Published online Mar 15, 2021. doi: 10.4239/wjd.v12.i3.292
Peer-review started: September 18, 2020
First decision: December 1, 2020
Revised: December 9, 2020
Accepted: December 23, 2020
Article in press: December 23, 2020
Published online: March 15, 2021
Processing time: 165 Days and 4.9 Hours
Poor sleep quality is a common clinical feature in patients with type 2 diabetes mellitus (T2DM), and often negatively related with glycemic control. Cognitive behavioral therapy (CBT) may improve sleep quality and reduce blood sugar levels in patients with T2DM. However, it is not entirely clear whether CBT delivered by general practitioners is effective for poor sleep quality in T2DM patients in community settings.
To test the effect of CBT delivered by general practitioners in improving sleep quality and reducing glycemic levels in patients with T2DM in community.
A cluster randomized controlled trial was conducted from September 2018 to October 2019 in communities of China. Overall 1033 persons with T2DM and poor sleep quality received CBT plus usual care or usual care. Glycosylated hemoglobin A1c (HbAlc) and sleep quality [Pittsburgh Sleep Quality Index (PSQI)] were assessed. Repeated measures analysis of variance and generalized linear mixed effects models were used to estimate the intervention effects on hemoglobin A1c and sleep quality.
The CBT group had 0.64, 0.50, and 0.9 lower PSQI scores than the control group at 2 mo, 6 mo, and 12 mo, respectively. The CBT group showed 0.17 and 0.43 lower HbAlc values than the control group at 6 mo and 12 mo. The intervention on mean ΔHbAlc values was significant at 12 mo (t = 3.68, P < 0.01) and that mean ΔPSQI scores were closely related to ΔHbAlc values (t = 7.02, P < 0.01). Intention-to-treat analysis for primary and secondary outcomes showed identical results with completed samples. No adverse events were reported.
CBT delivered by general practitioners, as an effective and practical method, could reduce glycemic levels and improve sleep quality for patients with T2DM in community.
Core Tip: Cognitive behavior therapy is recommended as the preferential intervention for insomnia. Cognitive behavior therapy could reduce hemoglobin A1c values at 6 mo and 12 mo following improved subjective sleep disturbance of patients with type 2 diabetes mellitus in the community-based randomized controlled trial. Cognitive behavior therapy should be included in the comprehensive management of diabetes and applied in community by general practitioners.
- Citation: Zhang HZ, Zhang P, Chang GQ, Xiang QY, Cao H, Zhou JY, Dong ZM, Qiao C, Xu CR, Qin Y, Lou PA. Effectiveness of cognitive behavior therapy for sleep disturbance and glycemic control in persons with type 2 diabetes mellitus: A community-based randomized controlled trial in China. World J Diabetes 2021; 12(3): 292-305
- URL: https://www.wjgnet.com/1948-9358/full/v12/i3/292.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i3.292
Diabetes is a major global public health problem. By 2040, an estimated 640 million people will suffer from diabetes mellitus globally[1]. China has above 100 million people with diabetes, and more than any other countries[2,3]. Owing to worry about uncontrolled glycemia and possible diabetic complications, etc., more than one-third of patients with type 2 diabetes mellitus (T2DM) experience subjective sleep disturbances[4]. Studies have shown that poor sleep quality is associated with higher glucose levels in diabetic patients[5-7]. Therefore, in theory, improvement of sleep quality in persons with T2DM may help to reduce glycemic levels[8].
Cognitive behavioral therapy (CBT) is a psychosocial intervention approach in which behavioral change is initiated by a therapist helping patients to confront and modify the irrational thoughts and beliefs that are most likely at the root of their maladaptive behaviors[9]. CBT is recommended as the first-line treatment for insomnia[8,10], and many studies have demonstrated its effect in improving sleep quality and reducing related sleep disorders[11-13]. There is also evidence that CBT improves sleep quality and reduces blood sugar levels in patients with T2DM[14]. However, most CBT studies have used relatively small sample sizes or have used websites through which patients receive online CBT from experts[10,12,13], rather than receiving face-to-face therapy from general practitioners in community settings. It is not entirely clear whether CBT delivered by general practitioners is effective for poor sleep quality in T2DM patients in community settings. Therefore, we conducted a community-based cluster randomized controlled trial to examine the effectiveness of CBT delivered for individuals with T2DM and comorbid sleep disturbances by general practitioners from September 2018 to October 2019 in Xuzhou City, China. We hypothesized that participants who received CBT would show better sleep quality at 2 mo, 6 mo, and 12 mo post-intervention than at the beginning of the intervention. We also predicted that the intervention would reduce glycemic levels at these time points.
This community-based cluster randomized controlled trial involving two groups of adults with T2DM were conducted from September 2018 to October 2019 in Xuzhou City. This area is in northern Jiangsu Province, eastern China, and is moderately well developed with a population of 10000000 across 3980 villages/communities. There are no geographical differences among these communities. Community health service centers are the sole health care units in each community.
In this study, 44 eligible clusters (villages/communities) were randomly assigned to receive CBT plus usual care intervention (CBT group) or usual care intervention [usual care (UC) group] with a 1:1 parallel design. A random number table is used to assign clusters to CBT or UC conditions at the individual level. The baseline survey was implemented from May to July 2018. All data collection was completed in August 2018.
Participants with T2DM and comorbid poor sleep quality were invited to participate in the study. The inclusion criteria were T2DM diagnosed at least 6 mo before recruitment, poor sleep quality, and normal cognitive function. Participants received face-to-face questionnaire interviews and questionnaire assessment in the same health care centers for both baseline and follow-up surveys.
