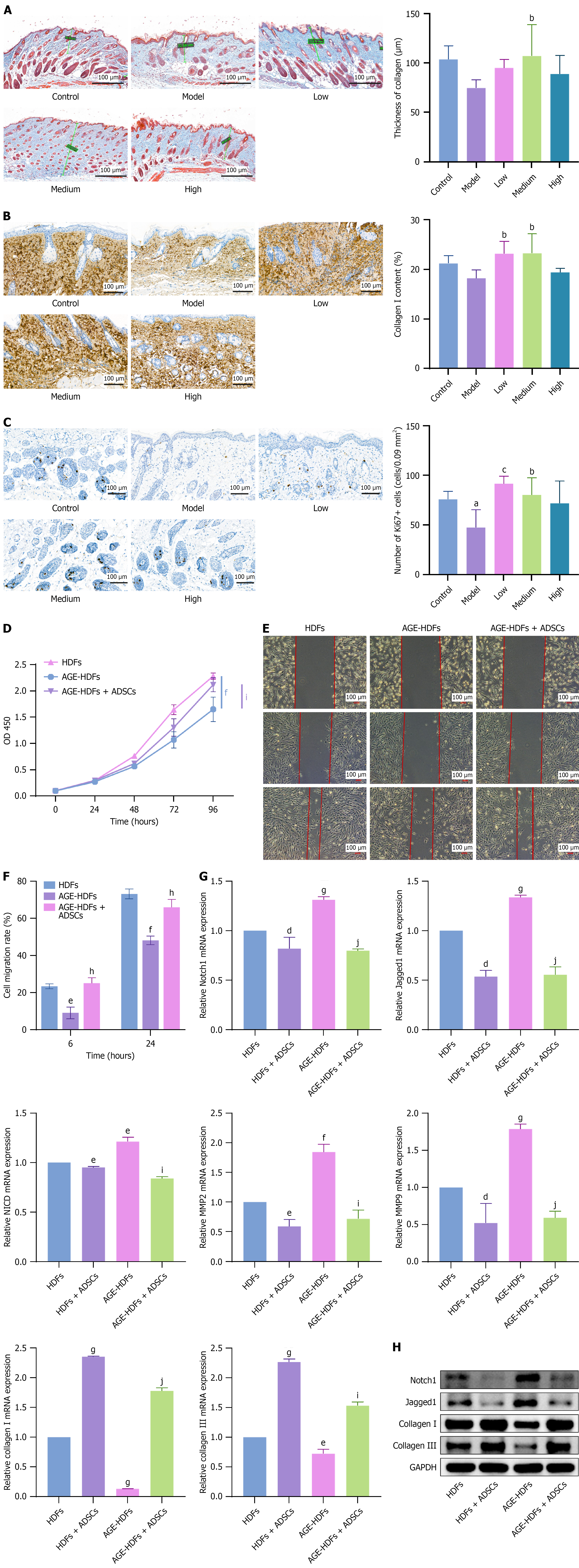
Tissue regeneration, including re-epithelialization and collagen remodeling, is a critical stage of wound healing[7]. To explore the regulatory effects of ADSCs on tissue regeneration, we observed collagen thickness via Masson’s trichrome staining of the wound beds of mice on days 7 and 14 after ADSC treatment and examined collagen I and the cell proliferation marker Ki67 by performing IHC analysis. The results indicated that ADSCs significantly enhanced collagen thickness in the wounds, increased collagen I levels, and stimulated cell proliferation in the wound beds on day 7 after subcutaneous ADSC injection (Figure 6A-C). By day 14, we observed a significant increase in collagen thickness in the medium-dose subcutaneous injection group, whereas a significant increase in the Ki67-positive cells was observed in the high-dose intramuscular injection group. Other results indicated that ADSCs had beneficial but not statistically significant effects (Supplementary Figure 4).
