Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.110722
Revised: August 1, 2025
Accepted: September 16, 2025
Published online: October 15, 2025
Processing time: 110 Days and 1.4 Hours
Cardiac autonomic neuropathy correlates intimately with cardiovascular com
To investigate how BG variability impacts heart rate (HR) dynamics in older adults with T2DM + coronary heart disease (CHD) and to evaluate the ability of functional myocardial ischemia to predict outcomes in this cohort.
We enrolled 143 older T2DM + CHD patients admitted to the First Affiliated Hospital, Hengyang Medical School, University of South China over a 3.5-year period (January 2018 to July 2021). Using a standard deviation of BG cutoff of 1.4 mmol/L, subjects were stratified into abnormal (n = 75) and normal (n = 68) fluctuation groups. All patients underwent 72-hour dynamic BG monitoring to detect BG fluctuation parameters. The time domain index of HR variability was measured by dynamic electrocardiogram. To determine how well glucose fluc
The abnormal fluctuation group showed greater levels of mean amplitude of glycemic excursions (MAGE), mean of daily differences (MODD), largest ampli
A negative correlation was found between BG variability and HR dynamics in older CHD + T2DM patients, and MAGE combined with MPPGE demonstrated better efficacy in predicting functional myocardial ischemia, which deserves clinical attention.
Core Tip: Recent evidence has linked cardio-cerebrovascular events in type 2 diabetes mellitus patients with concurrent coronary artery disease to blood glucose (BG) fluctuation, and the greater the BG fluctuation, the higher the incidence of chronic vascular complications of diabetes mellitus and acute cardiovascular events, and the worse the prognosis. This study analyzes how BG variability impacts heart rate dynamics in such patients and the predictive properties of functional myocardial ischemia.
- Citation: Li AQ, Zhang F. Blood glucose variability impacts heart rate dynamics in older type 2 diabetic and coronary heart disease patients. World J Diabetes 2025; 16(10): 110722
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/110722.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.110722
Coronary heart disease (CHD) is among the most prevalent cardiovascular issues in the older population, and its incidence is increasing, becoming an important factor affecting the life and health of this population[1]. Type 2 diabetes mellitus (T2DM) independently increases CHD risk in older patients, which can further aggravate their condition. For geriatric patients with T2DM-CHD comorbidity, improving condition assessment and prognosis prediction can provide an effective basis for rational diagnosis and treatment. The close connection between blood glucose (BG) variability and health status in older patients with both T2DM and CHD has been well established. It is expected to make significant progress in evaluating patients’ condition[2]. Although heart rate (HR) variability (HRV) is an important indicator for assessing CHD severity and prognosis, it is cumbersome to detect and difficult to widely adopt[3]. At this stage, how BG fluctuations correlate with HRV in older T2DM patients with CHD remains underexplored. Moreover, T2DM-CHD interactions in older patients can directly lead to functional myocardial ischemia. If the risk of this condition can be accurately assessed in advance, timely and targeted therapeutic intervention is crucial for enhancing patient clinical outcomes. However, the predictive effect of BG fluctuation parameters on functional myocardial ischemia remains to be determined. Therefore, our objective was to evaluate whether BG fluctuations influence HRV in senior patients with T2DM and CHD and the predictive performance of functional myocardial ischemia.
