Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.109815
Revised: July 18, 2025
Accepted: September 5, 2025
Published online: October 15, 2025
Processing time: 139 Days and 7.5 Hours
Glucotoxic pancreatic β cells impair glycogenesis of hepatocytes, with exosomes serving as novel mediators. miR-375-3p is the most abundant miRNA in the pancreas and critical for β-cell function, but whether it plays a role in pancreas-liver crosstalk remains unclear.
To investigate the role of miR-375-3p, a key regulator of pancreatic β cells, in remotely regulating hepatocyte glycogenesis via exosomes.
Mice fed a high-fat diet (HFD) served as animal models, and mouse primary pancreatic islet cells and the β-cell line MIN-6 were used as cellular models. miR-375-3p expression in pancreatic cells, hepatocytes and exosomes was detected in both animal and cellular models. Transwell assays, exosome treatment, and exosome-depleted supernatant culture were used to investigate the role of exosomal miR-375-3p in pancreatic-hepatocyte crosstalk. The AKT/GSK signaling pathway and hepatic glycogen content were used as indicators to evaluate hepatocyte glycogenesis. Luciferase reporter assays were used to evaluate the downstream targets of miR-375-3p.
Increased levels of miR-375-3p were observed in both the pancreas and liver of HFD-fed mice. In contrast to the in vivo results, high-glucose treatment exclusively increased the expression of miR-375-3p in pancreatic cells but had no effect on hepatocytes. Furthermore, hepatocytes treated with the supernatant and exo
Pancreatic cell-derived miR-375-3p can be delivered to hepatocytes via exosomes and inhibits hepatocyte gly
Core Tip: Glucotoxic pancreatic β cells impair glycogenesis of hepatocytes, with exosomes serving as novel mediators. Glucotoxicity increased the expression of miR-375-3p in pancreatic cells but had no effect on hepatocytes. Exosomal transfer of miR-375-3p from pancreatic β cells to hepatocytes enables it to directly target and inhibit the recombination signal binding protein for the immunoglobulin kappa J region (Rbpj). This suppression of Rbpj subsequently impairs the AKT/GSK-3 signaling pathway, thereby reducing hepatic glycogen content.
- Citation: Xu FZ, Dou L, Wu X, Xia CX, Yu DN, Man Y, Shen T, Huang XQ. Exosomal transfer of miR-375-3p from pancreatic β cells to hepatocytes impairs hepatic glycogenesis via Rbpj repression. World J Diabetes 2025; 16(10): 109815
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/109815.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.109815
The interaction between pancreatic β cells and hepatocytes plays a central role in regulating systemic glucose homeostasis[1,2]. Pancreatic β cells secrete insulin, which plays a key role in regulating blood glucose levels by acting on target cells[2]. The liver, a significant site for venous return, receives a rich supply of insulin through the portal vein. As one of the primary target organs of insulin, the process of glycogen synthesis in the liver under insulin stimulation is a critical determinant of systemic glucose homeostasis. The liver activates AKT/GSK signaling and promotes glycogenesis upon insulin stimulation, which plays an important role in reducing systemic glucose levels[3].
An in-depth study of the endocrine function of pancreatic β cells found that, in addition to secreting traditional hormones, pancreatic β cells can secrete novel molecular endocrine hormones, such as exosomes, to participate in the regulation of insulin signaling in the liver[4]. Exosomes are extracellular vesicles with a diameter of 30-150 nm that facilitate cell-organ communication by encapsulating and delivering a variety of biomolecules through a bilayer membrane structure[5]. In recent years, exosomal microRNAs have gained widespread recognition as a novel class of communication molecules. These microRNAs are selectively secreted in response to specific stimuli and participate in specific physiological or pathological processes in vivo by regulating the expression levels of related genes[6,7]. In the context of type 2 diabetes mellitus (T2DM), pancreatic β cells can increase the delivery of deleterious exosomal miRNAs[8-11] or decrease the delivery of protective exosomal miRNAs[12-15] to peripheral tissues, affecting the disease process[16]. However, the crosstalk between impaired pancreatic β cells and hepatocytes remains largely unexplored.
miR-375-3p is a small RNA that is highly expressed in pancreatic cells and plays crucial roles in the development and differentiation of β cells, their own proliferation, and regulation of insulin secretion[17]. Recent studies suggest that miR-375-3p has potential as a circulating biomarker for β-cell injury. In pancreatic β cells, expression of miR-375-3p is upregulated in response to high glucose stimulation and is subsequently released into the bloodstream[18]. In addition, elevated levels of miR-375-3p have been observed in animal models of T2DM as well as in patients with newly diagnosed T2DM, with a positive correlation to islet damage[19-22]. However, the role of miR-375-3p derived from glucotoxic pancreatic cells in systemic glucose homeostasis has been poorly investigated.
Previous studies have largely focused on the role of pancreatic β cells in reducing insulin secretion under glucotoxic conditions, thereby influencing hepatic gluconeogenesis. Here, our findings demonstrate that, in addition to decreased insulin level, glucotoxic pancreatic β cells can secrete miR-375-3p encapsulated within exosomes. These exosomes are subsequently transferred to hepatocytes, where they target the insulin signaling pathway by inhibiting recombination signal binding protein for the immunoglobulin kappa J region (Rbpj), thus modulating AKT/GSK-mediated hepatocyte glycogenesis. These results reveal new functions of miR-375-3p and provide fresh insights into the prevention and treatment of T2DM from the perspective of interactions between the pancreas and liver. The abbreviations are in Supplementary Table 1.
Eight-week-old male C57BL/6 mice were obtained from Sibefu Biotechnology Co. (Beijing, China). Twenty mice were equally divided into 2 groups and were fed ad libitum for 18 weeks with a standard chow diet (CD, H10010) or a high-fat diet (HFD, H10045) obtained from Beijing Huafukang Biotechnology Co. All the mice were housed under a 12:12 Light-dark cycle with lights on at 07:00 under a constant temperature of 25 °C. All mouse procedures were approved by the Laboratory Animal Welfare Ethics Branch of the Biomedical Ethics Committee of Peking University (No. LA2022012).
Preliminary pancreatic secretory cell clusters were obtained using in vivo perfusion, in vitro digestion and centrifugation. Pancreatic endocrine cells were then enriched by density gradient centrifugation using polysucrose solution, after which mouse pancreatic islet cell clusters were obtained by artificial cell sorting[23]. The cells were cultured in RPMI 1640 medium (Gibco, 11875135) supplemented with 7 mmol/L D-glucose (Solarbio Life Science, G8150), 10% FBS (Gibco, A5670701), 100 units/mL penicillin (Sigma, P4458), and 0.1 mg/mL streptomycin (Sigma, P4458). Morphological identification of pancreatic islet primary cells was accomplished by white light photography of the sorted islet cell clusters.
The mouse pancreatic β-cell line MIN-6 and hepatic cell line Hep1-6 were purchased from the American Type Culture Collection (Manassas, VA, United States). The cells were cultured in low-glucose Dulbecco's modified Eagle's medium (DMEM) (Gibco, Carlsbad, CA, United States) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Sigma, St. Louis, MO, United States) at 37 °C with humidified air and 5% CO2. A high glucose medium at a concentration of 33.5 mmol/L was prepared by adding 28 mmol/L D-glucose (Solarbio Life Science) to low-glucose DMEM to stimulate cell lines. After the cells were cultured in low-glucose medium for 1 day, they were stimulated with low-glucose medium and high-glucose medium for 24 hours. For the exosome secretion inhibition experiment, MIN-6 cells were pretreated with the exosome inhibitor GW4869 (MedChemExpress) at 2.5 μM, 5 μM, and 10 μM for 24 hours, followed by stimulation with 33.5 mmol/L glucose for a further 24 hours.