The exclusion criteria were shift work, other sleep disorders [such as obstructive sleep apnea (OSA), treated or untreated], harmful or hazardous alcohol use, having a medically unstable condition (e.g., cancer, stroke, cardiovascular disease, chronic obstructive pulmonary disease, and severe psychosis), receiving treatment for sleep problems, receiving psychological treatment, and sleep surroundings that interfered with regular night-time sleep patterns. Other exclusion criteria were unwillingness to participate in the study, inability to regularly attend sessions (being absent for more than two sessions), and experiencing severe crisis and stress before the study.
There are no cultural and belief differences among participants who were of Han ancestry with the same dietary habits. The guideline of Consolidated Standards of Reporting Trials extension for cluster trials (CONSORT) was followed[15]. The current study was conducted under protocols approved by Xuzhou Center for Disease Control and Prevention and the Xuzhou of Medical Sciences Ethics Committee (Approval No. 20151210). Written informed consent was obtained from all participants. The trial was registered in the Chinese Clinical Trials Registry on March 3, 2016 (reference: ChiCTR-IOP-16008045) and implemented according to the 2000 revised version of the Helsinki Declaration. Participants were lost to follow-up if we could not contact them or if they moved to another location, withdrew consent, refused to proceed, had invalid data, or were unable to complete the study.
Usual care (all participants): UC is a basic public health service requirement and was provided for T2DM patients by health care service general practitioners. Every 3 mo, general practitioners conducted a face-to-face visit in which they recorded the participant’s status; provided advice on diet, medication, and exercise; and monitored blood sugar. If the fasting blood sugar levels were ≥ 7.0 mmol/L, the general practitioners followed the patient once during the next 2 wk. If the fasting blood sugar levels were still ≥ 7.0 mmol/L, the general practitioners advised the patient to transfer to a higher level or specialized hospital for further treatment. After 2 wk, the general practitioners visited the patient again. The control group was only given UC by general practitioners.
Trained general practitioners: A total of 67 general practitioners from 22 health care centers attended a 2-d CBT training session for the intervention group. The training contents were adapted from previous studies[16-18], which comprised six sessions with 40-50 min of lectures and 10-15 min of discussions for six consecutive days within the first week. The six sessions included: (1) General information about type 2 diabetes management, the importance of sleep in type 2 diabetes management, and the benefits of CBT; (2) The importance and process of normal sleep; potential sleep barriers and difficulties of having diabetes; and the effect of environment and lifestyle factors on sleep, such as good light and ventilation in bedrooms and diet regulation; (3) Behavioral therapy, which included methods of establishing healthy sleeping habits, improving sleep quality, and reducing periods of waking; (4) Relaxation training (e.g., breathing techniques, progressive muscle relaxation, and mindfulness relaxation) and strategies to regulate emotions (e.g., self-improvement, and learning to give, to understand other people, to discriminate, to be self-possessed, to cherish, to use initiative, and to rest); (5) Learning strategies to cope with stress (e.g., physical fitness activities or relaxation activities, prayer or meditation, listening to classical music, watching a comedy movie, participating in social activities, and taking part in voluntary work); and (6) Communication with the doctor about problems with diagnosis, treatment, and follow-up during the study. Each general practitioner was administered a closed text examination at the end of each session.
All patients of the intervention group were asked to participate in the lectures. Every day in the first week, trained general practitioners gave participants a 40-50 min lecture followed by a 10-15 min discussion session.
The general practitioners reviewed all the discussed themes, received participant feedback about the course, were engaged in question-and-answer sessions, facilitated group discussions about the study and its conclusions, and tried to prevent the recurrence of poor sleep patterns. Subsequently, participants received two lectures plus discussion sessions each week for 6 wk. A member of our study group attended the lecture in the first week; after the class, they commented on the lectures and received questions from participants. A member of our study group also offered individual counseling after class. Participants were asked to establish routines comprising periods of activity and rest and a regular relaxing bedtime routine. General practitioners were encouraged to use CBT to help patients at the next follow-up when all sessions had been completed.
The endpoints were the last follow-up results of each participant. The primary outcome was hemoglobin A1c (HbA1c) levels at 2 mo, 6 mo, and 12 mo, measured using high-performance liquid chromatography (Bio-Rad D-10, glycated hemoglobin meter). Glycemic level is expressed as mean HbA1c value ± SD. The change in HbA1c value (ΔHbA1c value) is the difference between the mean HbA1C value at baseline and the mean HbA1c value after 12 mo.
The secondary outcome was sleep quality, measured using the Pittsburgh Sleep Quality Index (PSQI)[19]. The PSQI contains 19 items on seven dimensions. The global PSQI score is thus 0–21; higher scores indicate worse sleep quality. In the Chinese version of the PSQI, a score > 7 differentiates poor sleepers from good sleepers[20]. The change in sleep quality mean score (ΔPSQI score) is the difference between the mean score at baseline and the mean score after 12 mo.
We measured background participant characteristics, disease history, smoking, drinking, and anxiety/depression. Alcohol use was assessed using the Alcohol Use Disorders Identification Test[21]. Total scores are summed scores of ten items assessing the frequency and amount of alcohol use and symptoms of dependence. Scores ≥ 20 indicate harmful and hazardous levels of alcohol use that require treatment[22]. OSA was measured using the STOP-Bang questionnaire, and participants were defined as having OSA if they scored ≥ 3[23]. We assessed depression symptoms using the Patient Health Questionnaire-9 (PHQ-9), a nine-item scale with a 0-27 score range[24]. Anxiety symptoms were measured using the validated seven-item General Anxiety Disorder questionnaire (GAD-7; total score range 0-21)[25].
More than 240000 patients with T2DM were registered in all communities of Xuzhou City, an average number of 60 patients per community. Based on the principles of a cluster randomization design, and considering 90% power, a two-sided type I error of 5%, an intracluster correlation coefficient of 0.10, and a rate of loss to follow-up of 15%, 17 communities and a minimum of 501 T2DM patients with poor sleep quality in each intervention or control group (29 participants from each community) were needed to detect a 0.45% relative reduction in the levels of HbA1c between the two groups[26].
