Published online Mar 15, 2021. doi: 10.4239/wjd.v12.i3.278
Peer-review started: November 20, 2020
First decision: December 24, 2020
Revised: December 30, 2020
Accepted: February 11, 2021
Article in press: February 11, 2021
Published online: March 15, 2021
Processing time: 101 Days and 23.6 Hours
Diabetes is a common chronic disease. Given the increasing incidence of diabetes, more individuals are affected by diabetic optic neuropathy (DON), which results in decreased vision. Whether DON leads to abnormalities of other visual systems, including the eye, the visual cortex, and other brain regions, remains unknown.
To investigate the local characteristics of spontaneous brain activity using regional homogeneity (ReHo) in patients with DON.
We matched 22 patients with DON with 22 healthy controls (HCs). All subjects underwent resting-state functional magnetic resonance imaging. The ReHo technique was used to record spontaneous changes in brain activity. Receiver operating characteristic (ROC) curves were applied to differentiate between ReHo values for patients with DON and HCs. We also assessed the correlation between Hospital Anxiety and Depression Scale scores and ReHo values in DON patients using Pearson correlation analysis.
ReHo values of the right middle frontal gyrus (RMFG), left anterior cingulate (LAC), and superior frontal gyrus (SFG)/left frontal superior orbital gyrus (LFSO) were significantly lower in DON patients compared to HCs. Among these, the greatest difference was observed in the RMFG. The result of the ROC curves suggest that ReHo values in altered brain regions may help diagnose DON, and the RMFG and LAC ReHo values are more clinically relevant than SFG/LFSO. We also found that anxiety and depression scores of the DON group were extremely negatively correlated with the LAC ReHo values (r = -0.9336, P < 0.0001 and r =
Three different brain regions show ReHo changes in DON patients, and these changes could serve as diagnostic and/or prognostic biomarkers to further guide the prevention and treatment of DON patients.
Core Tip: Through the analysis of resting state functional magnetic resonance imaging, combined with the statistical calculation of all data, this study aimed to understand altered spontaneous brain activity in patients with diabetic optic neuropathy, and find out the differences of regional homogeneity values in different brain regions as well as the correlation of other clinical related behaviors, so as to guide the diagnosis and prevention of diabetes optic neuropathy.
- Citation: Guo GY, Zhang LJ, Li B, Liang RB, Ge QM, Shu HY, Li QY, Pan YC, Pei CG, Shao Y. Altered spontaneous brain activity in patients with diabetic optic neuropathy: A resting-state functional magnetic resonance imaging study using regional homogeneity. World J Diabetes 2021; 12(3): 278-291
- URL: https://www.wjgnet.com/1948-9358/full/v12/i3/278.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i3.278
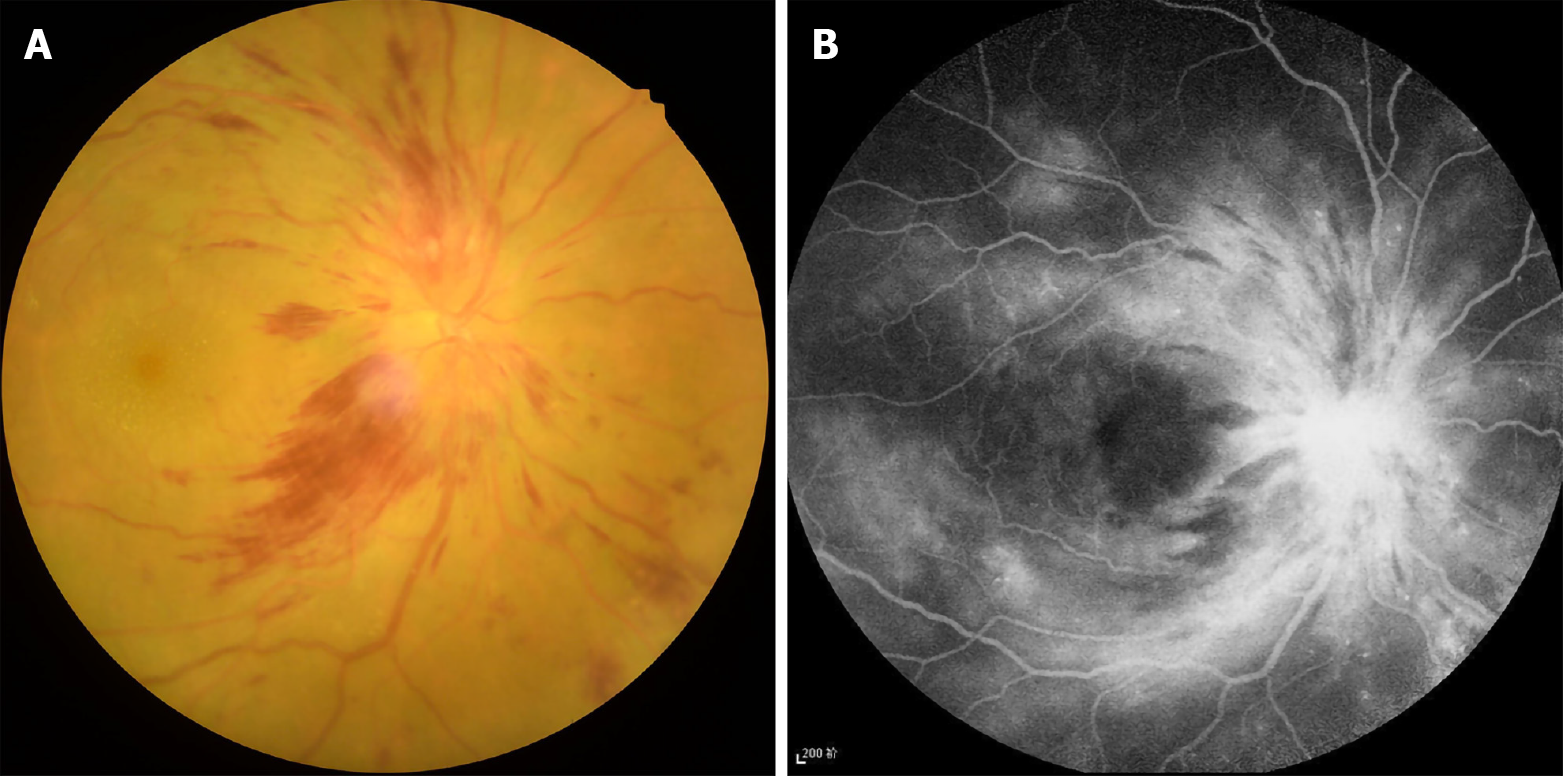
Diabetes is a common chronic disease. Given the increasing incidence of diabetes, more individuals are affected by diabetic optic neuropathy (DON)[1]. Diabetic neuropathy is one of the three major chronic complications of diabetes and can affect the central nervous system, peripheral nerves, and motor, sensory, and autonomic nerves. In the visual system, the main manifestations are corneal hypoperception, extraocular muscle paralysis, and DON. However, in clinical practice, the most familiar ocular complications in patients with diabetes are diabetic retinopathy and diabetic macular edema, so the prevalence of DON is often underestimated[2]. Research into related aspects is relatively extensive, but there are few studies of DON alone. Moreover, there are multiple clinical manifestations of DON, which makes it difficult to diagnose. Some of them can seriously threaten vision and affect patient quality of life, underscoring the need to take them seriously. According to current studies, optic neuropathy in diabetic patients includes optic disc neovascularization, anterior and posterior ischemic optic neuropathy, diabetic papillopathy, and Wolfram's syndrome[3]. The optic nerve is part of the central nervous system and comprises 1 to 1.2 million ganglion cell axons converging to form bundles of nerve fibers passing through the ethmoid plate. Like brain tissue, this structure is particularly sensitive to hypoxia, ischemia, and metabolic disorders. Hyperglycemia in diabetic patients leads to abnormal angiogenesis in the entire body, resulting in decreased local tissue blood flow that affects optic nerve metabolism[4]. The main mechanisms proposed to be associated with DON include chronic low-grade inflammation, abnormal activation of inflammatory factors, and oxidative stress[5]. As the disease progresses over time, the optic nerve irreversibly atrophies, eventually leading to blindness. Due to its anatomical specificity, the inspection and diagnosis of conventional optic neuropathy mainly rely on routine ophthalmic fundus examinations (Figure 1) such as fundus fluorescein angiography (FFA), visual evoked potential (VEP), and clinical symptoms and signs. However, some patients may have unclear optic disc boundaries, tortuous veins, telangiectasia, malformations, clusters of high fluorescence around the optic disc, and fluorescent leakage staining during FAA examination. This assessment may not be appropriate for all patients with diabetes if they have poor heart and kidney function and depending whether the contrast agent is sensitized. These limitations make it difficult to observe DON progression with FFA.
