Published online Jun 15, 2024. doi: 10.4239/wjd.v15.i6.1254
Revised: February 2, 2024
Accepted: March 19, 2024
Published online: June 15, 2024
Processing time: 179 Days and 6.3 Hours
The FreeStyle Libre flash glucose monitoring (FGM) system entered the Chinese market in 2017 to complement the self-monitoring of blood glucose. Due to its increased usage in clinics, the number of studies investigating its accuracy has increased. However, its accuracy has not been investigated in highland popu-lations in China.
To evaluate measurements recorded using the FreeStyle Libre FGM system compared with capillary blood glucose measured using the enzyme electrode method in patients with type 2 diabetes (T2D) who had migrated within 3 mo from highlands to plains.
Overall, 68 patients with T2D, selected from those who had recently migrated from highlands to plains (within 3 mo), were hospitalized at the Department of Endocrinology from August to October 2017 and underwent continuous glucose monitoring (CGM) with the FreeStyle Libre FGM system for 14 d. Throughout the study period, fingertip capillary blood glucose was measured daily using the enzyme electrode method (Super GL, China), and blood glucose levels were read from the scanning probe during fasting and 2 h after all three meals. Moreover, the time interval between reading the data from the scanning probe and collecting fingertip capillary blood was controlled to < 5 min. The accuracy of the FGM system was evaluated according to the CGM guidelines. Subsequently, the factors influencing the mean absolute relative difference (MARD) of this system were analyzed by a multiple linear regression method.
