©The Author(s) 2025.
World J Diabetes. Oct 15, 2025; 16(10): 109526
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.109526
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.109526
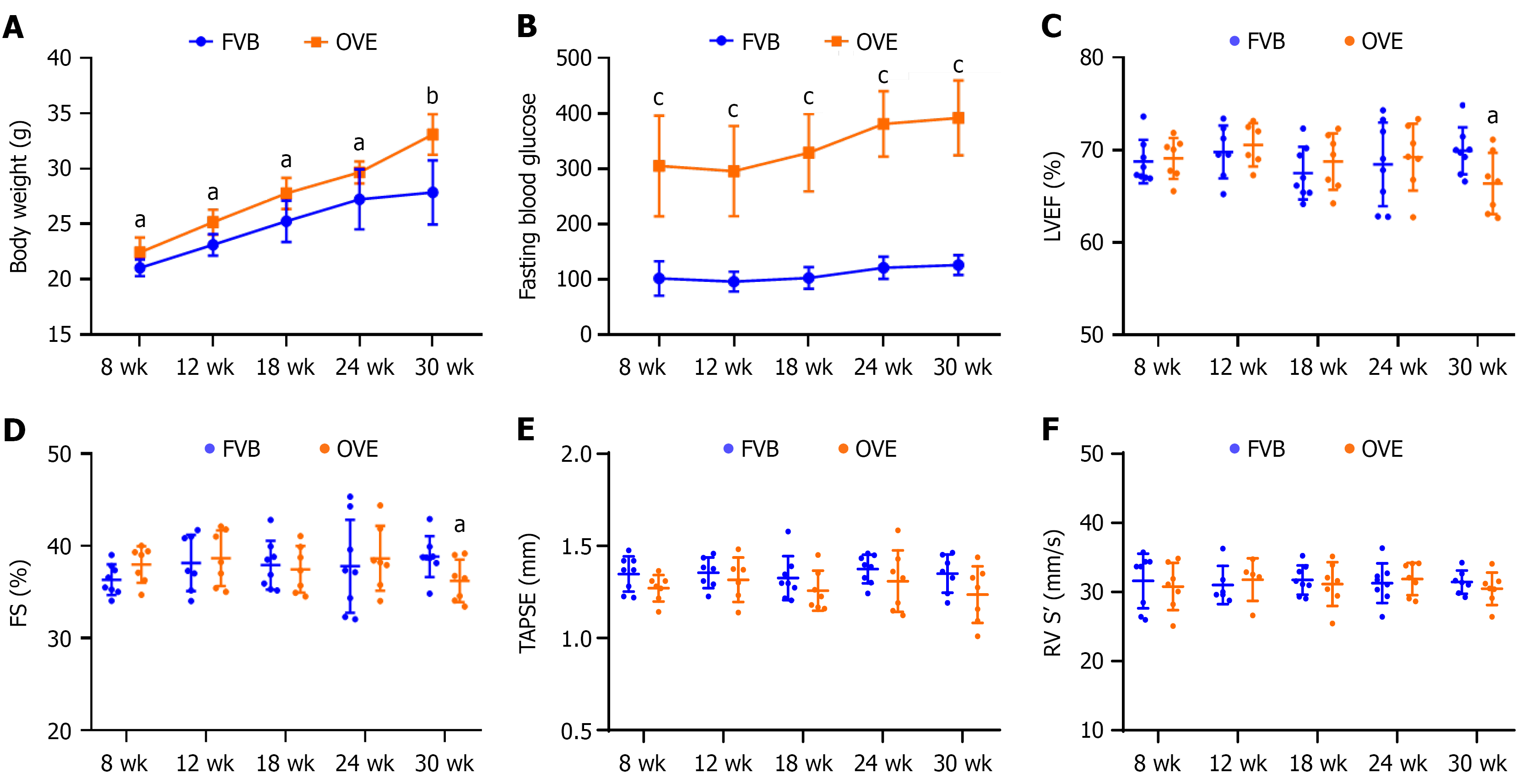
Figure 1 General features and cardiac functions of OVE26 vs control FVB mice.
A: Body weight; B: Fasting blood glucose; C: Left ventricle ejection fraction; D: Left ventricle fractional shortening; E: Right ventricle tricuspid valve annular plane systolic excursion; F: Right ventricle systolic velocity. Data are presented as mean ± SD (n = 6-8). aP < 0.05 vs control FVB mice; bP < 0.01 vs control FVB mice; cP = 0.06 vs control FVB mice. LVEF: Left ventricle ejection fraction; FS: Fractional shortening; TAPSE: Tricuspid valve annular plane systolic excursion; RV S’: Right ventricle systolic velocity.