Functional magnetic resonance imaging (fMRI)[6] is the main imaging modality applied to detect brain dysfunction. The basis for brain fMRI is deoxyhemoglobin in venous blood, which is an endogenous and noninvasive contrast agent. FMRI can reveal the oxygenation state of hemoglobin in the vasculature. When brain activity is high, there is an increase in local arterial oxygenated blood flow, which leads to a relative decrease in deoxyhemoglobin. When these hemodynamics occur, neurons are activated within a short period of time, even after a few seconds, and the generated signal is called the blood oxygen level-dependent (BOLD) signal. In technical BOLD studies, it was found to be useful for demonstrating abnormal cortical structure and function in patients with nonarterial anterior ischemic optic neuropathy (NAION). In addition to being noninvasive, fMRI also has advantages of high spatial resolution and recognition of neuronal activity[7]. fMRI has been applied to study cortical reorganization after NAION[8], and the results showed that neuronal activity in the occipital visual area plays an vital role in visual acuity, while a complete visual pathway depends on the normal activation of neurons in the visual cortex.
In recent years, resting-state fMRI (rs-fMRI) has been used to explore the relationships between brain regions[9]. Rs-fMRI is a relatively complex and diverse analysis technique that involves a number of methods, among which regional homogeneity (ReHo) is frequently employed. Notably, ReHo has been repeatedly utilized to assess local synchronization of neighboring voxels in the whole brain in the resting state[10]. ReHo assumes that for brain regions with the same function, the hemodynamics of each voxel are approximately the same, and the hemodynamics of the brain regions may change as a result of changes in function or tasks. ReHo values have been meaningfully applied to analyze many ophthalmologic diseases including neuromyelitis optica[11], retinal detachment[12], and glaucoma[13], as well as neurogenic diseases like sleep disorders[14] and Parkinson's disease[15]. The visual system is mainly composed of the eyeball, ocular adnexal, visual transmission pathways, and the visual center of the occipital lobe of the brain. External information is perceived by photoreceptors, imaged on the retina, and transmitted to the visual center of the brain through the optic nerve. Optic neuropathy can block visual information transmission and cause abnormal brain activity, but few studies have investigated altered spontaneous brain activity in DON patients. Consequently, we took advantage of ReHo analysis to test our hypothesis that DON is associated with abnormal neuronal activity in the visual cortex.
We enrolled 22 adult DON patients (11 males and 11 females) who had visited the Department of Ophthalmology of the First Affiliated Hospital of Nanchang University for the current study. According to Levin et al[16], the diagnostic criteria for DON were: (1) Clear history of diabetes; (2) Optic disc edema (nonspecific congestive edema) or ischemic optic neuropathy and optic nerve atrophy; (3) The degree of vision decline was different, the basis of visual impairment was not clinically evident, and no typical manifestations were seen on visual field examination, with some subjects having enlarged physiological blind spots or only decreased visual acuity; (4) FFA showing early lesions as partial or full optic papilla with obscure, leaky, or low fluorescence; (5) No other diseases that can cause optic disc edema (e.g., optic nerve trauma and systemic lesions); and (6) Optic disc edema was treated or recovered on its own after 6 mo (the recovered optic disc may appear pale).
We also recruited and enrolled 22 age- and sex-matched healthy controls (HCs) (11 males and 11 females) who met the following criteria: (1) No other eye diseases; (2) No neurological or psychiatric disorders; and (3) Normal brain parenchyma on MRI.
This study is in line with the principles specified by the Declaration of Helsinki. Before asking subjects to sign an informed consent form, we explained the purpose of the study and the possible risks in detail. The protocol was approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University (No. 2015029).
A 3-Tesla magnetic resonance scanner (Trio; Siemens AG, Munich, Germany) was used on all selected peoples. In order to reduce the error in the scanning process, the patients were requested to try to stay awake, keep their eyes closed, and relax their body until the end of the scan. Conventional T1 weighted image (T1WI) scans were gathered, and T2WI structural magnetic resonance database was used to rule out brain dysfunction, 3D high-resolution T1WI volume image data and rs-fMRI data. Among them, 176 3D high-resolution T1WI volume images were acquired by the T1-weighted 3D spoiled gradient sequence. The particular scanning parameters used are listed below: Repetition time (TR), 1900 msec; echo time (TE), 2.26 msec; thickness, 1.0 mm; gap, 0.5 mm; acquisition matrix, 256 × 256; flip angle, 9°; field-of-view (FOV), 250 mm × 250 mm. What’s more, a total of 240 functional images were obtained using the gradient-recalled echo-planar imaging sequence, based on the following particular scanning parameters: TR, 2000 msec; TE, 30 msec; thickness, 4.0 mm; gap, 1.2 mm; acquisition-matrix, 64 × 64; flip angle, 90°; and FOV, 220 mm × 220 mm. The scanning times of the two sequences were 5 min and 10 min, respectively.