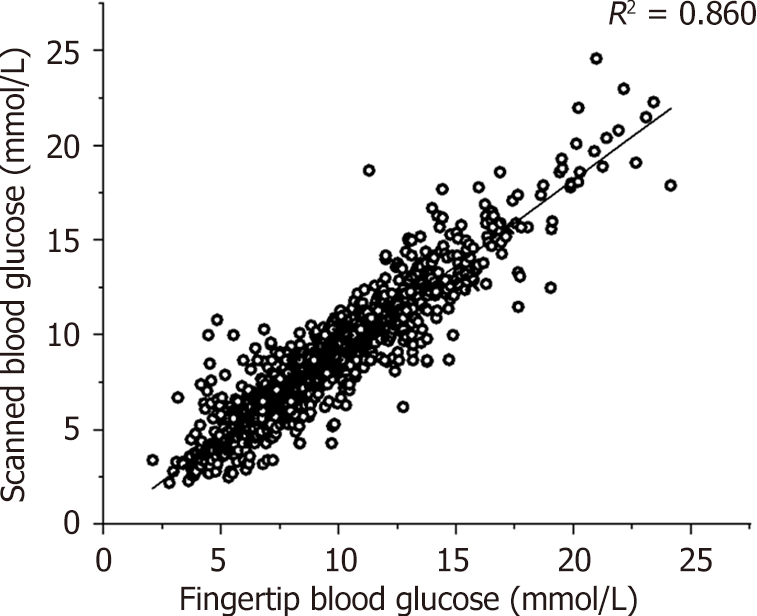
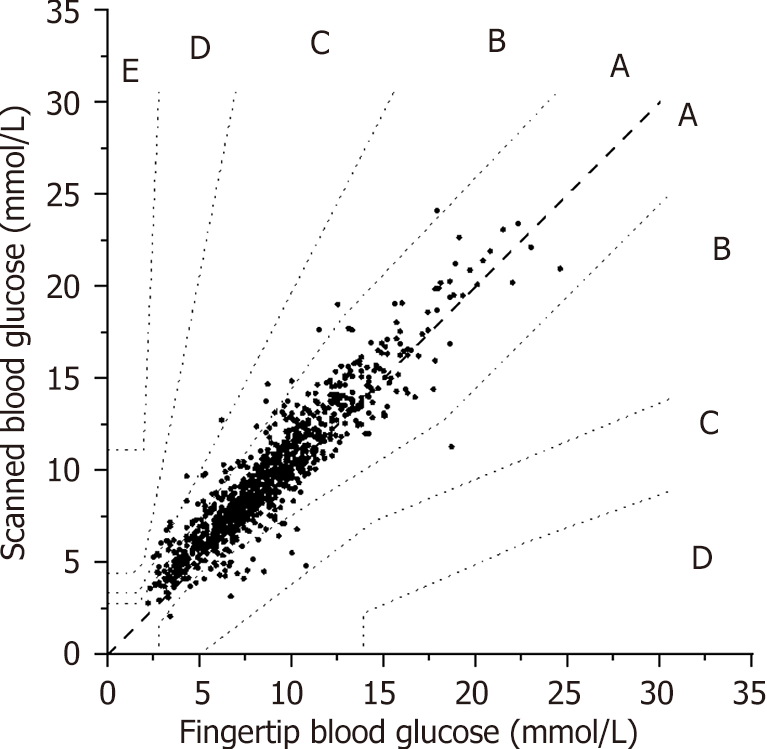
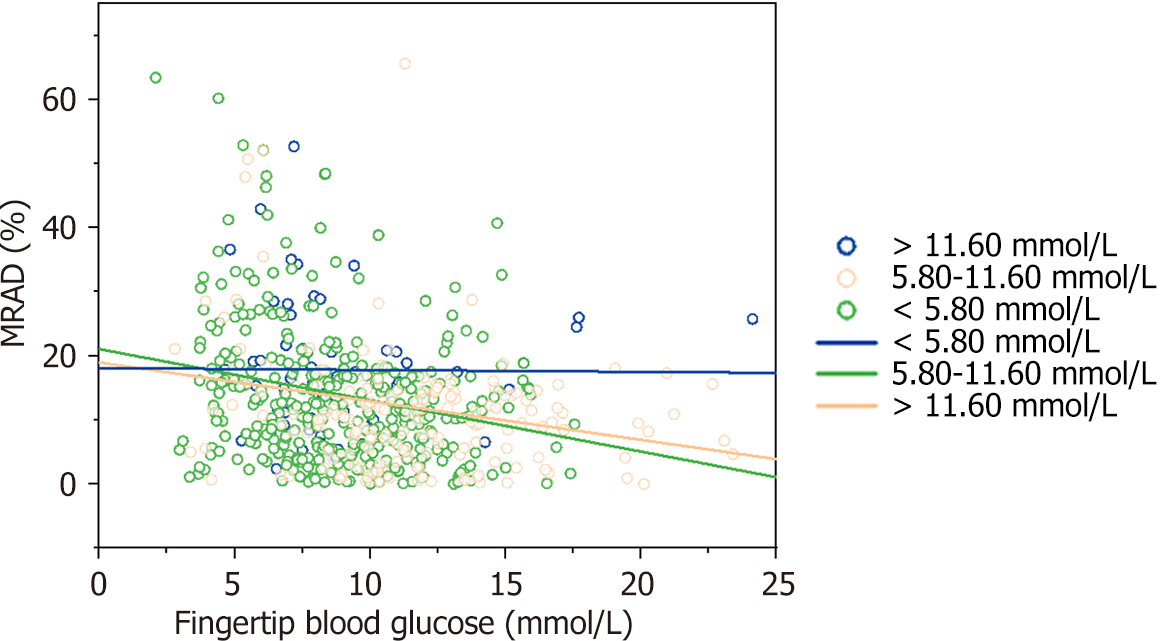
Pearson’s correlation analysis showed that the fingertip and scanned glucose levels were positively correlated (R = 0.86, P = 0.00). The aggregated MARD of scanned glucose was 14.28 ± 13.40%. Parker's error analysis showed that 99.30% of the data pairs were located in areas A and B. According to the probe wear time of the FreeStyle Libre FGM system, MARD1 d and MARD2–14 d were 16.55% and 14.35%, respectively (t = 1.23, P = 0.22). Multiple stepwise regression analysis showed that MARD did not correlate with blood glucose when the largest amplitude of glycemic excursion (LAGE) was < 5.80 mmol/L but negatively correlated with blood glucose when the LAGE was ≥ 5.80 mmol/L.
The FreeStyle Libre FGM system has good accuracy in patients with T2D who had recently migrated from highlands to plains. This system might be ideal for avoiding the effects of high hematocrit on blood glucose monitoring in populations that recently migrated to plains. MARD is mainly influenced by glucose levels and fluctuations, and the accuracy of the system is higher when the blood glucose fluctuation is small. In case of higher blood glucose level fluctuations, deviation in the scanned glucose levels is the highest at extremely low blood glucose levels.