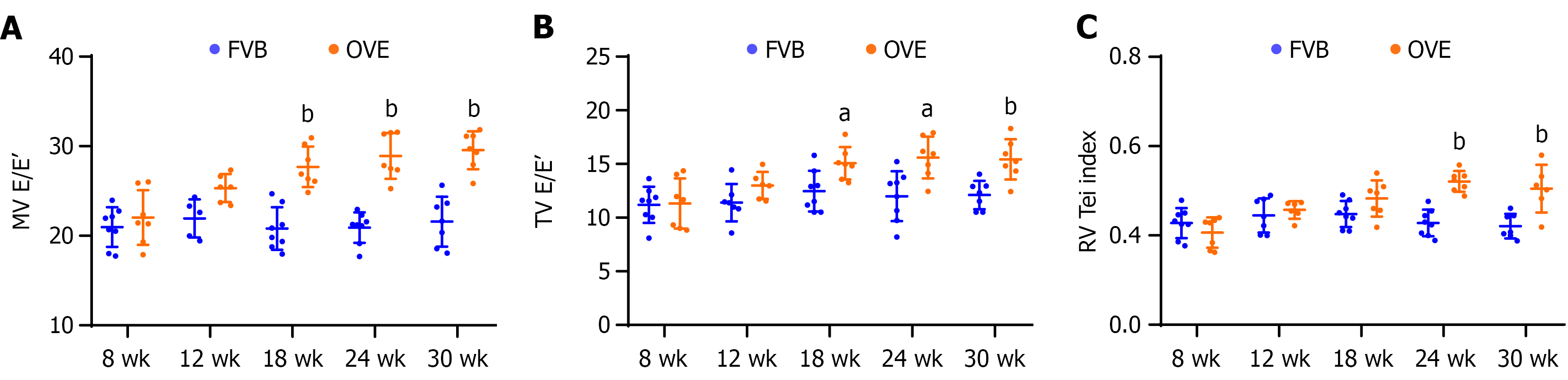
Figure 2 Left ventricular and right ventricular diastolic function in OVE26 mice vs FVB control.
A: Mitral valve E-to-E ratio for left ventricular diastolic dysfunction; B: Tricuspid valve E-to-E ratio for right ventricular diastolic dysfunction; C: Right ventricular myocardial performance index (right ventricular Tei index). Data are presented as mean ± SD (n = 6-8). aP < 0.05 vs control FVB mice; bP < 0.01 vs control FVB mice. MV E/E’: Mitral valve E-to-E ratio; TV E/E’: Tricuspid valve E-to-E ratio; RV: Right ventricular.
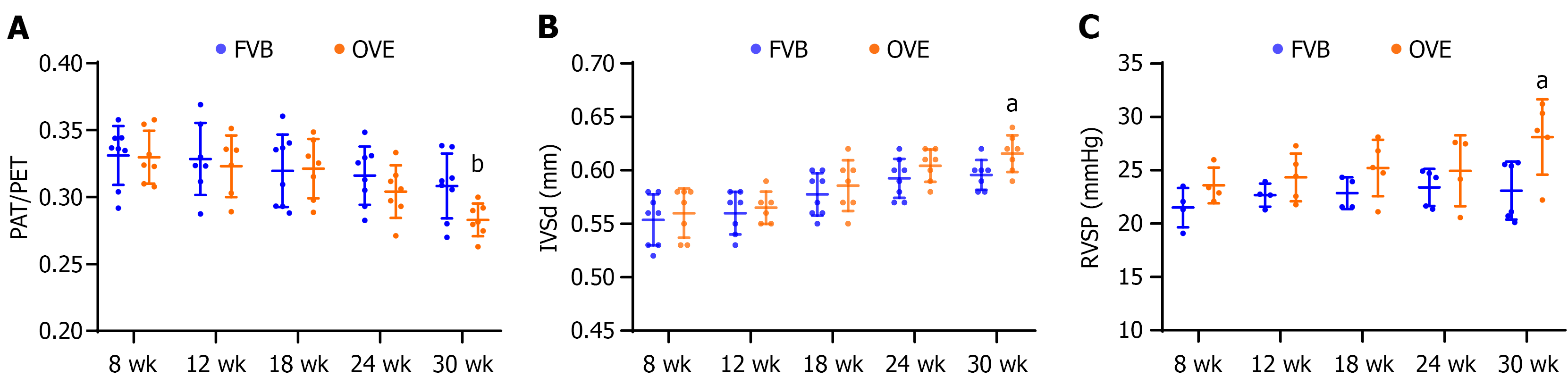
Figure 3 Index of pulmonary arterial hypertension.