To harvest the exosomes, the medium was replaced with fresh low-glucose DMEM, and cells were cultured for 72 hours. Then, the cell culture supernatants were centrifuged at 1000 × g for 20 minutes at 4 °C to remove cell debris and dead cells, centrifuged at 10000 × g for 30 minutes at 4 °C to eliminate large vesicles and finally centrifuged at 100000 × g for 4 hours at 4 °C to obtain exosome deposition. After washing with PBS (100000 × g, 2 hours, 4 °C), the exosome-containing pellet was resuspended in PBS. To obtain tissue-derived exosomes, fresh pancreatic tissue was sheared, digested, and filtered to obtain pancreatic tissue exosome pellets by differential centrifugation[24].
The size of the exosomes was analyzed via NTA (NanoSight, NS300), and the physical characterization of the exosomes was viewed by transmission electron microscopy (JEOL, JEM1400). The expression of exosome surface-specific marker proteins FLOT1 (ab41927, Abcam), CD63 (67605-1-Ig, Proteintech), and CD9 (60232-1-Ig, Proteintech) was detected by western blot. For exosome tracing, the exosomes were labeled with a PKH26 fluorescent tracer (MINI26-1KT, Sigma-Aldrich).
MIN-6 cells were seeded into Transwell chambers (3412, Corning) and cultured for 1-2 days. After being treated with low- or high-glucose medium for 1 day, cells were washed with PBS twice. The cells were subsequently cultured with Hep1-6 cells placed in the lower chamber at a 1:1 ratio in low-glucose medium for 48 hours. Finally, Hep1-6 cells were harvested for downstream analysis.
The recombinant plasmid pcDNA-Rbpj (Beijing Tsingke Biotech) was constructed by inserting mouse Rbpj cDNA into the pcDNA vector. The sequences of Rbpj 3'-UTR predicted to interact with miR-375-3p were cloned and inserted into the pmirGLO vector to construct a wild-type recombinant plasmid (pmirGLO-Rbpj-WT), and the binding site was mutated to construct a mutant recombinant plasmid (pmirGLO-Rbpj-MT).
The mimics and inhibitors of miR-375-3p, Rbpj siRNA and negative control were synthesized by GenePharma. Mimics and inhibitors were transfected into Hep1-6 cells for 48 hours using Lipofectamine RNAiMAX transfection reagent (13778150, Invitrogen), while recombinant plasmids were transfected using VigoFect High Performance Eukaryotic Transfection Reagent (T001, Beijing Jiangchen Wenxuan Biotechnology) for 48 hours.
Total RNA was extracted using TRIzol (15596026, Invitrogen) reagent. Then, the RNA was reverse transcribed to cDNA using a two-step reversal reagent (RR0307A, Takara) and finally analyzed by quantitative polymerase chain reaction (qPCR) (RR820A, Takara) using an IQ5 system (Bio-Rad). The primers used for reverse transcription were as follows: miR-375-3p: 5'-GTCGTATCCAGTGCCAGGGTCGAGGTATTCGCAC TGGATACGACTCAGC-3' and U6: 5'-GTCGTA
Total protein was extracted from mouse liver tissue and Hep1-6 cells using RIPA buffer. Protein samples were separated using SDS-PAGE gel and transferred to PVDF membranes (Millipore). After being blocked with 5% milk for 2 hours at room temperature, the protein blots were incubated with primary antibody overnight at 4 °C, followed by incubation with HRP-conjugated anti-IgG (115-035-003, 111-035-003, Jackson ImmunoResearch) for 2 hours at room temperature. Finally, the bands were detected using an enhanced chemiluminescence western blot detection kit (F010, Solarbio Life Science). Primary antibodies against phospho-AKT (Ser473, 9271, 1:1000), AKT (11E7, 4685, 1:1000), phospho-GSK-3β (Ser9, 9323, 1:1000) and GSK-3β (D5C5Z, 12456, 1:1000) were purchased from Cell Signaling Technology. Primary antibodies against Rbpj (30044-1-AP, 1:1000), β-Actin (66009-1-Ig, 1:2000) and β-Tubulin (10094-1-AP, 1:2000) were purchased from Proteintech.
The treated cells were incubated in the presence of insulin (0.25 IU/mL) for 3 hours before collection. The glycogen content in liver tissues and cells was measured using a glycogen assay kit (A043-1-1; Nanjing Jiancheng Bioengineering) according to the manufacturer’s instructions.
The mouse pancreas specimens were embedded in paraffin and serially sectioned at 6-μm intervals. After dewaxing to water, antigen repair, and blocking, the sections were incubated with a primary insulin antibody (GB15334-100, Servicebio, 1:200) and then incubated with the corresponding secondary antibody. After counterstaining with DAPI (C1002, Beyotime), the sections were sealed with an anti-fluorescence quenching agent (S2100, Solarbio Life Science).
The mouse pancreas samples were paraffin-embedded and sectioned. MIN-6 cells were seeded on glass cover slips for 24 hours and stimulated with low- or high-glucose medium for 24 hours. After fixation and permeabilization, tissue or cells were subjected to TUNEL detection using the In Situ Cell Death Detection Kit and fluorescein (Roche, 11684795910) according to the instructions.
The wild-type recombinant plasmid (pmirGLO-Rbpj-WT) or the mutant recombinant plasmid (pmirGLO-Rbpj-MT) was cotransfected with the miR-375-3p mimics or negative control into Hep1-6 cells using VigoFect for 48 hours. The binding of Rbpj to miR-375-3p was detected using the Dual-Luciferase Assay Kit (E1910, Promega) according to the manufacturer’s instructions.
The data are expressed as the mean ± SE. The two-tailed unpaired Student's t-test or analysis of variance was used to analyze the data, and a value of P < 0.05 was considered statistically significant.