Core Tip: Although blood glucose monitoring is the standard and most established approach for informing diabetes therapy, continuous glucose monitoring has seen a rapid increase in the number of users in recent years. Abnormal glucose metabolism in highland populations is a major health concern worldwide. This study focused on the accuracy of the FreeStyle Libre FGM system in highland populations.
- Citation: Sun ZM, Du YZ, Wang SY, Sun SY, Ye Y, Sun XP, Li MX, He H, Long WC, Zhang CH, Yao XY, Fan WY, Wang L, Wu YH. Accuracy of FreeStyle Libre flash glucose monitoring in patients with type 2 diabetes who migrated from highlands to plains. World J Diabetes 2024; 15(6): 1254-1262
- URL: https://www.wjgnet.com/1948-9358/full/v15/i6/1254.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i6.1254
The World Health Organization (WHO) has highlighted diabetes as a major global health threat[1]. Type 2 diabetes (T2D) accounts for 90%-99% of diabetes cases and is a complex illness associated with hyperglycemia and insulin resistance; long-term and costly medical care is usually required to prevent complications. Between 1980 and 2021, the number of adults with diabetes (90% of whom have T2D) has increased from 108 million to 537.00 million[1-3]. This phenomenon is global, and no nation has experienced a decline in diabetes incidence in the last 40 years[3]. According to the International Diabetes Federation, there were 536.60 million people with diabetes worldwide in 2021, with China accounting for approximately a quarter of these patients (140.90 million)[4]. The Tibetan population is a unique indigenous population that traditionally lives on the highest plateau in the world, with an average altitude > 4500 m above the sea level. The health burden of diabetes is also high in highland populations. Based on a recent study, the mortality rate associated with diabetes in 2018 in China was 19.1/1000000 in the Tibetan Autonomous Region (TAR)[5]. Although a high-altitude environment is a protective factor against glucose metabolism impairment, with the improvement in economy over the years, the prevalence of diabetes in highland populations has increased. Therefore, abnormal glucose metabolism in highland populations has become an important health concern in our society today.
The treatment of diabetes comprises five modules, of which blood glucose monitoring (BGM) is important for evaluating the effectiveness of the daily management of diabetes in the patients[6]. Hemoglobin A1c (HbA1c) and glycated albumin (GA) were found to be important indicators of blood glucose levels in patients over an average of 3 mo and 2 wk, respectively. Our team's previous research showed that hemoglobin levels were elevated in populations living at high altitudes for a long period of time due to adaptation to a low-oxygen environment; therefore, both HbA1c and GA cannot be used to accurately assess blood glucose levels in this population[7]. High red blood cell counts increase glucose fermentation in vitro. This phenomenon might result in falsely low blood glucose levels when venous glucose measurements are not performed in a timely manner. Self-monitoring of blood glucose (SMBG) is a common method for daily glucose monitoring in patients with abnormal glucose metabolism. Nevertheless, the accuracy of blood glucometers may be susceptible to high hematocrit (HCT) levels. In our previous study, we could not identify any blood glucometer whose accuracy in the plateau erythropoietic population met the International Organization for Standardization (ISO) requirements[8]. Therefore, identifying highly accurate BGM methods for use in high-altitude populations is an urgent need.
Currently, patients with diabetes may choose between two major types of systems for glucose measurement: BGM systems, which measure glucose within the capillary blood, and continuous glucose monitoring (CGM) systems, which measure glucose within the interstitial fluid[6]. While BGM is the standard and more established approach for informing diabetes therapy, CGM has seen a rapid increase in users in recent years. The FreeStyle Libr flash glucose monitoring (FGM) system was introduced in Europe in 2014; this was the first factory-calibrated device, and it has been proven to have good accuracy in patients with type 1 diabetes and T2D[9-12]. Some studies on hospitalizations for acute diabetes complications have also shown that the use of the FreeStyle Libre FGM system was associated with a significantly lower incidence of admissions for diabetes-related complications[13-15]. Nevertheless, research on the use of the FreeStyle Libre FGM system in patients residing at high altitudes in China is limited. Furthermore, the accuracy of this system in highland populations has not been investigated.