To ensure that enough patients with poor sleep quality were selected, based on the approximately 30% prevalence of poor sleep quality in T2DM patients[27], over 60% of the study communities that we selected contained ≥ 60 registered T2DM patients. Based on the registered numbers in each community, we selected 2156 participants from 28 communities with ≥ 60 registered patients, and 766 patients from 16 communities with < 60 registered patients.
After participants’ sleep quality was assessed, the 44 communities were sorted in descending order according to the number of participants with poor sleep quality by a research statistician who had no connection with the participants. Then, using a random number table, communities were randomly assigned (1:1) to either the CBT (intervention) group or the UC (control) group by one of the corresponding authors. The general practitioners arranged all participants for study intervention appointments. All assessments were conducted at community health service stations. The chief investigators and statisticians were masked to the group allocation.
We used EpiData 3.1 (The EpiData Association, Odense, Denmark) to input and manage the data. Continuous variables are presented as the mean ± SD and were analyzed using parametric or nonparametric tests, depending on whether the data were normally distributed. Categorical variables are presented as percentages and were analyzed using chi-square tests. Between-group differences in continuous and categorical variables at baseline were analyzed using t-tests and χ2 tests, respectively. Repeated measures analysis of variance was used to examine within-group variation before and after the intervention (time × group interaction effect).
Generalized linear mixed models (GLMMs) were used to estimate the effects of the intervention on the outcome variables. The models included two levels: Group membership and participants. The fixed parts of the models included the between-group comparisons for the pretreatment variables. Age, sex, and comorbidity were considered important variables and were included as covariates in all GLMMs. Other potentially confounding variables were also included as covariates in all GLMMs.
Intention-to-treat analysis was performed using the last PSQI and HbA1c assessments for the non-completers. All analyses were performed using Statistic Package for Social Science Statistics version 23.0 (Statistic Package for Social Science, Chicago, IL, United States), and the minimum statistical significance level was P < 0.05.
The two groups came from 44 communities and were similar in cluster and individual levels at baseline (Table 1). The number of groups for each community had not changed at 2 mo, 6 mo, and 12 mo in the CBT and UC groups. The number of group participants and general practitioners in each community were also similar between groups at the group level at these time points (Table 1).
| Variable | Baseline | 2 mo | 6 mo | 12 mo | |||||||||||||
| CBT | Control | χ2/t value | P value | CBT | Control | χ2/t value | P value | CBT | Control | χ2/t value | P value | CBT | Control | χ2/t value | P value | ||
| Cluster level (group) | |||||||||||||||||
| Number of groups | 22 | 22 | --- | --- | 22 | 22 | --- | --- | 22 | 22 | --- | --- | 22 | 22 | --- | --- | |
| Number of participants | |||||||||||||||||
| 10 | 6 | 10 | 1.62 | 0.45 | 7 | 11 | 1.58 | 0.45 | 13 | 14 | 1.348 | 0.51 | 18 | 20 | 0.77 | 0.38 | |
| 20 | 10 | 7 | 9 | 6 | 7 | 5 | 4 | 2 | |||||||||
| 30 | 6 | 5 | 6 | 5 | 1 | 3 | 0 | 0 | |||||||||
| Number of follow-up general practitioners in each community | |||||||||||||||||
| ≤ 5 | 6 | 7 | 0.55 | 0.76 | 6 | 7 | 0.55 | 0.76 | 6 | 7 | 0.553 | 0.758 | 6 | 7 | 0.55 | 0.76 | |
| 6-10 | 15 | 13 | 15 | 13 | 15 | 13 | 15 | 13 | |||||||||
| 10-15 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | |||||||||
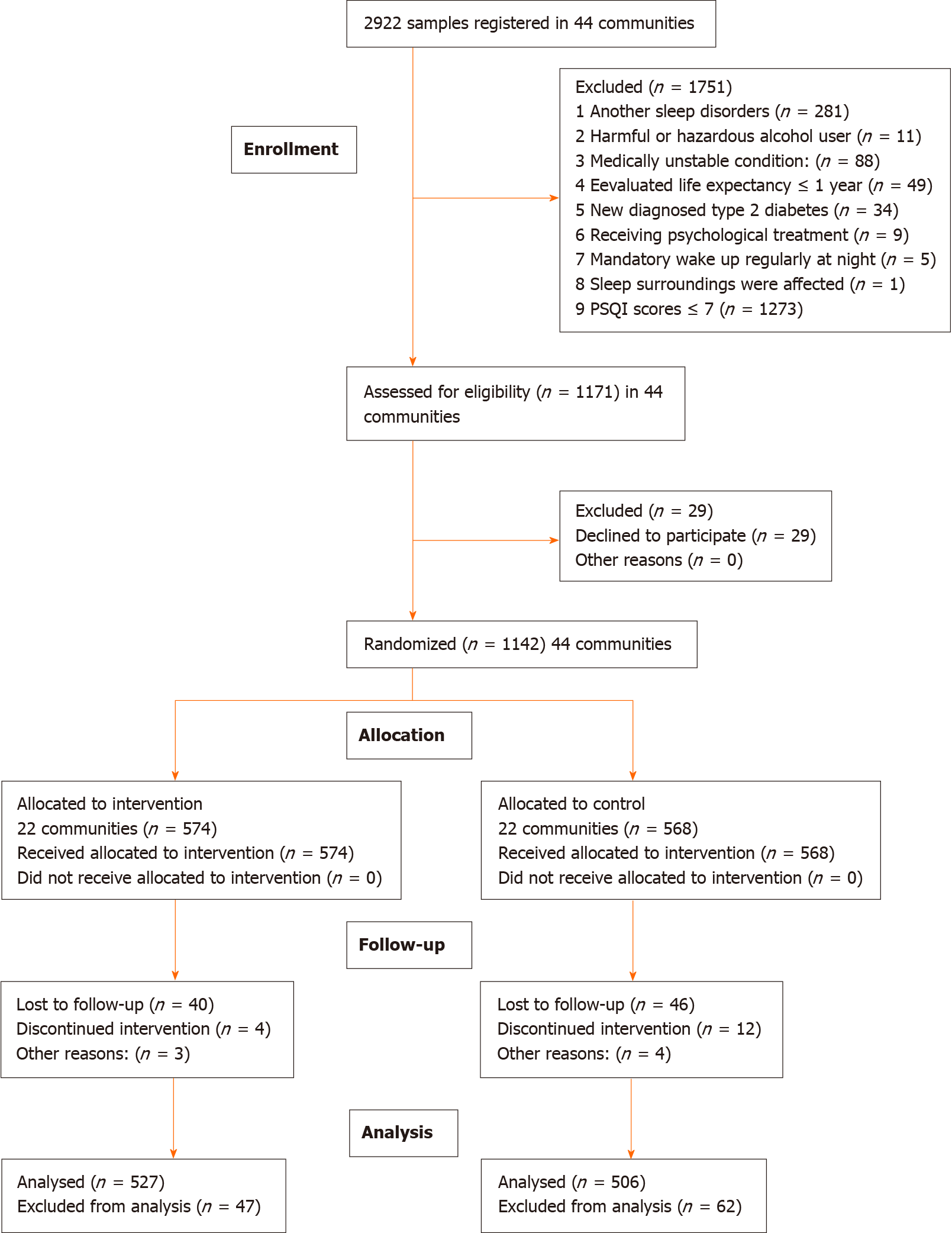