In vitro experiments revealed that AGE inhibited HDF proliferation and migration. When co-cultured with ADSCs in transwell chambers, HDFs showed enhanced proliferation and migration, even under AGE conditions (Figure 6D-F). Subsequent research revealed that AGE leads to Notch1, Jagged1, and NICD overexpression in HDFs, thereby increasing matrix metalloproteinase-2 (MMP2) and matrix metalloproteinase-9 (MMP9) levels and decreasing collagen I and III levels. Co-culturing with ADSCs significantly reduced Notch1, Jagged1, NICD, MMP2, and MMP9 expression and restored collagen I and III expression (Figure 6G and H). Additional results showed that high glucose levels inhibited HaCaT cell proliferation and migration, whereas ADSCs reversed these inhibitory effects (Supplementary Figure 5A-C). Interestingly, we observed that HaCaT cells overexpressed Notch1, Jagged1, NICD, MMP2, and MMP9 in the presence of AGE, whereas collagen I and III expression levels were very low in HaCaT cells (cycle threshold > 35). Co-culture with ADSCs significantly reduced Notch1, Jagged1, NICD, MMP2, and MMP9 expression (Supplementary Figure 5D and E). ADSCs can either directly differentiate into keratinocytes or indirectly enhance re-epithelialization by promoting keratinocyte proliferation and migration around the wound site[26]. Following in vitro induction, we observed that a small number of ADSCs differentiated into epithelial-like cells (Supplementary Figure 5F). Our results showed that ADSCs downregulated the excessively activated Notch signaling pathway in HDFs in an AGE environment, thereby promoting collagen deposition. Moreover, ADSCs promote re-epithelialization by regulating the Notch signaling pathway[27].
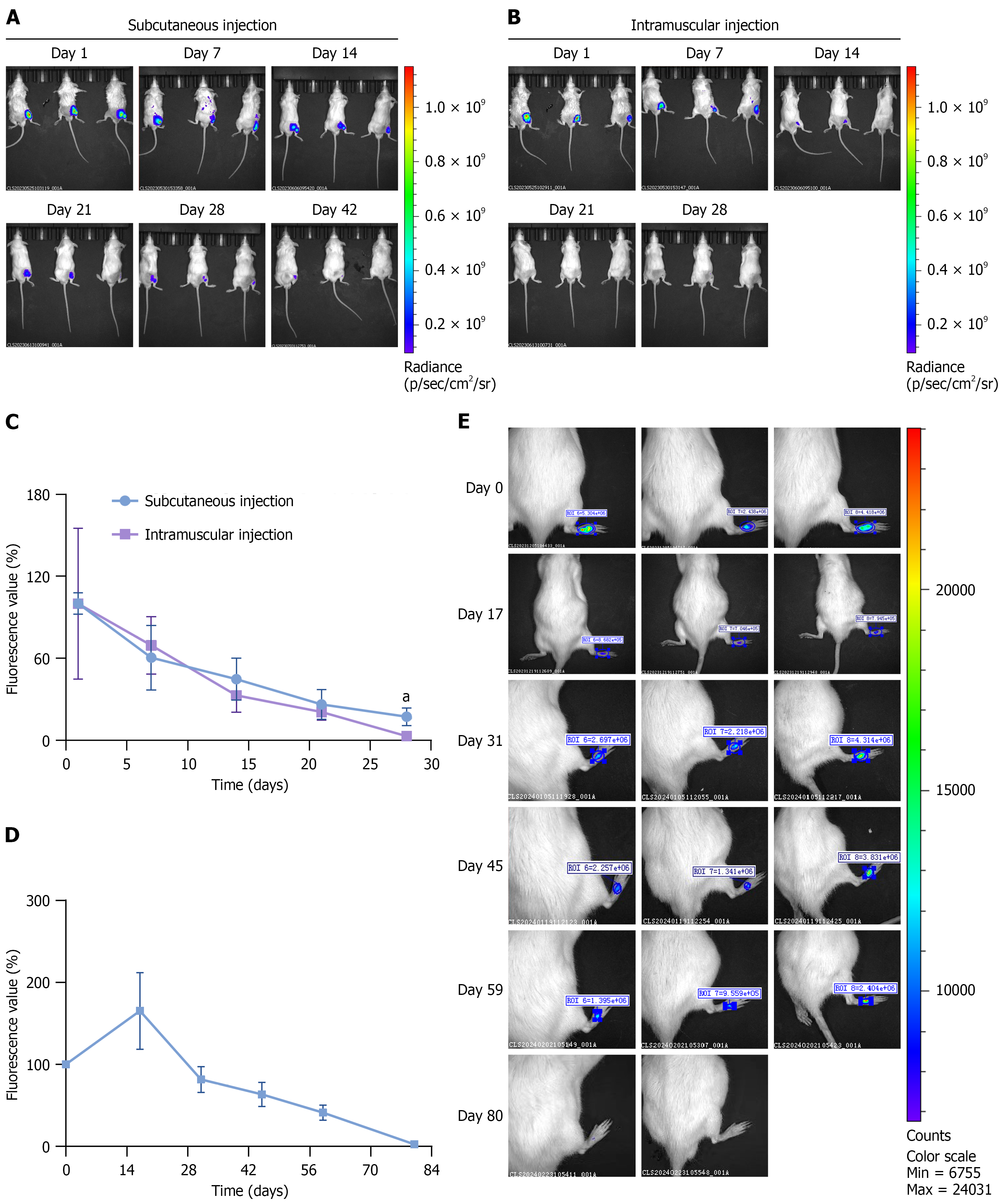
To determine the persistence and distribution of ADSCs in DFU model animals after injection, we labeled ADSCs with DiD fluorescent dye and used an in vivo imaging system to measure fluorescence intensity. The results showed that for all injection groups, most ADSCs were localized at the injection site. After subcutaneous injection into mouse thighs, ADSCs survived for up to 42 days, whereas they survived for only 28 days after intramuscular injection (Figure 7A and B). On day 28, the cell retention in the subcutaneous injection group was significantly greater than that in the intramuscular injection group (Figure 7C). Thus, subcutaneous ADSC injections can provide long-lasting and potentially improved therapeutic effects. ADSCs survived for up to 80 days after subcutaneous injection into rat feet, whereas they survived for 42 days after injection into mouse thighs (Figure 7D and E), indicating that the method and site of injection influenced the persistence and therapeutic effects of ADSCs in DFU models, likely due to the blood supply at the injection site.