To address these research gaps, we used the FreeStyle Libre FGM system to measure the glucose levels in patients from highlands. We aimed to analyze the blood glucose results of the FreeStyle Libre FGM system in patients with T2D who recently migrated from highlands to plains and evaluate the accuracy of this system in this highland population.
Overall, 68 patients with T2D were selected from those who resided in highlands, had recently migrated to plains, and were hospitalized at the Department of Endocrinology between August 2017 and October 2017. The inclusion criteria were as follows: (1) Adults aged over 18 years; (2) patients who had been clearly diagnosed with T2D according to the WHO standard introduced in 1999; (3) patients who could speak fluent Chinese and cooperate with the doctors in measuring their blood glucose using the FreeStyle Libre FGM system, and (4) patients who agreed to cooperate and provided written informed consent. The exclusion criteria were: (1) Shock, dehydration, diabetic ketosis, or other critical conditions; (2) inability to undergo continuous dynamic BGM because of conditions requiring frequent radiological examinations during hospitalization; (3) history of allergy to tape or alcohol; and (4) scars or skin damage at sensor implantation sites. This study was approved by the Ethics Committee of the Hospital of Chengdu Office of the People's Government of the TAR, China.
We used a self-designed questionnaire to collect data on sex, age, ethnicity, diabetes type, diabetes course, treatment, body mass index (BMI), HbA1c level, and the largest amplitude of glycemic excursion (LAGE).
Trained professional nurses measured the patients' blood glucose levels dynamically for 14 d using the FreeStyle Libre FGM system (Abbott Diabetes Care Ltd, United Kingdom). The nurse selected a flat skin area on the outer upper forearm, according to the operating procedures for the instrument, and implanted the probe under the skin. The probe recorded glucose values every 15 min, and the sensor was scanned at least every 8 h.
Fingertip blood was collected daily during fasting and 2 h after all three meals in patients wearing the FreeStyle Libre FGM system, and the enzyme electrode method (Super GL, China) was used to measure the fingertip capillary blood glucose level. Moreover, the glucose value measured using the FreeStyle Libre FGM system was read from the scanner of the probe within 5 min. Although BGM accuracy has been assessed in line with the ISO accuracy requirements and CGM accuracy has been assessed using the mean absolute relative difference (MARD) and mean absolute difference (MAD), the results from additional analyses, such as bias or error grid analyses, have also been reported[16]. The MARD was calculated as follows: (scanned blood glucose level - fingertip blood glucose level) × 100%/fingertip blood glucose level. The MAD was calculated by taking the absolute value of the relative error between the scanned and fingertip blood glucose levels and then calculating the mean. Smaller MAD and MARD values indicated better accuracy. Parker's error analysis was used to analyze the consistency of blood glucose levels between scanned and fingertip measurements.
IBM SPSS Statistics for Windows (version 27.0, IBM Corp., Armonk, NY, United States) was used for data analyses. Continuous variables are expressed as the mean and SD for normally distributed data or as median and interquartile ranges for non-normally distributed. Correlations between two variables were analyzed using Pearson’s correlation. Differences between the two groups were analyzed using the t-test. Categorical variables are expressed as counts and percentages, and consistency was analyzed using an error analysis. Multiple linear regression was used to analyze the factors influencing the MARD of the FreeStyle Libre FGM system, and P < 0.05 was considered statistically significant.
Of the 68 patients considered for inclusion in this study, two did not complete the fingertip blood tests. Of the final 66 patients included in this study, 40 were male, 26 were female, 30 were Tibetan, and 36 were Han. Their mean age was 53.20 ± 7.75 years, diabetes course was 7.59 ± 5.78 years, and HbA1c level was 9.22% ± 1.40%. A total of 1013 data pairs were obtained (Table 1 and Supplementary Table 1).
