Published online Oct 15, 2015. doi: 10.4251/wjgo.v7.i10.221
Peer-review started: May 7, 2015
First decision: June 2, 2015
Revised: June 12, 2015
Accepted: August 25, 2015
Article in press: August 28, 2015
Published online: October 15, 2015
Processing time: 173 Days and 20.7 Hours
The immune response to colorectal cancer has proven to be a reliable measure of patient outcome in several studies. However, the complexity of the immune response in this disease is not well understood, particularly the interactions between tumour-associated cells and cells of the innate and adaptive immune system. This review will discuss the relationship between cancer associated fibroblasts and macrophages, as well as between macrophages and T cells, and demonstrate how each population may support or prevent tumour growth in a different immune environment.
Core tip: The outcome of patients with colorectal cancer is influenced by the complex local immune system. Understanding how multiple relationships between immune cells may affect tumour growth or elimination will be key in designing new therapies to treat this disease.
- Citation: Norton SE, Ward-Hartstonge KA, Taylor ES, Kemp RA. Immune cell interplay in colorectal cancer prognosis. World J Gastrointest Oncol 2015; 7(10): 221-232
- URL: https://www.wjgnet.com/1948-5204/full/v7/i10/221.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v7.i10.221
Colorectal cancer (CRC) is the second and third most common cancer in women and men, respectively, worldwide[1]. In most cases, the disease occurs sporadically, but can also be caused by genetic predisposition or prior intestinal inflammation. While resection is often curative, approximately 45% of patients still die from the disease.
The recent introduction of successful immunotherapies against cancer, specifically checkpoint blockade antibodies, has increased attention on the immune response to tumours. These new treatments have provided opportunities for the development of new immune-based therapies for less responsive tumours, such as CRC.
The complexity of the anti-tumour immune response is vast - not only are there multiple cells, these cells interact with each other, and are plastic so can change phenotype and function in response to inflammatory or suppressive signals from the tumour and tumour associated cells[2]. Understanding the relationships between cancer cells and immune cells is critical to understanding and, ultimately, manipulating the tumour immune microenvironment.
The importance of local immunity is particularly true in CRC where the immune response in the gut has been “trained” to ignore commensal microflora, and yet retain the ability to induce an attack against a pathogen. The ability of the gut to do this relies on a series of signals and interactions between bacteria, epithelial cells, and innate cells such as dendritic cells, monocytes and gut resident macrophages. In CRC, there are local adaptive immune cells such as effector T cells likely to have an antitumor effect, and regulatory or inflammatory T cells predicted to have a pro-tumour effect[3].
Recent study of the immune response in CRC has resulted in the development of the Immunoscore, a means of measuring T cell infiltrate into CRCs[4]. The Immunoscore thus far has shown to be predictive of outcome and also superior to other methods for staging patients. Innate immune responses, particularly those involving tumour associated macrophages (TAMs), have been studied and data show that the frequency of these cells infiltrating the tumour can be associated with poor patient outcome, although this is controversial[5].
Immune responses against colorectal tumours can be detected in early stage cancers, indicating that the immune system is capable of recognizing a tumour[6]. However, the tumour produces molecules that inhibit immune cell infiltration, that reduce activity of immune cells, or that change the phenotype of immune cells to a less effective anti-tumour function, ultimately allowing tumour outgrowth[7].
The inflammatory immune environment underlying tumour initiation and progression in CRC has been reviewed extensively[8], although much of the supporting data relies on animal models of colitis-induced cancer[9]. However, colitis-associated cancer accounts for only a small percentage (1%-4%) of CRC cases in humans[10]. The influence of inflammation mediated by immune cells in established familial or sporadic human CRC has been much less studied. In addition, new data demonstrate an impressive complexity of innate and adaptive immune cells[11], suggesting that some associations with cancer progression may have been too simplistic in their interpretation.
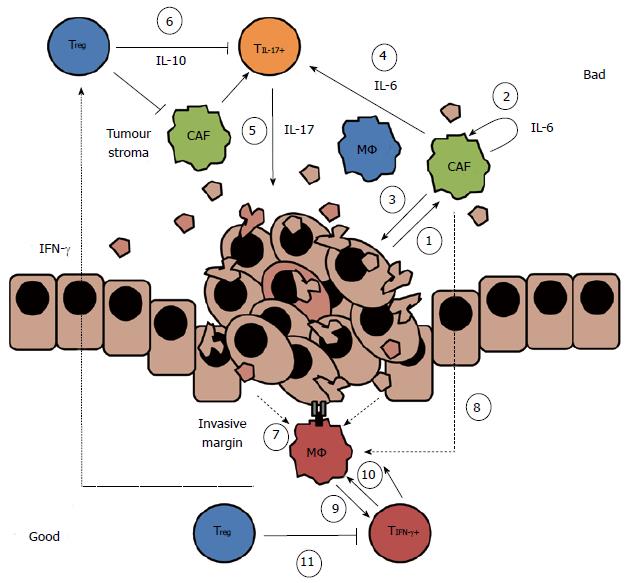
This review will concentrate on the networks of innate and adaptive immune cells, and tumour-associated immune cells in established CRC, and how these interactions can influence subsequent patient outcome (Figure 1). Despite recent interest in the immunology of CRC, there are limited experimental data studying the complexity of the immune response and the interactions between cancer cells and immune cells, particularly in humans. We will discuss (1) the interplay between the tumour stromal cells [particularly cancer-associated fibroblasts (CAFs)] and the macrophages infiltrating the tumour; and (2) the interactions between macrophages and T cells and how T cell populations may influence each other. We will attempt to describe the complexity and plasticity of these immune populations and discuss how they can be used to better understand the disease and to predict patient outcomes.