MRI data of 22 healthy controls and 22 adult DON patients included in the study were collected. MRIcro software was used to classify and filter the gained brain data. It must ensure the stability of the scanning signal and eliminate the impact of collection time on collected data. Hence, the first 15 scanned images were discarded without analysis. Previous studies analyzed the functions of the brain in detail[17]. As mentioned earlier, for the preprocessing of experimental data, the Data Processing Assistant for Resting-State fMRI (DPARSFA 4.0, http://rfmri.org/DPARSF) software and Statistical Parametric Mapping software (http://www.fil.ion.ucl.ac.uk/spm) can be used. The main steps of preprocessing comprised slice timing, head motion correction, making use of Friston six-head motion parameters to return head motion effects, spatial normalization with standard echo planar image templates to achieve Montreal Neurological institute standards, and ultimately smoothening with a Gaussian kernel of 6 mm × 6 mm × 6 mm full-width at half-maximum, so as to heighten the signal-to-noise ratio. REST software (http://sourceforge.net/projects/testing-fmri, Institute of Psychology, CAS) was used to calculate ReHo. The basic method of evaluation is Kendall consistency coefficient[10], whose main significance is to calculate the time series consistency of each voxel and its adjacent 26 voxels in the brain, to record the activity level of local neurons finally.
SPSS version 20.0 (IBM Corporation, Armonk, NY, United States) statistical software was used to perform calculations and analyses. To distinguish DON patients from HCs and identify differences, we applied independent two-sample t-tests. General linear model analysis was carried out using the SPM8 toolbox to study the differences in ReHo values between groups. For multiple comparisons, we employed Gaussian random field theory (z > 2.3; P < 0.05; cluster > 40 voxels, AlphaSim corrected). Based on the statistical results that ReHo values showed differences in identical brain regions, we generated receiver operating characteristic (ROC) curves to intuitively differentiate between DON patients and HCs. In all statistical analyses, P < 0.05 was considered statistically significant.