| Characteristic | Mean | SD | Minimum | Maximum |
| Age (yr) | 53.20 | 8.75 | 34.00 | 81.00 |
| Course (yr) | 7.59 | 5.78 | 0.17 | 30.00 |
| BMI (kg/m2) | 23.81 | 2.74 | 19.00 | 32.20 |
| HbA1c (%) | 9.22 | 2.40 | 5.70 | 13.80 |
| Hb (g/L) | 149.68 | 15.88 | 115 | 184 |
| HCT (%) | 43.99 | 4.42 | 35.5 | 54.2 |
| Fingertip blood glucose level (mmol/L) | 9.78 | 3.75 | 2.08 | 24.12 |
| Scanned blood glucose level (mmol/L) | 8.82 | 3.70 | 2.20 | 24.60 |
In this study, fingertip blood glucose levels were plotted on the x-axis, and scanned blood glucose levels obtained from the FreeStyle Libre FGM system were plotted on the y-axis. As presented in Figure 1, Pearson’s correlation analysis showed that fingertip and scanned blood glucose levels were positively correlated (R = 0.86, 95% confidence interval: 0.92-0.95, P = 0.00).
Considering the fingertip blood glucose values as the standard, the aggregated MAD of scanned blood glucose values was 1.27 ± 1.20 mmol/L, and the aggregated MARD was 14.28 ± 13.40%. The MAD and MARD for each subgroup grouped by the fingertip blood glucose level are shown in Table 2. The MARD met international standards (< 28%) in all groups, except for that in the extremely low blood glucose level group (< 2.77).
| Group | n | MAD (mean ± SD, mmol/L) | MARD (mean ± SD, %) |
| < 2.77 | 1 | 1.32 | 63.46 |
| 2.77-4.44 | 42 | 0.85 ± 0.82 | 21.85 ± 21.91 |
| 4.45-6.66 | 171 | 1.13 ± 0.94 | 20.56 ± 18.60 |
| 6.67-11.10 | 508 | 1.10 ± 0.94 | 12.72 ± 10.75 |
| 11.11-16.65 | 252 | 1.53 ± 1.14 | 11.66 ± 8.78 |
| 16.66-22.20 | 35 | 2.74 ± 3.24 | 14.71 ± 17.49 |
| > 22.20 | 4 | 3.11 ± 2.33 | 13.25 ± 9.61 |
According to the latest ISO standard, Parker's error analysis showed that 829 data pairs (81.84%), 177 (17.47%), 6 (0.59%), and 1 (0.10%) were in areas A, B, C, and D, respectively. As shown in Figure 2, 99.3% of the data pairs were located in areas A and B, which met the requirements of the latest ISO standard (99%).
The MARD obtained daily from the FreeStyle Libre FGM system measurement in each patient was used as the dependent variable. Patient probe wear time, sex, age, ethnicity, diabetes type, diabetes course, BMI, HbA1c level, treatment, fingertip blood glucose level, and LAGE were independent variables. Multiple linear regression showed that only the HbA1c and fingertip blood glucose levels were incorporated into the regression model (F = 24.20, P = 0.00, R2 = 0.41).
Patients were divided into three groups according to the LAGE of the FreeStyle Libre FGM system (< 5.80 mmol/L, 5.80-11.60 mmol/L, and > 11.60 mmol/L), and fingertip and scanned blood glucose levels were used to draw a trend plot. As presented in Figure 3, when LAGE was < 5.80 mmol/L, the MARD of the scanned blood glucose level obtained from the FreeStyle Libre FGM system did not correlate with the fingertip blood glucose level (R = 0.01, P = 0.92). However, when the patients' blood glucose levels fluctuated greatly (LAGE > 11.60 mmol/L and 5.80-11.60 mmol/L), the MARD of the scanned blood glucose level obtained from the FreeStyle Libre FGM system was inversely correlated with the fingertip blood glucose level (R1= -0.29, R2= 0.29, P = 0.00).
Patients were divided into two groups (1 d and 2–14 d) according to the FreeStyle Libre FGM system probe wear time, and MARD1 d and MARD2-14 d were calculated to be 16.55% and 14.35%, respectively (t = 1.23, P = 0.22). When patients were further divided into two groups based on 1 wk and 2 wk of wear time, the MARD1 wk and MARD2 wk were 14.57% and 14.53%, respectively (t = 0.02, P = 0.98).