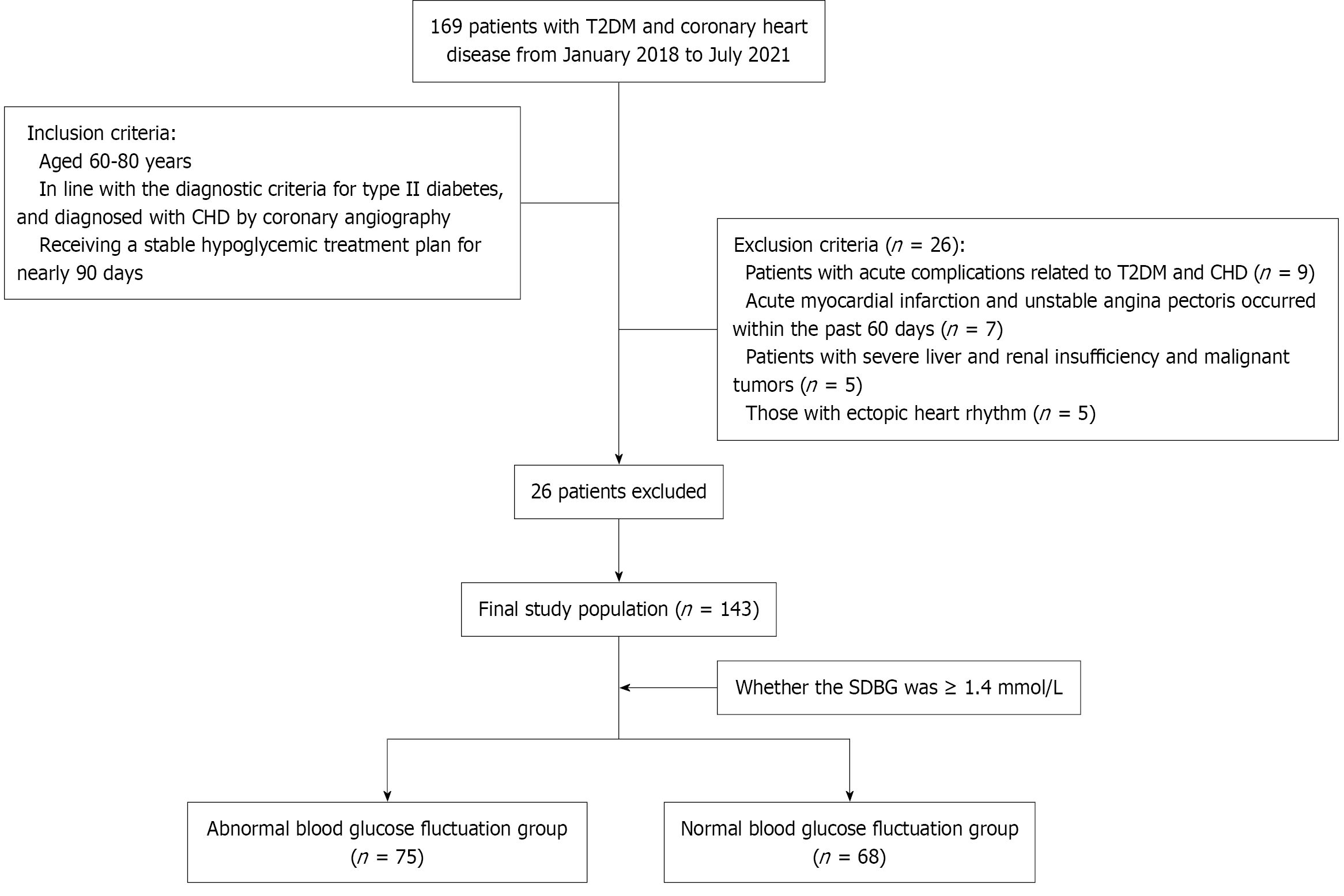
In this retrospective study, the study population included 143 older T2DM + CHD patients visiting The First Affiliated Hospital, Hengyang Medical School, University of South China, over a 3.5-year period (January 2018 to July 2021). Inclusion was restricted to patients who fulfilled these conditions: (1) Aged 60-80 years; (2) Met the diagnostic criteria for type 2 diabetes and diagnosed with CHD by coronary angiography; and (3) Received a stable hypoglycemic treatment plan for nearly 90 days. We applied the following exclusion criteria: (1) Acute complications related to T2DM and CHD; (2) Acute myocardial infarction and unstable angina pectoris occurred within the past 60 days; (3) Severe liver and renal insufficiency and malignant tumors; and (4) Ectopic heart rhythm.