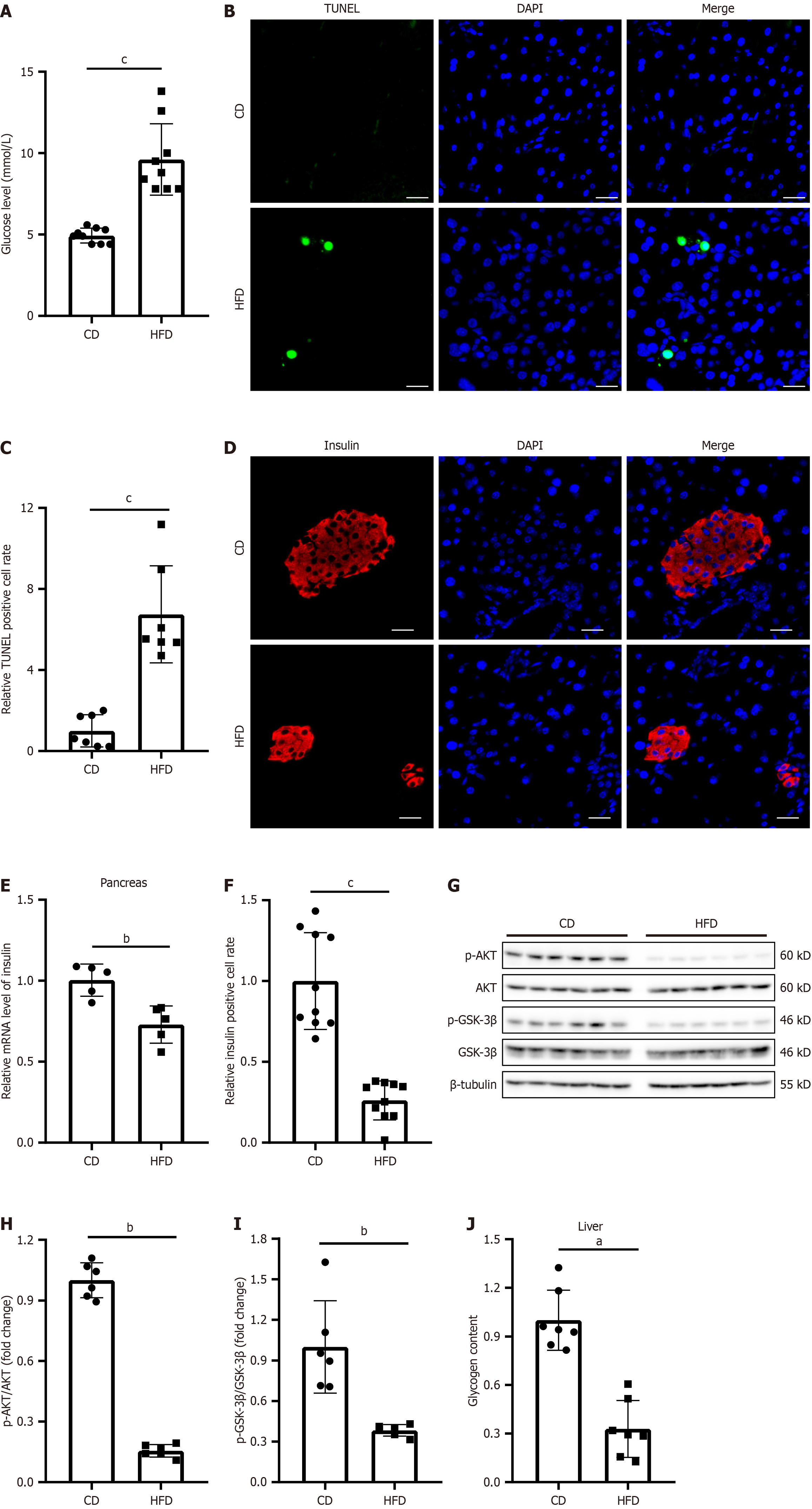
HFD is a commonly used diet in mouse models of T2DM. In line with results of previous studies, compared with CD-fed mice, HFD-fed mice exhibited markedly elevated fasting blood glucose levels (Figure 1A) and signs of pancreatic tissue damage, including increased apoptosis (Figure 1B and C) and reduced insulin levels (Figure 1D-F). In addition to pancreatic injury, the livers of HFD-fed mice also exhibited obvious characteristics of impaired glycogenesis, including suppression of the AKT/GSK signaling pathway (Figure 1G-I) and decreased liver glycogen content (Figure 1J).
First, we assessed whether glucotoxic pancreatic cells influenced glycogenesis of hepatocytes through the microenvironment. The MIN-6 mouse pancreatic β-cell line treated with high glucose (33.5 mmol/L) presented a decrease in cell viability (Figure 2A), elevated apoptosis (Figure 2B and C), and reduced insulin mRNA levels (Figure 2D), indicative of substantial glucose-induced cell damage. Subsequently, conditioned media from glucotoxic MIN-6 cells and control cells were collected and used to treat Hep1-6 cells. The results demonstrated that the medium from glucotoxic MIN-6 cells led to decreased AKT and GSK-3β phosphorylation levels in Hep1-6 cells (Figure 2E-G) and a reduction in glycogen content (Figure 2H). Similar results were obtained when Hep1-6 cells were treated with media from primary islet cells (IPCs) derived from HFD-fed or CD-fed mice (Figure 2I-L).
To better replicate the cellular microenvironment, we cocultured MIN-6 cells and hepatocytes in a Transwell plate. Our data revealed that AKT/GSK signaling was suppressed in hepatocytes cocultured with MIN-6 cells treated with high glucose (Figure 2M-O), and the glycogen content was also decreased (Figure 2P). Collectively, these results indicate that glucotoxic pancreatic cells can modulate the glycogenesis of hepatocytes via the microenvironment.
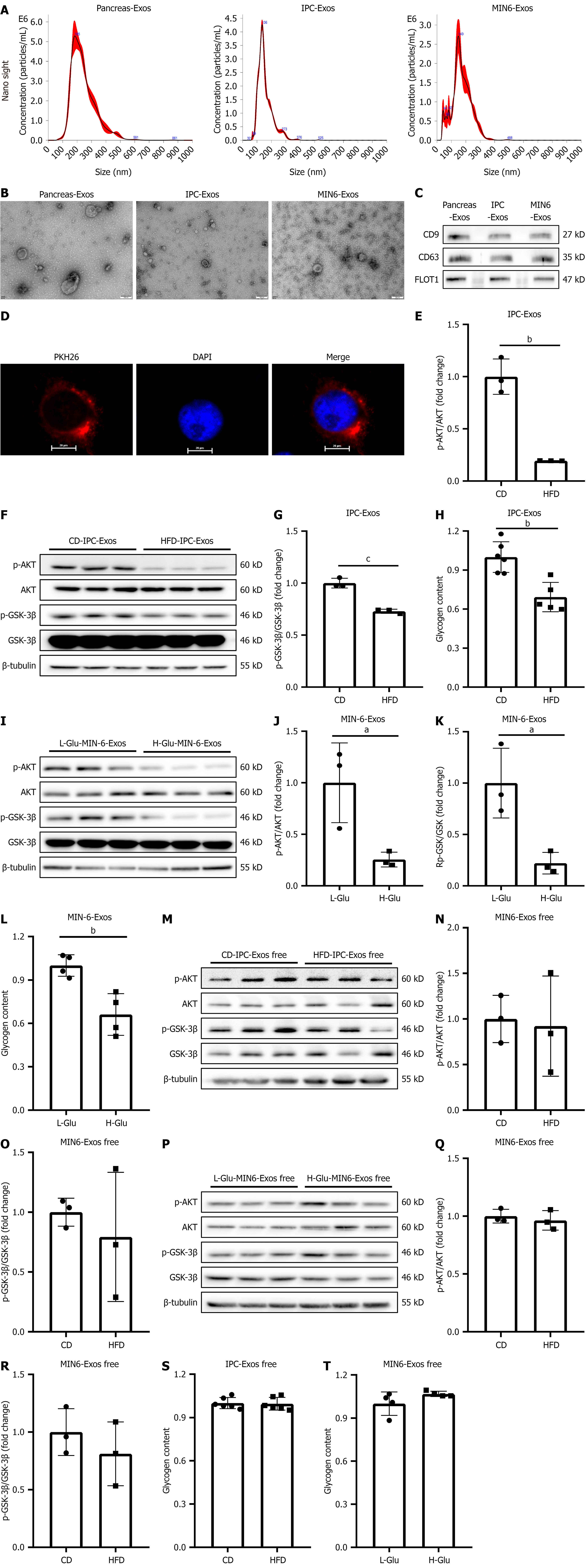
Exosomes are novel mediators that regulate the subtle interactions between cells. To evaluate whether exosomes mediate the crosstalk between glucotoxic pancreatic cells and hepatocytes, we first harvested exosomes from mouse pancreatic cells, IPCs, and MIN-6 cells. Then, we employed NanoSight technology to analyze the particle size distribution of the exosomes (Figure 3A), observed their morphology using electron microscopy (Figure 3B), and detected the surface markers of exosomal membrane proteins via western blotting (Figure 3C), which confirmed the successful isolation of the exosomes.