The FreeStyle Libre FGM system, a widely accepted CGM option for measuring interstitial glucose levels in patients with diabetes, has facilitated easy access to sensing technology with over two million patients using the device to manage their diabetes[17]. Compared with conventional SMBG options, this system can effectively avoid the effects of high HCT, a characteristic of highland populations. Avoiding the pain associated with frequent skin punctures adds to the popularity of this system. However, research on its use in patients dwelling at high altitudes is limited in China; therefore, healthcare workers still have concerns and queries regarding the accuracy of the system in the highland population.
In previous studies, fingertip blood glucose measured using blood glucose meters was used as a reference standard to evaluate the accuracy of the FreeStyle Libre FGM system[12,18,19]. However, to avoid the effect of high HCT levels on blood glucose measurements in highland populations, we used the enzyme electrode method to measure fingertip blood glucose levels in this study. A previous study has shown that the accuracy of this method is comparable to that of the FreeStyle Libre FGM system[16]. We chose this method as the gold standard in order to effectively improve the scientific validity of this study. Finally, on using the fingertip blood glucose level measured by the enzyme electrode method as the reference standard, it was found that the system had good accuracy when used in patients with T2D who recently migrated from highlands to plains. In contrast to the findings of our study, Schierenbeck et al found that the performance of the FreeStyle Libre FGM system in 24 patients who underwent cardiac surgery was poor[20].
MARD analysis is a widely used method to determine the accuracy of CGM systems; however, it is also sometimes applied to BGM systems[21,22], particularly when comparisons need to be made between the two types of systems[16,23]. In this study, the accuracy evaluation of scanned and fingertip blood glucose measurements revealed an R2 of 0.86, which exceeded the requirements outlined in the guidelines for dynamic BGM (0.79)[24]. The aggregated MARD of 14.28% ± 13.40% met the requirements of the guidelines. Ji et al[18] reported similar accuracy when capillary glucose measurement was used as the reference method. Analyses grouped by fingertip blood glucose levels showed that all the MARD values in groups 2-7 were < 28.00%, which also met the standards of the guidelines. The data for group 1 were insufficient, and the reference value was not considered[24]. Parker’s error analysis showed that 99.3% of the data pairs were located in areas A and B, which met the latest ISO standards[8].
We conducted an analysis to explore the factors influencing the accuracy of the FreeStyle Libre FGM system and found that the factors influencing MARD were blood glucose level fluctuations and blood glucose levels. The MARD of this system was maintained at 18 when LAGE was < 5.80 mmol/L and did not correlate with fingertip blood glucose levels. However, when a patient’s blood glucose level fluctuated greatly (LAGE ≥ 5.80 mmol/L), the MARD of this system negatively correlated with the fingertip blood glucose level. That is, the lower the blood glucose concentration, the higher the MARD and the worse the accuracy of the FreeStyle Libre FGM system. The scanned blood glucose levels in patients with hypoglycemia were significantly lower than the fingertip blood glucose levels, and these may be related to the principle behind the method used for calculation in the FreeStyle Libre FGM system. This system may be effective in reducing the incidence of hypoglycemic events[10,25].
There was no significant difference between MARD1 wk and MARD2 wk according to the probe wear time, which is consistent with previous research results[18,25]. Although MARD1 d was higher than MARD2–14 d, this difference was also not significant. This may be related to the small sample size of the present study. Previous research showed that MARD1 d was significantly higher than MARD2-5 d (11.50% vs 8.90%, P < 0.05)[18], which may be related to an insufficient reaction between the connecting enzyme and interstitial fluid glucose on early probe implantation.
Our study has several strengths. This study is the first to investigate the accuracy of the FreeStyle Libre FGM system in patients with T2D who recently migrated from highlands to plains. This was previously unexplored in this population. We also determined the clinical accuracy of different blood glucose measurements as recommended by the latest guidelines. This study also has some limitations. Only one patient with extreme hypoglycemia (< 2.77 mmol/L) was included; however, the overall number of patients included in the study was relatively small. Therefore, the accuracy of this system in hypoglycemic populations should be evaluated by further expanding the sample size. Long-term trials of CGM in a more general population of individuals with different glucose metabolism levels are also needed.