Figure 1 shows the CONSORT extension for cluster trial diagram of participant flow. In a word, 574 participants from 22 different communities were randomly allocated to receive CBT plus UC, and 568 participants from 22 different communities were randomly allocated to receive UC, with no significant differences in general characteristics between the two groups at baseline. The survey was started on September 1, 2018 and completed on October 31, 2019. Complete data for 1033 participants were included in the analysis, and 109 non-completers (47 and 62 in the intervention and control group, respectively) were omitted for attrition after 12-mo follow-up. The general characteristics did not differ significantly between the two groups of completers at baseline, 2 mo, and 6 mo in the individual level (Table 2), however, the GAD-7 scores and PHQ-9 scores were lower in the CBT group than in the UC group. No participants reported receiving treatment for sleep problems during the 12-mo follow-up.
| Variable | Baseline | 2 mo | 6 mo | 12 mo | ||||||||||||
| CBT | Control | χ2/t value | P value | CBT | Control | χ2/t value | P value | CBT | Control | χ2/t value | P value | CBT | Control | χ2/t value | P value | |
| Participant number | 568 | 574 | --- | --- | 550 | 559 | 541 | 549 | --- | --- | 506 | 527 | --- | --- | ||
| Gender (female) | ||||||||||||||||
| Female | 370 | 383 | 0.32 | 0.57 | 362 | 376 | 0.26 | 0.61 | 356 | 370 | 0.31 | 0.58 | 335 | 367 | 1.40 | 0.24 |
| Age (mean + SD) | 61.58 ± 9.17 | 61.83 ± 8.64 | -0.46 | 0.65 | 61.75 ± 9.12 | 62.10 ± 8.86 | -0.64 | 0.52 | 62.48 ± 9.14 | 62.75 ± 8.71 | -0.50 | 0.62 | 62.87 ± 9.25 | 62.74 ± 8.87 | 0.23 | 0.82 |
| Educational level | ||||||||||||||||
| High school and above | 53 | 56 | 0.42 | 0.81 | 51 | 56 | 0.38 | 0.83 | 47 | 56 | 1.09 | 0.58 | 41 | 50 | 1.00 | 0.61 |
| Junior high school | 240 | 251 | 234 | 243 | 231 | 240 | 208 | 223 | ||||||||
| High school and above | 275 | 267 | 265 | 260 | 263 | 253 | 257 | 254 | ||||||||
| Hypertension | 287 | 287 | 0.03 | 0.86 | 276 | 278 | 0.02 | 0.88 | 270 | 272 | 0.01 | 0.91 | 252 | 262 | 0.00 | 0.98 |
| Comorbidities | 109 | 118 | 0.34 | 0.56 | 109 | 114 | 0.06 | 0.81 | 107 | 110 | 0.01 | 0.92 | 91 | 110 | 1.37 | 0.24 |
| BMI | 24.77± 4.15 | 25.20± 4.94 | -1.60 | 0.11 | 24.99 ± 4.48 | 25.00 ± 4.94 | -0.06 | 0.96 | 24.71 ± 4.09 | 25.22 ± 5.01 | -1.84 | 0.07 | 24.76 ± 4.00 | 25.28 ± 5.02 | -1.84 | 0.07 |
| Drinking | 55 | 66 | 0.99 | 0.32 | 55 | 65 | 0.76 | 0.38 | 54 | 64 | 0.79 | 0.37 | 46 | 49 | 0.01 | 0.91 |
| Smoking | 80 | 92 | 0.84 | 0.36 | 79 | 88 | 0.41 | 0.52 | 76 | 86 | 0.56 | 0.45 | 64 | 69 | 0.05 | 0.83 |
| Course of the disease (yr) | 5.07 ± 5.25 | 5.21 ± 4.91 | 0.56 | 0.64 | 5.20 ± 5.06 | 5.41 ± 5.36 | -0.66 | 0.51 | 5.53 ± 5.21 | 5.66 ± 4.76 | -0.43 | 0.67 | 6.03 ± 5.28 | 6.20 ± 4.68 | -0.52 | 0.60 |
| Medication | ||||||||||||||||
| Oral hypoglycemic agents | 491 | 483 | 1.56 | 0.46 | 476 | 470 | 1.76 | 0.42 | 472 | 465 | 1.72 | 0.42 | 448 | 444 | 5.96 | 0.05 |
| Insulin | 45 | 49 | 42 | 46 | 39 | 44 | 37 | 43 | ||||||||
| Oral hypoglycemic agents plus insulin | 32 | 42 | 32 | 43 | 30 | 40 | 21 | 40 | ||||||||
| GAD-7 score | 7.56 ± 5.20 | 7.58 ± 5.25 | -0.04 | 0.97 | 7.47 ± 5.23 | 7.22 ± 5.48 | 0.76 | 0.45 | 7.53 ± 5.65 | 7.14 ± 5.93 | 1.10 | 0.27 | 7.14 ± 5.68 | 6.34 ± 5.56 | 2.27 | 0.02 |
| PHQ-9 score | 7.13 ± 5.53 | 6.94 ± 5.47 | 0.59 | 0.55 | 7.23 ± 5.36 | 7.00 ± 5.02 | 0.73 | 0.46 | 7.21 ± 5.48 | 6.74 ± 5.21 | 1.44 | 0.15 | 7.20 ± 5.45 | 6.10 ± 4.97 | 3.39 | 0.00 |
The results of the attrition analyses showed that the rates of female patients, those with smoking and drinking, and those with low education level among non-completers were higher in the UC group than in the CBT group (Table 3). Compared with completers, non-completers in both the CBT and UC groups were less educated and more likely to be male, smokers and drinkers, and insulin users (Table 3).
