©The Author(s) 2025.
World J Diabetes. Oct 15, 2025; 16(10): 110041
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.110041
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.110041
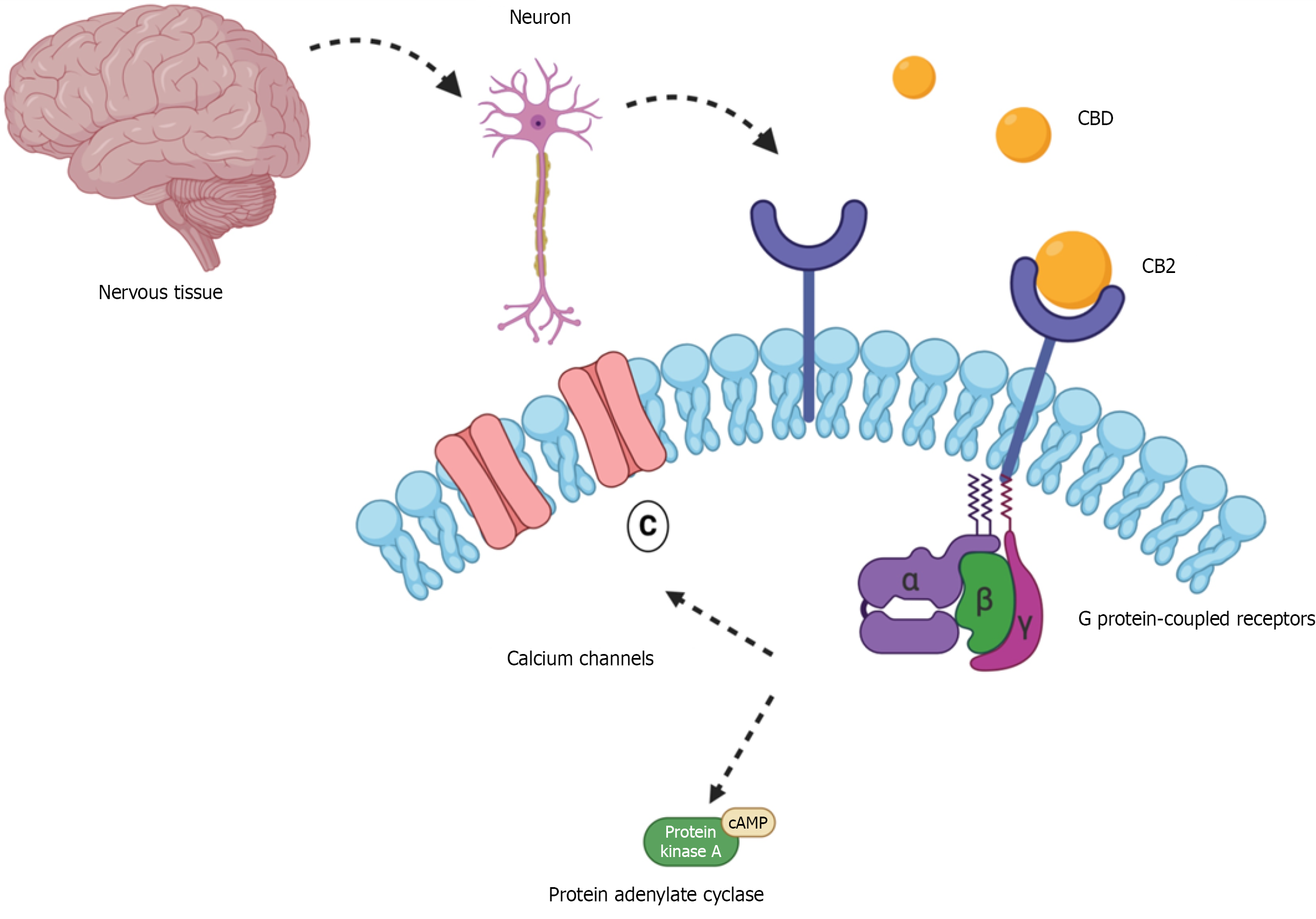
Figure 1 Cannabidiol activity on the cannabinoid type 1 receptor in nervous tissue.