Fibroblasts are a key component of the connective tissue and are found embedded in the extracellular matrix (ECM). Fibroblasts have important roles in tissue homeostasis and remodelling. They produce multiple cytokines and can therefore modulate the immune microenvironment. Fibroblasts found in tumour stroma are referred to as CAFs.
The exact origin of CAFs is not clear. It has been proposed that they are cancer cells that have undergone an epithelial-mesenchymal transition[12]. Other research suggests that fibroblasts mature from fibrocytes that, in turn, have differentiated from monocytes[13] and thus have a similar haematopoietic lineage to macrophages. It is then not surprising that there is significant phenotypic overlap between CAFs and macrophages. CAFs do not express the immune cell marker CD45, however they can express CD68, a marker commonly used to differentiate macrophages[14]. Madar et al[15] hypothesised that CAFs were the result of convergent differentiation from any one of multiple pathways within the tumour microenvironment, and that CAF is a description of a functional state rather than a defined lineage.
CAFS may have a direct role in promoting CRC cell growth. Primary CAFs cultured from human colorectal tumours developed into distinct populations, some inducing a pro-migratory effect on CRC cells[16]. These pro-tumour CAFS had a distinct genetic signature with significant prognostic value. In addition, CAFs have been shown to promote metastases in CRC[17].
Because of their role in in tissue homeostasis, CAFs are able to promote tumour growth via similar pathways, including via inflammatory mediators consistent with the wound healing process. These pathways were reviewed recently[12], so we will discuss the role of CAFs briefly, and focus on their influence on innate immune cells. CAF-derived inflammatory mediators can both promote tumour growth and tumour invasion (Figure 1). An important inflammatory cytokine produced by CAFs in the regulation of wound healing, interleukin (IL)-6, is also associated with disease progression in CRC.
IL-6 in patient serum has been associated with poor patient prognosis in many cancers, including CRC[18]. IL-6 promotes cell survival and supports the production of vascular endothelial growth factor (VEGF) from both tumour and immune cells. VEGF was associated with enhanced tumour progression and poor patient prognosis in CRC[19], likely through its role in angiogenesis[20]. CAFs produced more IL-6 than cancer cells, and CAF-derived IL-6 was increased in the presence of CRC cell lines[21]. In response to greater IL-6 production, CAFs up-regulated production of VEGF, leading to the proposal that the indirect effect of IL-6 on tumour growth via CAFs was more important that the direct effect of IL-6 on tumour cells[21].
Other inflammatory mediators produced by CAFs also increase IL-6 production, including IL-1β and TNFα[21]. In patients, high plasma levels of the TNFα receptor, TNFR-2, were associated with an increased relative risk of CRC[22]. Expression of both VEGF[23] and FSTL-1[24] (which enhances inflammatory cytokine and chemokine expression) was increased in CRC-associated CAFs. Chemotherapy, known to cause inflammation as cancer cells are killed[25], resulted in increased numbers of active CAFs in a cohort of CRC patients[26], and enhanced tumour growth in in vitro assays.
Fibroblasts both recruit, and are recruited by, monocytes/macrophages[12]. CAFs have been shown to recruit monocytes to the tumour microenvironment and thus may directly affect the local macrophage compartment. Indeed, Schellerer et al[27] showed there were more Intracellular Adhesion Molecule-1+ fibroblasts in tumour tissue than healthy bowel tissue from CRC patients, implying that cancer-associated cells have a higher affinity for monocytic cells. In an in vitro human breast cancer model, CAFs produced high levels of the chemokines CCL2 and CCL5 that attracted monocytes[28,29]. The production of these chemokines required IL-6, in a suggested IL-6-CCL2 auto-regulatory cycle[29]. CCL2 and CCL5 were also produced by tumour cells as well as the recruited monocyte/macrophages, creating a positive feedback loop and generating an inflammatory tumour microenvironment[28].
The prognostic significance of TAMs is controversial, particularly in CRC[30]. Macrophages are myeloid derived cells of the innate immune system. They are potent phagocytes and are involved in clearance of pathogens and cellular debris. They also initiate the adaptive response by functioning as antigen presenting cells (APCs). Macrophages reside in all tissues where they also maintain tissue integrity (reviewed in[31]). The phenotype and ontogeny of tissue resident macrophages varies between tissues. Some are freshly recruited bone marrow-monocyte derived macrophages, whereas others derive from the embryonic yolk sac (reviewed in[32]). In most adult tissue, however, resident macrophages are fetal liver derived. Both the ontogeny and microenvironment of resident macrophages influence their phenotype. As such, resident macrophage populations are often heterogeneous.
The phenotypic diversity of macrophages makes analysis of subpopulations challenging. A great deal of work has been undertaken assessing macrophage subsets using only one or two surface markers to determine function. However, a recent opinion suggests this approach to be misleading, due to the many causes of diversity[33]. Instead, multiple markers must be used to estimate the function of macrophage populations, or, where possible, primary functional data. It has been proposed that minimum reporting standards be introduced to allow better meta-analysis of macrophage data between research groups. This type of approach is paramount when assessing highly plastic macrophages, for example, human macrophages were shown to switch from anti-inflammatory to pro-inflammatory cytokine production within 24 h in response to IFNγ, Granulocyte-Monocyte Colony Stimulating Factor and lipopolysaccharide in vitro[34].