A: Pulmonary acceleration time/pulmonary ejection time ratio; B: Diastolic interventricular septum thickness. A and B were measured by echocardiography, data panels A and B are presented as mean ± SD (n = 6-8); C: Right ventricular systolic pressure was measured directly using a right ventricular pressure catheter. Data are presented as mean ± SD (n = 4-6). aP < 0.05 vs control FVB mice; bP = 0.09 vs control FVB mice. PAT: Pulmonary acceleration time; PET: Pulmonary ejection time; IVSd: Interventricular septum thickness; RVSP: Right ventricular systolic pressure.
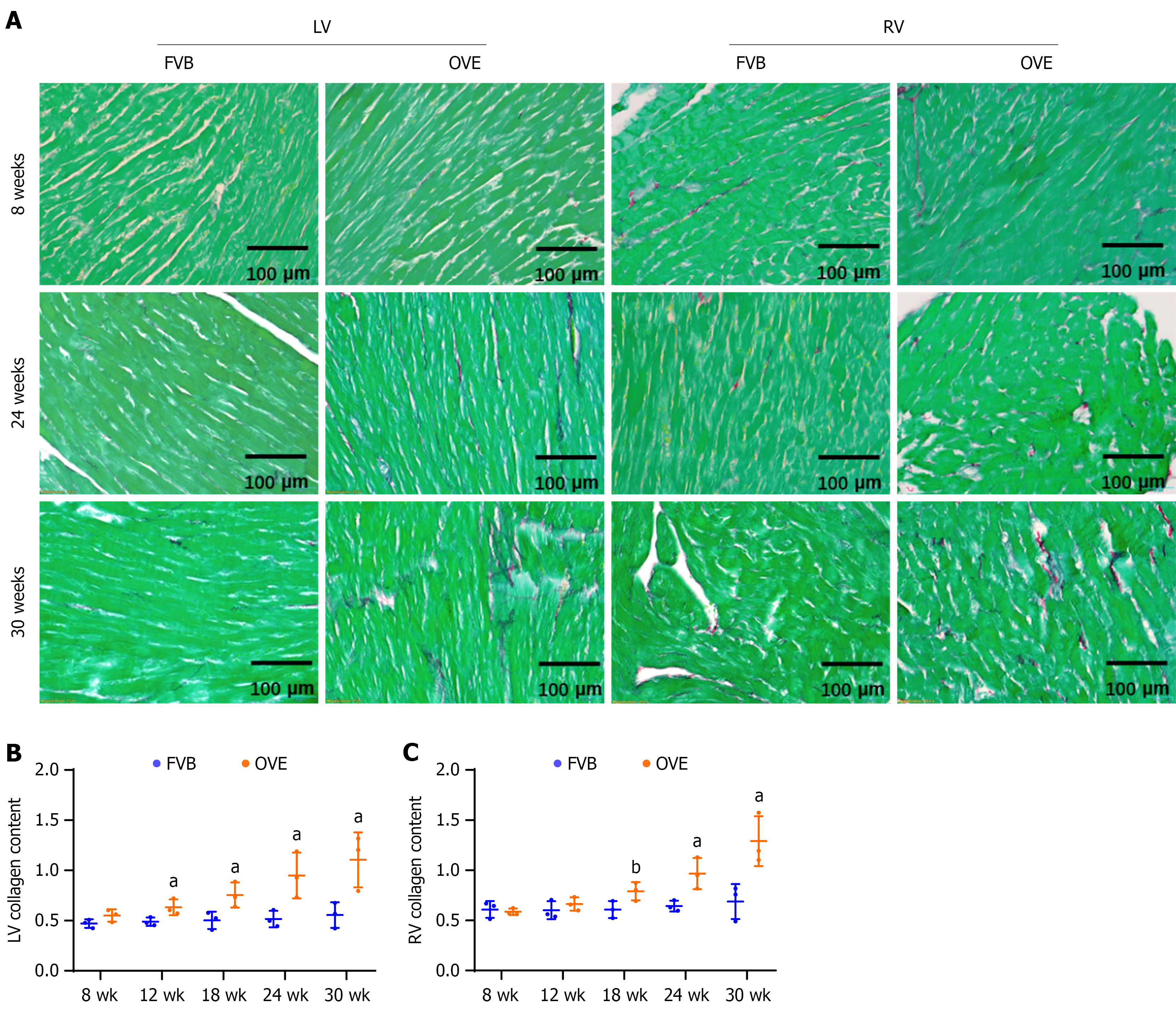
Figure 4 Fibrotic effects of type 1 diabetes on the left ventricle and right ventricle.