Based on whether the standard deviation of BG was ≥ 1.4 mmol/L[4], participants were categorized into a group with abnormal BG fluctuation (n = 75) and a group with normal BG fluctuation (n = 68). Patient selection flow diagram can be found in Figure 1.
BG level: All patients underwent 72-hour continuous BG monitoring; the Medtronic’s Guardian REAL-Time continuous BG monitoring system was used to monitor and record 288 BG values throughout the day, input finger BG correction probe information no less than four times during the monitoring period, and detect BG fluctuations parameters: Mean amplitude of glycemic excursions (MAGE), mean of daily differences (MODD), largest amplitude of glycemic excursions (LAGE), mean postprandial glucose excursions (MPPGE). All patients had completed BG monitoring.
When ensuring that the patient had stopped the intake of drugs (such as β-blockers) that may affect the HR and were emotionally stable, the United States Century 3000 24-hour ambulatory electrocardiograph was used to continuously record the 24-hour electrocardiogram signal, and detect the time-domain indicators of HRV, including the standard deviation of normal-to-normal (NN) interval (SDNN), standard deviation of the average NN interval (SDANN) every 5 minutes, standard deviation of NN intervals over every 5-minute period (SDNNindex), root mean square of successive differences (rMSSD), and the percentage of NN intervals differing by > 50 ms (pNN50). In all cases, 80% of the data were collected and recorded.
Inter-group comparisons were made regarding BG fluctuation parameters (MAGE, MODD, LAGE, and MPPGE) and time-domain HRV metrics (SDNN, SDANN, SDNNindex, rMSSD, and pNN50). Pearson correlation examined how BG fluctuation parameters correlated with HRV time-domain measures. To observe the occurrence of functional myocardial ischemia, in coronary angiography, the blood flow reserve fraction after inducing the maximum congestion of the culprit vessel is ≤ 0.8, which is the basis for judging functional myocardial ischemia[5]. Multivariate logistic regression analyzed key contributors to functional myocardial ischemia, with the predictive power of each BG fluctuation parameter for functional myocardial ischemia assessed by the area under the receiver operating characteristic (ROC) curve (AUC).
SPSS version 18.0 processed all the collected data. Measurements demonstrating normality appear as the mean ± SD, with inter-group differences identified by t-tests. Count data were analyzed by the χ2 test. The AUC under the ROC curve of the two groups was compared by the Delong test; Significant differences were noted at the P < 0.05 Level.
The abnormal BG fluctuation group included 42 male and 33 female patients aged between 62 years and 78 years (average: 65.63 ± 5.72 years). The body mass index ranged from 20-28 (23.55 ± 1.42) kg/m2. Moreover, 27, 11, and 40 patients had a history of smoking, drinking, and hypertension, respectively.
The normal blood sugar fluctuation group included 40 male and 28 female patients. They were 61-79 years old (average: 64.91 ± 5.68). The body mass index was 20-29 (24.01 ± 1.39) kg/m2. Moreover, 25, 10, and 37, 10, and 37 patients had a history of smoking, drinking, and hypertension, respectively. General data did not differ notably across groups (P > 0.05; Table 1).
| Normoglycemia group (n = 68) | Abnormal blood sugar fluctuation group (n = 75) | χ2/t | P value | |
| Age (years) | 64.91 ± 5.68 | 65.63 ± 5.72 | 0.754 | 0.452 |
| Gender | 0.116 | 0.733 | ||
| Male | 40 | 42 | ||
| Female | 28 | 33 | ||
| Body mass index (kg/m2) | 24.01 ± 1.39 | 23.55 ± 1.42 | 1.954 | 0.053 |
| Course of diabetes mellitus (years) | 5.27 ± 2.38 | 5.90 ± 2.29 | 1.610 | 0.110 |
| Smoking history | 25 | 27 | 0.009 | 0.924 |
| Drinking history | 10 | 11 | 0.007 | 0.995 |
| Hypertension | 37 | 40 | 0.017 | 0.897 |
BG and glycosylated hemoglobin (HbA1c) showed similar levels across groups (P > 0.05; Table 2).