Subjects in the DON group were required to accurately complete the Hospital Anxiety and Depression Scale (HADS) as much as possible, and clinical behavior differences were based on anxiety and depression scores. Pearson correlation was analyzed with GraphPad Prism 8 software (GraphPad Inc., San Diego, CA, United States) to evaluate and graph the linear correlation between HADS scores and ReHo values of the left anterior cingulate (LAC).
There were no significant differences in weight, age, handedness, or sex between the two groups (P > 0.05). However, the DON group had significant differences from the HC group in terms of best-corrected right and left eye visual acuities and bilateral VEP latency (ms) and amplitude (mv) (P < 0.05). In the DON group, the mean duration of disease was 56.76 ± 5.26 d. Detailed data on clinical characteristics are shown in Table 1.
| Condition | DON | HCs | t | P value1 |
| Male/female | 10/12 | 10/12 | N/A | > 0.99 |
| Age (years) | 54.74 ± 5.98 | 53.02 ± 5.12 | 0.274 | 0.914 |
| Weight (kg) | 65.32 ± 7.52 | 62.12 ± 8.57 | 0.197 | 0.943 |
| Handedness | 22R | 22R | N/A | > 0.99 |
| Duration of DON (days) | 56.76 ± 5.26 | N/A | N/A | N/A |
| Best-corrected VA-left eye | 0.35 ± 0.22 | 1.05 ± 0.25 | -3.581 | 0.014 |
| Best-corrected VA-right eye | 0.26 ± 0.19 | 1.05 ± 0.15 | -3.127 | 0.011 |
| Latency (ms)-right of the VEP | 121.01 ± 10.64 | 101.23 ± 5.42 | 3.291 | 0.002 |
| Amplitude (UV)-right of the VEP | 6.96 ± 2.15 | 13.29 ± 1.84 | -8.021 | 0.003 |
| Latency (ms)-left of the VEP | 110.42 ± 7.48 | 100.76 ± 3.29 | 5.597 | 0.012 |
| Amplitude (UV)-left of the VEP | 10.26 ± 3.34 | 16.24 ± 2.65 | -3.018 | 0.005 |
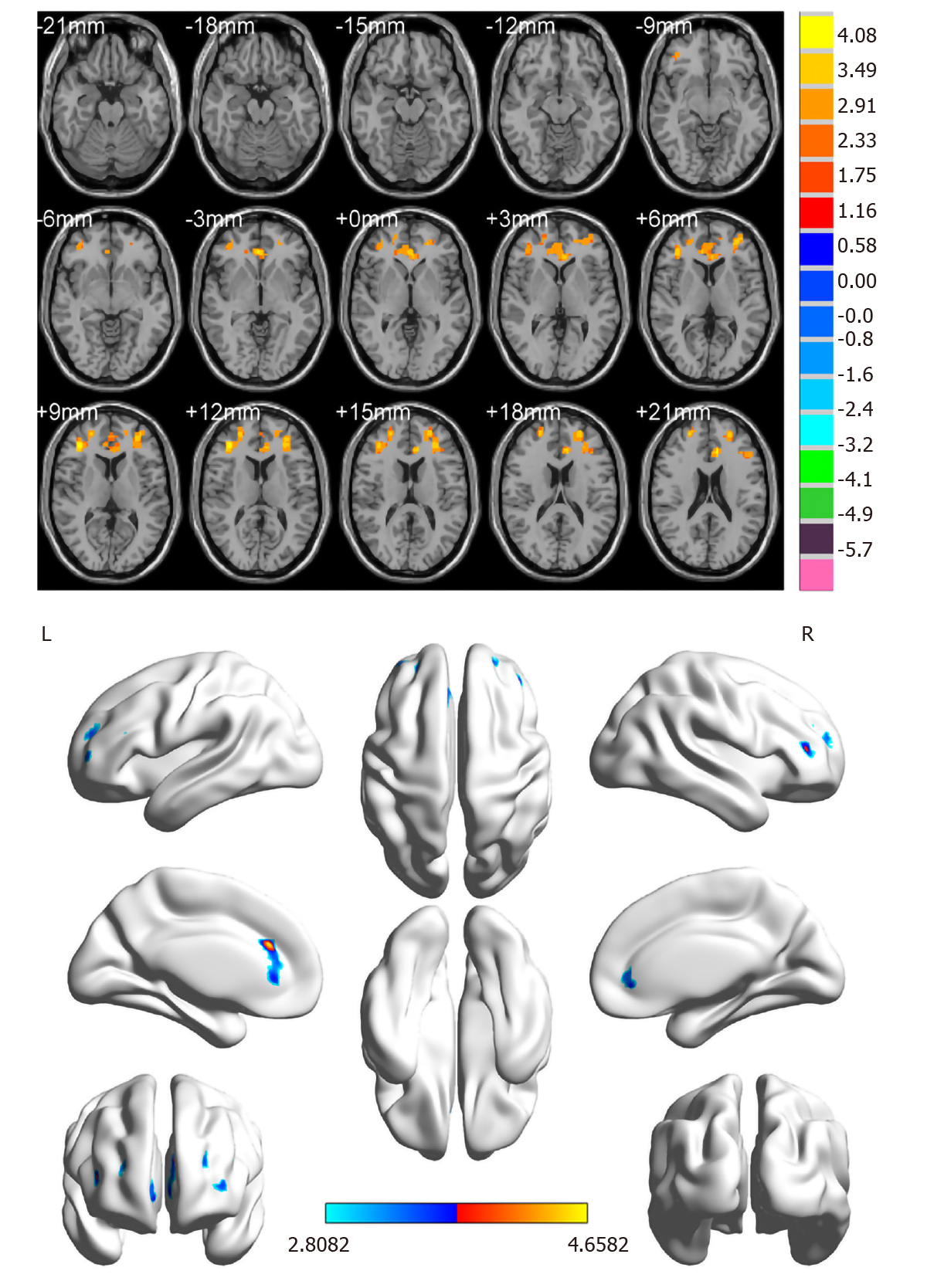
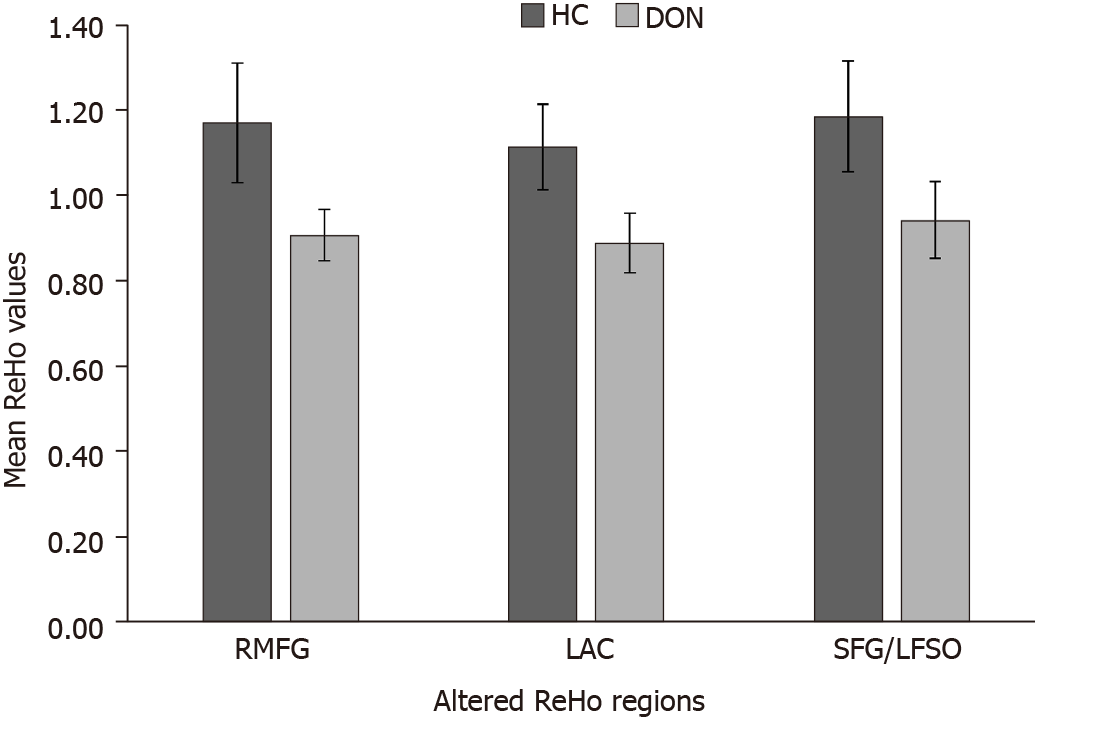
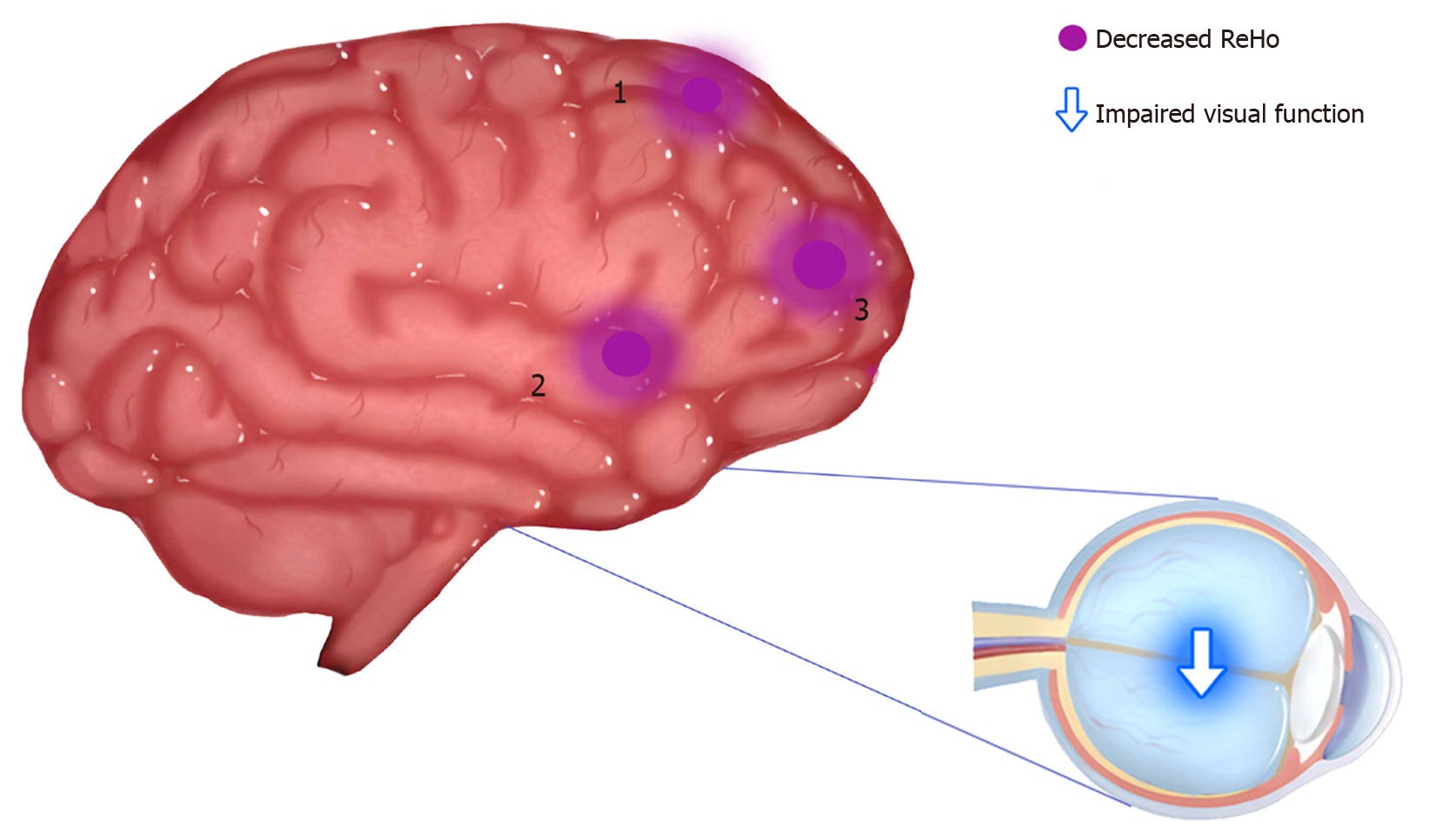
Compared with the HC group, ReHo values in the DON group were significantly (P < 0.05) decreased in specific brain areas including the right middle frontal gyrus (RMFG), LAC, and superior frontal gyrus/left frontal superior orbital gyrus (SFG/LFSO) (Figure 2 and Table 2). Figure 3 shows the mean ReHo values in brain regions in both groups, with the greatest difference between groups for the RMFG.
