©The Author(s) 2025.
World J Diabetes. Oct 15, 2025; 16(10): 108346
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.108346
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.108346
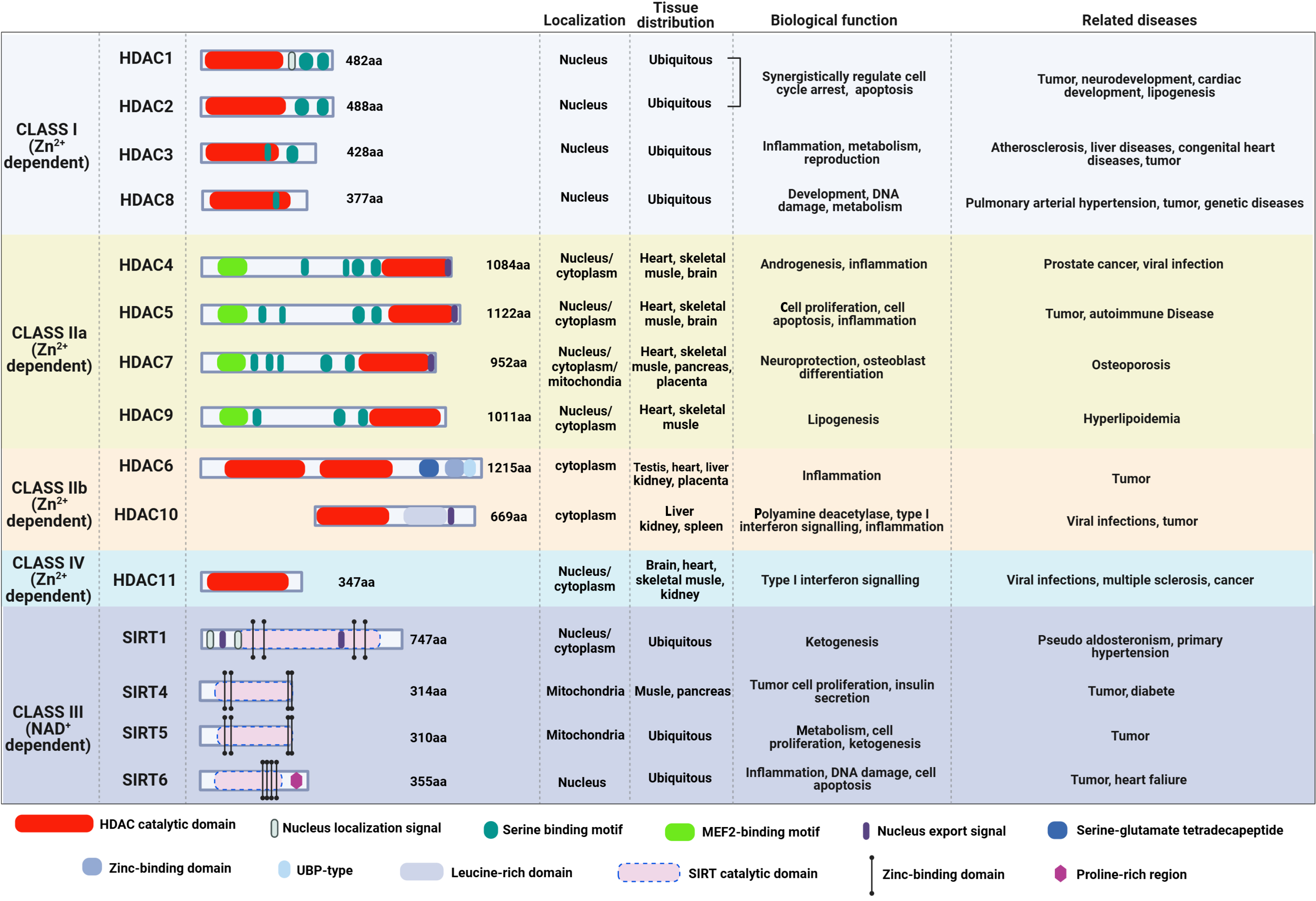
Figure 1 The structure, cellular localization, tissue distribution, biological functions, and related diseases of the four classes of histone deacetylases.