| Group | Number of cases | Fasting blood glucose (mmol/L) | HbA1c (%) |
| Normoglycemia group | 68 | 9.55 ± 1.26 | 9.75 ± 2.77 |
| Abnormal blood sugar fluctuation group | 75 | 9.92 ± 1.61 | 10.09 ± 1.26 |
| t value | 1.541 | 0.942 | |
| P value | 0.126 | 0.348 |
Patients with BG fluctuation abnormalities exhibited greater MAGE, MODD, LAGE and MPPGE relative to those with normal BG fluctuations (P < 0.05; Table 3).
| Group | Number of cases | MAGE | MODD | LAGE | MPPGE |
| Normoglycemia group | 68 | 3.07 ± 0.51 | 1.48 ± 0.33 | 5.71 ± 0.77 | 2.69 ± 0.85 |
| Abnormal blood sugar fluctuation group | 75 | 5.28 ± 1.24 | 2.45 ± 0.76 | 6.54 ± 1.24 | 3.97 ± 1.24 |
| t value | 4.685 | 4.235 | 4.514 | 4.387 | |
| P value | 0.041 | 0.047 | 0.044 | 0.046 |
Lower SDNN, SDANN, SDNNindex, rMSSD, and pNN50 were found in patients presenting BG fluctuation abnormalities vs the normal BG fluctuation group (P < 0.05; Table 4).
| Group | Number of cases | SDNN (ms) | SDANN (ms) | SDNNindex (ms) | rMSSD (ms) | pNN50 (%) |
| Normoglycemia group | 68 | 113.62 ± 24.83 | 101.47 ± 19.86 | 30.45 ± 10.64 | 24.15 ± 6.07 | 7.99 ± 4.06 |
| Abnormal blood sugar fluctuation group | 75 | 96.45 ± 15.87 | 85.74 ± 13.62 | 22.12 ± 6.40 | 17.42 ± 3.88 | 5.01 ± 2.25 |
| t value | 22.016 | 26.432 | 9.013 | 8.764 | 5.216 | |
| P value | 0.000 | 0.000 | 0.000 | 0.000 | 0.035 |
The Pearson correlation analysis showed that MAGE, MODD, LAGE, and MPPGE were negatively correlated with SDNN, SDANN, SDNNindex, rMSSD, and pNN50 in older patients with T2DM and CHD (P < 0.05; Table 5).
| Heart rate variability time domain indicator | MAGE | MODD | LAGE | MPPGE | ||||
| r value | P value | r value | P value | r value | P value | r value | P value | |
| SDNN | -0.231 | 0.033 | -0.198 | 0.042 | -0.224 | 0.035 | -0.291 | 0.000 |
| SDANN | -0.218 | 0.036 | -0.196 | 0.044 | -0.269 | 0.024 | -0.295 | 0.000 |
| SDNNindex | -0.259 | 0.029 | -0.195 | 0.045 | -0.302 | 0.000 | -0.289 | 0.000 |
| rMSSD | -0.263 | 0.026 | -0.206 | 0.040 | -0.189 | 0.047 | -0.214 | 0.036 |
| pNN50 | -0.201 | 0.039 | -0.213 | 0.037 | -0.254 | 0.021 | -0.239 | 0.034 |
Among 143 older patients with T2DM complicated by CHD, functional myocardial ischemia occurred in 26, accounting for 18.18%. Multivariate modeling revealed MAGE, MPPGE, and SDNN as independent predictors of functional myocar