A: Picro-Sirius red staining in left ventricle and right ventricle; B and C: Semi-quantification of cardiac collagen accumulation in left ventricle and right ventricle, respectively. Data are presented as mean ± SD (n = 3). aP < 0.05 vs control FVB mice; bP = 0.05 vs control FVB mice. LV: Left ventricle; RV: Right ventricle.
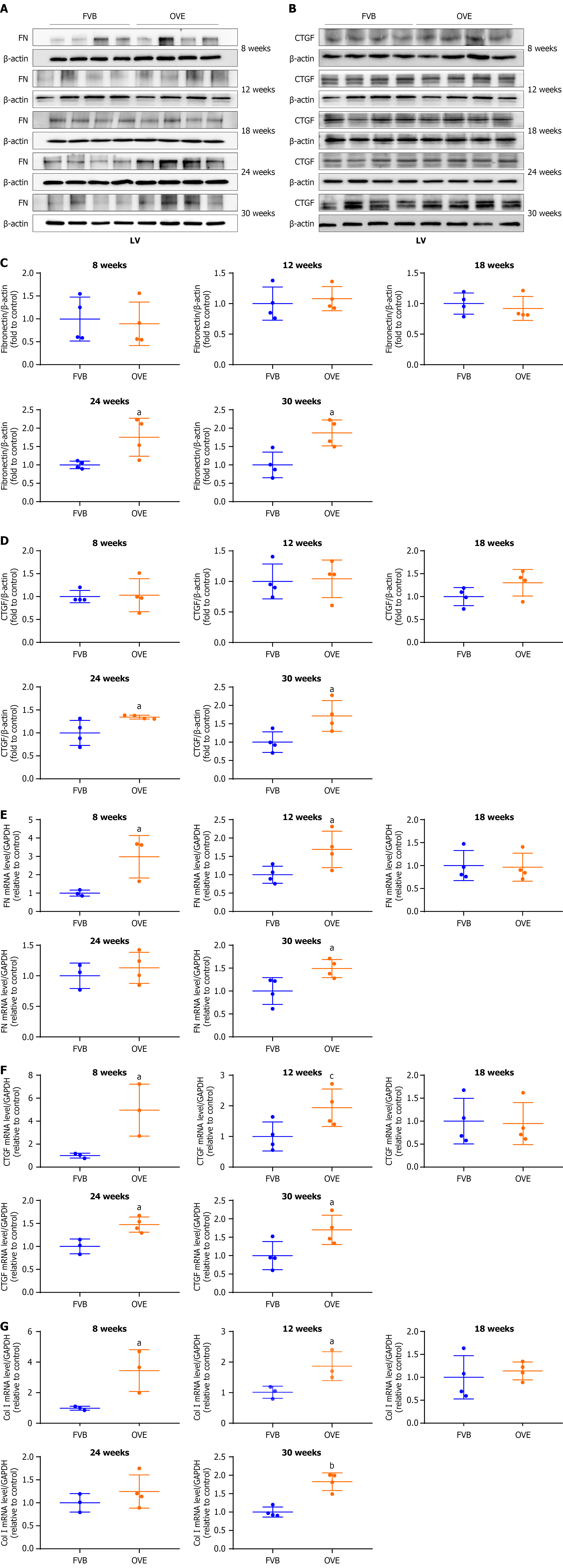
Figure 5 The impact of type 1 diabetes on cardiac fibrosis in the left ventricle of diabetic OVE26 mice.