DFUs remain one of the most intractable complications of diabetes, with impaired angiogenesis, persistent inflammation, and defective tissue regeneration as the major pathological barriers to healing[2,7]. In this study, we systematically evaluated the dose-response relationship, administration routes, persistence, and underlying mechanisms of ADSCs in DFU repair. Our results demonstrate that ADSCs accelerate wound closure, enhance angiogenesis, and modulate inflammatory responses, while the therapeutic efficacy is strongly influenced by dosage and delivery route. Subcutaneous administration of 5 × 105 ADSCs showed the most pronounced benefits and prolonged cellular persistence, providing an evidence-based foundation for optimizing ADSC therapy in DFUs.
The pro-angiogenic capacity of ADSCs is a key driver of DFU recovery. ADSC transplantation significantly increased CD31+ vessel density and VEGF levels in wound tissues, consistent with previous findings that ADSCs secrete VEGF and other angiogenic cytokines to promote neovascularization[18-21]. In vitro, ADSCs counteracted the inhibitory effects of AGEs on endothelial cells, restoring tube formation through activation of the PI3K/AKT/mTOR/HIF-1α/VEGF signaling pathway. These results are consistent with earlier reports demonstrating that MSCs or their exosomes enhance angiogenesis in diabetic wounds through the PI3K/AKT axis[28,29].
In addition to promoting angiogenesis, ADSCs exert immunomodulatory effects that are essential for transitioning wounds from the inflammatory to the proliferative phase. Chronic DFUs are often trapped in persistent inflammation, dominated by pro-inflammatory M1 macrophages[7,30]. Our data show that ADSCs attenuate inflammatory cell infiltration and reduce serum TNF-α, IL-1β, and IL-6 levels. In macrophage coculture systems, ADSCs inhibited M1 polarization while promoting anti-inflammatory M2 phenotypes, thereby facilitating wound resolution. Mechanistically, ADSCs suppressed the overactivated Notch1/Jagged1/NICD signaling triggered by AGEs, restoring macrophage balance. These findings corroborate prior studies indicating that hyperglycemia-induced Notch overactivation sustains inflammation in diabetic wounds[31,32], and that ADSCs can modulate this pathway to promote tissue repair[33].