| Indexes | B | SE | Wald | Sig. | Odds ratio | 95%CI |
| MAGE | 1.847 | 0.794 | 5.452 | 0.029 | 0.164 | 0.031-0.737 |
| MPPGE | 1.129 | 0.331 | 9.164 | 0.015 | 2.845 | 1.544-5.952 |
| SDNN | 1.543 | 0.552 | 6.535 | 0.031 | 4.442 | 1.405-13.841 |
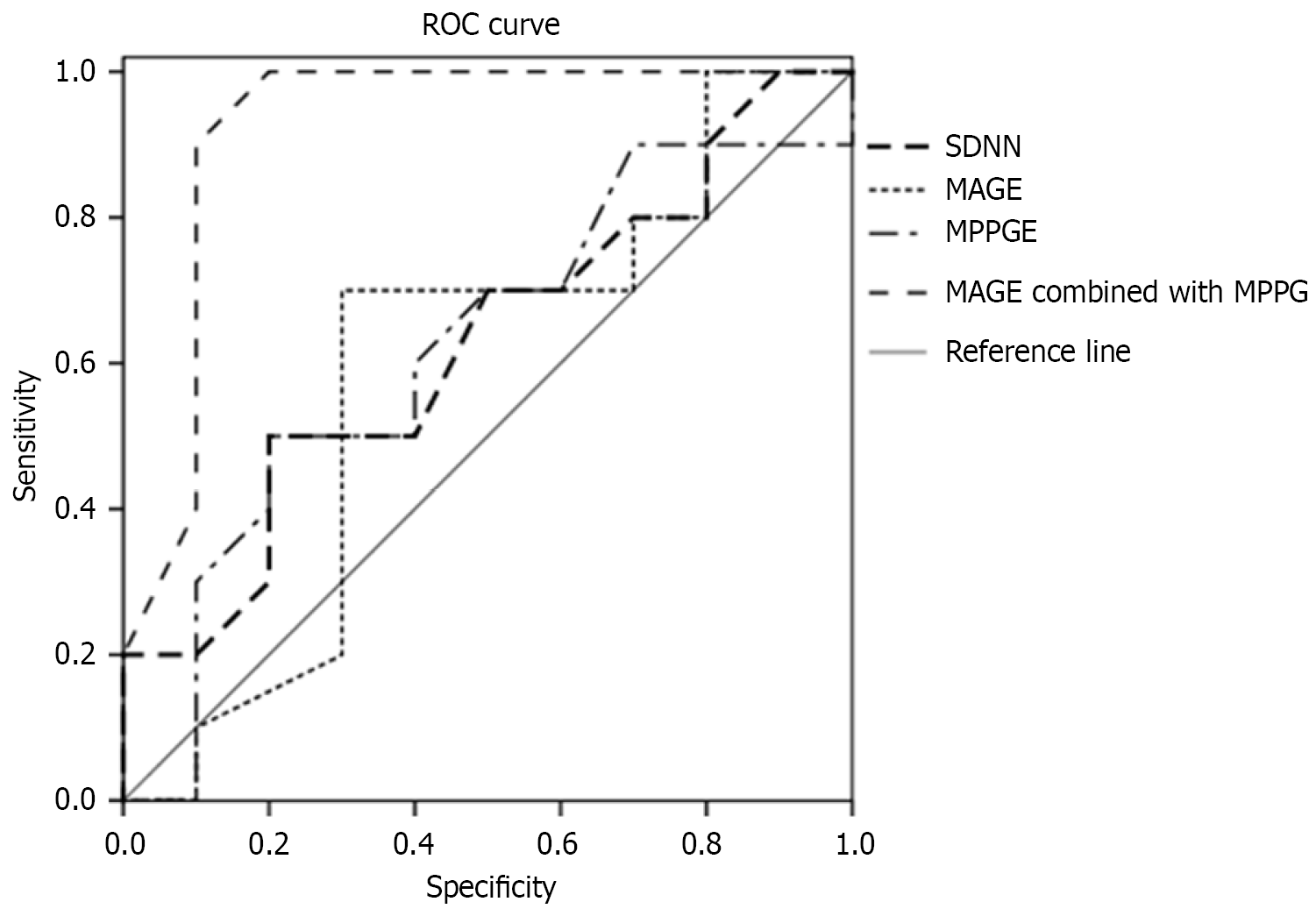
According to the ROC curve analysis, the AUC of MAGE + MPPGE in predicting functional myocardial ischemia was 0.912, which was significantly greater than that of SDNN, 0.694. The Delong test showed that the difference was signi
Patients with CHD often have impaired BG metabolism, particularly in older patients. Up to 70% of older patients with CHD have T2DM[6]. For older patients with T2DM and CHD, the hyperglycemia-related damage to the heart cannot be ignored, and it may be an important cause of disease progression. For a long time, HbA1c has been recognized as an effective indicator to measure the effect of blood sugar control, and its level is positively correlated with the risk of diabetes-related complications. However, recently, even with the same HbA1c, the occurrence of functional myocardial ischemia in older patients with T2DM and CHD varies[7]. International studies have found that changes in the cardiac structure and function in older patients with T2DM and CHD are closely related to BG fluctuations because both chronic persistent hyperglycemia and BG fluctuations can cause damage to target organs[8]. Zhang et al[9] indicated that fluctuating hyperglycemia has a more significant effect on cardiac structure and function. As shown in Table 1, all time-domain HRV measures were lower in the abnormal BG fluctuation group compared to the normal BG fluctuation group, which is similar to the research results of Shimabukuro et al[10], suggesting that patients with abnormal BG fluctuation have lower HRV, considering that abnormal blood sugar fluctuations can promote cardiac autonomic neuropathy. Li