The FreeStyle Libre FGM system is a feasible option for continuous BGM and offers the ability to measure blood glucose level every 15-60 min using a reader and without the need for skin puncture as in capillary testing. Our findings on using finger blood glucose levels measured by the enzyme electrode method as the standard suggest that the accuracy of the FreeStyle Libre FGM system in patients with T2D who recently migrated from highlands to plains, meets the national and international standards. Therefore, this system can be used to complement CGM in highland populations.
Abnormal glucose metabolism in highland populations is an important health concern in our society today.
Identifying highly accurate blood glucose monitoring methods for use in high-altitude populations is an urgent need.
To evaluate the accuracy of the FreeStyle Libre FGM system in highland populations.
A total of 66 patients with type 2 diabetes (T2D) were enrolled in this observational analysis. General information and blood glucose data were statistically analyzed.
The accuracy of the FreeStyle Libre FGM system in patients with T2D who recently migrated from highlands to plains met national and international standards when finger blood glucose levels measured using the enzyme electrode method were used as the standard.
The FreeStyle Libre FGM system complements continuous glucose monitoring in highland populations.
Based on the insights gained in this study, we aim to conduct an extended future study on the difference in the mean absolute relative difference values of FGM between patients with normal and elevated hemoglobin and hematocrit levels.
The authors would like to thank the staff in the Department of Endocrinology of the Hospital of Chengdu Office of the People's Government of the Tibetan Autonomous Region for their contributions.
| 1. | GBD 2019 Diabetes and Air Pollution Collaborators. Estimates, trends, and drivers of the global burden of type 2 diabetes attributable to PM(2·5) air pollution, 1990-2019: an analysis of data from the Global Burden of Disease Study 2019. Lancet Planet Health. 2022;6:e586-e600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 2. | Pierce DR, McDonald M, Merone L, Becker L, Thompson F, Lewis C, Ryan RYM, Hii SF, Zendejas-Heredia PA, Traub RJ, Field MA, Rahman T, Croese J, Loukas A, McDermott R, Giacomin PR. Effect of experimental hookworm infection on insulin resistance in people at risk of type 2 diabetes. Nat Commun. 2023;14:4503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | O'Hearn M, Lara-Castor L, Cudhea F, Miller V, Reedy J, Shi P, Zhang J, Wong JB, Economos CD, Micha R, Mozaffarian D; Global Dietary Database. Incident type 2 diabetes attributable to suboptimal diet in 184 countries. Nat Med. 2023;29:982-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 4. | Su H, Jiang C, Zhang W, Zhu F, Jin Y, Cheng K, Lam T, Xu L. Parity and incident type 2 diabetes in older Chinese women: Guangzhou Biobank Cohort Study. Sci Rep. 2023;13:9504. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Peng W, Li K, Yan AF, Shi Z, Zhang J, Cheskin LJ, Hussain A, Wang Y. Prevalence, Management, and Associated Factors of Obesity, Hypertension, and Diabetes in Tibetan Population Compared with China Overall. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16442] [Article Influence: 498.2] [Reference Citation Analysis (4)] |
| 7. | Sun ZM, Wang SY, He H, Sun SY, Ye Y, Yao RY, Long WC, Zhang HQ, Zhang CH, Li MX, Tang DM, Wu YH. The value of HbA1c for diagnosis of abnormal glucose metabolism in highland dwellers with different hemoglobin levels. Guoji Neifenmi Daixie Zazhi. 2019;39:73-76. [DOI] [Full Text] |
| 8. | Pokhrel A, Silvanus V, Pokhrel BR, Baral B, Khanal M, Gyawali P, Pokhrel L, Regmi D. Accuracy of Glucose Meter among Adults in a Semi-urban Area in Kathmandu, Nepal. JNMA J Nepal Med Assoc. 2019;57:104-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. |
Weiss RF.