ADSCs also support tissue regeneration by stimulating dermal fibroblasts and keratinocytes. Under AGE exposure, high glucose levels suppress fibroblast proliferation and collagen synthesis by activating the Notch1 pathway and increasing MMP-2 and MMP-9 expression[27,34,35]. ADSCs reversed these effects, downregulating Notch-related genes and restoring collagen I/III expression. The enhancement of re-epithelialization and collagen deposition observed in vivo further confirms that ADSCs promote remodeling through paracrine and cellular mechanisms. Together, these results suggest that ADSCs orchestrate wound healing by simultaneously restoring angiogenesis, suppressing chronic inflammation, and promoting dermal matrix regeneration.
Determining the optimal cell dosage is critical for effective stem cell therapy. Our findings indicate that moderate ADSC doses (5 × 105) achieved superior wound healing compared with lower or higher doses. Excessive cell loads may provoke immune reactions or experience poor survival under ischemic and hyperglycemic conditions, whereas moderate doses maintain paracrine activity and adaptability, echoing the “less-is-more” paradigm reported in other MSC studies[36,37]. These observations underscore the importance of dose optimization to balance efficacy and safety.
The administration route also critically affects ADSC distribution and therapeutic persistence. Intravenous delivery often results in pulmonary entrapment (“first-pass effect”) and reduced local concentration[38], while local injection enhances retention and efficacy[39,40]. In our study, subcutaneous injection outperformed intramuscular administration, sustaining ADSC survival for up to 42 days in mice and 80 days in rats. The superior outcome of subcutaneous injection may be attributed to improved local perfusion and a more favorable niche for cell survival, leading to prolonged paracrine signaling at the wound site.
Both mice and rats were employed to ensure the robustness of the findings. The mouse DFU model was used for dosage exploration due to its lower cost and well-characterized diabetic response, while rats were used for validation because their foot structure and wound-healing patterns more closely resemble those of human DFUs[41]. Biodistribution imaging confirmed that most transplanted ADSCs remained localized near the injection site, consistent with prior observations that locally injected MSCs exhibit limited migration but strong paracrine effects[42].
This study provides an experimental basis for optimizing ADSC therapy for chronic diabetic wounds. Subcutaneous administration of a moderate ADSC dose demonstrated sustained cell persistence and superior efficacy, suggesting that local delivery may represent a practical clinical approach. Nevertheless, several limitations should be acknowledged. First, although the preclinical data are encouraging, rodent wound healing relies more on contraction than re-epithelialization, which differs from human physiology[41]. Second, long-term safety of transplanted cells requires further clinical evaluation. Ongoing dose-exploration clinical trials approved by the National Medical Products Administration (No. 2024 LP00570) will address these issues. Finally, future studies should explore humanized wound models or 3D skin organoids to better simulate the human DFU microenvironment and verify the mechanistic pathways identified here. In conclusion, ADSCs facilitate DFU healing through enhanced angiogenesis, immunomodulation, and tissue regeneration. Optimizing cell dosage and administration routes maximizes therapeutic efficacy while minimizing risks. These findings lay a foundation for translational application of ADSC-based therapies and contribute to the development of regenerative strategies for chronic diabetic wounds.
ADSCs represent a potent therapeutic tool for managing DFUs by addressing multiple facets of wound healing through enhanced angiogenesis, immunomodulation, and tissue regeneration. Moderate subcutaneous administration achieved the best therapeutic balance between efficacy and safety. These preclinical findings suggest that optimizing ADSC dosage and delivery route may provide a promising strategy for improving chronic DFU healing.