The effect of BG fluctuations on HRV in older T2DM patients with concurrent CHD has only received attention in recent years, and related research is still in its infancy. Cardiovascular autonomic neuropathy (CAN), a prevalent but underdiagnosed DM-induced long-term complication, is associated with elevated disease burden and death rates[12]. Although some studies have shown higher glycemic variability in CAN[13], other studies have found no relationship between glycemic variability and CAN[14]. In this study, a 72-hour dynamic BG monitoring system was used, which can accurately reflect BG fluctuations throughout the day[15]. As shown in Table 1, BG fluctuation parameters were higher in patients with BG fluctuation abnormalities compared to normal counterparts, suggesting that the above indicators can effectively reflect changes in BG fluctuations and do not depend on the overall BG level. Studies have shown significantly greater BG fluctuation parameters in older patients with T2DM-CHD comorbidity than those with simple T2DM or CHD, indicating that the former has greater blood sugar fluctuations[16].
In this study, Pearson correlation analysis was conducted, and the BG fluctuation parameters in older T2DM + CHD patients were negatively correlated with the time domain indicators of HRV (P < 0.05). This finding supports the inverse connection between BG fluctuation degree and HRV reduction in T2DM + CHD patients, indicating that BG fluctuations may affect the degree of HRV reduction in patients. In the broadest sense, variability serves as a crucial element in the body’s major control systems[17]. Insulin action and glucose tolerance are related to circadian rhythms of hormone synthesis[18]. Quantifiable glucose variability (GV) occurs during physiologic glucose tolerance[19]; however, GV shows significant elevation in diabetic and glucose dysregulation conditions[20]. Long-term abnormal BG fluctuations can cause a series of metabolic disorders, trigger oxidative stress, lead to cardiac autonomic neuropathy, mainly vagus nerve damage, and then reduce HRV. Monnier et al[21] conducted a highly cited clinical study involving 21 patients with T2DM, showing a strong positive correlation between 24-hour urinary excretion rates of free 8-iso prostaglandin F2alpha (8-iso PGF2a), an oxidative stress marker, and MAGE. The BG volatility of older patients with T2DM and CHD should be widely concerned. By strengthening BG control and maintaining BG balance, to delay HRV reduction, it is expected to reduce the occurrence of functional myocardial ischemia.