This figure illustrates how cannabidiol (CBD) interacts with cannabinoid type 1 receptor (CB1) located on neuronal membranes in the nervous system – an interaction relevant to its neuroprotective potential in type 1 diabetes. When CBD binds to the cannabinoid type 1 receptor, it activates associated G proteins that inhibit calcium ion channels, and the enzyme adenylate cyclase. As a result, cyclic adenosine monophosphate (cAMP) levels and protein kinase A activity are reduced, ultimately suppressing neurotransmitter release. Beyond this, CBD may also modulate key inflammatory mediators such as nuclear factor kappa B, interleukin 6, and tumor necrosis factor alpha, all of which are linked to neuroinflammation in type 1 diabetes. These combined effects suggest that CBD may help reduce oxidative stress and protect neuronal integrity.
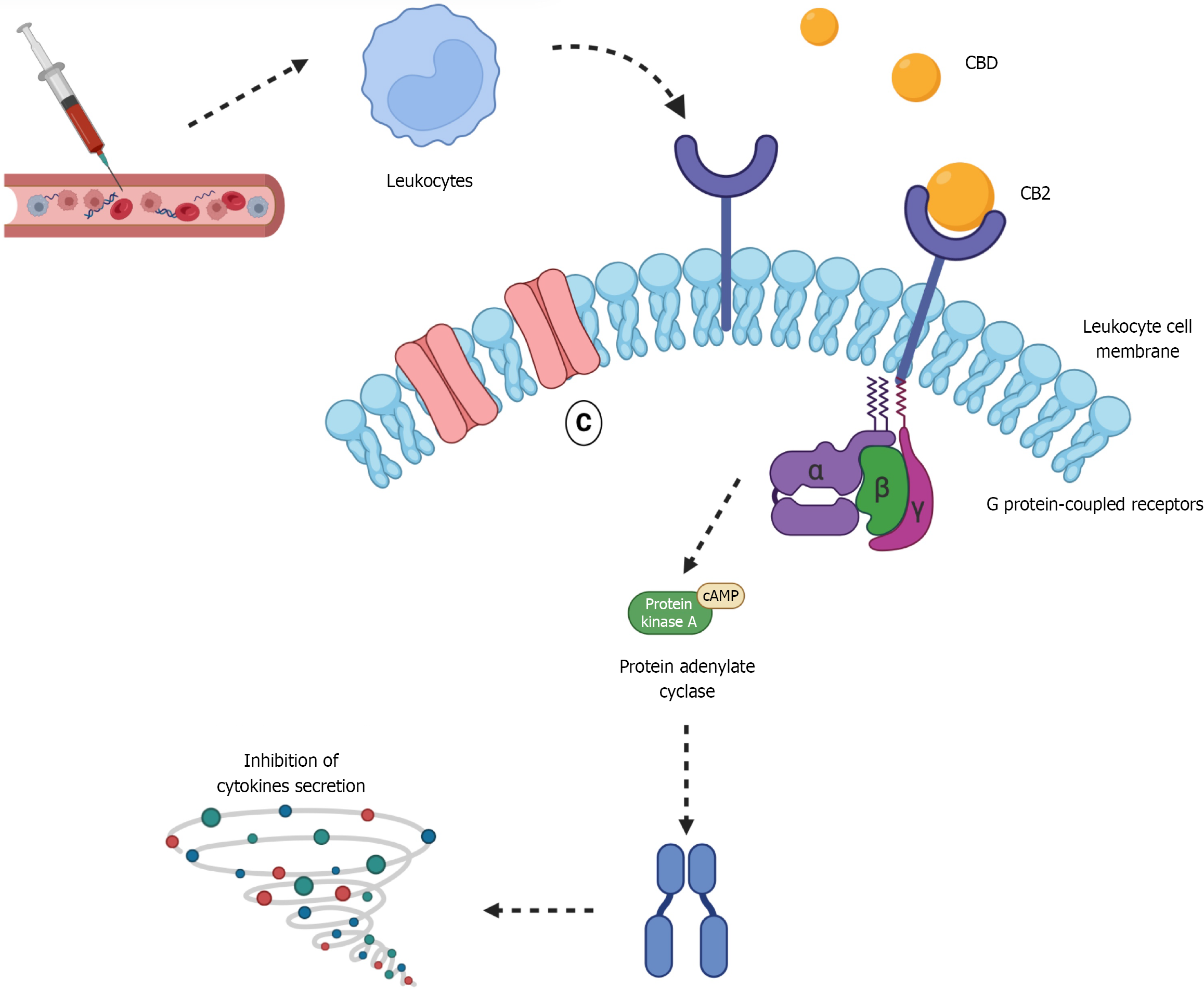
Figure 2 Cannabidiol activity on the cannabinoid receptor type 2 in leukocytes this figure illustrates the mechanism by which cannabidiol interacts with cannabinoid receptor type 2 Located on leukocyte membranes, emphasizing its role in modulating inflammation in type 1 diabetes mellitus.