| ReHo | DON group and HCs | MNI coordinates | |||||
| Brain areas | BA | Peak t-values | Voxels | X | Y | Z | |
| DON < HCs | |||||||
| 1 | RMFG | 45 | 4.4606 | 185 | 39 | 36 | 9 |
| 2 | LAC | 32 | 4.6582 | 195 | -9 | 30 | 24 |
| 3 | SFG/LFSO | 48 | 4.0066 | 214 | -30 | 36 | 15 |
There were significant differences in ReHo values between the DON and HC groups. We therefore assumed that the ReHo value could be applied to distinguish DON patients from healthy subjects. To test this hypothesis, ROC curves were generated to analyze mean ReHo values in distinct brain regions. The area under the ROC curve (AUC) represents the diagnostic rate. Values of 0.5 to 0.7 indicate low accuracy, 0.7 to 0.9 are middle accuracy, and > 0.9 is high accuracy. The AUCs for ReHo values were as follows: RMFG 0.984, LAC 0.984, and SFG/LFS 0.929 (P < 0.001; Figure 4). Collectively, these results demonstrate that ReHo values in altered brain regions may help diagnose DON. Moreover, the ROC curves suggest that the RMFG and LAC ReHo values were more clinically relevant than SFG/LFSO.
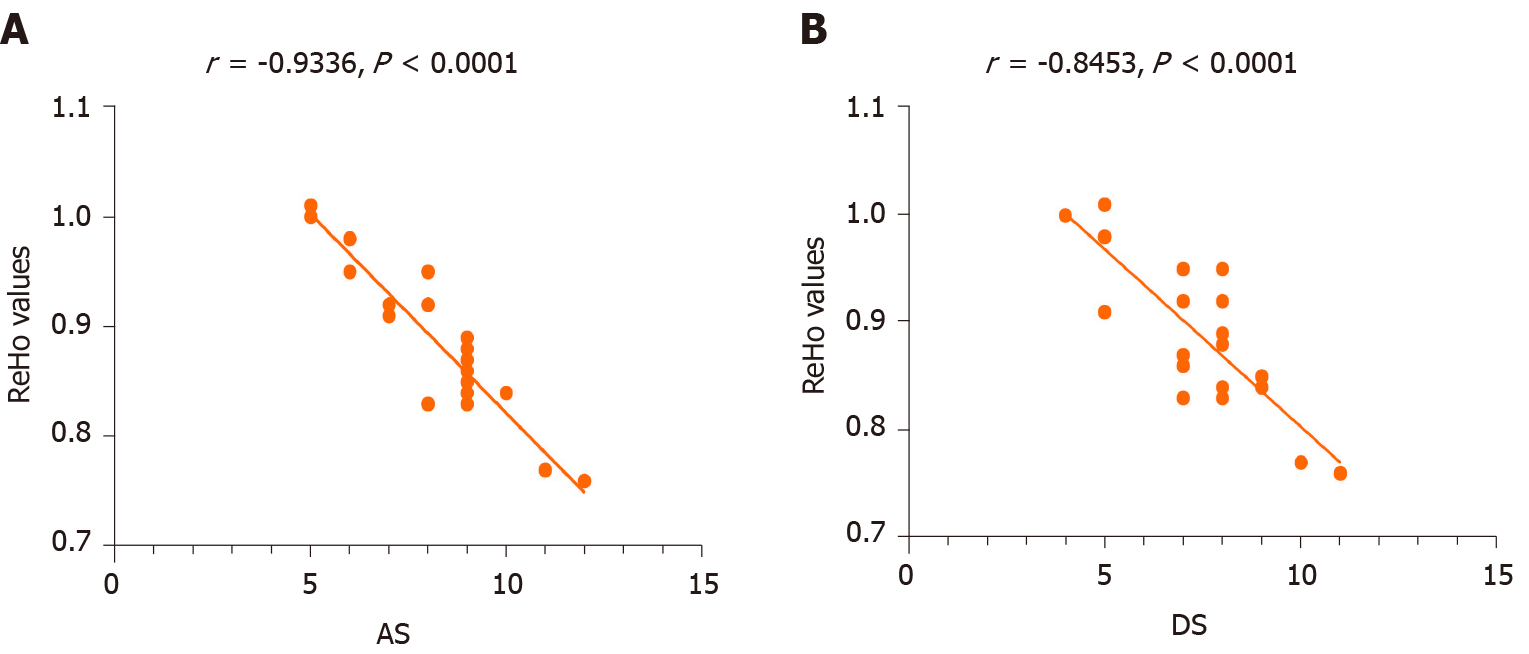
There were highly significant negative correlations between anxiety and depression scores and LAC ReHo values in the DON group (r = -0.9336, P < 0.0001 and r = -0.8453, P < 0.0001, respectively; Figure 5).