Class I, class II and class IV histone deacetylases (HDACs) are zinc ion-dependent, whereas class III HDACs are nicotinamide adenine dinucleotide-dependent. Class I HDACs are primarily distributed in the nucleus, and they are related to the pathogenesis of tumors, genetic diseases, and metabolic diseases. The Class IV HDAC is distributed in both the nucleus and cytoplasm, and it participates in the development of infectious diseases, nervous system diseases and tumors. Class II and class III HDACs are distributed in the nucleus, cytoplasm, and mitochondria, and they are involved in the pathogenesis of tumors, infectious diseases, metabolic diseases, and cardiovascular diseases. HDAC: Histone deacetylase; Zn2+: Zinc ion; SIRT: Sirtuin; NAD: Nicotinamide adenine dinucleotide; MEF2: Myocyte enhancer factor 2; UBP: Ubiquitin-specific proteases.
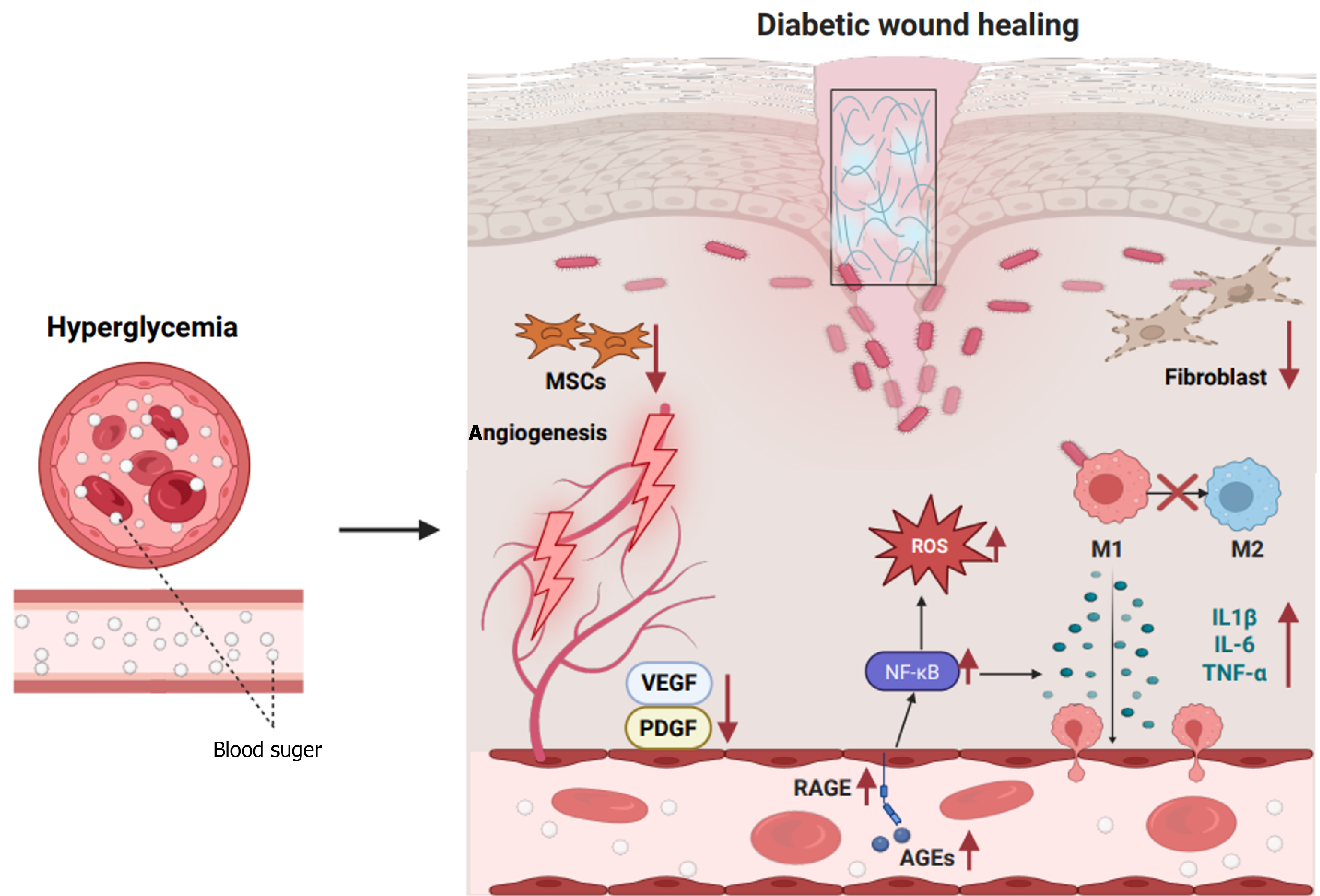
Figure 2 Characteristics of wound healing in diabetes.