| Variable | Completers | Lost to follow-up | χ2/t value | P value | ||||
| Intervention group | Control group | χ2/t value | P value | Total | ||||
| Participant number | 1142 | 62 | 47 | —— | —— | 109 | —— | —— |
| Gender (Female) | 753 | 35 | 16 | 5.39 | 0.02 | 51 | 15.88 | < 0.01 |
| Age | 62.44 (9.02) | 62.95 (7.74) | 62.23 (8.51) | 0.46 | 0.65 | 62.64 (8.05) | -0.22 | 0.82 |
| Educational level | ||||||||
| High school and above | 109 | 12 | 6 | 1.03 | 0.60 | 18 | 15.9 | < 0.01 |
| Junior high school | 491 | 32 | 28 | 60 | ||||
| Elementary school and below | 542 | 18 | 13 | 31 | ||||
| Hypertension | 574 | 35 | 25 | 0.11 | 0.74 | 60 | 0.91 | 0.34 |
| Comorbidities | 227 | 18 | 8 | 2.12 | 0.15 | 26 | 0.75 | 0.32 |
| BMI | 25.02 (4.17) | 24.77 (4.53) | 24.43 (4.73) | 0.75 | 0.46 | 24.62 (4.83) | 0.94 | 0.35 |
| Drinking | 121 | 9 | 17 | 6.9 | 0.009 | 26 | 16.87 | < 0.001 |
| Smoking | 172 | 16 | 23 | 6.22 | 0.01 | 39 | 30.46 | < 0.001 |
| Course of the disease (yr) | 6.12 (4.98) | 5.22 (4.53) | 6.14 (5.17) | -1.001 | 0.32 | 5.62 (4.81) | 1.004 | 0.32 |
| Medication | ||||||||
| Oral hypoglycemic agents | 974 | 43 | 39 | 4.74 | 0.09 | 82 | 7.89 | 0.019 |
| Insulin | 94 | 8 | 6 | 14 | ||||
| Oral hypoglycemic agents plus insulin | 74 | 11 | 2 | 13 | ||||
| GAD-7 score | 6.75 (6.64) | 7.31 (4.64) | 7.53 (5.94) | -0.22 | 0.82 | 7.40 (5.22) | -0.99 | 0.32 |
| PHQ-9 score | 6.63 (5.24) | 7.81 (5.39) | 6.30 (4.94) | 0.78 | 0.44 | 6.74 (5.19) | -0.21 | 0.83 |
| PSQI score | 9.45 (2.55) | 9.83 (2.73) | 9.51 (2.54) | 0.64 | 0.52 | 9.35 (2.63) | 0.39 | 0.70 |
| HbA1c value | 7.36 (1.14) | 7.20 (1.31) | 7.39 (1.45) | -0.7 | 0.48 | 7.28 (1.36) | 0.69 | 0.49 |
The CBT group maintained better sleep quality compared with the UC group (F = 12.75, P < 0.001). The main effects for time were significant (F = 13.61, P < 0.001), indicating the time of treatment continuation to 12 mo. The interaction effect was significant (F = 11.97, P < 0.001), indicating that the effects for time were significantly different between the CBT and UC groups (Table 4).
| Variable | Baseline | 2 mo | 6 mo | 12 mo | Significance level | ||||||
| CBT | UC | CBT | UC | CBT | UC | CBT | UC | Condition | Time | Interaction | |
| PSQI score | 10.07 (2.38) | 10.04 (2.37) | 9.35 (2.79) | 9.99(2.41) | 9.42 (2.75) | 9.92 (2.54) | 9.01 (2.67) | 9.91 (2.34) | < 0.001 | < 0.001 | < 0.001 |
| HbA1c value | 7.44 (1.12) | 7.50 (1.13) | 7.46 (1.10) | 7.50 (1.15) | 7.27 (1.18) | 7.44 (1.20) | 7.22 (1.14) | 7.52 (1.12) | < 0.001 | 0.001 | < 0.001 |
The GLMMs showed that the effect of the intervention on PSQI scores remained significant at 2 mo (t = 3.93, P < 0.01), 6 mo (t = 3.03, P = 0.002), and 12 mo (t = 5.77, P < 0.01). The CBT group had a 0.64 lower PSQI score than the control group at 2 mo [95% confidence interval (CI): 0.32-0.96, P < 0.01], 0.50 lower PSQI score at 6 mo (95%CI: 0.18-0.82, P = 0.002), and 0.90 lower PSQI score at 12 mo (95%CI: 0.60-1.21, P < 0.01).