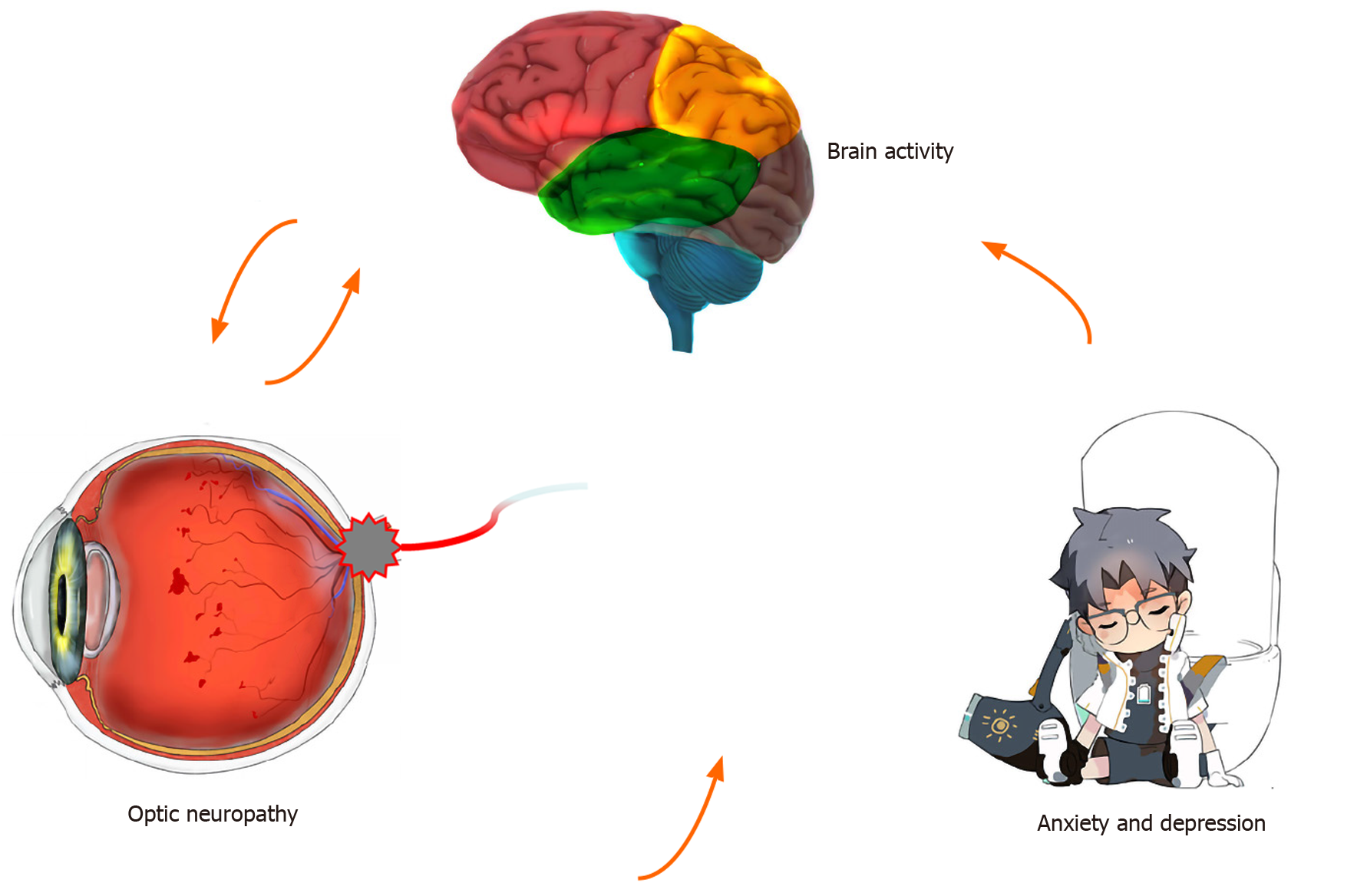
Rs-fMRI signals are related to resting-state networks and used to evaluate individual’s cognitive, emotional, and executive function[18]. Different signals are thought to reflect spontaneous neural activity in the brain[19]. Changes in cortical signals can be used to locate the central cortical functional area and further study other brain functions. ReHo is a method for processing fMRI results, and Zang et al[10] found a noteworthy correlation between ReHo abnormalities and changes of neuronal activity in local functional brain regions. In other words, ReHo abnormalities indicate that the synchronous activity of local neurons has changed. To our knowledge, this is the first study to measure synchronous neural activity in DON patients using the ReHo method. This approach has been applied in patients with a variety of ophthalmic and neurogenic diseases (Table 3). It is a crucial and valuable method to research functional changes in brain regions and investigate the underlying pathophysiological mechanisms of DON. In contrast to the HC group, ReHo values obviously decreased in the RMFG, LAC, and SFG/LFSO (Figure 6), suggesting that these areas may be dysfunctional in patients with DON. It is noteworthy that DON can lead to visual impairment that affects normal life and easily cause emotional changes in patients, such as anxiety and depression. Therefore, abnormal neural activity may also occur in brain regions related to emotional processing (Figure 7).
| Ref. | Year | Disease | |
| Ophthalmologic diseases | Song et al[13] | 2014 | Glaucoma |
| Chen et al[38] | 2017 | Primary angle-closure glaucomas | |
| Huang et al[12] | 2017 | Retinal detachment | |
| Guo et al[11] | 2019 | Neuromyelitis optica | |
| Wang et al[39] | 2019 | High-tension glaucoma | |
| Shi et al[40] | 2019 | Constant exotropia | |
| Yang et al[17] | 2019 | Strabismus amblyopia | |
| Liao et al[41] | 2019 | Diabetic retinopathy | |
| Lu et al[42] | 2020 | Anisometropic amblyopia | |
| Guo et al[7] | 2020 | Nonarteritic anterior ischemic optic neuropathy | |
| Neurogenic diseases | Dai et al[14] | 2012 | Sleep disorders |
| Li et al[15] | 2016 | Parkinson disease | |
| Russo et al[43] | 2020 | Bipolar mania and depression |
Diabetes can damage multiple organs throughout the body. Large fluctuations in blood sugar and long disease duration can also cause significant deterioration in brain function and structure, eventually leading to dysfunction and cognitive decrements associated with alterations in resting state neural connectivity[20]. When retinopathy causes optic nerve ischemia, the nerve fibers undergo axon demyelination and degeneration, which damage their integrity[21]. One study reported that that nerve conduction defects are obviously related to hyperglycemia[22]. The primary causes of optic neuropathology in diabetic retinopathy were decreased optic nerve blood flow and increased optic nerve microvascular permeability[23], which led to decreased numbers and diameters of distal optic axons and myelin sheath abnormalities.
In this study, almost all patients in the DON group cannot control blood sugar well, and DON patients with poor glycemic control may experience optic disc inflammation and edema, resulting in delayed VEP latency and decreased amplitude. VEP values demonstrate the mechanism of optic nerve axon damage and retinal ganglion cell apoptosis; they also intuitively show the extent of the optic nerve damage[24]. VEP examination can fully reflect the transmission function and shape information from the retinal ganglion cells to the brain’s visual center, but this method has nonspecific characteristics. It is common knowledge that VEP waves represent transmission to subcortical nuclei and visual cortices. The visual pathway conveys information from retinal photoreceptors to the visual center of the occipital lobe. Damage to this lobe can cause visual impairment, as well as memory deficits and poor motor perception.
The functions of the brain regions of interest in this study are shown in Table 4, and they are closely related to the formation of vision. The frontal lobe includes the head-eye movement area and can regulate optic nerve function. When this area is damaged, the patient may have enlarged eyelids, dilated pupils, and vision impairment. The middle frontal gyrus belongs to the prefrontal cortex and participates in various advanced cognitive functions including contingency awareness and executive attention[12,25]. A case report, based on rs-fMRI results, suggests that the right middle frontal gyrus plays a role in reorienting of attention[26]. The orbitofrontal cortex is the portion of the prefrontal cortex located at the anterior and lower part of the frontal lobe. It receives direct input from multiple brain regions including the amygdala, dorsolateral thalamus, temporal lobe, ventral tegmental area, and olfactory system. Its efferent nerves project to the amygdala, lateral hypothalamus, hippocampus, cingulate gyrus, and temporal lobe. The orbitofrontal cortex participates in many types of information transmission such as taste, smell, and vision, and it controls behaviors related to rewards and punishments and is also involved in emotion[27]. Animal and human MRI studies have demonstrated how the orbitofrontal cortex works in concert with other brain regions[28-30]. Orbitofrontal gyrus fiber bundles merge into the anterior medial part of the thalamus tract through the lateral caudate nucleus, which plays a significant part in visual perception. The cingulate gyrus[31] is part of the limbic system and consists of anterior and posterior regions with different functions and morphological structures. The anterior cingulate gyrus is associated with afferent fibers from dorsolateral frontal, orbitofrontal, and insular areas and thalamic nuclei[32], while the right dorsal anterior cingulate gyrus has vital roles in visual function[33]. Clinical and experimental results suggested that the anterior cingulate primarily performs cognitive functions and regulates emotional change[34]. One study of patients with poor emotional control and anxiety and depression reported improvements after they underwent anterior cingulotomy[35]. According to recent functional imaging studies, the prefrontal cortex and the adjacent anterior cingulate gyrus are now considered to play a central role in emotion regulation[36]. In addition, the patients with anterior cingulate gyrus lesions also have significant changes in subjective emotional state and social behavior, and are prone to show anxiety and depression[37]. The DON group had significantly decreased ReHo values in the LAC, and correlation analysis with HADS findings showed that anxiety and depression scores were negatively correlated with LAC ReHo values (Figure 5). Therefore, our study suggests that the lesions of anterior cingulate gyrus may be related to the anxiety and depression of DON patients.