In diabetic wounds, persistent hyperglycemia leads to the chaotic release of cytokines and abnormal activation of pathways that play critical roles in wound healing, such as receptor of the advanced glycation end-products, nuclear factor kappa-B, and vascular endothelial growth factor signaling pathway. Thus, chronic inflammation and dysfunction of macrophages, fibroblasts, and mesenchymal stem cells leads to abnormal epithelial regeneration and impaired angiogenesis and extracellular matrix formation, contributing to non-healing wounds and ulcer formation. MSCs: Mesenchymal stem cells; VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor; RAGE: Receptor of the advanced glycation end-products; AGEs: Advanced glycation end-products; ROS: Reactive oxygen species; IL: Interleukin; TNF-α: Tumor necrosis factor-α; NF-κB: Nuclear factor kappa-B.
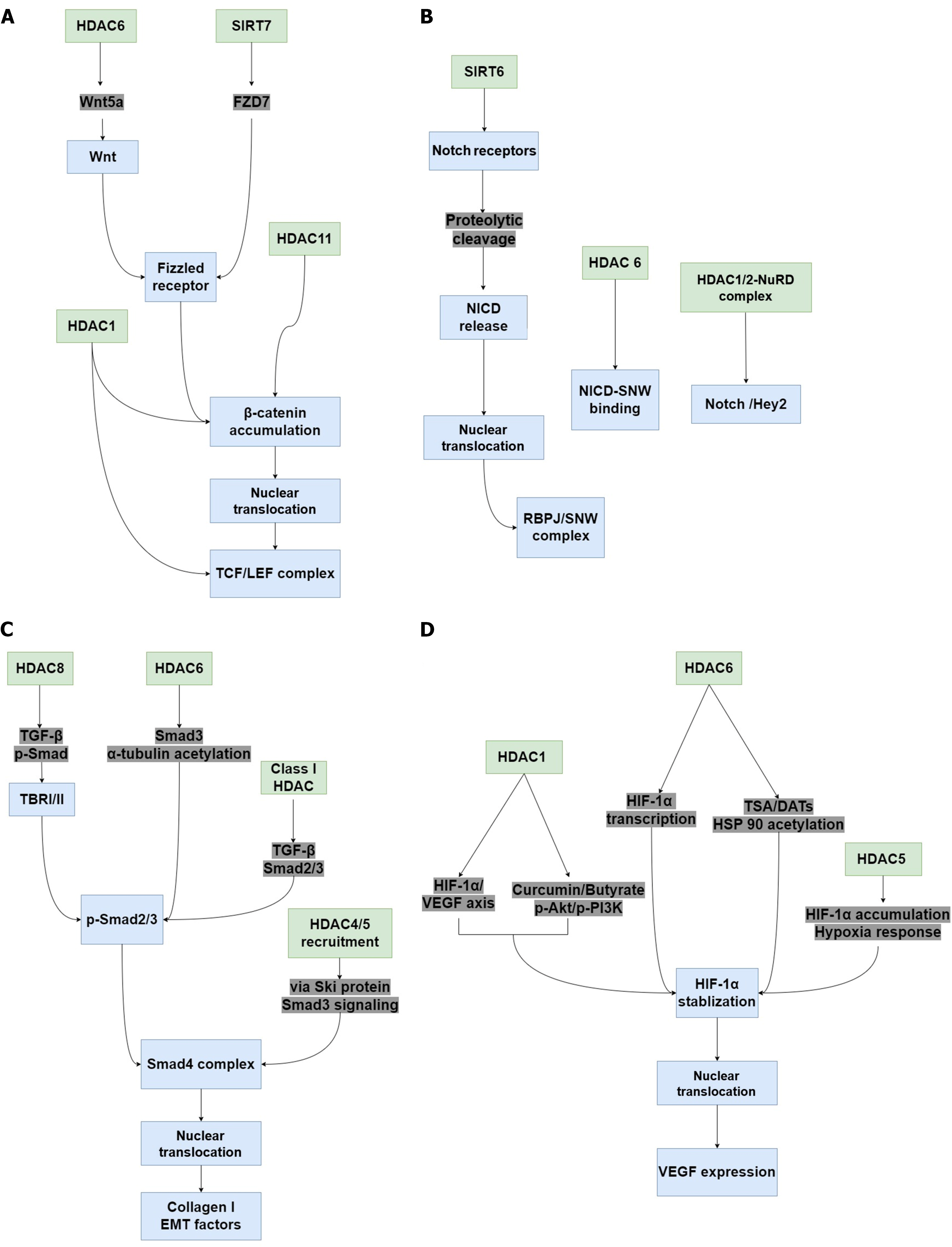
Figure 3 The potential signaling pathways associated with histone deacetylases in diabetic wound healing.