| 1. | Chhillar A, Jaiswal A. Hyaluronic Acid-Based Self-Healing Hydrogels for Diabetic Wound Healing. Adv Healthc Mater. 2025;14:e2404255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 2. | Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. N Engl J Med. 2017;376:2367-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 2572] [Article Influence: 285.8] [Reference Citation Analysis (2)] |
| 3. | Armstrong DG, Tan TW, Boulton AJM, Bus SA. Diabetic Foot Ulcers: A Review. JAMA. 2023;330:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 798] [Article Influence: 266.0] [Reference Citation Analysis (2)] |
| 4. | Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1590] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 5. | Petersen BJ, Linde-Zwirble WT, Tan TW, Rothenberg GM, Salgado SJ, Bloom JD, Armstrong DG. Higher rates of all-cause mortality and resource utilization during episodes-of-care for diabetic foot ulceration. Diabetes Res Clin Pract. 2022;184:109182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Ruke MG, Selva Elavarasan S, Devarajan A, Viswanathan V. Progress and challenges in diabetic foot care in South-East Asia and India - Current scenario. Diabetes Res Clin Pract. 2025;226:112293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Monaghan MG, Borah R, Thomsen C, Browne S. Thou shall not heal: Overcoming the non-healing behaviour of diabetic foot ulcers by engineering the inflammatory microenvironment. Adv Drug Deliv Rev. 2023;203:115120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 8. | Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1737] [Article Influence: 82.7] [Reference Citation Analysis (1)] |
| 9. | Liu ZJ, Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10:1869-1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Shao Y, Zhou X, Zhou S, Long J, Jin L, Shi X, Zhou L, Zhang Y, Fan D. Injectable DMM/GelMA hydrogel for diabetic wound healing via regulating mitochondrial metabolism and macrophage repolarization. Colloids Surf B Biointerfaces. 2025;248:114488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Mehnath S, Karthikeyan K, Jeyaraj M. Mechanical Force on Hydrogel Implication on Enhanced Drug Release, Antibacterial, and M2 Macrophage Polarization: New Insights Alleviate Diabetic Wound Healing. ACS Appl Mater Interfaces. 2024;16:55166-55180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Su X, Geng X, Li F, Song M, Lv R, Zhang Y, Yuan J, Dong J, Shi Y, Zhao L. Microneedles loaded with l-arginine-modified puerarin-derived carbon nanoparticles improved treatment of diabetic wound via photothermal and nitric oxide-based gas therapy. J Colloid Interface Sci. 2025;691:137353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 13. | Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 506] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 14. | Cao Y, Yan J, Dong Z, Wang J, Jiang X, Cui T, Huang Y, Liu H. Adipose-derived Mesenchymal Stem Cells are Ideal for the Cell-based Treatment of Refractory Wounds: Strong Potential for Angiogenesis. Stem Cell Rev Rep. 2024;20:313-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856-1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 469] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 16. | Nambu M, Kishimoto S, Nakamura S, Mizuno H, Yanagibayashi S, Yamamoto N, Azuma R, Nakamura S, Kiyosawa T, Ishihara M, Kanatani Y. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg. 2009;62:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Zhang S, Chen L, Zhang G, Zhang B. Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res Ther. 2020;11:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, Sung JH. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 19. | Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 638] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 20. | Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1649] [Cited by in RCA: 1730] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 21. | Shi R, Jin Y, Cao C, Han S, Shao X, Meng L, Cheng J, Zhang M, Zheng J, Xu J, Li M. Localization of human adipose-derived stem cells and their effect in repair of diabetic foot ulcers in rats. Stem Cell Res Ther. 2016;7:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3887] [Cited by in RCA: 3392] [Article Influence: 212.0] [Reference Citation Analysis (0)] |