Upon binding to cannabinoid receptor type 2 (CB2), cannabinoid (CBD) activates G protein-coupled signaling pathways that inhibit adenylate cyclase, without impacting calcium-dependent ion channels. This inhibition lowers intracellular cyclic adenosine monophosphate (cAMP) levels and reduces protein kinase A activity, ultimately leading to decreased production of proinflammatory cytokines (represented by the spiral icon), such as tumor necrosis factor alpha, interleukin 6 (IL-6), and IL-1β. Additionally, CBD may influence other inflammatory mediators, including nuclear factor kappa B and chemokines such as C-C motif chemokine ligand 2 and C-X-C motif chemokine ligand 10, suggesting a broader anti-inflammatory effect. Through these mechanisms, CBD contributes to the attenuation of pancreatic inflammation and the preservation of β-cell function in type 1 diabetes mellitus.
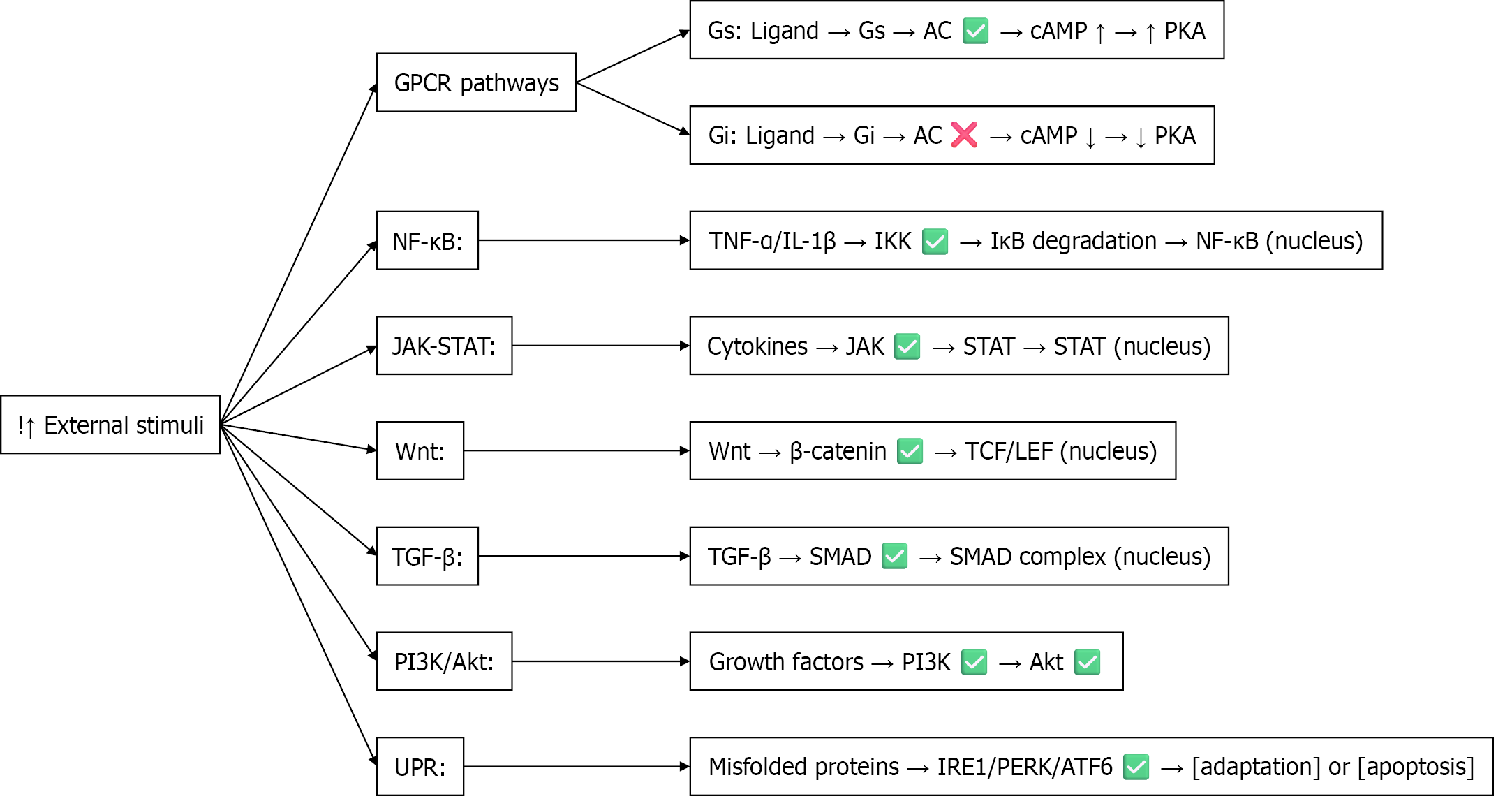
Figure 3 Overview of calcium-independent signaling pathways activated by external stimuli this diagram summarizes key calcium-independent signaling pathways triggered by external stimuli, including G protein-coupled receptors, nuclear factor kappa B, Janus kinase/signal transducer and activator of transcription, wingless/integrated, transforming growth factor beta, phosphoinositide 3-kinase/protein kinase B, and the unfolded protein response.