The CBT group maintained superior glycemic control compared with the CONTROL group (F = 16.60, P < 0.001). The interaction effect was significant (F = 7.02, P < 0.001), indicating that the effects for time were significantly different between the CBT and UC groups (Table 3).
The GLMMs showed that the effect of the intervention on HbAlc values was not significant at 2 mo (t = 0.03, P = 0.98). The effect of the intervention on HbAlc values was significant between the two groups at 6 mo (t = 2.31, P = 0.02) and at 12 mo (t = 6.11, P < 0.01). The CBT group had a 0.17 lower HbAlc value than the control group at 6 mo (95%CI: 0.02-0.31, P = 0.02), and 0.43 lower HbAlc value than at 12 mo (95%CI: 0.30-0.57, P < 0.01).
The GLMMs showed that the effect of the intervention on mean ΔHbAlc values was significant at 12 mo (t = 3.68, P < 0.01) and that mean ΔPSQI scores were closely related to ΔHbAlc values (t = 7.02, P < 0.01). For each increase of 1 score on ΔPSQI scores, the ΔHbAlc values increased 0.11.
All participants who completed the assessment were included to perform the intention-to-treat analyses, and the results agreed with the completer sample analysis (Table 5). The GLMMs showed that the effect of the intervention on mean ΔHbAlc values was significant at 12 mo (t = 2.61, P < 0.01) and that mean ΔPSQI scores were closely related to ΔHbAlc values (t = 7.41, P < 0.01). For each increase of 1 score on ΔPSQI scores, the ΔHbAlc values increased 0.10.
| Variable | Baseline | 2 mo | 6 mo | 12 mo | Significance level | ||||||
| CBT | Control | CBT | Control | CBT | Control | CBT | Control | Condition | Time | Interaction | |
| PSQI score | 10.07 (2.38) | 10.04 (2.37) | 9.36 (2.77) | 9.92 (2.44) | 9.45(2.75) | 9.95 (2.59) | 9.07 (2.69) | 9.94 (2.40) | < 0.001 | < 0.001 | < 0.001 |
| HbA1c value | 7.44 (1.12) | 7.50 (1.11) | 7.46 (1.10) | 7.49 (1.15) | 7.27 (1.18) | 7.44 (1.20) | 7.23 (1.17) | 7.61 (1.16) | < 0.001 | 0.028 | < 0.001 |
None of adverse events that were related to the study procedures or intervention occurred.
This community-based randomized controlled trial first evaluated the effect of group CBT on sleep quality in patients with T2DM in China. The findings indicated that group CBT intervention improved sleep quality. The mean sleep quality scores in the CBT group were significantly lower at 2 mo, 6 mo, and 12 mo post-intervention than in the UC group. The HbA1c values were reduced at 6 mo and 12 mo following the improving sleep quality, but not at 2 mo. Moreover, HbA1c levels were affected by changes in sleep quality, and were closely associated with changes in PSQI scores. However, the reduction of HbA1c values did not follow with the improvement of sleep quality at 2 mo.
Our results showed that CBT improved sleep quality, consistent with previous study findings[10,12,28]. CBT had not only a short-term (2 mo) but also a medium and long-term (6 and 12 mo) lasting in improving sleep quality in patients with T2DM. But the PSQI scores in our study decreased less than that in other studies[28,29]. Differences in diseases and intervention schedules may explain these contrasting findings.
Our findings suggest that poor sleep quality is independently associated with changes in HbA1c. Higher PSQI scores are associated with higher HbA1c values. Poor sleep quality may affect HbA1c by increasing insulin resistance and leading to isletβcell dysfunction[30,31]. Diabetes causes secondary physiological and pathological changes in various tissues and organs, leading to a decline in sleep quality and a significant decline in quality of life. Complications of diabetes may lead to sleep disorders such as restless leg syndrome, nocturia, and hypoglycemic awakening. In summary, poor sleep quality increases HbA1c values and HbA1c fluctuation. Improvement of sleep quality contributes to the improvement in HbA1c values. Carroll et al[32] have reported that improvements in sleep quality resulting from CBT can reduce HbA1c levels in older adults with insomnia. Another study also found that CBT can reduce HbA1c levels of adults with uncontrolled type 2 diabetes[14]. The results of the current study are consistent with these previous findings. However, we recruited patients with T2DM and poor sleep quality from communities, so our data are perhaps more representative of the real world. Our findings suggest that CBT for poor sleep quality is effective in improving long-term glycemic control. A possible mechanism is that CBT improves slow-wave sleep[33], which might be beneficial for daytime glucose control[34,35]. In addition, good sleep quality may help to reduce the intake of fat, carbohydrates, and free sugars[36], and a better lifestyle may further reduce β-cell damage and decrease glycemic levels[37]. Another possible reason for the maintenance of the effect is that general practitioners in our study were encouraged to use CBT to help patients at the next follow-up when all sessions were completed. At 6 mo and 12 mo post-intervention assessment, participants who received CBT had HbA1c values that were 0.17% and 0.43% lower than control group participants, indicating that better sleep quality can decrease blood sugar levels. Although there was a significant improvement in sleep quality after 2 mo, there was no change in HbA1c values, which may be due to the fact that HbA1c values reflected the mean value of blood glucose in the period of 90-120 d[38]. Previous studies have shown that an improvement in sleep quality is associated with reduced depression in people with T2DM[39]. We found that GAD-7 and PHQ-9 scores decreased after the CBT intervention. However, the relationships among HbA1c values, anxiety and depression, and sleep quality need to be studied further.