The link between macrophage infiltration and prognosis in CRC is still poorly understood. While some studies have shown a positive correlation between macrophage infiltration and patient prognosis, others have shown the opposite[30]. For example, Forssell et al[35] demonstrated that a dense macrophage infiltration at the tumour invasive margin was associated with improved patient prognosis, and that macrophage inhibition of tumour spread and growth required direct cell-to-cell contact in an in vitro CRC model. In contrast, Kang et al[36] demonstrated that intra-tumoural TAM count correlated with parameters of worse disease progression (depth of invasion, lymph node metastasis and stage). Using an in vitro co-culture macrophage and CRC cell lines these researchers also demonstrated that macrophages increased cancer cell invasiveness and migration. It may be that the conflicting data relating to the role of macrophages in CRC prognosis is due to inaccuracies of reporting culture conditions or a lack of detailed phenotype[33].
Regular interaction between immune cells and microbes in the gut creates an immune environment that must be tightly regulated. Gut resident macrophages provide an important role in regulating this commensal barrier. These particular macrophages have an anergic phenotype; they destroy any bacteria that breach the epithelial barrier but do not initiate an immune reaction against them under homeostatic conditions[37,38].
Unlike most tissue resident macrophage populations, gut resident macrophages are bone marrow derived[32,37]. Newly recruited monocytes undergo a conditioning process, mediated by the gut epithelia, that matures them into the resident anergic phenotype. However, upon acute inflammatory insult, such as that seen in inflammatory bowel disorders, this conditioning process becomes dysregulated, resulting in a mature macrophage population that acquires and maintains migratory and inflammatory characteristics[37,39].
In the context of CRC, monocyte conditioning is unlikely to be modulated only by inflammation, but also factors actively produced by the tumour[40], hypoxic conditions[41] and glucose starvation[28]. As a result, unique macrophage populations will exist depending strongly on the context of the local microenvironment. Hence, describing a homogeneous macrophage population in CRC can be misleading.
It is well documented that TAMs can promote tumour growth, both directly on tumour cells, and indirectly via cells in the tumour microenvironment (reviewed in[42]). The human monocytic cell line, THP-1, produced IL-6 in the presence of a colorectal cell line[43], and macrophage-derived IL-6 induced expression of IL-6 by the HT29 CRC cell line[44]. TAMs also upregulated the expression of metalloproteinase (MMP)-2 and MMP-9 on cancer cells, molecules associated with lymph node metastasis[42,45]. TAM-derived IL-6 promoted STAT-3 mediated IL-10 production in CRC cells, a cytokine that has also been associated with poor patient prognosis[46]. In fact, p-STAT3 overexpression in the tumours of CRC patients is significantly correlated with tumour specific mortality[47]. Together, these studies demonstrate that TAMs and CAFs promote an environment to support tumour progression in CRC.
Macrophages have been shown to preferentially migrate to hypoxic regions of tumours[48]. In a mouse model of colitis-associated CRC, repression of hypoxia inducible factor 1 led to decreased macrophage infiltration in tumours[49]. Interestingly, under hypoxic conditions, macrophages can acquire a phenotype similar to that seen in macrophages involved in wound-healing role - a phenotype likely to promote tumour growth. More specifically, human macrophages in hypoxic conditions (0.5% oxygen) up-regulated expression of both VEGF and glucose transporter (GLUT)-1 compared to normoxia[50]. GLUT-1 is the primary rate limiting glucose transporter in inflammatory macrophages[51]. Using transgenic RAW264.7 macrophages that stably overexpressed GLUT-1, it was shown that high glucose trafficking via GLUT-1 promoted a pro-inflammatory macrophage phenotype[51]. It is then possible to hypothesise that under hypoxic conditions such as those in a tumour, macrophages up-regulate GLUT-1 in an attempt to scavenge more glucose in a low glucose environment.
Beyond the production of inflammatory modulators, colorectal tumours also cause barrier defects, which allow for contact between immune cells and microbial products. Myeloid cells showed an increase in production of the inflammatory cytokine IL-23 under inflammatory conditions compared with homeostatic conditions in the APCmin mouse model of CRC[52]. IL-23 stimulates and maintains IL-17 production from both tumour cells and T cells. In a mouse model of colitis associated CRC, IL-23- and IL-17-mediated inflammation disrupted the commensal microflora, and created a population of microbes that promoted tumour progression[53]. Furthermore, confocal microscopy of human CRC patient samples revealed that IL-17 production was not limited to T cells, but was also co-expressed with the myeloid cell marker, CD68[54]. These findings indicate that myeloid cells such as macrophages may be capable of producing IL-17 in CRC in vivo.
A high infiltrate of macrophages at the invasive margin of colorectal tumours has been associated with improved patient prognosis[35], and macrophages at the invasive margin of patients with CRC displayed characteristics of an anti-tumour phenotype[55]. These cells expressed the co-stimulatory molecules CD80 and CD86, and apoptotic signalling molecule FasL at greater levels than stromal macrophages. Moreover, macrophages have been closely associated with apoptotic cancer cells along the invasive margin[56] and, using cell lines, CRC TAMs have been observed to be highly phagocytic[57]. In an in vitro model of macrophage differentiation, with either human peripheral blood mononuclear cells or murine bone marrow derived macrophages, IL-6 promoted maintenance of the established macrophage phenotype, even when the original cytokine stimuli were removed[58]. Because macrophages themselves also produce IL-6, as well as respond to CAF-produced IL-6, they are especially sensitive to the conditioning signals in their immediate environment. For example, macrophages pre-exposed to IL-4/13, acquired a phenotype characterised by increased IL-10 production in response to IL-6. However, macrophages pre-exposed to IFNγ, acquired a phenotype characterised by production of IL-1β and TNFα in the presence of IL-6. We propose that, in CRC, IL-6 both promotes and inhibits tumour growth via uniquely located macrophage populations (Figure 1).