| Brain region | Experimental result | Brain function | Anticipated results |
| Middle frontal gyrus | DONs < HCs | Contingency awareness and executive attention | Dysfunction of cognitive activities |
| Anterior cingulate | DONs < HCs | Cognition and emotion | Depression and anxiety |
| Superior frontal gyrus/frontal superior orbital gyrus | DONs < HCs | Visual transmission and emotional expression | Impaired visual function |
Figure 3 shows that compared with the HC group, ReHo values in the RMFG, LAC, and SFG/LFSO were significantly lower in the DON group. The ROC curves demonstrate the clinical diagnostic utilities of these three regions (Figure 4). They also show that the different signal values of these brain regions demonstrate acceptable sensitivity and excellent specificity to help distinguish between the groups. The AUC is often used to assess diagnostic value. We calculated the average ReHo values of the altered regions and created ROC curves (Figure 4) to evaluate their accuracy for diagnosing DON. The results showed that the diagnostic accuracies of the RMFG and LAC were higher than that of the SFG/LFSO. This supports our hypothesis that the ReHo method can be used to diagnose DON. Besides, many brain regions in patients with DON exhibited abnormal spontaneous activities that might indicate altered synchronous neural activity in the visual cortex and other vision-related brain regions. Our findings should be considered in the context of the study’s limitations. First, we included a relatively small sample size, and there were differences between different samples. In addition, some subjects may have made small body movements that affected the rs-fMRI results. Finally, these results do not explain the specific pathogenesis of DON. In the future, we plan to study the mechanism of nerve damage and subsequent changes in brain activity.
In summary, we have identified three different regions of brain dysfunction in DON patients, including the RMFG, LAC, and SFG/LFSO. The RMFG brain region has the most significant decrease and the highest diagnostic value. It could be used to study how optic neuropathy develops in patients with diabetes, and further guide the prevention and treatment of DON patients.
Diabetes is a common chronic disease. Given the increasing incidence of diabetes, more individuals are affected by diabetic optic neuropathy (DON), resulting in decreased vision. Whether DON leads to abnormalities of other visual systems, including the eye, the visual cortex, and other brain regions, remains unknown.
We are more concerned about the damage of DON to the optic disc and vision nowadays, and whether DON leads to abnormalities of other visual systems, including the eye, the visual cortex, and other brain regions remains unknown. In this study, we investigated the underlying regional homogeneity (ReHo) of brain-activity abnormalities in patients with DON and their relationship with clinical features. Our study may contribute to understanding altered neural mechanisms present in patients with DON.
The aim of the current study was to use ReHo to investigate the local characteristics of spontaneous brain activity in patients with diabetic optic neuropathy and brain activity deficits and to identify the underlying pathophysiological mechanisms of DON.
We matched 22 patients with DON with 22 healthy controls (HCs). All subjects underwent resting-state functional magnetic resonance imaging. The ReHo technique was used to record spontaneous changes in brain activity. Receiver operating characteristic (ROC) curves were applied to differentiate between ReHo values for patients with DON and HCs. We also assessed the correlation between Hospital Anxiety and Depression Scale scores and ReHo values in DON patients using Pearson correlation analysis.
ReHo values of the right middle frontal gyrus (RMFG), left anterior cingulate (LAC), and superior frontal gyrus (SFG)/left frontal superior orbital gyrus (LFSO) were significantly lower in DON patients compared to HCs. Among these, the greatest difference was observed in the RMFG. The result of the ROC curves suggest that ReHo values in altered brain regions may help diagnose DON, and the RMFG and LAC ReHo values are more clinically relevant than SFG/LFSO. We also found that anxiety and depression scores of the DON group were extremely negatively correlated with the LAC ReHo values (r = -0.9336, P < 0.0001 and r = -0.8453, P < 0.0001, respectively).
Three different brain regions show ReHo changes in DON patients, and these changes could serve as diagnostic and/or prognostic biomarkers for patients with DON. They could also guide the development of new measures to prevent and treat optic neuropathy.
We have identified three different regions of brain dysfunction in DON patients, including the RMFG, LAC, and SFG/LFSO. The RMFG brain region has the most significant decrease and the highest diagnostic value, suggesting that it could be used to study how optic neuropathy develops in patients with diabetes. Changes of spontaneous brain activity in different brain regions show different ReHo values, thus revealing brain activity in DON patients.