Most of the time, patients with diabetes are managed by the general practitioner in the community. The present results show that CBT can be delivered by general practitioners in the communities for poor sleep quality of patients with T2DM, which provides an example for managing sleep disturbances of patients with diabetes in communities. However, there are several reasons why there are difficulties in using CBT to treat sleep disturbances in China. First, many clinicians do not consider poor sleep as a health problem. Second, many patients would feel uncomfortable in terms of receiving a “psychological” intervention[40]. In addition, the numbers of psychiatrists are limited. Community-based health services in China cannot provide sophisticated, individual-based psychological services to individuals owing to the lack of psychiatric health care providers[41]. Therefore, there is an urgent need for CBT training for general practitioners at community-based health services to further help manage sleep problems in T2DM patients in China.
This is the first study to assess the effect of CBT on sleep quality in patients with T2DM in China using a community-based, cluster randomized controlled design. However, some limitations should be addressed. First, we used self-report questionnaires to assess sleep quality, which can lead to response bias. Second, the trained general medical practitioners who delivered the CBT intervention used their additional professional skills in daily follow-ups, which might have improved glycemic control in the CBT intervention group. Third, participants were drawn from a limited area, so caution is needed before generalizing from these results.
In summary, general practitioners can learn and use CBT in communities. CBT reduces glycemic level with the improvement of sleep quality, which suggests that better sleep quality may be associated with better glycemic control in patients with T2DM. The rate of poor sleep quality is as high as 33.6% in T2DM patients and only 49.2% of T2DM patients treated in China have adequate glycemic control[3,27]. Therefore, compre-hensive strategies are needed to improve sleep quality and reduce glycemic levels in diabetic patients in China.
Poor sleep quality is a common clinical feature in patients with type 2 diabetes mellitus (T2DM), and often negatively related with glycemic control. Cognitive behavioral therapy (CBT) may improve sleep quality and reduces blood sugar levels in patients with T2DM.
It is not entirely clear whether CBT delivered by general practitioners is effective for poor sleep quality in T2DM patients in community settings.
To test the effect of CBT delivered by general practitioners in improving sleep quality and reducing glycemic levels in patients with T2DM in community.
Repeated measures analysis of variance and generalized linear mixed effects models were used to estimate the intervention effects on hemoglobin A1c and sleep quality in this cluster randomized controlled trial.
CBT could improve subjective sleep disturbance of patients with T2DM at 2 mo, 6 mo, and 12 mo in the community-based randomized controlled trial. CBT could reduce hemoglobin A1c values at 6 mo and 12 mo following improved subjective sleep disturbance of patients with T2DM in the community-based randomized controlled trial.
CBT should be included in the comprehensive management of diabetes and applied in community by general practitioners.
There is an urgent need for CBT training for general practitioners at community-based health services to further help manage sleep problems in type 2 diabetes patients in China.
The authors thank all the participants involved in the intervention. The help of Centers for Disease Control and Prevention and clinics in Xuzhou City in the field intervention and data collection is very much appreciated.
| 1. | Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1798] [Article Influence: 199.8] [Reference Citation Analysis (1)] |
| 2. | in China: mapping the road ahead. Lancet Diabetes Endocrinol. 2014;2:923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317:2515-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1399] [Article Influence: 155.4] [Reference Citation Analysis (0)] |
| 4. | Sridhar GR, Madhu K. Prevalence of sleep disturbances in diabetes mellitus. Diabetes Res Clin Pract. 1994;23:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 5. | Sakamoto R, Yamakawa T, Takahashi K, Suzuki J, Shinoda MM, Sakamaki K, Danno H, Tsuchiya H, Waseda M, Takano T, Minagawa F, Takai M, Masutani T, Nagakura J, Shigematsu E, Ishikawa M, Nakajima S, Kadonosono K, Terauchi Y. Association of usual sleep quality and glycemic control in type 2 diabetes in Japanese: A cross sectional study. Sleep and Food Registry in Kanagawa (SOREKA). PLoS One. 2018;13:e0191771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34:1171-1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Trento M, Broglio F, Riganti F, Basile M, Borgo E, Kucich C, Passera P, Tibaldi P, Tomelini M, Cavallo F, Ghigo E, Porta M. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008;45:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Tan X, van Egmond L, Chapman CD, Cedernaes J, Benedict C. Aiding sleep in type 2 diabetes: therapeutic considerations. Lancet Diabetes Endocrinol. 2018;6:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Gatchel RJ, Rollings KH. Evidence-Based Management of Low Back Pain. 2012; chapter 21: 286. [DOI] [Full Text] |
| 10. | Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163:191-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 687] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 11. | Sadler P, McLaren S, Klein B, Harvey J, Jenkins M. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Ritterband LM, Thorndike FP, Ingersoll KS, Lord HR, Gonder-Frederick L, Frederick C, Quigg MS, Cohn WF, Morin CM. Effect of a Web-Based Cognitive Behavior Therapy for Insomnia Intervention With 1-Year Follow-up: A Randomized Clinical Trial. JAMA Psychiatry. 2017;74:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 225] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 13. | Christensen H, Batterham PJ, Gosling JA, Ritterband LM, Griffiths KM, Thorndike FP, Glozier N, O'Dea B, Hickie IB, Mackinnon AJ. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 14. | Whitehead LC, Crowe MT, Carter JD, Maskill VR, Carlyle D, Bugge C, Frampton CMA. A nurse-led education and cognitive behaviour therapy-based intervention among adults with uncontrolled type 2 diabetes: A randomised controlled trial. J Eval Clin Pract. 2017;23:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Moher D, Schulz KF, Altman DG. The CONSORT statement: Revised commendations for improving the quality of reports of parallel-group randomized trials. Lancet. 2001;357:1191-4. [PubMed] |