While considerable evidence on the role of T cells in preventing tumour growth in animal models has been acquired over decades, it was not until 2005 that a definitive role for T cells in CRC outcome was shown in patients[59]. Galon et al[60] demonstrated, in 2006, that a high infiltrate of CD3+ CD8+ CD45RO+ T cells at the invasive margin and the centre of the tumour was predictive of improved Overall Survival and Disease-Free Survival in a large cohort of people with CRC. Since then, these data have been confirmed by other groups, and have led to the introduction of the Immunoscore to quantify infiltrating T cells in clinical practice[61].
The Immunoscore uses immunohistochemistry techniques to quantify the CD3+ CD8+ T cell infiltrate cell analysis at the centre of the tumour and at the invasive margin in people with CRC[4]. To date, the Immunoscore has proven to provide an accurate staging diagnosis as well as to predict patient outcome[62]. Although the Immunoscore is an improvement on the current staging methods for CRC, its efficacy may be hindered by the interference of T cell subsets that are not associated with good prognosis.
Although it remains clear that the infiltrate of CD3+ CD8+ CD45RO+ T cells is associated with good patient prognosis in CRC, some T cell subsets have been associated with poor prognosis. Specifically, inflammatory CD4+ T cells (Th17 cells), usually measured via production of the cytokine IL-17; and regulatory CD4+ T cells (Tregs), often quantified by expression of the transcription factor, FoxP3; have been associated with both good and bad outcomes (reviewed in[63]). In addition, a low ratio of CD4+ to CD8+ T cells is associated with improved outcome[64]. Interestingly, Väyrynen et al[65] measured infiltrates of innate cells and adaptive cells in 117 CRC patients and found three parameters associated with Disease Free Survival at 24 mo: High infiltration of CD3+ cells at the invasive margin and high infiltration of FoxP3+ cells at the invasive margin and at the tumour stroma. Taken together, these findings indicate that that CD8+ T cells may be more effective than CD4+ T cells in an anti-tumour immune response, or that beneficial CD4+ T cell subsets are masked by subsets associated with poor outcome[64]. The phenotype of T cells resident in the tumour is controlled by the local cytokine environment, particularly APCs such as macrophages. The efficacy of the T cell response against the tumour is therefore dependent on interactions with other cells (Figure 1).
T cells respond to specific antigens expressed by pathogens or tumours. These antigens are presented by a subset of immune cells, APCs, including dendritic cells and macrophages, but also non-immune cells such as epithelial cells or tumour cells. The T cell infiltrate in CRC is likely to be maximally effective if those cells are specific for tumour antigens.
Nagorsen et al[66] used HLA tetramer analysis to show that tumour specific CD8+ T cells in the blood were not correlated with improved clinical outcome in people with CRC or breast cancer, highlighting the need to study the tumour microenvironment. In a separate study, tumour-associated-antigen specific T cells were detected in 30%-40% of patients with CRC[67]. This study also showed that only a small subpopulation of infiltrating T cells could respond to tumour-associated antigens, indicating that not all infiltrating T cells were tumour-specific. Recently, Reissfelder et al[68] proposed that a subpopulation of tumour antigen-specific T cells infiltrating the tumours of people with CRC was responsible for the prognostic impact of T cells shown by other studies.
Multiple studies in animals have shown that cytotoxic T cells, via IFNγ, perforin and granzymes, can destroy established tumours. Gene cluster analysis of a large cohort of 602 patients with early stage CRC revealed that those patients with high CD8+ and CD45RO+ T cell infiltrates into the tumour also had increased expression of genes associated with anti-tumour responses compared with those patients with low CD8+ and CD45RO+ T cell infiltrates into the tumour[69]. The up-regulated anti-tumour gene signature included genes encoding for granzymes and perforin, as well as effector molecules such as IFNγ and the related transcription factor T-bet. The expression of Granzyme B protein in tumours from CRC patients was also associated with improved survival[70]. These, and many other data, support a role for CD8+ T cells and T cells producing the effector molecules IFNγ and granzymes in eliminating CRC.
Effective T cells must become activated by interactions with APCs presenting antigen in the context of an appropriate cytokine milieu. TAMs were shown to express higher levels of the co-stimulatory molecule, CD80, than tumour stromal cells, indicating that these cells could activate T cells within the tumour[55]. In addition, using a multi-cellular tumour spheroid model, Ong et al[71] showed that TAMs up-regulated the expression of CD25 and IFNγ in T cells better than in vitro macrophages did. They also showed that the frequency of TAMs in human CRC tumours correlated with the frequency of infiltrating IFNγ-producing T cells in vivo. These data indicate that TAMs may be able to promote effector T cell responses within the tumour microenvironment (Figure 1). We propose that effective anti-tumour immunity is determined by TAM-T cell interactions occurring at the invasive margin in CRC.
Inflammatory T cells [defined here as IL-17-producing (or Th17) cells] are important in antimicrobial responses in the gut (reviewed in[72]). The acquisition of an IL-17-producing phenotype occurs when naïve T cells are activated in the presence of IL-6, IL-1β, TGFβ and IL-23; the maintenance of the phenotype is regulated by these same cytokines. Inflammatory IL-17 responses involve production of cytokines (especially IL-17) that recruit monocytes and neutrophils to sites of inflammation[73]. These innate cells in turn produce the same cytokines to promote ongoing Th17 responses[74].