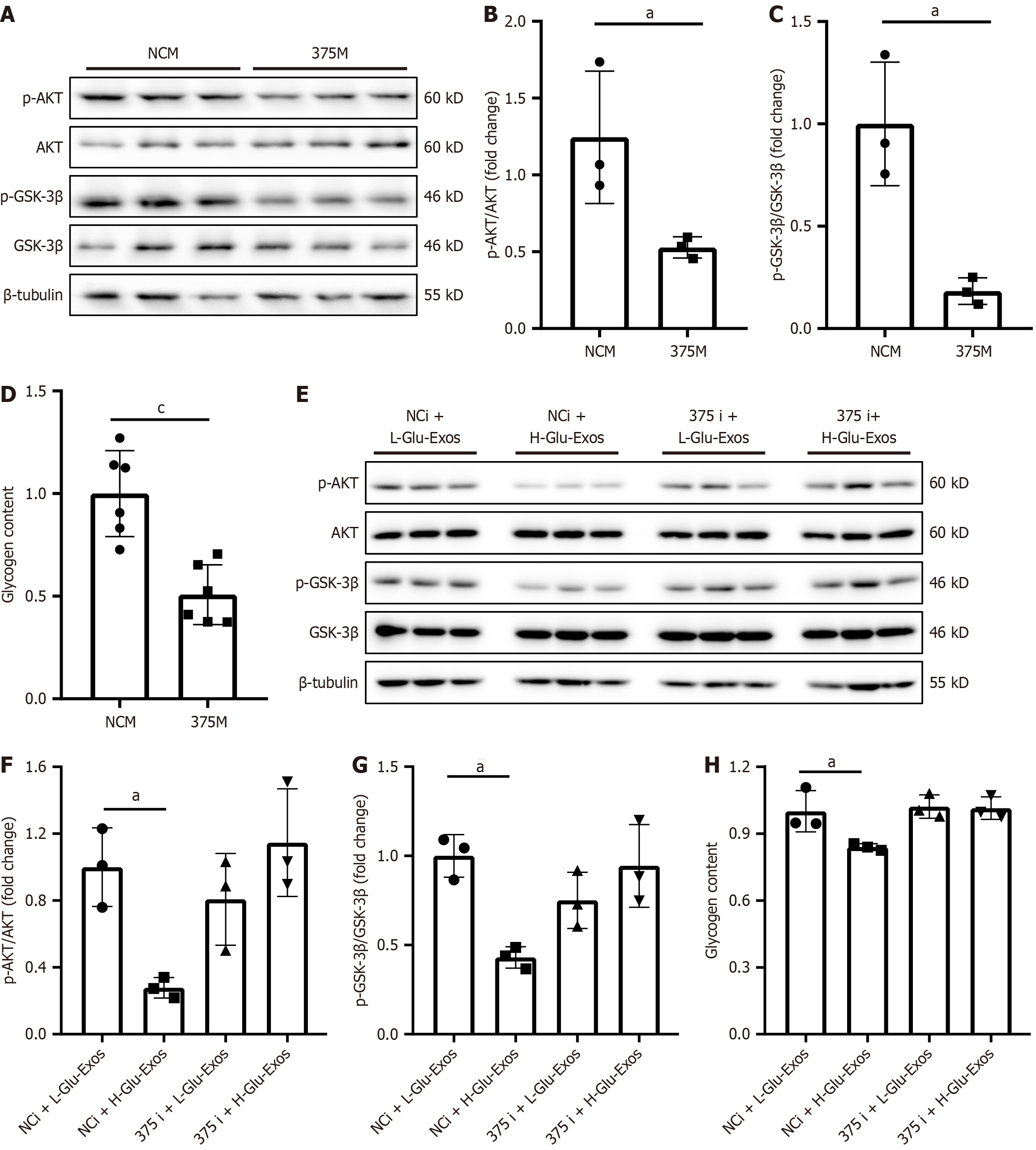
We investigated whether exosomes derived from glucotoxic pancreatic cells could enter hepatocytes and participate in the regulation of glycogenesis in hepatocytes. We treated hepatocytes with the collected exosomes and labeled them with PKH26 dye, and immunofluorescence tracking confirmed that the exosomes were efficiently internalized by the hepatocytes (Figure 3D). Next, we explored whether exosomes are involved in the impairment of hepatocyte glycogenesis induced by glucotoxic pancreatic cells. As shown in Figure 3E-H, exosomes released from the IPCs of HFD-fed mice decreased the phosphorylation levels of AKT and GSK-3β and inhibited glycogen synthesis in hepatocytes. Similarly, exosomes derived from glucotoxic MIN-6 cells also suppressed the activation of AKT and GSK-3β in hepatocytes (Figure 3I-K) and reduced the glycogen content (Figure 3L). In contrast, treatment of hepatocytes with cell culture supernatant devoid of exosomes did not alter the phosphorylation levels of AKT and GSK-3β (Figure 3M-R) or glycogen content (Figure 3S and T). These findings imply that exosomes originating from glucotoxic pancreatic cells serve as the primary mediators, contributing to the decline in glycogenesis of hepatocytes.
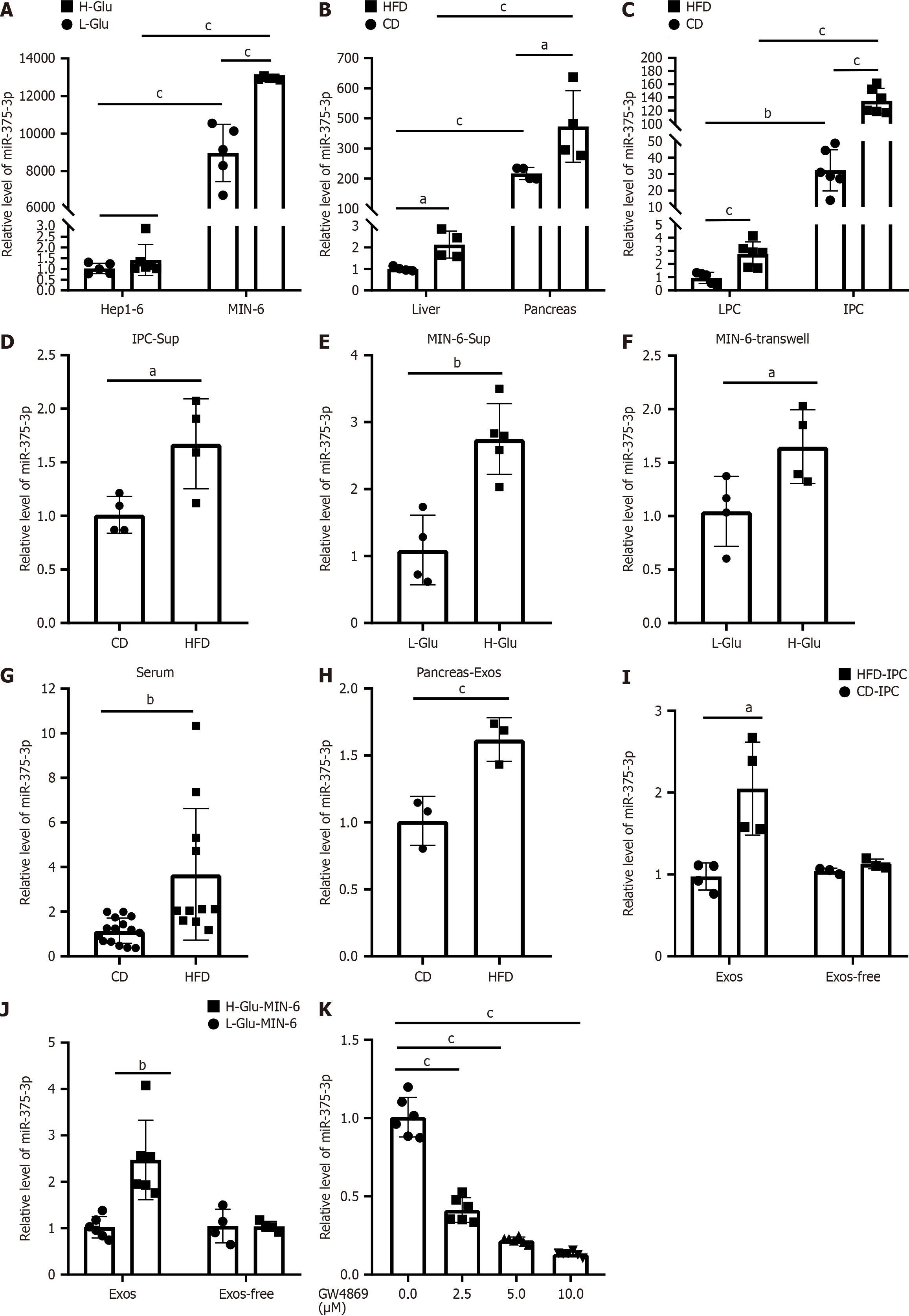
miR-375-3p is a miRNA that is highly expressed in islets. We conducted a comparative analysis of miR-375-3p expression levels in the mouse pancreas and liver, as well as in IPCs and liver parenchymal cells. In accordance with results of previous studies, our findings confirmed that miR-375-3p was highly abundant in pancreatic cells and the expression level of miR-375-3p in pancreatic cells was much higher than that in hepatocytes (Figure 4A-C).