Functional myocardial ischemia is an important complication causing adverse prognosis in older T2DM + CHD cases[22]. Numerous studies have shown that endothelial cell dysfunction and damage is one of the main mechanisms resulting in functional myocardial ischemia onset and progression, and long-term BG fluctuations can seriously damage endothelial cell function[23-25]. Farabi et al’s study[26] have shown that large blood GV can lead to more severe endo
This study still has some limitations. First, the modest cohort size may underlie the absence of statistical significance in some subgroup comparisons. Second, this was a retrospective, and a selection bias might be present. This might limit the ability to establish causal relationships. Third, although we have adjusted for some potential confounding factors, other confounders are at play. Fourth, lacking long-term follow-up data, we were unable to assess the long-term impacts of BG fluctuations on HRV and functional myocardial ischemia. Fifth, how glycemic variability impacts the severity of coronary artery lesions remains to be explored. All in all, a multicenter prospective design with expanded sample sizes and long-term follow-up research to validate correlations and explore causality.
In conclusion, BG fluctuations and HRV in older patients with T2DM-CHD comorbidity are negatively correlated, and MAGE combined with MPPGE demonstrated better performance in predicting functional myocardial ischemia, which is worthy of clinical attention. Of course, this study is constrained by its limited case recruitment, lack of extended follow-up results, and absence of analysis of the relationship between BG fluctuations and the degree of coronary artery disease. Expanding the research scale in the future, improving the research design, and deeply analyzing the mechanism of BG fluctuation in the occurrence and development of CHD is necessary.
| 1. | Soares-Miranda L, Sattelmair J, Chaves P, Duncan GE, Siscovick DS, Stein PK, Mozaffarian D. Physical activity and heart rate variability in older adults: the Cardiovascular Health Study. Circulation. 2014;129:2100-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Schroeder EB, Chambless LE, Liao D, Prineas RJ, Evans GW, Rosamond WD, Heiss G; Atherosclerosis Risk in Communities (ARIC) study. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2005;28:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Salmoirago-Blotcher E, Hovey KM, Andrews CA, Allison M, Brunner RL, Denburg NL, Eaton C, Garcia L, Sealy-Jefferson SM, Zaslavsky O, Kang J, López L, Post SG, Tindle H, Wassertheil-Smoller S. Psychological Traits, Heart Rate Variability, and Risk of Coronary Heart Disease in Healthy Aging Women-The Women's Health Initiative. Psychosom Med. 2019;81:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Zhou J, Li H, Ran X, Yang W, Li Q, Peng Y, Li Y, Gao X, Luan X, Wang W, Jia W. Establishment of normal reference ranges for glycemic variability in Chinese subjects using continuous glucose monitoring. Med Sci Monit. 2011;17:CR9-C13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 497] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 6. | Hillis GS, Woodward M, Rodgers A, Chow CK, Li Q, Zoungas S, Patel A, Webster R, Batty GD, Ninomiya T, Mancia G, Poulter NR, Chalmers J. Resting heart rate and the risk of death and cardiovascular complications in patients with type 2 diabetes mellitus. Diabetologia. 2012;55:1283-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Salmani Mood M, Yavari Z, Bahrami Taghanaki H, Mahmoudirad G. The effect of acupressure on fasting blood glucose, glycosylated hemoglobin and stress in patients with type 2 diabetes. Complement Ther Clin Pract. 2021;43:101393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Aronov D, Bubnova M, Iosseliani D, Orekhov A. Clinical Efficacy of а Medical Centre- and Home-based Cardiac Rehabilitation Program for Patients with Coronary Heart Disease After Coronary Bypass Graft Surgery. Arch Med Res. 2019;50:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Zhang J, Yang J, Liu L, Li L, Cui J, Wu S, Tang K. Significant abnormal glycemic variability increased the risk for arrhythmias in elderly type 2 diabetic patients. BMC Endocr Disord. 