A-D: Changes in fibronectin and connective tissue growth factor protein levels in the left ventricle of 12-week-old mice were assayed by Western blot; E-G: Changes of left ventricle mRNA expression of fibronectin, connective tissue growth factor, and type I collagen were measured by quantitative reverse transcription polymerase chain reaction. Data are presented as mean ± SD (n = 3-4). aP < 0.05 vs control FVB mice; bP < 0.01 vs control FVB mice; cP = 0.06 vs control FVB mice. FN: Fibronectin; CTGF: Connective tissue growth factor; LV: Left ventricle; Col I: Type I collagen.
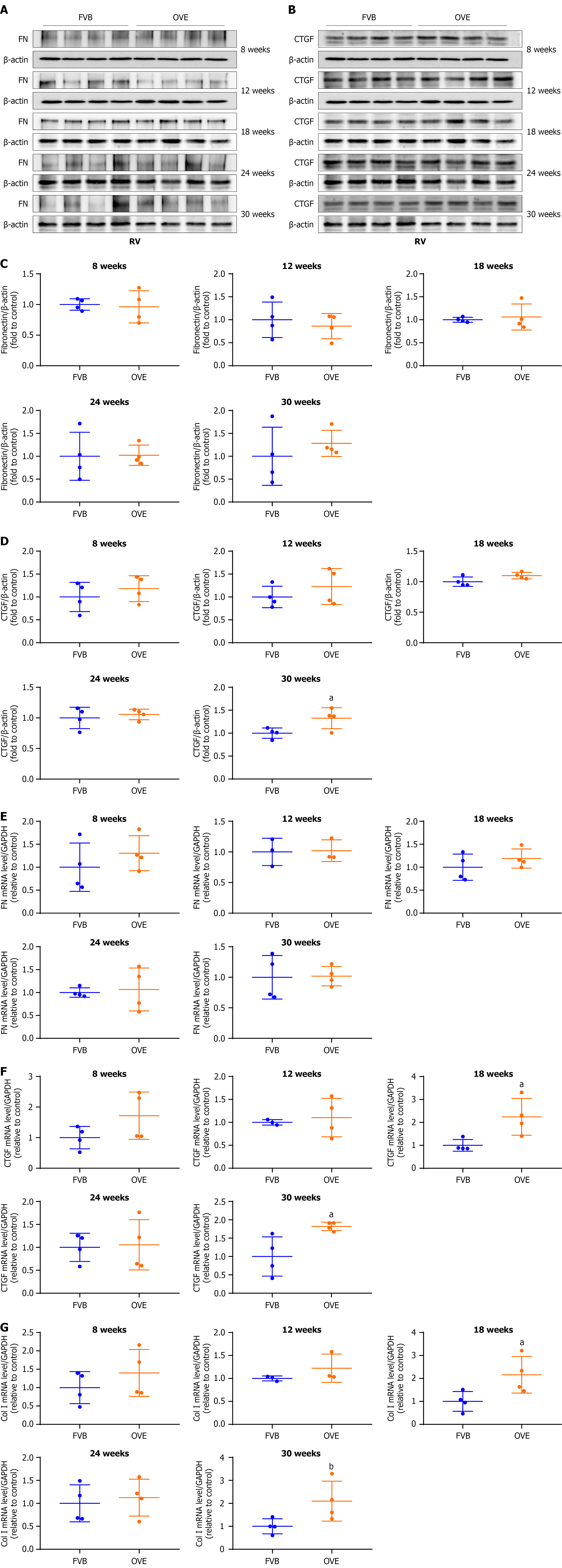
Figure 6 The impact of type 1 diabetes on cardiac fibrosis in the right ventricle of OVE26 mice.
A-D: Changes in fibronectin and connective tissue growth factor protein levels in the right ventricle were assayed by Western blot; E-G: Changes of right ventricle mRNA expression of fibronectin, connective tissue growth factor, type I collagen were measured by quantitative reverse transcription polymerase chain reaction. Data are presented as mean ± SD (n = 3-4). aP < 0.05 vs control FVB mice; bP = 0.06 vs control FVB mice. FN: Fibronectin; CTGF: Connective tissue growth factor; RV: Right ventricle; Col I: Type I collagen.