Next, we compared the effects of glucotoxicity on miR-375-3p expression in both pancreatic cells and hepatocytes. In vivo, our results revealed significant upregulation of miR-375-3p expression in both the liver and pancreas following HFD feeding (Figure 4B and C). Unlike the in vivo data, high-glucose treatment upregulated miR-375-3p expression in the MIN-6 mouse pancreatic β cell line but had no effect on miR-375-3p expression in Hep1-6 cells (Figure 4A). Notably, similar results were observed in pancreatic cells and hepatocytes, both of which were treated with high palmitate concentrations (data not shown). These findings indicate that glucotoxicity induces increased expression of miR-375-3p in pancreatic cells but not in hepatocytes. These observations suggest that the elevated levels of miR-375-3p in the liver of HFD-fed mice may originate from the pancreas.
To confirm that miR-375-3p from pancreatic cells can be transferred to hepatocytes via the microenvironment, we treated hepatocytes with supernatants collected from IPCs and MIN-6 cells. Conditioned medium from both HFD-derived IPCs and high-glucose-treated MIN-6 cells significantly increased miR-375-3p levels in hepatocytes (Figure 4D and E). Similar results were obtained from Transwell coculture experiments (Figure 4F). Additionally, we observed a marked increase in miR-375-3p expression in the plasma of HFD-fed mice (Figure 4G). Collectively, these findings demonstrate that miR-375-3p can be transmitted from pancreatic cells to hepatocytes through the microenvironment.
Next, we investigated whether miR-375-3p can be transferred via exosomes. Notably, we detected a substantial increase in miR-375-3p expression in exosomes derived from the pancreas, IPCs of HFD-fed mice, and high-glucose-stimulated MIN-6 cells (Figure 4H-J). Importantly, hepatocytes treated with exosomes from HFD-derived pancreatic IPCs or high-glucose-stimulated MIN-6 cells presented marked upregulation of miR-375-3p, whereas exosome-depleted supernatants failed to alter its expression (Figure 4I-J). Collectively, these results demonstrate that exosomes act as essential carriers for miR-375-3p delivery under glucotoxic conditions. This conclusion was further strengthened by experiments showing that blocking exosome secretion with GW4869 diminished miR-375-3p transfer from pancreatic β cells to hepatocytes in a concentration-dependent manner (Figure 4K).
First, we explored the role of miR-375-3p in hepatocyte glycogenesis. Transfection of Hep1-6 cells with miR-375-3p mimics reduced the levels of AKT and GSK-3β phosphorylation (Figure 5A-C) and decreased glycogen content (Figure 5D). These findings suggest that miR-375-3p acts as a negative regulator of glycogenesis in hepatocytes.
We subsequently investigated whether miR-375-3p mediated the effects of pancreatic cell-derived exosomes on hepatocytes. Transfection of Hep1-6 cells with an miR-375-3p inhibitor reversed the inhibitory effects of exosomes on AKT and GSK-3β activation (Figure 5E-G) and restored glycogen content (Figure 5H) in hepatocytes. These findings confirm that glucotoxic pancreatic β cells reduce hepatocyte glycogenesis through exosomal delivery of miR-375-3p.
We used the miRNA target gene prediction database to identify potential targets of miR-375-3p related to insulin signaling. TargetScan analysis revealed that there was a binding site for miR-375-3p in the 3' untranslated region (3'-UTR) of Rbpj (Figure 6A).
Rbpj is a key regulator of AKT signaling. We investigated the role of Rbpj in hepatocyte glycogenesis. Following the transfection of Hep1-6 cells with siRNA targeting Rbpj, we observed a reduction in the phosphorylation levels of AKT and GSK-3β (Figure 6B-E) and a decrease in glycogen content (Figure 6F) in hepatocytes. In addition, overexpression of Rbpj in hepatocytes reversed the impairment of the AKT/GSK pathway (Figure 6G-J) and glycogen synthesis (Figure 6K) induced by miR-375-3p overexpression.
Next, we investigated whether miR-375-3p affects the expression of Rbpj. Transfection with miR-375-3p mimics reduced both protein and mRNA levels of Rbpj (Figure 6 L-N) in Hep1-6 cells. The protein and mRNA levels of Rbpj were significantly reduced in the liver of HFD-fed mice (Figure 6O-Q), In addition, treatment of Hep1-6 with exosomes derived from high-glucose-stimulated MIN-6 cells decreased Rbpj protein expression (Figure 6R and S). Transfection of a miR-375-3p inhibitor abolished the influence of glucotoxic pancreatic-cell-derived exosomes on Rbpj protein expression in Hep1-6 cells (Figure 6T and U).
To confirm that Rbpj is a target gene of miR-375-3p, we constructed a wild-type Rbpj recombinant plasmid (pmirGLO-Rbpj-WT) and a mutant recombinant plasmid (pmirGLO-Rbpj-MT) (Figure 6V). The results of the luciferase reporter assay demonstrated that the overexpression of miR-375-3p suppressed the luciferase activity of Rbpj-WT but not that of Rbpj-MT (Figure 6W and X). These findings confirmed that miR-375-3p can directly bind to the 3'-UTR of Rbpj.
All these results suggest that miR-375-3p regulates hepatocyte glycogenesis through Rbpj.