Each pathway involves specific signaling molecules and culminates in transcriptional regulation, cellular adaptation, or apoptosis. Akt: Protein kinase B; ATF6: Activating transcription factor 6; cAMP: Cyclic adenosine monophosphate; Gi: Inhibitory G proteins; GPCR: G protein-coupled receptor; Gs: Stimulatory G proteins; IκB: Inhibitor of nuclear factor kappa B; IL: Interleukin; IRE1: Inositol-requiring enzyme 1; JAK: Janus kinase; LEF: Lymphoid enhancer-binding factor; NF-κB: Nuclear factor kappa B; PERK: Protein kinase RNA-like endoplasmic reticulum kinase; PI3K: Phosphoinositide 3-kinase; PKA: Protein kinase A; SMAD: Small mothers against decapentaplegic homolog; STAT: Signal transducer and activator of transcription; TCF: T-cell factor; TGF-β: Transforming growth factor beta; TNF: Tumor necrosis factor; Wnt: Wingless/integrated; UPR: Unfolded protein response.
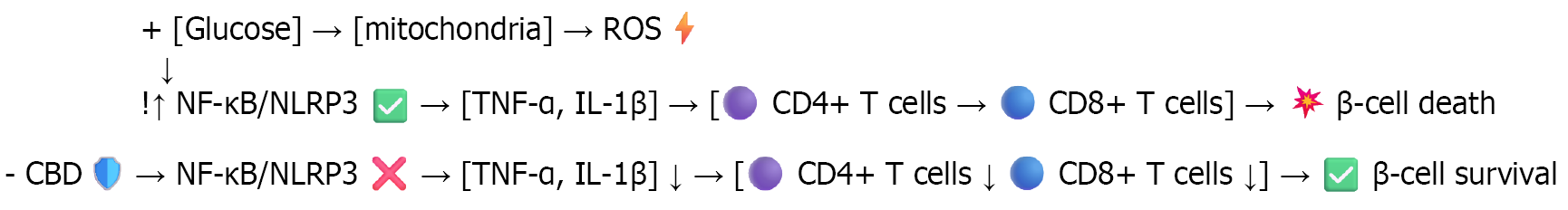
Figure 4 High glucose levels increase mitochondrial activity, leading to the production of reactive oxygen species, which activates the nuclear factor kappa B/nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing protein 3 inflam
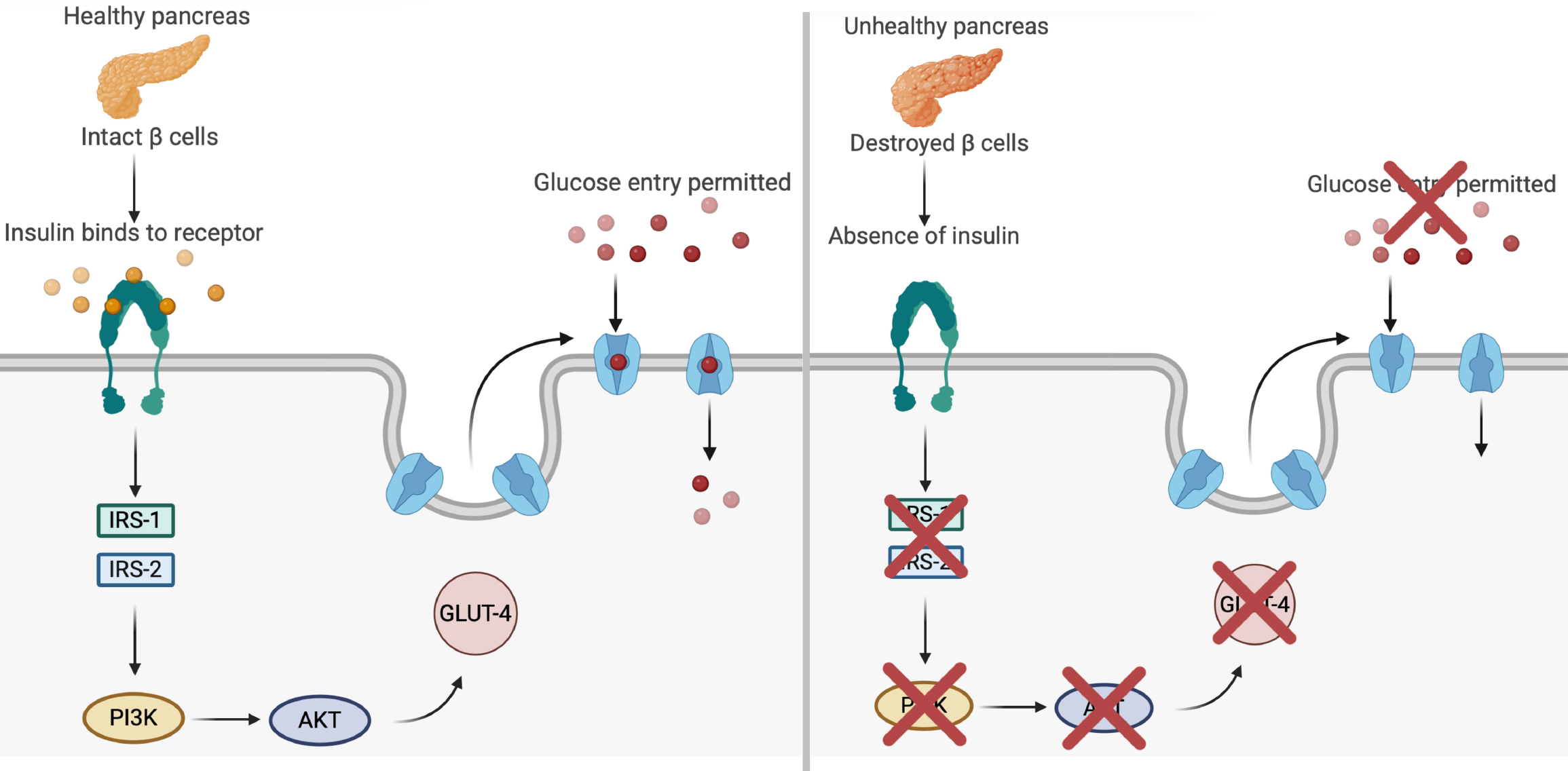
Figure 5 Insulin signaling cascade and glucose transporter type 4 translocation in type 1 diabetes.