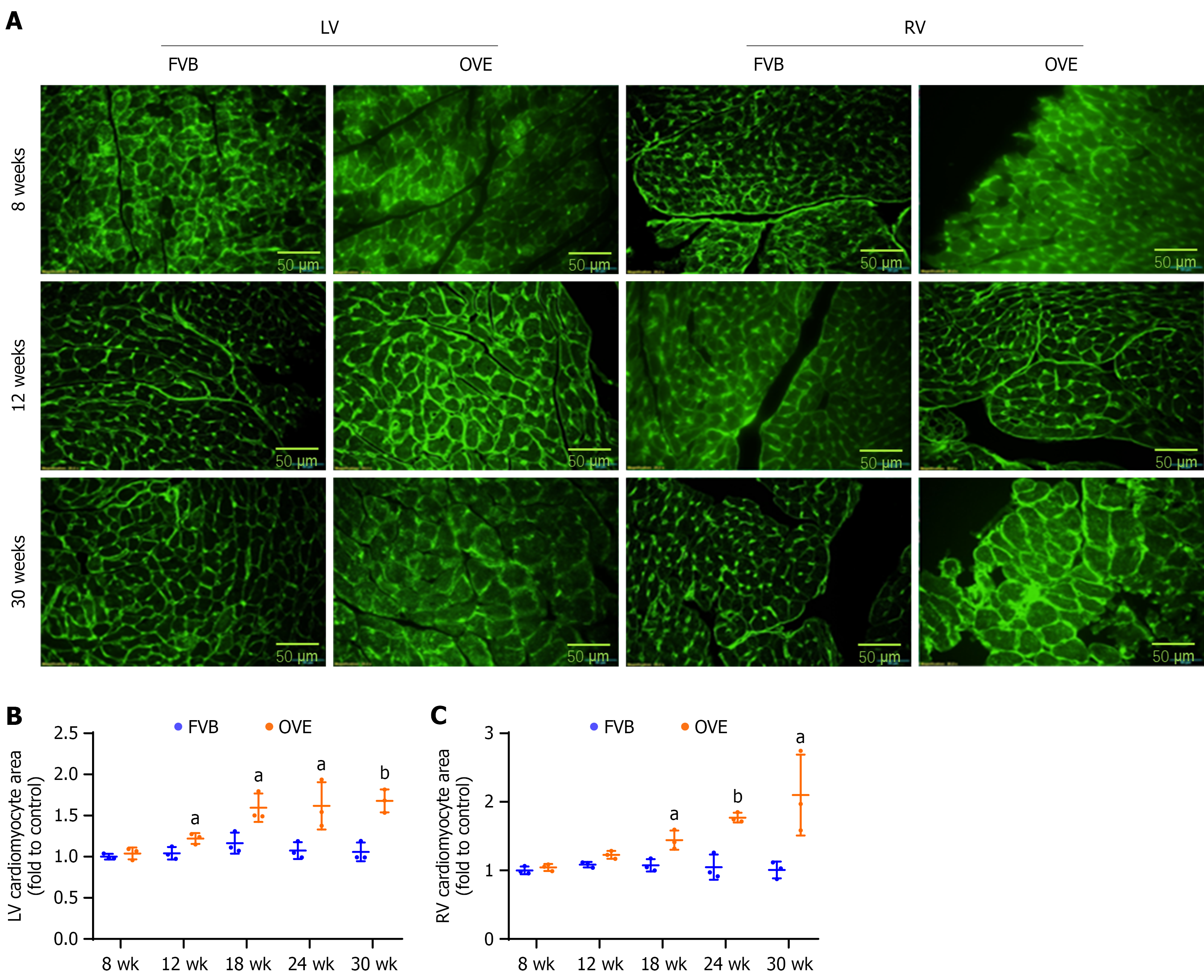
Figure 7 Changes in cardiomyocyte size in the left and right ventricles of OVE26 and FVB mice.
A: Wheat germ agglutinin staining in left ventricle and right ventricle; B: Left ventricle cardiomyocyte cross-sectional area; C: Right ventricle cardiomyocyte cross-sectional area. Data are presented as mean ± SD (n = 3). aP < 0.05 vs control FVB mice; bP < 0.01 vs control FVB mice. LV: Left ventricle; RV: Right ventricle.