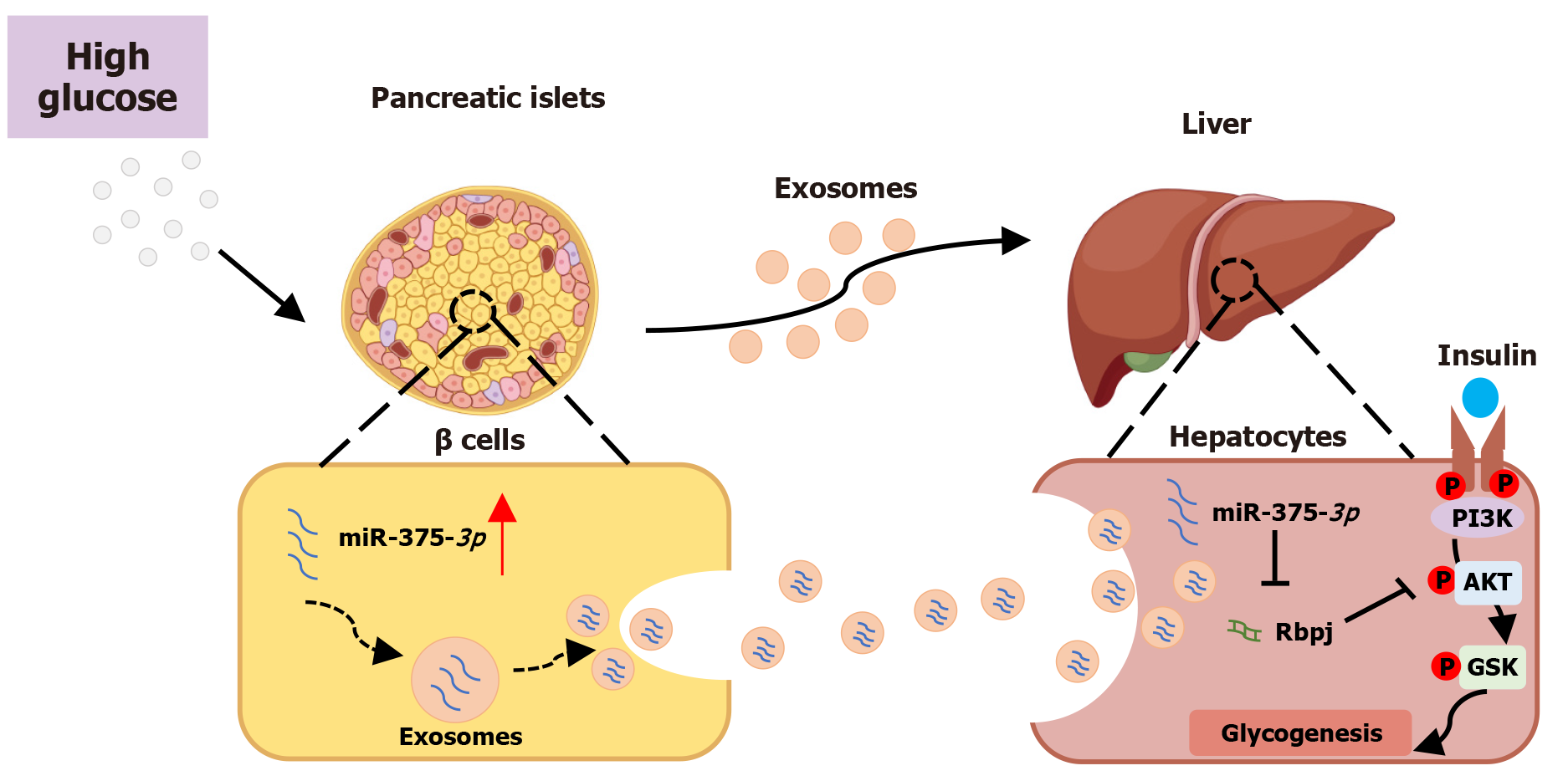
In this study, we revealed a new role of miR-375-3p in addition to its role in pancreatic β cells. We elucidated that glucotoxicity-damaged pancreatic β cells can secrete miR-375-3p in the form of exosomes, which are then transferred to hepatocytes. Exosomal transfer of miR-375-3p from pancreatic β cells affects AKT/GSK-mediated hepatocyte glycogenesis by inhibiting Rbpj (Figure 7). These results demonstrate for the first time the regulatory role of pancreatic-β-cell-derived miR-375-3p in hepatic glycogenesis via exosomes, revealing a novel mechanism by which intracellular regulators in the pancreas remotely modulate glucose metabolic homeostasis.
As an important endocrine organ, the pancreas plays a crucial role in regulating glucose homeostasis in the body. Pancreatic β cells play a decisive role in the body's glucose metabolic homeostasis because the decline in insulin levels caused by cell loss and functional failure is highly correlated with the occurrence and deterioration of DM[24,25]. Glucotoxicity is one of the important factors causing β cell damage. Consistent with previous results, we found that high glucose levels led to decreased insulin secretion and increased apoptosis in the pancreas as well as in the β-cell line MIN-6.
The liver is one of the major target organs of insulin, and impaired glycogenesis is a hallmark of hepatic insulin resistance. Previous studies have shown that glucotoxic pancreatic β cells impair the glycogenesis of hepatocytes, which impairs systemic glucose homeostasis. By using Transwell assays and supernatant culture, we revealed that glucotoxicity not only induces damage to the pancreatic cells themselves but also influences hepatocyte glycogenesis through the microenvironment.
What mediates the interaction between glucotoxic pancreatic cells and hepatocytes in the microenvironment? Insulin, the primary effector molecule regulating insulin target organ actions, was initially identified. Additionally, soluble cytokines such as interleukin-6 have been reported to participate in the functional regulation of target organs. With the deepening of research into the endocrine function of pancreatic β cells, it has been found that these cells can also regulate insulin signaling pathways in insulin target organs such as the liver through novel molecules such as exosomes[26]. Since Transwell and supernatant experiments cannot completely rule out the influence of soluble cytokines on hepatocytes, we isolated and purified pancreatic cell-derived exosomes using ultracentrifugation. PKH26 Labeling experiments demonstrated that pancreatic cell-derived exosomes can enter hepatocytes. Subsequent experiments with conditioned media depleted of exosomes and exosome-treated hepatocytes revealed that exosomes indeed mediate the regulation of glycogenesis in hepatocytes by glucotoxic pancreatic cells. Notably, although high-glucose treatment can lead to alterations in insulin secretion from pancreatic cells, we did not detect any influence of pancreatic-cell-secreted insulin on insulin signaling pathways and hepatic glycogen synthesis in hepatocytes cultured with pancreatic cells with exosome-depleted conditioned medium. One possible reason is that all the aforementioned assays, including AKT/GSK signaling and glycogen synthesis, required the addition of exogenous insulin (0.25 IU/mL) in vitro, which provides a concentration much greater than the level of insulin secreted by pancreatic cells themselves. Although our results did not confirm the role of insulin in the microenvironment, they clearly demonstrated the role of exosomes in microenvironmental cell-cell communication.
Various miRNA molecules, such as miR-9, miR-124a, miR-143, miR-338-3p, miR-7, and miR-184, are involved in the regulation of insulin secretion and apoptotic injury in pancreatic β cells[27-33]. miR-375-3p, the most abundant miRNA in the pancreas[17,34], is involved in the regulation of pancreatic cell apoptosis and is closely related to pancreas injury[35]. El Ouaamari et al[36] reported that miR-375-3p promotes pancreatic β-cell apoptosis through the inhibition of phosphatidylinositol 3-phosphate-dependent protein kinase 1, whereas Li et al[37] reported that, in the presence of palmitic acid stimulation, miR-375-3p promotes pancreatic β-cell apoptosis through the downregulation of myotrophin. Consistent with previous studies, our results revealed that pancreatic cell injury was accompanied by a significant increase in miR-375-3p expression in pancreatic cells.
Recently, several studies have shown that some important regulators in pancreatic β cells not only function within pancreatic β cells but also act in an exosomal manner to regulate insulin signaling in insulin target cells. For example, peripheral insulin sensitivity is induced by injured β cells through decreasing the exosomal translocation of protective miR-26a[14]. Pancreatic cell-derived exosomal miR-15a translocates to retinal cells and causes retinal damage by inducing oxidative stress, leading to diabetic complications[38]. Exosomal miR-29s released by pancreatic β cells can control glucose homeostasis by targeting hepatic insulin signaling[26]. As a pancreas-enriched miRNA critical for β-cell function, whether miR-375-3p is released via exosomes to act on other insulin target organs remains unclear.