| 23. | Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 24. | Uzun E, Güney A, Gönen ZB, Özkul Y, Kafadar İH, Günay M, Mutlu M. Intralesional allogeneic adipose-derived stem cells application in chronic diabetic foot ulcer: Phase I/2 safety study. Foot Ankle Surg. 2021;27:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Yin Q, Yang G, Su R, Bu J, Li Y, Zhang H, Zhang Y, Zhuang P. Zi Shen Wan Fang repaired blood-brain barrier integrity in diabetic cognitive impairment mice via preventing cerebrovascular cells senescence. Chin Med. 2024;19:169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 27. | Zhu P, Chen C, Wu D, Chen G, Tan R, Ran J. AGEs-induced MMP-9 activation mediated by Notch1 signaling is involved in impaired wound healing in diabetic rats. Diabetes Res Clin Pract. 2022;186:109831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Jere SW, Houreld NN, Abrahamse H. Role of the PI3K/AKT (mTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019;50:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 29. | Liang ZH, Pan NF, Lin SS, Qiu ZY, Liang P, Wang J, Zhang Z, Pan YC. Exosomes from mmu_circ_0001052-modified adipose-derived stem cells promote angiogenesis of DFU via miR-106a-5p and FGF4/p38MAPK pathway. Stem Cell Res Ther. 2022;13:336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 30. | Tan H, Xu W, Ding X, Ye H, Hu Y, He X, Ming Y, Zheng L. Notch/NICD/RBP-J signaling axis regulates M1 polarization of macrophages mediated by advanced glycation end products. Glycoconj J. 2022;39:487-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 31. | Zheng X, Narayanan S, Sunkari VG, Eliasson S, Botusan IR, Grünler J, Catrina AI, Radtke F, Xu C, Zhao A, Ekberg NR, Lendahl U, Catrina SB. Triggering of a Dll4-Notch1 loop impairs wound healing in diabetes. Proc Natl Acad Sci U S A. 2019;116:6985-6994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 32. | Ebrahim N, Dessouky AA, Mostafa O, Hassouna A, Yousef MM, Seleem Y, El Gebaly EAEAM, Allam MM, Farid AS, Saffaf BA, Sabry D, Nawar A, Shoulah AA, Khalil AH, Abdalla SF, El-Sherbiny M, Elsherbiny NM, Salim RF. Adipose mesenchymal stem cells combined with platelet-rich plasma accelerate diabetic wound healing by modulating the Notch pathway. Stem Cell Res Ther. 2021;12:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 33. | Kimball AS, Joshi AD, Boniakowski AE, Schaller M, Chung J, Allen R, Bermick J, Carson WF 4th, Henke PK, Maillard I, Kunkel SL, Gallagher KA. Notch Regulates Macrophage-Mediated Inflammation in Diabetic Wound Healing. Front Immunol. 2017;8:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 34. | Tardáguila-García A, García-Morales E, García-Alamino JM, Álvaro-Afonso FJ, Molines-Barroso RJ, Lázaro-Martínez JL. Metalloproteinases in chronic and acute wounds: A systematic review and meta-analysis. Wound Repair Regen. 2019;27:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 35. | Krishnan N, Velramar B, Ramatchandirin B, Abraham GC, Duraisamy N, Pandiyan R, Velu RK. Effect of biogenic silver nanocubes on matrix metalloproteinases 2 and 9 expressions in hyperglycemic skin injury and its impact in early wound healing in streptozotocin-induced diabetic mice. Mater Sci Eng C Mater Biol Appl. 2018;91:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 786] [Cited by in RCA: 908] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 37. | Maria AT, Toupet K, Bony C, Pirot N, Vozenin MC, Petit B, Roger P, Batteux F, Le Quellec A, Jorgensen C, Noël D, Guilpain P. Antifibrotic, Antioxidant, and Immunomodulatory Effects of Mesenchymal Stem Cells in HOCl-Induced Systemic Sclerosis. Arthritis Rheumatol. 2016;68:1013-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 963] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 39. | Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1574] [Cited by in RCA: 1481] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 40. | Torres-Torrillas M, Rubio M, Damia E, Cuervo B, Del Romero A, Peláez P, Chicharro D, Miguel L, Sopena JJ. Adipose-Derived Mesenchymal Stem Cells: A Promising Tool in the Treatment of Musculoskeletal Diseases. Int J Mol Sci. 2019;20:3105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 41. | Kato J, Kamiya H, Himeno T, Shibata T, Kondo M, Okawa T, Fujiya A, Fukami A, Uenishi E, Seino Y, Tsunekawa S, Hamada Y, Naruse K, Oiso Y, Nakamura J. Mesenchymal stem cells ameliorate impaired wound healing through enhancing keratinocyte functions in diabetic foot ulcerations on the plantar skin of rats. J Diabetes Complications. 2014;28:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Zhang R, Luo W, Zhang Y, Zhu D, Midgley AC, Song H, Khalique A, Zhang H, Zhuang J, Kong D, Huang X. Particle-based artificial three-dimensional stem cell spheroids for revascularization of ischemic diseases. Sci Adv. 2020;6:eaaz8011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/