| 16. | Perfect MM, Elkins GR. Cognitive-behavioral therapy and hypnotic relaxation to treat sleep problems in an adolescent with diabetes. J Clin Psychol. 2010;66:1205-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Abbasi S Ms, Alimohammadi N PhD, Pahlavanzadeh S Ms. Effectiveness of Cognitive Behavioral Therapy on the Quality of Sleep in Women with Multiple Sclerosis: A Randomized Controlled Trial Study. Int J Community Based Nurs Midwifery. 2016;4:320-328. [PubMed] |
| 18. | Sousa A, Kalra S. Sleep hygiene and diabetes: Suggestions for primary care. J Pak Med Assoc. 2017;67:814-815. [PubMed] |
| 19. | Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17520] [Cited by in RCA: 23452] [Article Influence: 633.8] [Reference Citation Analysis (0)] |
| 20. | Liu XC, Tang MQ, Hu L, Wang AZ, Wu HX, Zhao GF, Gao CN, Li WS. Reliability and validity of the Pittsburgh sleep quality index. Zhonghua Jingshenke Zazhi. 1996;29:103-7. [DOI] [Full Text] |
| 21. | World Health Organization. AUDIT: the alcohol use disorders identification test, guidelines for use in primary care. 2nd ed. Geneva: World Health Organization 2001. Available from: https://www.who.int/publications/i/item/audit-the-alcohol-use-disorders-identification-test-guidelines-for-use-in-primary-health-care. |
| 22. | Zhang C, Yang GP, Li Z, Li XN, Li Y, Hu J, Zhang FY, Zhang XJ. [Reliability and validity of the Chinese version on Alcohol Use Disorders Identification Test]. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38:1064-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108:768-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 630] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 24. | Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21545] [Cited by in RCA: 31136] [Article Influence: 1245.4] [Reference Citation Analysis (0)] |
| 25. | Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11947] [Cited by in RCA: 20648] [Article Influence: 1032.4] [Reference Citation Analysis (0)] |
| 26. | Reutens AT, Hutchinson R, Van Binh T, Cockram C, Deerochanawong C, Ho LT, Ji L, Khalid BA, Kong AP, Lim-Abrahan MA, Tan CE, Tjokroprawiro A, Yoon KH, Zimmet PZ, Shaw JE. The GIANT study, a cluster-randomised controlled trial of efficacy of education of doctors about type 2 diabetes mellitus management guidelines in primary care practice. Diabetes Res Clin Pract. 2012;98:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Lou P, Qin Y, Zhang P, Chen P, Zhang L, Chang G, Li T, Qiao C, Zhang N. Association of sleep quality and quality of life in type 2 diabetes mellitus: a cross-sectional study in China. Diabetes Res Clin Pract. 2015;107:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | McCurry SM, Guthrie KA, Morin CM, Woods NF, Landis CA, Ensrud KE, Larson JC, Joffe H, Cohen LS, Hunt JR, Newton KM, Otte JL, Reed SD, Sternfeld B, Tinker LF, LaCroix AZ. Telephone-Based Cognitive Behavioral Therapy for Insomnia in Perimenopausal and Postmenopausal Women With Vasomotor Symptoms: A MsFLASH Randomized Clinical Trial. JAMA Intern Med. 2016;176:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Nguyen S, Wong D, McKay A, Rajaratnam SMW, Spitz G, Williams G, Mansfield D, Ponsford JL. Cognitive behavioural therapy for post-stroke fatigue and sleep disturbance: a pilot randomised controlled trial with blind assessment. Neuropsychol Rehabil. 2019;29:723-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 30. | Byberg S, Hansen AL, Christensen DL, Vistisen D, Aadahl M, Linneberg A, Witte DR. Sleep duration and sleep quality are associated differently with alterations of glucose homeostasis. Diabet Med. 2012;29:e354-e360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Arora T, Chen MZ, Omar OM, Cooper AR, Andrews RC, Taheri S. An investigation of the associations among sleep duration and quality, body mass index and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. Ther Adv Endocrinol Metab. 2016;7:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Carroll JE, Seeman TE, Olmstead R, Melendez G, Sadakane R, Bootzin R, Nicassio P, Irwin MR. Improved sleep quality in older adults with insomnia reduces biomarkers of disease risk: pilot results from a randomized controlled comparative efficacy trial. Psychoneuroendocrinology. 2015;55:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Krystal AD, Edinger JD. Sleep EEG predictors and correlates of the response to cognitive behavioral therapy for insomnia. Sleep. 2010;33:669-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Armitage R, Lee J, Bertram H, Hoffmann R. A preliminary study of slow-wave EEG activity and insulin sensitivity in adolescents. Sleep Med. 2013;14:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci. 2008;105:1044-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 657] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 36. | Al Khatib HK, Hall WL, Creedon A, Ooi E, Masri T, McGowan L, Harding SV, Darzi J, Pot GK. Sleep extension is a feasible lifestyle intervention in free-living adults who are habitually short sleepers: a potential strategy for decreasing intake of free sugars? Am J Clin Nutr. 2018;107:43-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 37. | Kolb H, Martin S. Environmental/Lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 458] [Article Influence: 50.9] [Reference Citation Analysis (1)] |
| 38. | Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM. Tests of glycemia in diabetes. Diabetes Care. 1995;18:896-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Uchendu C, Blake H. Effectiveness of cognitive-behavioural therapy on glycaemic control and psychological outcomes in adults with diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabet Med. 2017;34:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Kathol RG, Arnedt JT. Cognitive Behavioral Therapy for Chronic Insomnia: Confronting the Challenges to Implementation. Ann Intern Med. 2016;165:149-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Zeng Q, He Y, Shi Z, Liu W, Tao H, Bu S, Miao D, Liu P, Zhang X, Li X, Qi X, Zhou Q. A community-based controlled trial of a comprehensive psychological intervention for community residents with diabetes or hypertension. Shanghai Arch Psychiatry. 2016;28:72-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Health care sciences and services
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wan TT, Zhou M S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Ma YJ