We compared the expression of miR-375-3p in the pancreas and liver. As expected, as a miRNA highly expressed in the pancreas, miR-375-3p expression levels were hundreds of times higher than those in the liver before and after high-glucose treatment, whether in MIN-6 vs Hep1-6 cells, primary pancreatic cells vs liver cells, or pancreas vs liver tissues. Notably, high glucose levels increased miR-375-3p in pancreatic cells but not in hepatocytes in vitro. Conversely, HFD feeding upregulated miR-375-3p in mouse livers in vivo. These observations suggest that hepatic miR-375-3p elevation in HFD-fed mice originates from the pancreas.
To address this issue, we used pancreatic cell supernatant to culture hepatocytes and carried out a Transwell assay to coculture MIN-6 cells with hepatocytes to simulate the in vivo environment. The results showed that miR-375-3p produced by pancreatic cells could be delivered to hepatocytes through the microenvironment. Further treatment with exosomes or exosome-depleted culture supernatants revealed that miR-375-3p produced by pancreatic cells was delivered to hepatocytes via exosomes.
What effects does exosomal miR-375-3p exert when it enters the liver? Impaired glycogenesis is a hallmark of reduced insulin sensitivity in hepatocytes, where insulin promotes hepatic glycogen synthesis through the phosphoinositide 3 kinase/AKT/GSK signaling pathway. We treated hepatocytes with supernatants from cultured pancreatic cells, Transwell assays, exosomes and exosome-depleted supernatants to measure p-AKT and p-GSK-3β levels in the insulin signaling pathway and glycogen content. The results showed that glucotoxicity-induced damage to pancreatic cells could reduce hepatocyte glycogenesis through exosomal delivery.
To clarify the role of miR-375-3p during this process, we overexpressed miR-375-3p in hepatocytes or inhibited its expression in hepatocytes. The results revealed that the overexpression of miR-375-3p led to impaired hepatocyte glycogenesis, whereas the inhibition of miR-375-3p expression ameliorated the effect of pancreatic-cell-derived exosomes on hepatocyte glycogenesis. Considering these findings together, exosomal miR-375-3p derived from pancreatic cells mediated the reduction in hepatocyte glycogenesis.
miRNAs are able to bind to the 3'-UTR of target gene mRNAs to negatively regulate protein expression, thereby participating in physiological and pathological processes[39]. To further explore the specific mechanism by which miR-375-3p participates in the glycogenesis of hepatocytes, we identified Rbpj as a potential target of miR-375-3p using TargetScan bioinformatics analysis and confirmed by luciferase reporter analysis that miR-375-3p targeted Rbpj and significantly downregulated its expression. Rbpj is a key transcription factor involved in cell proliferation, differentiation and development. Previous studies have demonstrated that Rbpj can positively regulate AKT signaling, thereby affecting the transmission and effects of the insulin signaling pathway[40]. In vitro experiments demonstrated that the inhibition of Rbpj likewise inhibited hepatic glycogenesis, while overexpression of Rbpj reversed the damage to the insulin signaling pathway caused by miR-375-3p overexpression. Collectively, these results demonstrate that miR-375-3p regulates hepatocyte glycogenesis by inhibiting Rbpj expression.
Nevertheless, it is imperative to acknowledge the limitations of our study. Firstly, there was a lack of human clinical validation. Secondly, the in vivo delivery efficiency remained low due to the insufficient hepatocyte-targeting specificity of natural exosomes in systemic circulation. Future research should concentrate on verifying clinical relevance and optimizing delivery strategies. This includes establishing a dynamic association between miR-375-3p and liver glycogen synthesis in T2DM, and developing engineered targeting vectors (such as galactose modification or GPC3 affinity peptides) to enhance liver enrichment efficiency. Additionally, this study identified an exosome-mediated pancreas-liver axis. Beyond pancreatic exosomes, extracellular vesicles from other sources (e.g., adipose tissue and intestinal epithelium) may also regulate hepatic and systemic metabolism[41,42]. Exploring the cross-talk network of these multi-tissue extracellular vesicles could deepen understanding of metabolic regulation and aid in identifying new therapeutic targets for metabolic diseases[43].
This study revealed a novel mechanism of intercellular communication between the pancreas and the liver. We demonstrated that under glucotoxic conditions, pancreatic β-cells secreted exosomes containing miR-375-3p. These exosomes acted as vehicles to deliver miR-375-3p to hepatocytes, where it impaired hepatic glycogenesis by directly targeting and inhibiting its downstream gene, Rbpj. This discovery not only expands our understanding of the endocrine function of exosomes in regulating systemic glucose metabolism but also identifies miR-375-3p as a critical mediator in pancreas-liver crosstalk, offering a potential therapeutic target for metabolic diseases such as T2DM.
| 1. | Adeva-Andany MM, Pérez-Felpete N, Fernández-Fernández C, Donapetry-García C, Pazos-García C. Liver glucose metabolism in humans. Biosci Rep. 2016;36:e00416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 260] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Hudish LI, Reusch JE, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 2019;129:4001-4008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 3. | Khalid M, Alkaabi J, Khan MAB, Adem A. Insulin Signal Transduction Perturbations in Insulin Resistance. Int J Mol Sci. 2021;22:8590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 4. | Wang YD, Wu LL, Qi XY, Wang YY, Liao ZZ, Liu JH, Xiao XH. New insight of obesity-associated NAFLD: Dysregulated "crosstalk" between multi-organ and the liver? Genes Dis. 2023;10:799-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 2794] [Article Influence: 399.1] [Reference Citation Analysis (0)] |
| 6. | Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol Metab. 2017;28:3-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 290] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 7. | Samuelson I, Vidal-Puig AJ. Fed-EXosome: extracellular vesicles and cell-cell communication in metabolic regulation. Essays Biochem. 2018;62:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Salama A, Fichou N, Allard M, Dubreil L, De Beaurepaire L, Viel A, Jégou D, Bösch S, Bach JM. MicroRNA-29b modulates innate and antigen-specific immune responses in mouse models of autoimmunity. PLoS One. 2014;9:e106153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Giri KR, de Beaurepaire L, Jegou D, Lavy M, Mosser M, Dupont A, Fleurisson R, Dubreil L, Collot M, Van Endert P, Bach JM, Mignot G, Bosch S. Molecular and Functional Diversity of Distinct Subpopulations of the Stressed Insulin-Secreting Cell's Vesiculome. Front Immunol. 2020;11:1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Sou YL, Chilian WM, Ratnam W, Zain SM, Syed Abdul Kadir SZ, Pan Y, Pung YF. Exosomal miRNAs and isomiRs: potential biomarkers for type 2 diabetes mellitus. Precis Clin Med. 2024;7:pbae021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 11. | Sun Y, Zhou Y, Shi Y, Zhang Y, Liu K, Liang R, Sun P, Chang X, Tang W, Zhang Y, Li J, Wang S, Zhu Y, Han X. Expression of miRNA-29 in Pancreatic β Cells Promotes Inflammation and Diabetes via TRAF3. Cell Rep. 2021;34:108576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 12. | Nielsen LB, Wang C, Sørensen K, Bang-Berthelsen CH, Hansen L, Andersen ML, Hougaard P, Juul A, Zhang CY, Pociot F, Mortensen HB. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res. 2012;2012:896362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Kim H, Bae YU, Lee H, Kim H, Jeon JS, Noh H, Han DC, Byun DW, Kim SH, Park HK, Ryu S, Kwon SH. Effect of diabetes on exosomal miRNA profile in patients with obesity. BMJ Open Diabetes Res Care. 2020;8:e001403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Xu H, Du X, Xu J, Zhang Y, Tian Y, Liu G, Wang X, Ma M, Du W, Liu Y, Dai L, Huang W, Tong N, Wei Y, Fu X. Pancreatic β cell microRNA-26a alleviates type 2 diabetes by improving peripheral insulin sensitivity and preserving β cell function. PLoS Biol. 2020;18:e3000603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Shyu KG, Wang BW, Fang WJ, Pan CM, Lin CM. Exosomal MALAT1 Derived from High Glucose-Treated Macrophages Up-Regulates Resistin Expression via miR-150-5p Downregulation. Int J Mol Sci. 2022;23:1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Carciero L, Di Giuseppe G, Di Piazza E, Parand E, Soldovieri L, Ciccarelli G, Brunetti M, Gasbarrini A, Nista EC, Pani G, Pontecorvi A, Giaccari A, Mezza T. The interplay of extracellular vesicles in the pathogenesis of metabolic impairment and type 2 diabetes. Diabetes Res Clin Pract. 2024;216:111837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Eliasson L. The small RNA miR-375 - a pancreatic islet abundant miRNA with multiple roles in endocrine beta cell function. Mol Cell Endocrinol. 2017;456:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Zhang A, Li D, Liu Y, Li J, Zhang Y, Zhang CY. Islet β cell: An endocrine cell secreting miRNAs. Biochem Biophys Res Commun. 2018;495:1648-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Calvano J, Edwards G, Hixson C, Burr H, Mangipudy R, Tirmenstein M. Serum microRNAs-217 and -375 as biomarkers of acute pancreatic injury in rats. Toxicology. 2016;368-369:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Rouse R, Rosenzweig B, Shea K, Knapton A, Stewart S, Xu L, Chockalingam A, Zadrozny L, Thompson K. MicroRNA biomarkers of pancreatic injury in a canine model. Exp Toxicol Pathol. 2017;69:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Fu Q, Jiang H, Wang Z, Wang X, Chen H, Shen Z, Xiao L, Guo X, Yang T. Injury factors alter miRNAs profiles of exosomes derived from islets and circulation. Aging (Albany NY). 2018;10:3986-3999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Wei J, Wang Z, Han T, Chen J, Ou Y, Wei L, Zhu X, Wang K, Yan Z, Han YP, Zheng X. Extracellular vesicle-mediated intercellular and interorgan crosstalk of pancreatic islet in health and diabetes. Front Endocrinol (Lausanne). 2023;14:1170237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Wang K, Wang X, Han CS, Chen LY, Luo Y. Scaffold-supported Transplantation of Islets in the Epididymal Fat Pad of Diabetic Mice. J Vis Exp. 2017;54995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16:349-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 571] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 25. | Campbell JE, Newgard CB. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol. 2021;22:142-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 413] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 26. | Li J, Zhang Y, Ye Y, Li D, Liu Y, Lee E, Zhang M, Dai X, Zhang X, Wang S, Zhang J, Jia W, Zen K, Vidal-Puig A, Jiang X, Zhang CY. Pancreatic β cells control glucose homeostasis via the secretion of exosomal miR-29 family. J Extracell Vesicles. 2021;10:e12055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Ramachandran D, Roy U, Garg S, Ghosh S, Pathak S, Kolthur-Seetharam U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic β-islets. FEBS J. 2011;278:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Sebastiani G, Po A, Miele E, Ventriglia G, Ceccarelli E, Bugliani M, Marselli L, Marchetti P, Gulino A, Ferretti E, Dotta F. MicroRNA-124a is hyperexpressed in type 2 diabetic human pancreatic islets and negatively regulates insulin secretion. Acta Diabetol. 2015;52:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Jordan SD, Krüger M, Willmes DM, Redemann N, Wunderlich FT, Brönneke HS, Merkwirth C, Kashkar H, Olkkonen VM, Böttger T, Braun T, Seibler J, Brüning JC. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol. 2011;13:434-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 432] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 30. | Jacovetti C, Abderrahmani A, Parnaud G, Jonas JC, Peyot ML, Cornu M, Laybutt R, Meugnier E, Rome S, Thorens B, Prentki M, Bosco D, Regazzi R. MicroRNAs contribute to compensatory β cell expansion during pregnancy and obesity. J Clin Invest. 2012;122:3541-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Jacovetti C, Jimenez V, Ayuso E, Laybutt R, Peyot ML, Prentki M, Bosch F, Regazzi R. Contribution of Intronic miR-338-3p and Its Hosting Gene AATK to Compensatory β-Cell Mass Expansion. Mol Endocrinol. 2015;29:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Latreille M, Hausser J, Stützer I, Zhang Q, Hastoy B, Gargani S, Kerr-Conte J, Pattou F, Zavolan M, Esguerra JL, Eliasson L, Rülicke T, Rorsman P, Stoffel M. MicroRNA-7a regulates pancreatic β cell function. J Clin Invest. 2014;124:2722-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 33. | Nesca V, Guay C, Jacovetti C, Menoud V, Peyot ML, Laybutt DR, Prentki M, Regazzi R. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia. 2013;56:2203-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 34. | Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foà R, Schliwka J, Fuchs U, Novosel A, Müller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3103] [Cited by in RCA: 3008] [Article Influence: 158.3] [Reference Citation Analysis (1)] |
| 35. | Zhou J, Song S, He S, Zhu X, Zhang Y, Yi B, Zhang B, Qin G, Li D. MicroRNA-375 targets PDK1 in pancreatic carcinoma and suppresses cell growth through the Akt signaling pathway. Int J Mol Med. 2014;33:950-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | El Ouaamari A, Baroukh N, Martens GA, Lebrun P, Pipeleers D, van Obberghen E. miR-375 targets 3'-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells. Diabetes. 2008;57:2708-2717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 360] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 37. | Li Y, Xu X, Liang Y, Liu S, Xiao H, Li F, Cheng H, Fu Z. miR-375 enhances palmitate-induced lipoapoptosis in insulin-secreting NIT-1 cells by repressing myotrophin (V1) protein expression. Int J Clin Exp Pathol. 2010;3:254-264. [PubMed] |
| 38. | Kamalden TA, Macgregor-Das AM, Kannan SM, Dunkerly-Eyring B, Khaliddin N, Xu Z, Fusco AP, Yazib SA, Chow RC, Duh EJ, Halushka MK, Steenbergen C, Das S. Exosomal MicroRNA-15a Transfer from the Pancreas Augments Diabetic Complications by Inducing Oxidative Stress. Antioxid Redox Signal. 2017;27:913-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 39. | Ghelani HS, Rachchh MA, Gokani RH. MicroRNAs as newer therapeutic targets: A big hope from a tiny player. J Pharmacol Pharmacother. 2012;3:217-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat Med. 2013;19:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 41. | Grossini E, Ola Pour MM, Venkatesan S. The Role of Extracellular Vesicles in the Pathogenesis of Metabolic Dysfunction-Associated Steatotic Liver Disease and Other Liver Diseases. Int J Mol Sci. 2025;26:5033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 42. | Wang J, Bao S, An Q, Li C, Feng J. Roles of extracellular vesicles from different origins in metabolic-associated fatty liver disease: progress and perspectives. Front Immunol. 2025;16:1544012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Xourafa G, Korbmacher M, Roden M. Inter-organ crosstalk during development and progression of type 2 diabetes mellitus. Nat Rev Endocrinol. 2024;20:27-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 140] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/