This figure illustrates the effects of healthy vs impaired pancreatic function on glucose metabolism in type 1 diabetes. In the healthy pancreas (panel A, left), functional β cells secrete insulin, which binds to its receptor on the cell membrane and activates a signaling cascade via insulin receptor substrate 1 (IRS-1) and IRS-2. This triggers the phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB) pathway, promoting the translocation of glucose transporter type 4 (GLUT4) to the membrane and facilitating glucose entry into muscle cells. By contrast, panel B (right) depicts the absence of insulin secretion due to β-cell destruction, which disrupts activation of the IRS-1/IRS-2 and PI3K/PKB pathways. As a result, GLUT4 translocation is impaired, preventing glucose uptake and leading to hyperglycemia. This dysfunction is closely associated with inflammatory and oxidative stress in type 1 diabetes, reinforcing the importance of therapeutic strategies that preserve β-cell function or modulate inflammation.
- Citation: Ramos Fernandes VA, Mendes LR, Franco Netto ROR, Belozo FL, Bezerra AA, dos Santos CPC, Cruel PTE, Buchaim DV, Buchaim RL, da Cunha MR. Anti-inflammatory effects of cannabidiol in the treatment of type 1 diabetes: A mini review. World J Diabetes 2025; 16(10): 110041
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/110041.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.110041