IL-17 production in CRC has been associated with low Disease-Free Survival and Overall Survival[75] but the exact role of Th17 cells in CRC is not understood. Liu et al[54] showed that Th17 induced production of VEGF in CRC cell lines in vitro, which decreased T cell production of IFNγ and Granzyme B. This study also showed that in human CRC tumours, high expression of IL-17 correlated with high VEGF expression. VEGF expression has been inversely correlated with CD8+ CD45RO+ T cell infiltrate in tumours of CRC patients[69].
CAFs may be activated via microbial products that cross the compromised epithelial barrier and promote IL-23 secretion[52], further supporting Th17 responses. Using a mouse model of CRC, Numasaki et al[76] showed that tumour cells engineered to express IL-17 led to increased production of angiogenic factors, including VEGF, not only by tumour cells, but also by CAFs. Th17 responses may therefore directly aid in the inflammatory responses of innate cells in CRC.
Liu et al[54] showed that IL-17 was increased in tumour tissue compared to healthy bowel tissue in a cohort of CRC patients, and that it was strongly correlated with overall survival. IL-17 added to human CRC cells ex vivo stimulated glucose metabolism by the tumour cells[77]. IL-17 promoted tumour growth through a STAT3-mediated pathway in CRC patients[78]; this result has also been shown in other models of cancer[79]. Together, these data indicate that the presence of intra-tumoural IL-17 may support tumour angiogenesis via VEGF and IL-6, and directly promote tumour cell proliferation (Figure 1).
Regulatory T cells (Tregs) suppress inflammatory responses in the healthy gut and regulate normal immune responses by inhibiting proliferation and activity of effector T cells. Induced Tregs acquire a suppressive phenotype in the presence of cytokines such as TGFβ; the regulatory phenotype is characterised by up-regulation of the transcription factor FoxP3 and the production of IL-10, amongst other cytokines (reviewed in[80]). Dysregulated immune responses of the gut, for example inflammatory bowel diseases, are often typified by a high infiltrate of Tregs. In the presence of excess inflammatory cytokines from innate and adaptive immune cells, particularly IL-6, Tregs can convert into IL-17 inflammatory cells, or maintain their regulatory function while co-producing IL-17 (reviewed in[81]). Conversely, Treg differentiation can also inhibit the generation of Th17 cells.
In many human cancers an accumulation of Tregs is associated with poor patient outcome, presumably by suppressing effector T cell responses against the tumour[63]. Controversially, in CRC, Tregs have been associated with both good and poor outcomes for patients[82]. It is possible that because Tregs suppress other T cells, they could impair the function of anti-tumour effector cells as well as pro-tumour inflammatory Th17 cells.
Using a complex library of tumour associated antigen-polypeptides, tumour-antigen specific Tregs were identified in the blood of CRC patients[83] providing evidence that these cells have the potential to inhibit specific anti-tumour immune responses. Therefore, the nature of the tumour immune microenvironment may influence the action of infiltrating Tregs.
Tumour-specific Tregs isolated from ovarian tumours suppressed effector CD8+ T cell production of IFNγin vitro after stimulation with tumour antigen[84]. The infiltrate of Tregs correlated with poor patient prognosis. In CRC patients with recurrent disease, specific T cell responses to the tumour antigens CEA and 5T4 were also suppressed[85]. In the same study, tumour specific Tregs and effector T cells were required to have the same specificity in order for Tregs to suppress the T cell response. Indeed, in an independent study, while tumour-antigen specific Tregs were identified in the tumours of CRC patients, the specificity of the majority of these cells was distinct from that of the effector and memory T cells in the same patients[83]. By depleting Tregs ex vivo in culture, only the effector anti-tumour T cells with the same specificity as the Tregs were increased.
The mechanism of Treg mediated suppression in tumour environments is not clear. In a mouse model of transplantable CRC using CMT93 cells, TAMs were able to recruit CCR6+ Tregs to the tumour via production of the chemokine CCL20[86]. The infiltrate of Treg cells was associated with tumour development. Similarly, in breast cancer patients, the infiltrate of CCR6+ Tregs into the tumour was inversely correlated with IFNγ production from tumour infiltrating CD8+ T cells[87]. Using flow cytometry, the authors showed that CCR6+ Tregs, but not CCR6- Tregs were associated with poor survival in breast cancer patients. This leads us to hypothesise that, in CRC, tumour-antigen specific Treg populations are actively recruited to the tumour by TAMs and inhibit the anti-tumour immune response, leading to poor prognosis of patients.
Tregs recovered from blood of CRC patients were shown to inhibit the proliferation of Th17 cells sorted from blood and to suppress IL-17 production[88]. It is possible, therefore, that an accumulation of Tregs in the tumour of some CRC patients suppresses the inflammatory Th17 cell response rather than the anti-tumour effector response, leading to improved patient outcome.