| 1. | Hua R, Qu L, Ma B, Yang P, Sun H, Liu L. Diabetic Optic Neuropathy and Its Risk Factors in Chinese Patients With Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2019;60:3514-3519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3524] [Cited by in RCA: 3253] [Article Influence: 232.4] [Reference Citation Analysis (3)] |
| 3. | Moro F, Doro D. Diabetic optic neuropathies: clinical features. Metab Pediatr Syst Ophthalmol (1985). 1986;9:65-70. [PubMed] |
| 4. | Ciulla TA, Harris A, Latkany P, Piper HC, Arend O, Garzozi H, Martin B. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand. 2002;80:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Icel E, Icel A, Uçak T, Karakurt Y, Elpeze B, Keskin Çimen F, Süleyman H. The effects of lycopene on alloxan induced diabetic optic neuropathy. Cutan Ocul Toxicol. 2019;38:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Young KD, Zotev V, Phillips R, Misaki M, Drevets WC, Bodurka J. Amygdala real-time functional magnetic resonance imaging neurofeedback for major depressive disorder: A review. Psychiatry Clin Neurosci. 2018;72:466-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Guo P, Zhao P, Lv H, Su Y, Liu M, Chen Y, Wang Y, Hua H, Kang S. Abnormal Regional Spontaneous Neural Activity in Nonarteritic Anterior Ischemic Optic Neuropathy: A Resting-State Functional MRI Study. Neural Plast. 2020;2020:8826787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Aguirregomozcorta M, Mancini L, Jenkins TM, Hickman SJ, Ciccarelli O, Plant GT, Thompson AJ, Toosy AT. A longitudinal functional MRI study of non-arteritic anterior ischaemic optic neuropathy patients. J Neurol Neurosurg Psychiatry. 2011;82:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Lützen N, Urbach H. [Magnetic Resonance Imaging of the Optic Pathway]. Klin Monbl Augenheilkd. 2018;235:1259-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1520] [Cited by in RCA: 2101] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 11. | Guo X, Zhu J, Zhang N, Zhang L, Qi Y, Cai H, Zhang X, Sun J, Wang Q, Yang L, Shi FD, Yu C. Altered neurovascular coupling in neuromyelitis optica. Hum Brain Mapp. 2019;40:976-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Huang X, Li D, Li HJ, Zhong YL, Freeberg S, Bao J, Zeng XJ, Shao Y. Abnormal regional spontaneous neural activity in visual pathway in retinal detachment patients: a resting-state functional MRI study. Neuropsychiatr Dis Treat. 2017;13:2849-2854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Song Y, Mu K, Wang J, Lin F, Chen Z, Yan X, Hao Y, Zhu W, Zhang H. Altered spontaneous brain activity in primary open angle glaucoma: a resting-state functional magnetic resonance imaging study. PLoS One. 2014;9:e89493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Dai XJ, Gong HH, Wang YX, Zhou FQ, Min YJ, Zhao F, Wang SY, Liu BX, Xiao XZ. Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI study. Sleep Med. 2012;13:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Li Y, Liang P, Jia X, Li K. Abnormal regional homogeneity in Parkinson's disease: a resting state fMRI study. Clin Radiol. 2016;71:e28-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Levin LA, Arnold AC, Levin LA, Arnold & Anthony C. Neuro-ophthalmology: the practical guide. Thieme, New York, Stuttgart: Thieme Medical Publishers Inc; 2005. |
| 17. | Yang X, Lu L, Li Q, Huang X, Gong Q, Liu L. Altered spontaneous brain activity in patients with strabismic amblyopia: A resting-state fMRI study using regional homogeneity analysis. Exp Ther Med. 2019;18:3877-3884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2151] [Cited by in RCA: 2242] [Article Influence: 140.1] [Reference Citation Analysis (1)] |
| 19. | Lv H, Wang Z, Tong E, Williams LM, Zaharchuk G, Zeineh M, Goldstein-Piekarski AN, Ball TM, Liao C, Wintermark M. Resting-State Functional MRI: Everything That Nonexperts Have Always Wanted to Know. AJNR Am J Neuroradiol. 2018;39:1390-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 256] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 20. | van Duinkerken E, Schoonheim MM, Sanz-Arigita EJ, IJzerman RG, Moll AC, Snoek FJ, Ryan CM, Klein M, Diamant M, Barkhof F. Resting-state brain networks in type 1 diabetic patients with and without microangiopathy and their relation to cognitive functions and disease variables. Diabetes. 2012;61:1814-1821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Zhang C, Guo Y, Slater BJ, Miller NR, Bernstein SL. Axonal degeneration, regeneration and ganglion cell death in a rodent model of anterior ischemic optic neuropathy (rAION). Exp Eye Res. 2010;91:286-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Mendonca HR, Carpi-Santos R, da Costa Calaza K, Blanco Martinez AM. Neuroinflammation and oxidative stress act in concert to promote neurodegeneration in the diabetic retina and optic nerve: galectin-3 participation. Neural Regen Res. 2020;15:625-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Lee CS, Larson EB, Gibbons LE, Latimer CS, Rose SE, Hellstern LL, Keene CD, Crane PK; Adult Changes in Thought (ACT) Study. Ophthalmology-Based Neuropathology Risk Factors: Diabetic Retinopathy is Associated with Deep Microinfarcts in a Community-Based Autopsy Study. J Alzheimers Dis. 2019;68:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Norcia AM, Appelbaum LG, Ales JM, Cottereau BR, Rossion B. The steady-state visual evoked potential in vision research: A review. J Vis. 2015;15:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 673] [Article Influence: 61.2] [Reference Citation Analysis (1)] |
| 25. | Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2006;29:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci. 2015;9:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 27. | Shott ME, Cornier MA, Mittal VA, Pryor TL, Orr JM, Brown MS, Frank GK. Orbitofrontal cortex volume and brain reward response in obesity. Int J Obes (Lond). 2015;39:214-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 827] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 29. | Cavada C, Compañy T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suárez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 691] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 30. | Kahnt T, Chang LJ, Park SQ, Heinzle J, Haynes JD. Connectivity-based parcellation of the human orbitofrontal cortex. J Neurosci. 2012;32:6240-6250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 31. | San Pedro EC, Mountz JM, Ojha B, Khan AA, Liu HG, Kuzniecky RI. Anterior cingulate gyrus epilepsy: the role of ictal rCBF SPECT in seizure localization. Epilepsia. 2000;41:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Unnwongse K, Wehner T, Foldvary-Schaefer N. Mesial frontal lobe epilepsy. J Clin Neurophysiol. 2012;29:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Shinoura N, Yamada R, Tabei Y, Shiode T, Itoi C, Saito S, Midorikawa A. The right dorsal anterior cingulate cortex may play a role in anxiety disorder and visual function. Neurol Res. 2013;35:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4219] [Cited by in RCA: 4336] [Article Influence: 166.8] [Reference Citation Analysis (0)] |
| 35. | Whitty CW, Duffield JE, Tov' PM, Cairns H. Anterior cingulectomy in the treatment of mental disease. Lancet. 1952;1:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 2376] [Article Influence: 108.0] [Reference Citation Analysis (2)] |
| 37. | Seitz RJ, Nickel J, Azari NP. Functional modularity of the medial prefrontal cortex: involvement in human empathy. Neuropsychology. 2006;20:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Chen W, Zhang L, Xu YG, Zhu K, Luo M. Primary angle-closure glaucomas disturb regional spontaneous brain activity in the visual pathway: an fMRI study. Neuropsychiatr Dis Treat. 2017;13:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Wang Y, Lu W, Yan T, Zhou J, Xie Y, Yuan J, Liu G, Teng Y, Han W, Chen D, Qiu J. Functional MRI reveals effects of high intraocular pressure on central nervous system in high-tension glaucoma patients. Acta Ophthalmol. 2019;97:e341-e348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Shi H, Wang Y, Liu X, Xia L, Chen Y, Lu Q, Nguchu BA, Wang H, Qiu B, Wang X, Feng L. Cortical Alterations by the Abnormal Visual Experience beyond the Critical Period: A Resting-state fMRI Study on Constant Exotropia. Curr Eye Res. 2019;44:1386-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Liao XL, Yuan Q, Shi WQ, Li B, Su T, Lin Q, Min YL, Zhu PW, Ye L, Shao Y. Altered brain activity in patients with diabetic retinopathy using regional homogeneity: A resting-state fMRI study. Endocr Pract. 2019;25:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Lu W, Yu X, Zhao L, Zhang Y, Zhao F, Wang Y, Qiu J. Enhanced Gray Matter Volume Compensates for Decreased Brain Activity in the Ocular Motor Area in Children with Anisometropic Amblyopia. Neural Plast. 2020;2020:8060869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Russo D, Martino M, Magioncalda P, Inglese M, Amore M, Northoff G. Opposing Changes in the Functional Architecture of Large-Scale Networks in Bipolar Mania and Depression. Schizophr Bull. 2020;46:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Găman MA, Popovic DS S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Ma YJ