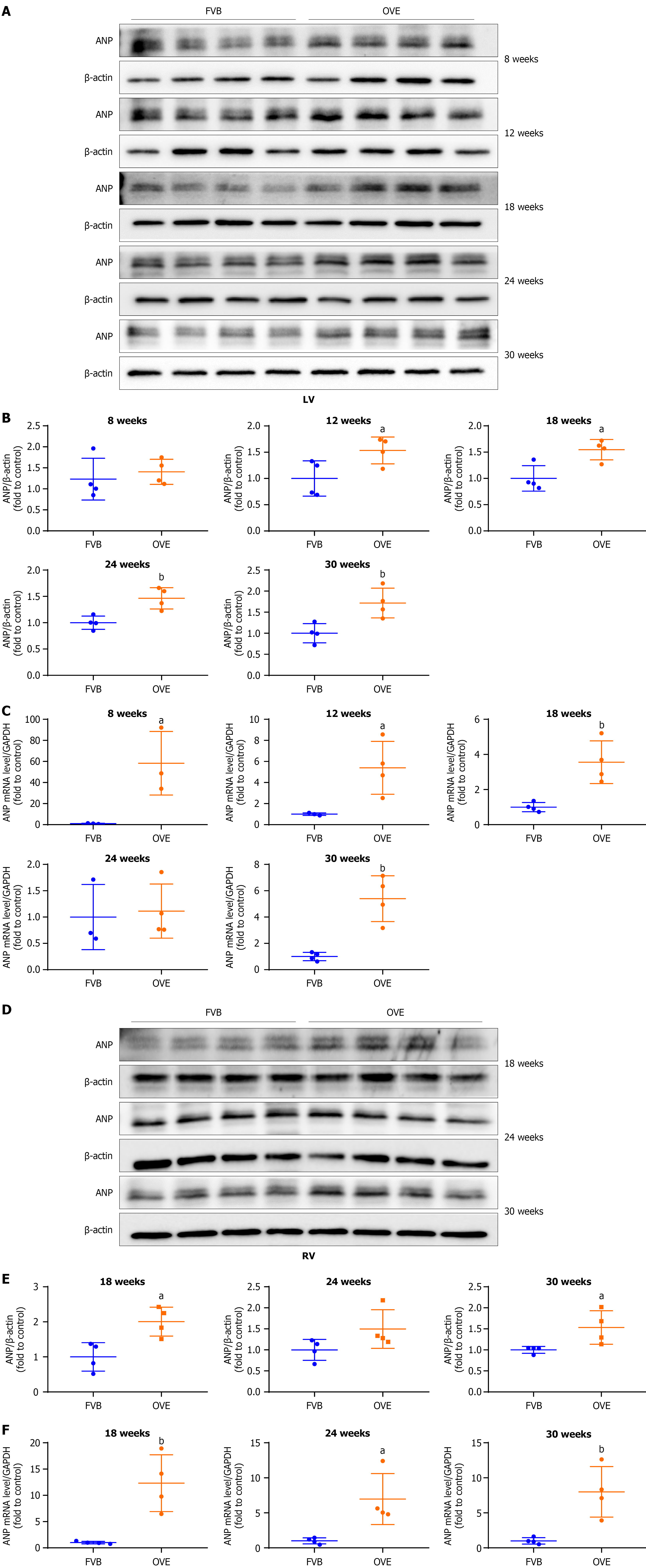
Figure 8 The impact of type 1 diabetes on cardiac hypertrophy in the left and right ventricles of OVE26 and FVB mice.
A and B: Changes in atrial natriuretic peptide protein levels in the left ventricle were assayed by Western blot; C: Changes in atrial natriuretic peptide mRNA expression in the left ventricle were measured by quantitative reverse transcription polymerase chain reaction; D and E: Changes in atrial natriuretic peptide protein levels in the right ventricle were assayed by Western blot; F: Changes in atrial natriuretic peptide mRNA expression in the right ventricle were measured by quantitative reverse transcription polymerase chain reaction. Data are presented as mean ± SD (n = 4). aP < 0.05 vs control FVB mice; bP < 0.01 vs control FVB mice. LV: Left ventricle; ANP: Atrial natriuretic peptide; RV: Right ventricle.