2021;21:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Shimabukuro M, Tanaka A, Sata M, Dai K, Shibata Y, Inoue Y, Ikenaga H, Kishimoto S, Ogasawara K, Takashima A, Niki T, Arasaki O, Oshiro K, Mori Y, Ishihara M, Node K; Collaborators on the Effect of Miglitol on Glucose Metabolism in Acute Coronary Syndrome (MACS) Study. α-Glucosidase inhibitor miglitol attenuates glucose fluctuation, heart rate variability and sympathetic activity in patients with type 2 diabetes and acute coronary syndrome: a multicenter randomized controlled (MACS) study. Cardiovasc Diabetol. 2017;16:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Li WL, Sang H, Xu X, Zhang YY, Gao J, Chen BH, Meng XY. The correlation study on homocysteine, blood lipids and blood glucose levels in patients with cerebral infarction. Am J Transl Res. 2021;13:5659-5664. [PubMed] |
| 12. | Eleftheriadou A, Spallone V, Tahrani AA, Alam U. Cardiovascular autonomic neuropathy in diabetes: an update with a focus on management. Diabetologia. 2024;67:2611-2625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 13. | Gad H, Elgassim E, Mohammed I, Alhaddad AY, Aly HAHZ, Cabibihan JJ, Al-Ali A, Sadasivuni KK, Petropoulos IN, Ponirakis G, Abuhelaiqa W, Jayyousi A, AlMohanadi D, Baagar K, Malik RA. Cardiovascular autonomic neuropathy is associated with increased glycemic variability driven by hyperglycemia rather than hypoglycemia in patients with diabetes. Diabetes Res Clin Pract. 2023;200:110670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 14. | Racca C, Bouman EJ, Van Beers CAJ, Smits MM, van Raalte DH, Serné EH. Association between hypoglycaemic glucose variability and autonomic function in type1 diabetes with impaired hypoglycaemia awareness. Diabetes Res Clin Pract. 2022;189:109964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Huang ES, Zhang Q, Gandra N, Chin MH, Meltzer DO. The effect of comorbid illness and functional status on the expected benefits of intensive glucose control in older patients with type 2 diabetes: a decision analysis. Ann Intern Med. 2008;149:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Young HA, Benton D. Heart-rate variability: a biomarker to study the influence of nutrition on physiological and psychological health? Behav Pharmacol. 2018;29:140-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 17. | Serhiyenko VA, Serhiyenko LM, Sehin VB, Serhiyenko AA. Pathophysiological and clinical aspects of the circadian rhythm of arterial stiffness in diabetes mellitus: A minireview. Endocr Regul. 2022;56:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Hariri A, Mirian M, Zarrabi A, Kohandel M, Amini-Pozveh M, Aref AR, Tabatabaee A, Prabhakar PK, Sivakumar PM. The circadian rhythm: an influential soundtrack in the diabetes story. Front Endocrinol (Lausanne). 2023;14:1156757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 19. | Belli M, Bellia A, Sergi D, Barone L, Lauro D, Barillà F. Glucose variability: a new risk factor for cardiovascular disease. Acta Diabetol. 2023;60:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 20. | Onaolapo AY, Onaolapo OJ. Circadian dysrhythmia-linked diabetes mellitus: Examining melatonin's roles in prophylaxis and management. World J Diabetes. 2018;9:99-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1689] [Cited by in RCA: 1836] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 22. | Wang EY, Dixson J, Schiller NB, Whooley MA. Causes and Predictors of Death in Patients With Coronary Heart Disease (from the Heart and Soul Study). Am J Cardiol. 2017;119:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 315] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | Manzella D, Paolisso G. Cardiac autonomic activity and Type II diabetes mellitus. Clin Sci (Lond). 2005;108:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Xhyheri B, Manfrini O, Mazzolini M, Pizzi C, Bugiardini R. Heart rate variability today. Prog Cardiovasc Dis. 2012;55:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 26. | Farabi SS, Quinn L, Phillips S, Mihailescu D, Park C, Ali M, Martyn-Nemeth P. Endothelial Dysfunction is Related to Glycemic Variability and Quality and Duration of Sleep in Adults With Type 1 Diabetes. J Cardiovasc Nurs. 2018;33:E21-E25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Buchner T. A quantitative model of relation between respiratory-related blood pressure fluctuations and the respiratory sinus arrhythmia. Med Biol Eng Comput. 2019;57:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/