Tregs are characterised by production of IL-10, a multifunctional cytokine generally believed to support anti-inflammatory immune responses. CRC patients had elevated levels of serum IL-10, and IL-10 remained high in those patients who had recurrent disease following tumour resection[89]. However, it has become clear that treatment of cancer with IL-10 could lead to improved anti-tumour responses (reviewed in[90]). In human CRC, the amount of IL-17 was inversely correlated with the amount of IL-10 produced[91]. Interestingly, it has been shown that IL-10 mediated suppression of IL-17 responses was dependent on type-I IFN signalling[92]. Further, Mumm et al[93] showed that IL-10 production induced the production of IFNγ and granzymes from human effector CD8+ T cells in vitro. Together these data suggest that IL-10 production from Tregs may, in fact, inhibit pro-tumour inflammatory responses as well as promote anti-tumour immune responses. Phase 1 clinical trials have now begun in advanced solid tumours using recombinant human IL-10 as a therapy (https://clinicaltrials.gov/show/NCT02009449).
Studying the immune response to CRC is difficult because of the complexity of both the gut immune response and the tumour microenvironment. As with most human studies, much of what has been studied has been observational and compounded by individual patient variation and individual tumour variation. The vast majority of CRC cases in humans are sporadic and the mutations that lead to tumour initiation and progression, and therefore immune responses, differ from person to person. Further, while animal models of CRC have provided useful information, their ability to truly mimic human disease is limited (reviewed in[94]). The two most commonly used models represent colitis-associated CRC (1%-4% of human CRC) or APCmin mice representing familial CRC (about 20% of human CRC)[95]. We (and others[96,97]) have developed orthotopic surgical murine models of CRC that result in a tumour immune microenvironment more similar to that seen in sporadic human CRC than other mouse models. It is possible these models may be used to test new immune-based interventions.
Two new immune-based drugs have recently been introduced in the treatment of cancer - anti-CTLA-4 (ipilimumab) and anti-PD-L1/anti-PD-1 (nivolumab or pembrolizumab). Both types of drugs act to prevent the tumour-mediated suppression of effector T cell responses, and have been successful in melanoma (reviewed in[98]). However, both checkpoint blockade drugs have shown much less success in CRC[99-102]. The reasons behind this are unclear but it has been shown that many colorectal tumours do not express PD-L1, the ligand for PD-1. Therefore, if the suppressive effect of PD-L1 on anti-tumour T cells is absent, then therapy targeting the PD-1 pathway is unlikely to be successful[101]. However, it has recently been shown that microsatellite instability (MSI) high CRC tumours (15% of CRC tumours that have mutations in mismatch repair genes and are more immunogenic) expressed more PDL1 than MSI low tumours, indicating that checkpoint blockade may be more successful in the MSI high subset of CRC patients[103]. Clinical trials using anti-PD1 therapy in such a subset of patients are now underway to exploit this possibility.
Adoptive cell therapy (ACT) has been trialled in CRC to some success. Karlsson et al[104] used ex vivo T cells (recovered from tumour-draining lymph nodes) of CRC patients as a therapy. No side effects were observed and complete responses were seen in 4 out of 9 patients with metastatic disease. A Phase II trial is currently being undertaken to further test ACT in patients with metastatic CRC (https://clinicaltrials.gov/ct2/show/NCT01174121). The use of genetically engineered tumour-antigen specific T cells has been less successful in CRC. T cells genetically engineered to target carcinogenic embryonic antigen (CEA) caused a measurable decrease in serum CEA levels in 4/4 CRC patients treated but also induced severe colitis in all patients[105], consistent with studies in other cancers. Targeting neo-antigens in tumours and individualising therapy may be the way forward in ACT of CRC.
Recent technological breakthroughs have allowed the analysis of single cells, providing enormous amounts of data on the immune system (reviewed in[11]). These data provide novel insights into the function and complex connectivity of immune cells. This new network approach to studying immunology is likely to transform our understanding of the immune microenvironment of individuals with CRC. The immune response to CRC in humans is complex and involves a panoply of cells interacting with each other and the tumour. Patient outcome is unlikely to be accurately predicted by measuring one immune parameter independently. Moreover, any new immune-based therapies will need to take into account the pro- as well as anti-tumour activities of specific innate and adaptive immune cells.
| 1. | Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, Kuipers EJ. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 954] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 2. | Hölzel M, Bovier A, Tüting T. Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat Rev Cancer. 2013;13:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 3. | Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3003] [Cited by in RCA: 3078] [Article Influence: 219.9] [Reference Citation Analysis (7)] |
| 4. | Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 632] [Cited by in RCA: 645] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 5. | Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 3008] [Article Influence: 188.0] [Reference Citation Analysis (0)] |
| 6. | Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, Laghi L, Allavena P, Mantovani A, Marchesi F. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20:2147-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 7. | Devaud C, John LB, Westwood JA, Darcy PK, Kershaw MH. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology. 2013;2:e25961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Djaldetti M, Bessler H. Modulators affecting the immune dialogue between human immune and colon cancer cells. World J Gastrointest Oncol. 2014;6:129-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Antoniou E, Margonis GA, Angelou A, Zografos GC, Pikoulis E. Cytokine networks in animal models of colitis-associated cancer. Anticancer Res. 2015;35:19-24. [PubMed] |
| 10. | Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53-61. [PubMed] |
| 11. | Chattopadhyay PK, Gierahn TM, Roederer M, Love JC. Single-cell technologies for monitoring immune systems. Nat Immunol. 2014;15:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 12. | Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211:1503-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 719] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 13. | Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331-4339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 732] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 14. | Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 403] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 15. | Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’--more than meets the eye. Trends Mol Med. 2013;19:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 16. | Herrera M, Islam AB, Herrera A, Martín P, García V, Silva J, Garcia JM, Salas C, Casal I, de Herreros AG. Functional heterogeneity of cancer-associated fibroblasts from human colon tumors shows specific prognostic gene expression signature. Clin Cancer Res. 2013;19:5914-5926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Tommelein J, Verset L, Boterberg T, Demetter P, Bracke M, De Wever O. Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer. Front Oncol. 2015;5:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 18. | Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients--a summary of published results. Int J Colorectal Dis. 2010;25:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer. 2003;97:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW. Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res. 2002;62:7042-7049. [PubMed] |