When histone deacetylase (HDAC) activity is altered in tissue, many pathways with critical roles in wound healing, such as Wnt/β-catenin, Notch, transforming growth factor (TGF)-β/suppressor of mother against decapentaplegic (Smad), and hypoxia-inducible factor-1α (HIF-1α)/vascular endothelial growth factor (VEGF) signaling are regulated. A: Inhibiting HDAC1 upregulates TCF and LEF expression, promotes the accumulation and nuclear translocation of β-catenin, and activates the Wnt/β-catenin pathway. HDAC6 increases Wnt5a expression and activates the Wnt signaling pathway. Sirtuin (SIRT) 7 promotes FZD7 expression, contributing to β-catenin stability and activation, thereby activating the Wnt/β-catenin pathway. HDAC11 suppression also promotes β-catenin stability and activates Wnt/β-catenin signaling in vascular smooth muscle cells; B: Silencing SIRT6 Leads to dysregulation of the RBPJ/SNW complex and inhibits Notch signaling. HDAC6 inhibition represses the binding of Notch intracellular cytoplasmic domain and SNW domain containing 1, thereby inhibiting Notch transcriptional responses. Recruitment of HDAC1/2-nucleosome remodeling and deacetylase also inhibits the Notch/Hey2 signaling axis; C: Inhibition of HDAC6 and HDAC8 inactivates Smad3, resulting in α-tubulin acetylation and impairing TGF-β-induced epithelial-mesenchymal transition (EMT). Class I HDAC inhibition inactivates the TGF-β-Smad2/3 signaling axis and decreases type 1 collagen expression induced by TGF-β1. The oncoprotein Sloan-kettering institute recruits HDAC4 and HDAC5, thereby inhibiting TGF-β/Smad3 signaling, leading to the decrease of EMT factors; D: Curcumin and sodium butyrate-induced HDAC1 inhibition suppresses the phospho-protein kinase B/phospho-phosphatidylinositol 3-kinase/HIF-1α/VEGF axis, whereas HDAC1 inhibition by 1,3-diphenylurea induces VEGF expression via HIF-1α, thereby increasing the migration and wound healing effects. HDAC5 overexpression enhances HIF-1α stabilization and nuclear translocation in response to hypoxia. Selective HDAC6 inhibition reduces HIF-1-mediated transcription by deacetylating heat-shock protein 90. HDAC: Histone deacetylase; SIRT: Sirtuin; NICD: Notch intracellular cytoplasmic domain; 2-NuRD: 2-nucleosome remodeling and deacetylase; TGF: Transforming growth factor; p-Smad: Phospho-suppressor of mother against decapentaplegic; TBR: Transforming growth factor-β receptor; Ski: Sloan-kettering institute; EMT: Epithelial-mesenchymal transition; VEGF: Vascular endothelial growth factor; HIF-1α: Hypoxia-inducible factor-1α; p-Akt: Phospho-protein kinase B; p-PI3K: Phospho-phosphatidylinositol 3-kinase; TSA: Trichostatin A; DATs: Diallyl trisulfide; HSP90: Heat-shock protein 90.
- Citation: Zhang F, Ma HG, Zhang B, Jiang LL, Nie KY, Deng CL, Liu Y. Potential roles of histone deacetylases in diabetic wound healing. World J Diabetes 2025; 16(10): 108346
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/108346.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.108346