Regarding "The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System," by Bailey |
| 10. | Ancona P, Eastwood GM, Lucchetta L, Ekinci EI, Bellomo R, Mårtensson J. The performance of flash glucose monitoring in critically ill patients with diabetes. Crit Care Resusc. 2017;19:167-174. [PubMed] |
| 11. | Alva S, Bailey T, Brazg R, Budiman ES, Castorino K, Christiansen MP, Forlenza G, Kipnes M, Liljenquist DR, Liu H. Accuracy of a 14-Day Factory-Calibrated Continuous Glucose Monitoring System With Advanced Algorithm in Pediatric and Adult Population With Diabetes. J Diabetes Sci Technol. 2022;16:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 12. | Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The Performance and Usability of a Factory-Calibrated Flash Glucose Monitoring System. Diabetes Technol Ther. 2015;17:787-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 511] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 13. | Roussel R, Riveline JP, Vicaut E, de Pouvourville G, Detournay B, Emery C, Levrat-Guillen F, Guerci B. Important Drop in Rate of Acute Diabetes Complications in People With Type 1 or Type 2 Diabetes After Initiation of Flash Glucose Monitoring in France: The RELIEF Study. Diabetes Care. 2021;44:1368-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 14. | Guerci B, Roussel R, Levrat-Guillen F, Detournay B, Vicaut E, De Pouvourville G, Emery C, Riveline JP. Important Decrease in Hospitalizations for Acute Diabetes Events Following FreeStyle Libre System Initiation in People with Type 2 Diabetes on Basal Insulin Therapy in France. Diabetes Technol Ther. 2023;25:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 15. | Guerci B, Levrat-Guillen F, Vicaut E, De Pouvourville G, Detournay B, Emery C, Riveline JP. Reduced Acute Diabetes Events After FreeStyle Libre System Initiation in People 65 Years or Older with Type 2 Diabetes on Intensive Insulin Therapy in France. Diabetes Technol Ther. 2023;25:384-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 16. | Freckmann G, Pleus S, Grady M, Setford S, Levy B. Measures of Accuracy for Continuous Glucose Monitoring and Blood Glucose Monitoring Devices. J Diabetes Sci Technol. 2019;13:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 17. | Park C, Le QA. The Effectiveness of Continuous Glucose Monitoring in Patients with Type 2 Diabetes: A Systematic Review of Literature and Meta-analysis. Diabetes Technol Ther. 2018;20:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Ji L, Guo X, Guo L, Ren Q, Yu N, Zhang J. A Multicenter Evaluation of the Performance and Usability of a Novel Glucose Monitoring System in Chinese Adults With Diabetes. J Diabetes Sci Technol. 2017;11:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Zschornack E, Schmid C, Pleus S, Link M, Klötzer HM, Obermaier K, Schoemaker M, Strasser M, Frisch G, Schmelzeisen-Redeker G, Haug C, Freckmann G. Evaluation of the performance of a novel system for continuous glucose monitoring. J Diabetes Sci Technol. 2013;7:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Schierenbeck F, Franco-Cereceda A, Liska J. Accuracy of 2 Different Continuous Glucose Monitoring Systems in Patients Undergoing Cardiac Surgery. J Diabetes Sci Technol. 2017;11:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Freckmann G, Link M, Pleus S, Westhoff A, Kamecke U, Haug C. Measurement Performance of Two Continuous Tissue Glucose Monitoring Systems Intended for Replacement of Blood Glucose Monitoring. Diabetes Technol Ther. 2018;20:541-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Wadwa RP, Laffel LM, Shah VN, Garg SK. Accuracy of a Factory-Calibrated, Real-Time Continuous Glucose Monitoring System During 10 Days of Use in Youth and Adults with Diabetes. Diabetes Technol Ther. 2018;20:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 23. | Bedini JL, Wallace JF, Pardo S, Petruschke T. Performance Evaluation of Three Blood Glucose Monitoring Systems Using ISO 15197: 2013 Accuracy Criteria, Consensus and Surveillance Error Grid Analyses, and Insulin Dosing Error Modeling in a Hospital Setting. J Diabetes Sci Technol. 2015;10:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Bao Y, Zhu D; Chinese Diabetes Society. Clinical application guidelines for blood glucose monitoring in China (2022 edition). Diabetes Metab Res Rev. 2022;38:e3581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Ólafsdóttir AF, Attvall S, Sandgren U, Dahlqvist S, Pivodic A, Skrtic S, Theodorsson E, Lind M. A Clinical Trial of the Accuracy and Treatment Experience of the Flash Glucose Monitor FreeStyle Libre in Adults with Type 1 Diabetes. Diabetes Technol Ther. 2017;19:164-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0