| 21. | Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. 2014;110:469-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 328] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 22. | Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140:799-808, quiz e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Koshida Y, Kuranami M, Watanabe M. Interaction between stromal fibroblasts and colorectal cancer cells in the expression of vascular endothelial growth factor. J Surg Res. 2006;134:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Torres S, Bartolomé RA, Mendes M, Barderas R, Fernandez-Aceñero MJ, Peláez-García A, Peña C, Lopez-Lucendo M, Villar-Vázquez R, de Herreros AG. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin Cancer Res. 2013;19:6006-6019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 25. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8397] [Article Influence: 524.8] [Reference Citation Analysis (7)] |
| 26. | Lotti F, Jarrar AM, Pai RK, Hitomi M, Lathia J, Mace A, Gantt GA, Sukhdeo K, DeVecchio J, Vasanji A. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J Exp Med. 2013;210:2851-2872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 27. | Schellerer VS, Langheinrich M, Hohenberger W, Croner RS, Merkel S, Rau TT, Stürzl M, Naschberger E. Tumor-associated fibroblasts isolated from colorectal cancer tissues exhibit increased ICAM-1 expression and affinity for monocytes. Oncol Rep. 2014;31:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 692] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 29. | Silzle T, Kreutz M, Dobler MA, Brockhoff G, Knuechel R, Kunz-Schughart LA. Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur J Immunol. 2003;33:1311-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 677] [Cited by in RCA: 824] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 31. | Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1215] [Cited by in RCA: 1618] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 32. | Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1364] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 33. | Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4289] [Cited by in RCA: 4761] [Article Influence: 396.8] [Reference Citation Analysis (1)] |
| 34. | Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1045] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 35. | Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 443] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 36. | Kang JC, Chen JS, Lee CH, Chang JJ, Shieh YS. Intratumoral macrophage counts correlate with tumor progression in colorectal cancer. J Surg Oncol. 2010;102:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 603] [Cited by in RCA: 725] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 38. | Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, Wahl SM. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 39. | Zigmond E, Jung S. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol. 2013;34:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 40. | Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1459] [Cited by in RCA: 1496] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 41. | Obeid E, Nanda R, Fu YX, Olopade OI. The role of tumor-associated macrophages in breast cancer progression (review). Int J Oncol. 2013;43:5-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 42. | Erreni M, Mantovani A, Allavena P. Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron. 2011;4:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 43. | Xu H, Lai W, Zhang Y, Liu L, Luo X, Zeng Y, Wu H, Lan Q, Chu Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer. 2014;14:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | Li YY, Hsieh LL, Tang RP, Liao SK, Yeh KY. Interleukin-6 (IL-6) released by macrophages induces IL-6 secretion in the human colon cancer HT-29 cell line. Hum Immunol. 2009;70:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Illemann M, Bird N, Majeed A, Sehested M, Laerum OD, Lund LR, Danø K, Nielsen BS. MMP-9 is differentially expressed in primary human colorectal adenocarcinomas and their metastases. Mol Cancer Res. 2006;4:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Herbeuval JP, Lelievre E, Lambert C, Dy M, Genin C. Recruitment of STAT3 for production of IL-10 by colon carcinoma cells induced by macrophage-derived IL-6. J Immunol. 2004;172:4630-4636. [PubMed] |
| 47. | Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clin Cancer Res. 2011;17:1452-1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 48. | Tripathi C, Tewari BN, Kanchan RK, Baghel KS, Nautiyal N, Shrivastava R, Kaur H, Bhatt ML, Bhadauria S. Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines Oncostatin M and Eotaxin. Oncotarget. 2014;5:5350-5368. [PubMed] |
| 49. | Shay JE, Imtiyaz HZ, Sivanand S, Durham AC, Skuli N, Hsu S, Mucaj V, Eisinger-Mathason TS, Krock BL, Giannoukos DN. Inhibition of hypoxia-inducible factors limits tumor progression in a mouse model of colorectal cancer. Carcinogenesis. 2014;35:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, Lewis CE. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 2003;163:1233-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 218] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 51. | Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, Macintyre AN, Goraksha-Hicks P, Rathmell JC, Makowski L. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289:7884-7896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 749] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 52. | Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1066] [Article Influence: 76.1] [Reference Citation Analysis (2)] |
| 53. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1735] [Article Influence: 123.9] [Reference Citation Analysis (1)] |
| 54. | Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 55. | Ohtani H, Naito Y, Saito K, Nagura H. Expression of costimulatory molecules B7-1 and B7-2 by macrophages along invasive margin of colon cancer: a possible antitumor immunity? Lab Invest. 1997;77:231-241. [PubMed] |
| 56. | Sugita J, Ohtani H, Mizoi T, Saito K, Shiiba K, Sasaki I, Matsuno S, Yagita H, Miyazawa M, Nagura H. Close association between Fas ligand (FasL; CD95L)-positive tumor-associated macrophages and apoptotic cancer cells along invasive margin of colorectal carcinoma: a proposal on tumor-host interactions. Jpn J Cancer Res. 2002;93:320-328. [PubMed] |
| 57. | Edin S, Wikberg ML, Rutegård J, Oldenborg PA, Palmqvist R. Phenotypic skewing of macrophages in vitro by secreted factors from colorectal cancer cells. PLoS One. 2013;8:e74982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Fernando MR, Reyes JL, Iannuzzi J, Leung G, McKay DM. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One. 2014;9:e94188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 59. | Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1646] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 60. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 5007] [Article Influence: 250.4] [Reference Citation Analysis (19)] |