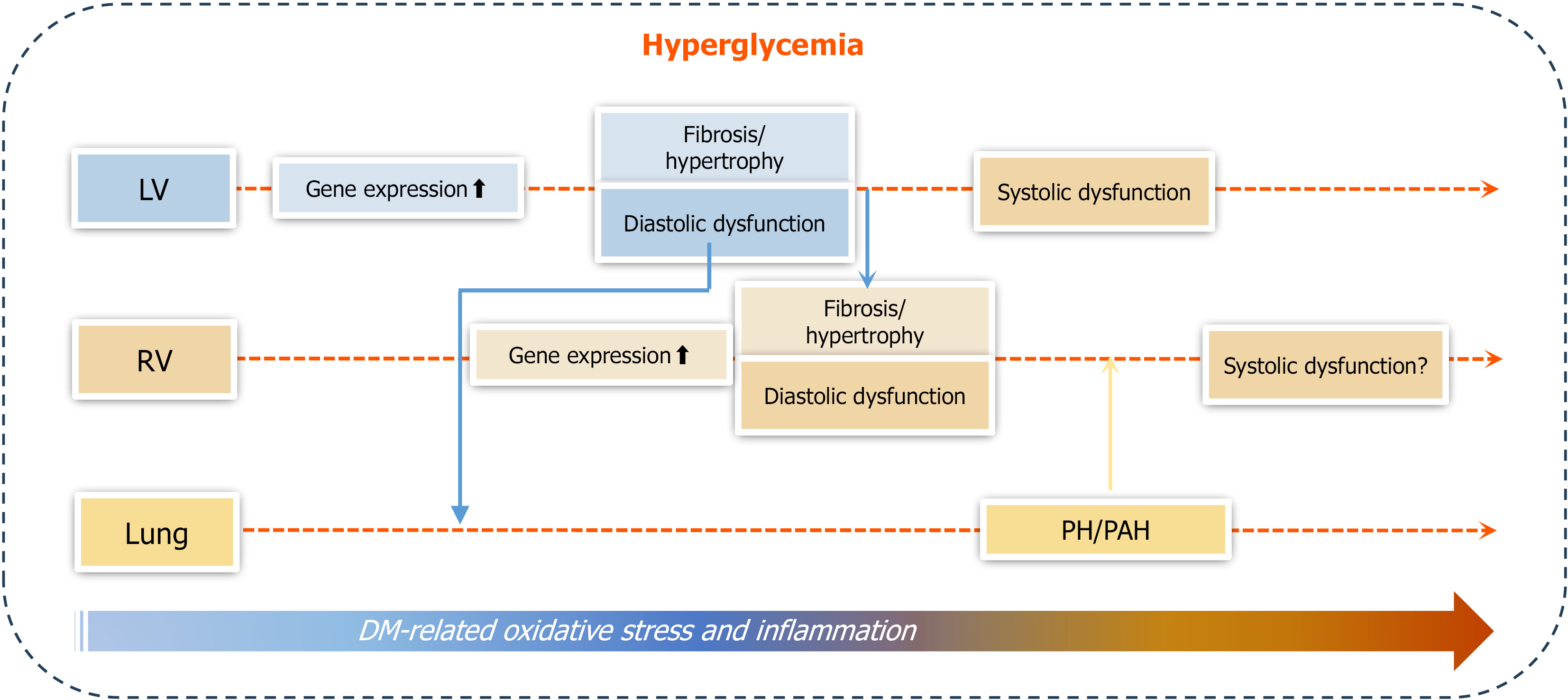
Figure 9 Outline of the main findings of the study.
Under hyperglycemic conditions that promote progressive cardiac oxidative stress and inflammation, right ventricular (RV) systolic function remained comparable to controls, whereas left ventricular (LV) systolic function declined in type 1 diabetes (T1D) mice by 30 weeks of age. RV diastolic dysfunction became evident by 18 weeks and worsened by 30 weeks, while LV diastolic dysfunction showed an increasing trend at 12 weeks, reached significance by 18 weeks, and further progressed by 30 weeks. Additionally, RV diastolic dysfunction was accompanied by RV cardiac fibrosis and hypertrophy, which occurred later than the corresponding changes in the LV. Pulmonary arterial hypertension (PAH) developed in T1D mice, evidenced by increased pulmonary acceleration time to pulmonary ejection time ratio and RV peak systolic pressure at 30 weeks. In conclusion, T1D can cause diabetic cardiomyopathy, including both RV and LV dysfunctions, which are mediated by pathological remodeling (hypertrophy and fibrosis) of each ventricle; however, diastolic dysfunction occurs earlier than systolic dysfunction. Mild PAH is present at later stages of T1D, most likely derived from LV dysfunction. The development of PAH may contribute to RV systolic impairment and remodeling. DM: Diabetes mellitus; LV: Left ventricle; RV: Right ventricle; PH: Pulmonary hypertension; PAH: Pulmonary arterial hypertension.
- Citation: Yu JJ, Han JG, Tan Y, Xu JX, LeBlanc A, Keller BB, Huang J, Cai L. Right ventricular dysfunctions in type 1 diabetic mice: A longitudinal study. World J Diabetes 2025; 16(10): 109526
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/109526.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.109526