| 61. | Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 500] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 62. | Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 433] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 63. | Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3108] [Cited by in RCA: 3732] [Article Influence: 266.6] [Reference Citation Analysis (1)] |
| 64. | Diederichsen AC, Hjelmborg Jv, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 65. | Väyrynen JP, Tuomisto A, Klintrup K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Detailed analysis of inflammatory cell infiltration in colorectal cancer. Br J Cancer. 2013;109:1839-1847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 66. | Nagorsen D, Scheibenbogen C, Schaller G, Leigh B, Schmittel A, Letsch A, Thiel E, Keilholz U. Differences in T-cell immunity toward tumor-associated antigens in colorectal cancer and breast cancer patients. Int J Cancer. 2003;105:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Koch M, Beckhove P, Op den Winkel J, Autenrieth D, Wagner P, Nummer D, Specht S, Antolovic D, Galindo L, Schmitz-Winnenthal FH. Tumor infiltrating T lymphocytes in colorectal cancer: Tumor-selective activation and cytotoxic activity in situ. Ann Surg. 2006;244:986-992; discussion 992-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 68. | Reissfelder C, Stamova S, Gossmann C, Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M, Saadati M. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125:739-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 69. | Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944-5951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 752] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 70. | Salama P, Phillips M, Platell C, Iacopetta B. Low expression of Granzyme B in colorectal cancer is associated with signs of early metastastic invasion. Histopathology. 2011;59:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Ong SM, Tan YC, Beretta O, Jiang D, Yeap WH, Tai JJ, Wong WC, Yang H, Schwarz H, Lim KH. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur J Immunol. 2012;42:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 72. | Blaschitz C, Raffatellu M. Th17 cytokines and the gut mucosal barrier. J Clin Immunol. 2010;30:196-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 73. | Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 1010] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 74. | Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 75. | Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 911] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 76. | Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 647] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 77. | Straus DS. TNFα and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells. Mol Cancer. 2013;12:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 78. | De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493-3503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 487] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 79. | Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848-862. [PubMed] |
| 80. | Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259:88-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 501] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 81. | Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013;25:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 82. | Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 266] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 83. | Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, Juenger S, Vlodavsky I, Khazaie K, Jaeger D. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119:3311-3321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 84. | Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3582] [Cited by in RCA: 3943] [Article Influence: 179.2] [Reference Citation Analysis (0)] |
| 85. | Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, Kumar M, Jones S, Rees B, Williams G. Suppression of tumour-specific CD4⁺ T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. 2012;61:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 86. | Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, Wang H, Chen J, Wang H. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One. 2011;6:e19495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 87. | Xu L, Xu W, Qiu S, Xiong S. Enrichment of CCR6+Foxp3+ regulatory T cells in the tumor mass correlates with impaired CD8+ T cell function and poor prognosis of breast cancer. Clin Immunol. 2010;135:466-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Crome SQ, Clive B, Wang AY, Kang CY, Chow V, Yu J, Lai A, Ghahary A, Broady R, Levings MK. Inflammatory effects of ex vivo human Th17 cells are suppressed by regulatory T cells. J Immunol. 2010;185:3199-3208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 89. | Galizia G, Orditura M, Romano C, Lieto E, Castellano P, Pelosio L, Imperatore V, Catalano G, Pignatelli C, De Vita F. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol. 2002;102:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 90. | Mumm JB, Oft M. Pegylated IL-10 induces cancer immunity: the surprising role of IL-10 as a potent inducer of IFN-γ-mediated CD8(+) T cell cytotoxicity. Bioessays. 2013;35:623-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, Weitz J, Koch M, Halverson AL, Bentrem DJ. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci USA. 2010;107:6430-6435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 92. | Stewart CA, Metheny H, Iida N, Smith L, Hanson M, Steinhagen F, Leighty RM, Roers A, Karp CL, Müller W. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Invest. 2013;123:4859-4874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 93. | Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J. IL-10 elicits IFNγ-dependent tumor immune surveillance. Cancer Cell. 2011;20:781-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 351] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 94. | Karim BO, Huso DL. Mouse models for colorectal cancer. Am J Cancer Res. 2013;3:240-250. [PubMed] |
| 95. | Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18 Suppl 2:1-5. [PubMed] |
| 96. | Tseng W, Leong X, Engleman E. Orthotopic mouse model of colorectal cancer. J Vis Exp. 2007;484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 97. | Terracina KP, Aoyagi T, Huang WC, Nagahashi M, Yamada A, Aoki K, Takabe K. Development of a metastatic murine colon cancer model. J Surg Res. 2015;Apr 15; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer. 2014;111:2214-2219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 99. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6397] [Article Influence: 456.9] [Reference Citation Analysis (0)] |
| 100. | Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 101. | Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8900] [Cited by in RCA: 10124] [Article Influence: 723.1] [Reference Citation Analysis (5)] |
| 102. | Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1801] [Cited by in RCA: 1843] [Article Influence: 153.6] [Reference Citation Analysis (0)] |
| 103. | Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1200] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 104. | Karlsson M, Marits P, Dahl K, Dagöö T, Enerbäck S, Thörn M, Winqvist O. Pilot study of sentinel-node-based adoptive immunotherapy in advanced colorectal cancer. Ann Surg Oncol. 2010;17:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 822] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Huang ZH S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL