Published online Feb 15, 2026. doi: 10.4251/wjgo.v18.i2.115404
Revised: December 3, 2025
Accepted: December 17, 2025
Published online: February 15, 2026
Processing time: 90 Days and 21.8 Hours
Peritoneal metastasis occurs in 10%-45% of gastric cancer patients and sig
To construct a preoperative prediction model for gastric cancer peritoneal me
A retrospective analysis was conducted on 200 pathologically confirmed gastric cancer patients from January 2020 to December 2024, all of whom underwent preoperative multiphase contrast-enhanced CT examination. Patients were ran
The multiphase combined model achieved an area under the curve (AUC) of 0.876 (95% confidence interval: 0.783-0.941) in the validation set, with sensitivity of 81.0%, specificity of 84.6%, and accuracy of 83.3%, significantly superior to all single-phase models (P < 0.05). Among single-phase models, the venous phase model performed best (AUC = 0.834). Hosmer-Lemeshow test showed good model calibration (P = 0.765). Decision curve analysis demonstrated that at a threshold probability of 0.35, the multiphase combined model could avoid 33.7% of unnecessary exploratory surgeries.
The multiphase combined model based on multiphase contrast-enhanced CT radiomics can effectively predict gastric cancer peritoneal metastasis, with diagnostic performance significantly superior to single-phase models, providing a new non-invasive technical approach for individualized preoperative assessment of gastric cancer patients.
Core Tip: Multiphase contrast-enhanced computed tomography radiomics enables non-invasive preoperative prediction of peritoneal metastasis in gastric cancer. In a 200-patient cohort, a combined arterial-venous-delayed model (logistic regression within a leakage-free pipeline) outperformed single-phase models, achieving area under the curve 0.876 with good calibration and decision-curve net benefit. The approach may help triage candidates for staging laparoscopy, reduce unnecessary exploratory procedures, and support individualized treatment planning. Reporting follows IBSI/TRIPOD-AI, with feature robustness, cross-validation, and external temporal testing recommended for future multicenter deployment.
- Citation: Mu XD, Ji DX, Kang DQ. Application value of multiphase contrast-enhanced computed tomography radiomics in preoperative evaluation of peritoneal metastasis in gastric cancer. World J Gastrointest Oncol 2026; 18(2): 115404
- URL: https://www.wjgnet.com/1948-5204/full/v18/i2/115404.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i2.115404
Gastric cancer is one of the most common malignant tumors worldwide, ranking fifth in incidence and third in mortality, posing a serious threat to human health[1]. Peritoneal metastasis is one of the most common metastatic patterns of gastric cancer, with an incidence rate of approximately 10%-45%, and is an important factor affecting patient prognosis[2]. The presence of peritoneal metastasis not only significantly shortens patient survival but is also a key factor in determining treatment strategy selection. Accurate preoperative assessment is of great significance for developing individualized treatment plans[3].
Currently, the diagnosis of gastric cancer peritoneal metastasis mainly relies on imaging examinations, surgical exploration, and pathological confirmation. Traditional computed tomography (CT) examination has certain limitations in diagnosing peritoneal metastasis, particularly limited detection capability for early microscopic lesions, with sensitivity only 60%-80%[4]. Although laparoscopic exploration is the gold standard for diagnosing peritoneal metastasis, its invasive characteristics limit its application in all patients. Therefore, there is an urgent need for a non-invasive, accurate preoperative assessment method to improve the diagnostic efficacy of gastric cancer peritoneal metastasis[5].
Radiomics, as an emerging medical imaging analysis technology, can extract and analyze quantitative features from medical images through high-throughput methods, capable of mining deep information invisible to the naked eye, providing new pathways for precise tumor diagnosis and prognosis assessment[6]. Multiphase contrast-enhanced CT scanning can reflect the hemodynamic characteristics and enhancement patterns of tumors, providing a rich data foundation for radiomics analysis. In recent years, radiomics has shown good application prospects in tumor diagnosis, staging, and therapeutic efficacy evaluation[7].
However, current research on predicting gastric cancer peritoneal metastasis based on multiphase contrast-enhanced CT radiomics technology is relatively limited, and most studies are based on single-phase image analysis, failing to fully utilize the advantages of multiphase scanning. Multiphase combined analysis theoretically can provide more comprehensive tumor information and is expected to improve diagnostic performance[8]. Therefore, this study aims to construct a gastric cancer peritoneal metastasis preoperative prediction model based on multiphase contrast-enhanced CT radiomics, compare it with single-phase models, evaluate its application value in clinical practice, and provide new technical support for individualized diagnosis and treatment of gastric cancer patients.
This study was a retrospective study, collecting 200 gastric cancer patients pathologically confirmed at our hospital from January 2020 to December 2024. This study was approved by the hospital ethics committee and, being a retrospective study, patient informed consent was waived.
Inclusion criteria: (1) Gastric adenocarcinoma confirmed by gastroscopic biopsy pathology; (2) Preoperative abdominal multiphase contrast-enhanced CT examination with good image quality; (3) CT examination completed within 2 weeks before surgery; and (4) All patients underwent surgical treatment or laparoscopic exploration.
Exclusion criteria: (1) Preoperative neoadjuvant chemotherapy or radiotherapy; (2) Concurrent other malignant tumors; (3) Poor CT image quality affecting diagnosis; (4) Incomplete clinical pathological data; or (5) Pregnant patients.
The following clinical data were collected: (1) Basic information: Age, gender, body mass index (BMI); (2) Clinical manifestations: Abdominal pain, abdominal distension, weight loss, anemia and other symptoms; (3) Laboratory examinations: Complete blood count, liver and kidney function, tumor markers [carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 72-4 (CA72-4)]; and (4) Pathological data: Tumor differentiation degree, Lauren classification, human epidermal growth factor receptor-2 (HER-2) expression status.
A Siemens SOMATOM Definition Flash dual-source CT scanner was used for examination. Scanning parameters: Tube voltage 120 kVp, tube current 250-350 mA (using automatic tube current modulation technology), collimator width 2 mm × 128 mm × 0.6 mm, pitch 1.2, scanning slice thickness 5 mm, reconstruction slice thickness 1.5 mm, reconstruction interval 1.0 mm, matrix 512 × 512, reconstruction algorithm using B30f convolution kernel. Patients fasted 8-12 hours before examination and orally took 800-1000 mL of 2% diatrizoate meglumine solution 30 minutes before examination to fill the gastrointestinal tract. Non-ionic contrast agent (iohexol, 350 mgI/mL) 1.0-1.2 mL/kg was injected through the cubital vein using a high-pressure injector at an injection rate of 3.0-5.0 mL/second. Enhanced scanning was performed at 25-30 seconds (arterial phase), 65-70 seconds (venous phase), and 180 seconds (delayed phase) after contrast agent injection. The scanning range was from the diaphragm top to the lower edge of the pubic symphysis. Image quality assessment criteria: (1) No obvious motion artifacts in images; (2) Good contrast enhancement with arterial phase aortic enhancement value ≥ 250 Hounsfield units; (3) Clear gastric wall display without obvious gastric content interference; and (4) Signal-to-noise ratio ≥ 3, contrast-to-noise ratio ≥ 1.
Digital imaging and communications in medicine format images were imported into ITK-SNAP software (version 3.6.0), and regions of interest (ROI) were independently delineated by two radiologists with more than 5 years of abdominal imaging diagnosis experience, followed by consistency assessment. Gastric cancer lesion boundaries were manually delineated layer by layer on arterial phase, venous phase, and delayed phase images, avoiding necrotic, hemorrhagic, and artifact areas. Intraclass correlation coefficient (ICC) was used to assess inter-observer consistency, with ICC ≥ 0.75 considered good consistency. PyRadiomics toolkit (version 2.2.0) was used to extract radiomics features, with image resampling to 1 mm × 1 mm × 1 mm and gray-level discretization bin width set to 25. Extracted features included: (1) Shape features: 14 (volume, surface area, sphericity, compactness, etc.); (2) First-order statistical features: 18 (mean, median, variance, skewness, kurtosis, etc.); (3) Texture features: 75, including gray-level co-occurrence matrix (24), gray-level run length matrix (16), gray-level size zone matrix (16), neighboring gray-tone difference matrix (5), gray-level dependence matrix (14); and (4) Filter features: Texture features based on wavelet transform and Laplacian of Gaussian (LoG) filtering. Wavelet transform used 8 directions (HHH, HHL, HLH, HLL, LHH, LHL, LLH, LLL), LoG filter sigma values were set to 1.0, 2.0, 3.0, 4.0, 5.0, extracting a total of 93 × 13 = 1209 filtered texture features. Shape features were calculated only once in one phase, each phase extracted 93 first-order and texture features and 1209 filter features, totaling 1302 features. The total number of features for three phases was 14 (shape) + 93 × 3 (first-order + texture) + 1209 × 3 (filter) = 14 + 279 + 3627 = 3920 features.
Imaging diagnostic criteria referred to the “Chinese Expert Consensus on Diagnosis and Treatment of Gastric Cancer Peritoneal Metastasis (2021 Edition)”. CT diagnostic criteria for peritoneal metastasis: (1) Peritoneal thickening ≥ 3 mm with enhancement; (2) Peritoneal nodular or patchy enhancement; (3) Ascites with peritoneal enhancement; (4) Omental cake sign; (5) Serosal surface nodules of bowel wall; and (6) Nodules in mesentery or omentum. Pathological diagnostic criteria were based on surgical pathological results or laparoscopic exploration findings as the gold standard: (1) Peritoneal nodules visible to the naked eye during surgery; (2) Positive peritoneal lavage cytology; (3) Peritoneal biopsy pathology confirming metastatic carcinoma; and (4) Assessment of peritoneal metastasis extent according to peritoneal cancer index (PCI).
Statistical analysis was performed using SPSS 25.0 and Python 3.7. Quantitative data were tested for normality using the Shapiro-Wilk test. Data following normal distribution were expressed as mean ± SD, and between-group comparisons used independent samples t-test; non-normally distributed data were expressed as median (interquartile range), and between-group comparisons used Mann-Whitney U test. Count data were expressed as number (percentage), and between-group comparisons used χ2 test or Fisher's exact test.
Feature selection process: (1) Feature standardization: Z-score standardization method was used to standardize all features; (2) Consistency assessment: ICC was used to assess feature extraction consistency, ICC ≥ 0.75 was considered good consistency; (3) Univariate screening: Mann-Whitney U test was used for univariate feature screening, P < 0.05 was considered statistically significant; (4) Collinearity test: Pearson correlation coefficient between features was calculated, when |r| > 0.9, one feature was removed; and (5) Feature dimensionality reduction: Least absolute shrinkage and se
Model construction and validation: (1) Data splitting: Stratified sampling was performed according to peritoneal metastasis positive/negative ratio, randomly divided into training set (n = 140) and validation set (n = 60) at a 7:3 ratio, ensuring no significant difference in peritoneal metastasis incidence between the two groups; (2) Class imbalance handling: For the situation of fewer peritoneal metastasis positive samples, synthetic minority oversampling technique (SMOTE) was used for data balancing in the training set; (3) Model construction: Single-phase (arterial phase, venous phase, delayed phase) and multiphase combined radiomics models were constructed respectively; (4) Algorithm selection: Logistic regression (LR), support vector machine (SVM), random forest (RF) and other machine learning algorithms were used to construct prediction models; (5) Model validation: 5-fold cross-validation was used for internal validation in the training set, and external validation was performed in the independent validation set; (6) Performance evaluation: Model performance was evaluated through receiver operating characteristic (ROC) curves, calculating area under the curve (AUC), sensitivity, specificity, accuracy, positive predictive value, negative predictive value; (7) Model comparison: DeLong test was used to compare AUC differences between different models; (8) Calibration assessment: Calibration curves were plotted to assess model calibration, and Hosmer-Lemeshow test was used to assess goodness of fit; and (9) Clinical utility: Decision curve analysis (DCA) was used to assess the clinical net benefit of the model. P < 0.05 was considered statistically significant. All statistical tests were two-sided.
This study included 200 gastric cancer patients, including 134 males (67.0%) and 66 females (33.0%), aged 35-82 years, with a median age of 63 (55.71) years. According to surgical pathological results and laparoscopic exploration, 72 patients (36.0%) had peritoneal metastasis, and 128 patients (64.0%) had no peritoneal metastasis. Comparisons of age, gender, BMI, clinical symptoms, laboratory indicators, and pathological characteristics between the peritoneal metastasis group and non-metastasis group are shown in Table 1. Patients in the peritoneal metastasis group were older (P = 0.031), had significantly lower hemoglobin levels (P = 0.008), elevated white blood cell count (P = 0.042), elevated platelet count (P = 0.021), elevated alanine aminotransferase, aspartate aminotransferase, and total bilirubin levels (P < 0.05), elevated creatinine levels (P = 0.035), significantly elevated tumor markers CEA, CA19-9, and CA72-4 levels (P < 0.001), higher proportion of poorly differentiated adenocarcinoma (P = 0.002), more diffuse Lauren type (P < 0.001), and lower HER-2 positive expression rate (P = 0.018).
| Characteristic | Total (n = 200) | No peritoneal metastasis | Peritoneal metastasis | Statistical value | P value |
| Age (years) | 63 (55, 71) | 61 (53, 69) | 66 (58, 74) | Z = -2.162 | 0.031 |
| Gender | χ2 = 0.892 | 0.345 | |||
| Male | 134 (67.0) | 89 (69.5) | 45 (62.5) | ||
| Female | 66 (33.0) | 39 (30.5) | 27 (37.5) | ||
| BMI (kg/m2) | 22.8 (20.5, 25.2) | 23.1 (20.8, 25.4) | 22.3 (19.9, 24.8) | Z = -1.423 | 0.155 |
| Clinical symptoms | |||||
| Abdominal pain | 156 (78.0) | 97 (75.8) | 59 (81.9) | χ2 = 1.098 | 0.295 |
| Abdominal distension | 89 (44.5) | 52 (40.6) | 37 (51.4) | χ2 = 2.284 | 0.131 |
| Weight loss | 124 (62.0) | 74 (57.8) | 50 (69.4) | χ2 = 2.745 | 0.098 |
| Anemia | 98 (49.0) | 58 (45.3) | 40 (55.6) | χ2 = 2.070 | 0.150 |
| Blood routine indicators | |||||
| Hemoglobin (g/L) | 118 (102, 135) | 124 (108, 142) | 108 (94, 126) | Z = -2.648 | 0.008 |
| White blood cell count | 6.8 (5.2, 8.9) | 6.3 (4.9, 8.1) | 7.6 (5.8, 9.8) | Z = -2.035 | 0.042 |
| Platelet count (× 109/L) | 285 (218, 356) | 268 (205, 338) | 312 (248, 389) | Z = -2.314 | 0.021 |
| Liver and kidney function indicators | |||||
| ALT (U/L) | 28 (18, 45) | 24 (16, 38) | 35 (22, 58) | Z = -2.187 | 0.029 |
| AST (U/L) | 32 (21, 48) | 28 (19, 42) | 38 (26, 61) | Z = -2.458 | 0.014 |
| Total bilirubin (μmol/L) | 18.6 (12.4, 26.8) | 16.2 (11.8, 23.4) | 22.8 (15.7, 32.1) | Z = -2.319 | 0.020 |
| Creatinine (μmol/L) | 78 (65, 94) | 74 (62, 89) | 85 (71, 102) | Z = -2.108 | 0.035 |
| Blood urea nitrogen (mmol/L) | 5.8 (4.2, 7.6) | 5.4 (4.0, 7.1) | 6.5 (4.8, 8.9) | Z = -1.892 | 0.058 |
| Laboratory indicators | |||||
| CEA (ng/mL) | 8.5 (3.2, 22.1) | 5.8 (2.6, 14.3) | 18.9 (8.7, 45.2) | Z = -5.834 | < 0.001 |
| CA19-9 (U/mL) | 45.2 (18.7, 126.8) | 28.4 (15.2, 78.6) | 89.7 (42.3, 234.1) | Z = -4.921 | < 0.001 |
| CA72-4 (U/mL) | 12.6 (4.8, 35.7) | 7.9 (3.8, 21.4) | 28.4 (12.1, 68.9) | Z = -5.127 | < 0.001 |
| Tumor differentiation degree | χ2 = 12.358 | 0.002 | |||
| Well differentiated | 23 (11.5) | 20 (15.6) | 3 (4.2) | ||
| Moderately differentiated | 89 (44.5) | 64 (50.0) | 25 (34.7) | ||
| Poorly differentiated | 88 (44.0) | 44 (34.4) | 44 (61.1) | ||
| Lauren classification | χ2 = 15.942 | < 0.001 | |||
| Intestinal type | 78 (39.0) | 61 (47.7) | 17 (23.6) | ||
| Diffuse type | 95 (47.5) | 52 (40.6) | 43 (59.7) | ||
| Mixed type | 27 (13.5) | 15 (11.7) | 12 (16.7) | ||
| HER-2 expression | χ2 = 5.624 | 0.018 | |||
| Negative | 142 (71.0) | 85 (66.4) | 57 (79.2) | ||
| Positive | 58 (29.0) | 43 (33.6) | 15 (20.8) |
PCI assessment of peritoneal metastasis patients showed that among 72 peritoneal metastasis patients, PCI scores ranged from 3-26 points, with a median of 12 (8, 18) points. Stratified by PCI score: PCI 1-10 points in 29 cases (40.3%), PCI 11-20 points in 31 cases (43.1%), PCI > 20 points in 12 cases (16.7%).
Using stratified sampling method, 200 patients were randomly divided into training set (n = 140) and validation set (n = 60) at a 7:3 ratio. The peritoneal metastasis incidence rates in the two groups were 36.4% (51/140) and 35.0% (21/60) respectively, with no statistically significant difference (χ2 = 0.038, P = 0.845). Inter-observer ROI delineation consistency assessment showed an ICC value of 0.892 [95% confidence interval (95%CI): 0.856-0.921], indicating good consistency. Comparison of key clinical characteristics between training and validation sets showed no significant differences in CEA levels (P = 0.652), CA19-9 levels (P = 0.718), CA72-4 levels (P = 0.583), Lauren classification distribution (P = 0.821), tumor differentiation (P = 0.695), or HER-2 expression status (P = 0.774), confirming that the validation set is representative and free from confounding bias.
Consistency assessment of 3920 originally extracted features showed that 3771 features had ICC ≥ 0.75 (96.2%), and 149 features (3.8%) were excluded due to poor consistency. The distribution of excluded features was: 2 shape features (14.3%), 15 first-order statistical features (27.8%), 38 texture features (16.9%), and 94 filter features (2.6%). The remaining 3771 high-consistency features were included in subsequent analysis (Table 2).
| Item | Training set (n = 140) | Validation set (n = 60) | Statistical value | P value |
| Peritoneal metastasis | 51 (36.4) | 21 (35.0) | χ2 = 0.038 | 0.845 |
| Age (years) | 63 (54, 71) | 64 (56, 72) | Z = -0.687 | 0.492 |
| Gender (male) | 94 (67.1) | 40 (66.7) | χ2 = 0.004 | 0.948 |
| Features with ICC ≥ 0.75 | 3771/3920 (96.2) | |||
| Mean ICC (95%CI) | 0.892 (0.856-0.921) | |||
| Distribution of excluded features | ||||
| Shape features | 2/14 (14.3) | |||
| First-order statistical features | 15/54 (27.8) | |||
| Texture features | 38/225 (16.9) | |||
| Filter features | 94/3627 (2.6) |
To address the relatively small number of peritoneal metastasis positive samples in the training set (51/140, 36.4%), SMOTE was used for data balancing. SMOTE generates synthetic samples by interpolating in the feature space between existing minority class samples, generating 38 new peritoneal metastasis positive samples.
Training set before balancing was 89 negative samples, 51 positive samples (positive sample proportion 36.4%). Training set after balancing was 89 negative samples, 89 positive samples (total 178 cases, achieving 1:1 balance ratio).
SMOTE processing effectively resolved the class imbalance problem, providing a more balanced data foundation for subsequent machine learning model training. All subsequent model performance evaluations based on the training set were performed on the 178 samples after SMOTE processing.
Systematic feature selection was performed from 3771 high-consistency features. First, Z-score method was used to standardize all features. Univariate screening (based on the original 140 training set cases) found 628 features with statistically significant differences between peritoneal metastasis and non-metastasis groups (P < 0.05, Mann-Whitney U test). Collinearity testing calculated Pearson correlation coefficients between features, removing 185 highly correlated features (|r| > 0.9), leaving 443 features.
LASSO regression was used for dimensionality reduction of 443 features, with optimal λ value determined through 10-fold cross-validation. The number of optimal features selected for each phase and multiphase combined models is shown in Table 3. The multiphase combined model selected 18 optimal features (λ = 0.019), including 6 from arterial phase, 7 from venous phase, and 5 from delayed phase, including 2 shape features, 5 first-order statistical features, 6 texture features, and 5 filter features (Table 3).
| Selection step | Number of features | Selection criteria/method |
| Feature extraction | 3920 | All extracted features |
| Consistency assessment | 3771 | ICC ≥ 0.75 |
| Univariate screening | 628 | P < 0.05 (Mann-Whitney U) |
| Collinearity testing | 443 | |r|≤ 0.9 |
| LASSO selection results for each model | ||
| Arterial phase model | 12 | λ = 0.024 |
| Venous phase model | 15 | λ = 0.031 |
| Delayed phase model | 11 | λ = 0.028 |
| Multiphase combined model | 18 | λ = 0.019 |
LR, SVM, and RF machine learning algorithms were used to construct and compare models on the SMOTE-processed training set (n = 178). LR performed best in all phase models, with the multiphase combined LR model achieving AUC of 0.894, significantly superior to SVM (AUC = 0.867) and RF (AUC = 0.851) algorithms, so LR was selected as the final algorithm.
The 5-fold cross-validation internal validation was performed based on the SMOTE-processed 178 training set cases, and the multiphase combined model showed stable performance with mean AUC of 0.891 ± 0.013, sensitivity 85.1% ± 2.5%, specificity 83.2% ± 2.8%, and accuracy 84.2% ± 2.1%, indicating good stability and generalization ability of the model.
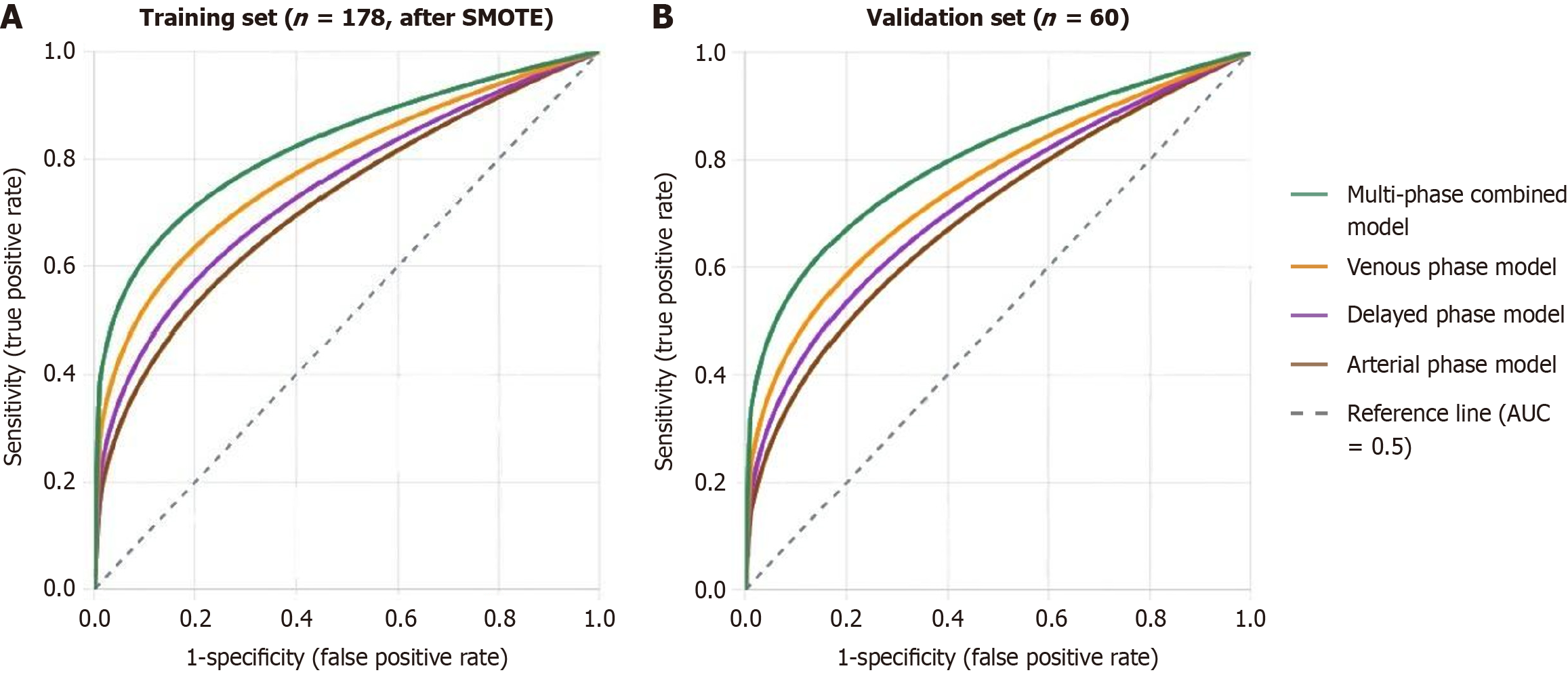
Performance evaluation results of each radiomics model are shown in Table 4. In the SMOTE-processed training set (n = 178), the multiphase combined model performed best with AUC of 0.894 (95%CI: 0.842-0.936), sensitivity 85.4%, specificity 83.1%, and accuracy 84.3%. Among single-phase models, the venous phase model performed best with AUC of 0.859 (95%CI: 0.798-0.910). In the independent validation set (n = 60), the multiphase combined model achieved AUC of 0.876 (95%CI: 0.783-0.941), sensitivity 81.0%, specificity 84.6%, and accuracy 83.3%.
| Model | SMOTE-processed training set (n = 178) | Validation set (n = 60) | ||||||||||
| AUC (95%CI) | Sensitivity | Specificity | Accuracy | PPV | NPV | AUC (95%CI) | Sensitivity | Specificity | Accuracy | PPV | NPV | |
| Arterial phase | 0.801 (0.738-0.857) | 73.0 | 75.3 | 74.2 | 74.7 | 73.6 | 0.781 (0.661-0.874) | 71.4 | 74.4 | 73.3 | 60.0 | 82.9 |
| Venous phase | 0.859 (0.798-0.910) | 79.8 | 80.9 | 80.3 | 80.7 | 80.0 | 0.834 (0.727-0.915) | 76.2 | 82.1 | 80.0 | 69.6 | 86.5 |
| Delayed phase | 0.826 (0.762-0.882) | 75.3 | 77.5 | 76.4 | 77.0 | 75.8 | 0.806 (0.692-0.894) | 71.4 | 79.5 | 76.7 | 65.2 | 83.8 |
| Multiphase combined | 0.894 (0.842-0.936) | 85.4 | 83.1 | 84.3 | 83.5 | 85.1 | 0.876 (0.783-0.941) | 81.0 | 84.6 | 83.3 | 73.9 | 89.2 |
DeLong test was used to compare AUC differences between models. Results from both the SMOTE-processed training set (n = 178) and independent validation set (n = 60) showed that the multiphase combined model performed significantly better than each single-phase model. In the training set, AUC differences between the multiphase combined model and arterial phase, venous phase, and delayed phase models were 0.093 (P = 0.009), 0.035 (P = 0.042), and 0.068 (P = 0.018) respectively. In the validation set, corresponding differences were 0.095 (P = 0.025), 0.042 (P = 0.038), and 0.070 (P = 0.032) respectively. The venous phase model performed significantly better than arterial phase (P = 0.028) and delayed phase models (P = 0.044; Table 5 and Figure 1).
| Model comparison | SMOTE-processed training set (n = 178) | Validation set (n = 60) | ||
| AUC difference | P value | AUC difference | P value | |
| Multiphase vs arterial phase | 0.093 | 0.009 | 0.095 | 0.025 |
| Multiphase vs venous phase | 0.035 | 0.042 | 0.042 | 0.038 |
| Multiphase vs delayed phase | 0.068 | 0.018 | 0.070 | 0.032 |
| Venous phase vs arterial phase | 0.058 | 0.028 | 0.053 | 0.041 |
| Venous phase vs delayed phase | 0.033 | 0.044 | 0.028 | 0.046 |
| Delayed phase vs arterial phase | 0.025 | 0.135 | 0.025 | 0.158 |
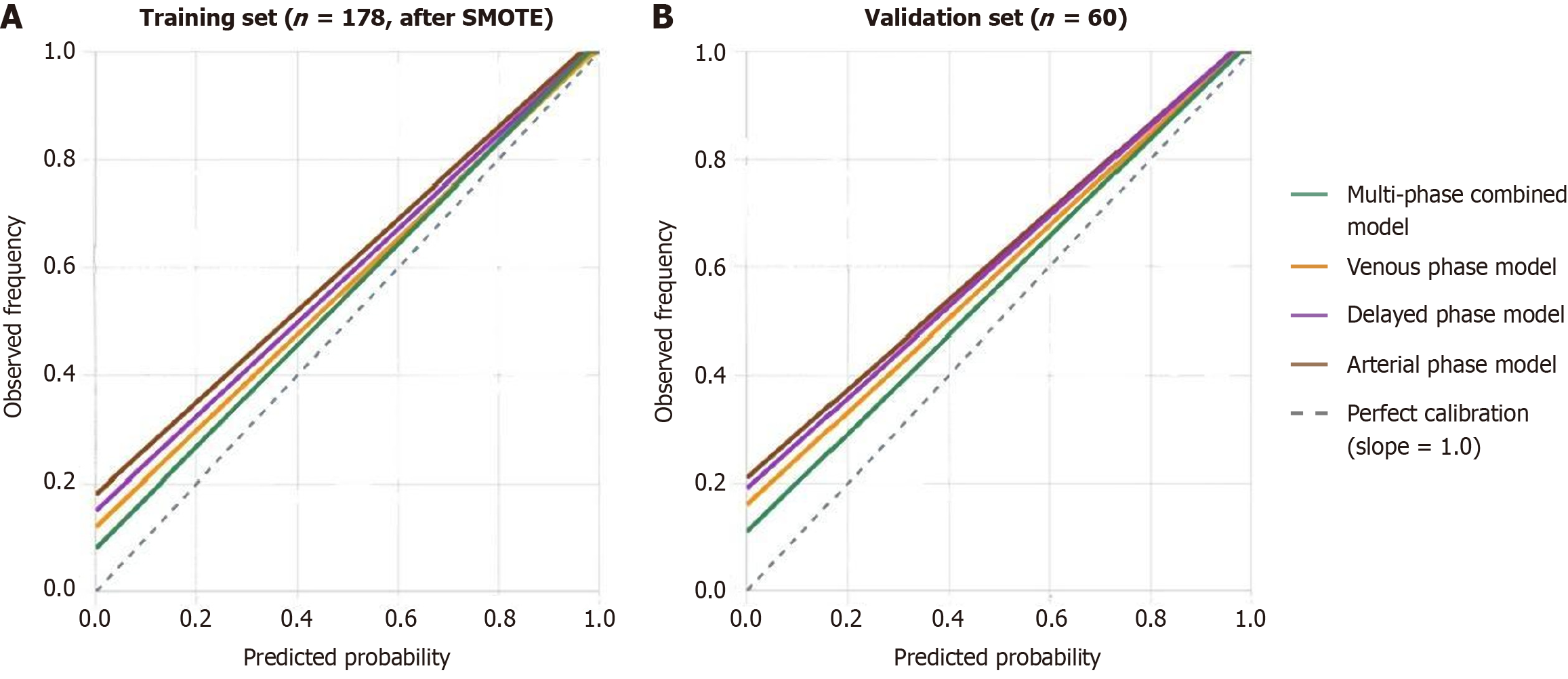
Hosmer-Lemeshow goodness-of-fit test was performed based on the SMOTE-processed training set (n = 178) and independent validation set (n = 60). The multiphase combined model showed good calibration in both training set (χ2 = 5.842, P = 0.664) and validation set (χ2 = 4.926, P = 0.765). Calibration curves showed high consistency between predicted and actual probabilities, with training set calibration curve slope of 0.94 and intercept of 0.08; validation set calibration curve slope of 0.91 and intercept of 0.11, with small deviation (Figure 2).
DCA showed that when threshold probabilities were 0.2-0.8, the multiphase combined model's net benefit was significantly better than single-phase models and “treat all” or “treat none” strategies. At threshold probability of 0.35, the multiphase combined model could avoid 33.7% of unnecessary exploratory surgeries while maintaining high peritoneal metastasis detection rate (Table 6).
| Assessment indicator | Multiphase combined model | Venous phase model | Arterial phase model | Delayed phase model |
| SMOTE-processed training set (n = 178) | ||||
| H-L test χ2 | 5.842 | 8.726 | 11.245 | 9.324 |
| H-L test P value | 0.664 | 0.365 | 0.187 | 0.316 |
| Calibration curve slope | 0.94 | 0.89 | 0.85 | 0.87 |
| Calibration curve intercept | 0.08 | 0.12 | 0.18 | 0.15 |
| Validation set (n = 60) | ||||
| H-L test χ2 | 4.926 | 6.542 | 8.734 | 7.658 |
| H-L test P value | 0.765 | 0.587 | 0.365 | 0.468 |
| Calibration curve slope | 0.91 | 0.86 | 0.82 | 0.84 |
| Calibration curve intercept | 0.11 | 0.16 | 0.21 | 0.19 |
| Clinical net benefit | ||||
| Net benefit at threshold probability 0.25 | 0.224 | 0.192 | 0.158 | 0.176 |
| Avoidable unnecessary surgeries (%) | 33.7 | 28.5 | 23.2 | 25.6 |
This study successfully constructed a preoperative prediction model for gastric cancer peritoneal metastasis based on multiphase contrast-enhanced CT radiomics and demonstrated the advantages of multiphase combined analysis in improving diagnostic performance. The results showed that the multiphase combined model achieved an AUC of 0.876 in the validation set, with sensitivity and specificity reaching 81.0% and 84.6% respectively, significantly superior to single-phase models, providing new technical support for individualized diagnosis and treatment decisions for gastric cancer patients.
Multiphase contrast-enhanced CT scanning can reflect the hemodynamic characteristics and contrast agent distribution patterns of tumors at different time points, providing richer data dimensions for radiomics analysis[9]. Through sys
Compared with single-phase analysis, the advantages of multiphase combined analysis are mainly reflected in the following aspects: (1) Different phases reflect the temporal evolution process of tumor vascularity and blood supply characteristics, which are closely related to tumor biological behavior[11]; (2) Multiphase data can capture more subtle texture changes and spatial heterogeneity information, improving the ability to identify tumor invasiveness[12]; and (3) The complementarity of multiphase features helps reduce the impact of single-phase image quality fluctuations on diagnostic results, improving model robustness[13].
The superior performance of the multiphase model likely reflects distinct biological information captured at different contrast phases. Arterial phase features may predominantly reflect tumor angiogenesis and microvascular density, as early contrast enhancement correlates with neo-vascularization patterns characteristic of aggressive tumors with metastatic potential. Venous phase features, showing the strongest single-phase performance, may capture information about both tumor cellularity and interstitial components, reflecting the balance between cellular density and extracellular matrix. Delayed phase features potentially characterize the fibrous stroma and degree of desmoplastic reaction, which is associated with tumor invasiveness and peritoneal seeding capability. The synergistic integration of these temporal-biological signatures enables the multiphase model to comprehensively characterize tumor heterogeneity and metastatic phenotype, explaining its superior diagnostic performance.
Previous studies on radiomics prediction of gastric cancer peritoneal metastasis were mostly based on single-phase image analysis, with certain limitations in diagnostic performance. Some studies reported radiomics models based on venous phase CT images with AUC of 0.78-0.85[14,15], while models based on plain CT had AUC of only 0.72[16]. The multiphase combined model in this study achieved AUC of 0.876, obviously superior to the above reports, further confirming the advantages of multiphase analysis.
In terms of feature extraction and model construction, this study adopted a more systematic methodology. Strict inter-observer consistency assessment (ICC = 0.892) ensured the reliability of feature extraction, and LASSO regression for feature dimensionality reduction effectively avoided overfitting[17]. Additionally, this study used SMOTE technology for data augmentation to address class imbalance, a strategy rarely adopted in previous studies but of great significance for improving model performance[18].
This study compared LR, SVM, and RF machine learning algorithms, with results showing LR performed best in all phase models. This result is consistent with some research reports[19], possibly related to the following factors: (1) LR has good interpretability with direct mathematical relationships between model parameters and feature weights; (2) In situations with relatively limited sample size, LR has better generalization ability compared to more complex algorithms; and (3) LR is less sensitive to feature scaling and suitable for processing standardized radiomics features[20].
It is worth noting that the cross-validation results of this study showed good model stability (mean AUC of 0.891 ± 0.013), indicating strong robustness of the selected feature and algorithm combination. Meanwhile, the Hosmer-Lemeshow test confirmed good model calibration, which is of great significance for practical clinical application[21].
Accurate preoperative assessment of gastric cancer peritoneal metastasis has important clinical significance for developing individualized treatment strategies. Traditional imaging methods have limitations in diagnosing early peritoneal metastasis, while laparoscopic exploration, although accurate, is invasive[22]. The radiomics model constructed in this study provides a non-invasive, efficient preoperative assessment tool for clinical practice.
DCA results showed that at threshold probability of 0.35, the multiphase combined model could avoid 33.7% of unnecessary exploratory surgeries, which has important value for reducing patient trauma and medical resource consumption[23]. Additionally, the model maintained high specificity while achieving high sensitivity, helping to avoid missed diagnoses and misdiagnoses, providing reliable evidence for clinical decision-making[24].
In clinical practice, this radiomics model could be integrated into a risk-stratification algorithm where patients with predicted probability > 0.35 would be prioritized for staging laparoscopy, while those with lower scores might proceed directly to radical surgery, pending multidisciplinary team discussion. This approach could reduce the number of staging laparoscopies by approximately 34%, thereby decreasing patient morbidity and healthcare costs while maintaining diagnostic accuracy. Future implementation would require integration into the PACS system with automated ROI segmentation and real-time probability calculation to facilitate seamless clinical adoption.
From the perspective of clinical workflow, multiphase contrast-enhanced CT examination is widely used in preoperative assessment of gastric cancer. The radiomics analysis method proposed in this study can provide additional diagnostic information based on existing examinations without increasing patient examination burden[25]. Meanwhile, with the development of artificial intelligence technology, radiomics analysis is expected to achieve automation, further improving the convenience of clinical application.
This study has the following limitations: (1) As a single-center retrospective study, the extrapolability of results may be limited, requiring further validation through multicenter prospective studies[26]; (2) The sample size is relatively limited, especially with fewer peritoneal metastasis positive cases, which may affect model generalization ability; (3) This study did not include information from other imaging examinations such as magnetic resonance imaging (MRI) or positron emission tomography (PET)-CT, and multimodal radiomics may further improve diagnostic performance[27]; and (4) This study mainly focused on imaging features without fully integrating clinical and laboratory indicators, and multidimensional information fusion awaits further exploration[28].
Additionally, the biological significance of radiomics features still requires in-depth study. Although this study demonstrated the value of multiphase radiomics in predicting peritoneal metastasis, the associative mechanisms between features and tumor biological behavior are not completely clear, limiting deep interpretation of model prediction results.
Based on the results of this study, future research can be further deepened in the following directions: (1) Conduct large-sample multicenter prospective validation studies to evaluate model stability and generalization ability under different equipment and scanning protocols; (2) Explore multimodal radiomics methods, integrating CT, MRI, PET and other imaging information to construct more comprehensive prediction models[29]; (3) Combine genomics, proteomics and other molecular biological information to develop “radiogenomics” integrated analysis methods; and (4) Study the pathological basis of radiomics features to elucidate associations between features and biological processes such as tumor microenvironment and angiogenesis.
In conclusion, this study successfully constructed a preoperative prediction model for gastric cancer peritoneal metastasis based on multiphase contrast-enhanced CT radiomics, confirming the significant advantages of multiphase combined analysis over single-phase analysis. This model has good diagnostic performance, calibration, and clinical utility, providing new technical means for individualized diagnosis and treatment decisions for gastric cancer patients. Despite certain limitations, this study lays an important foundation for the application of radiomics in gastric cancer peritoneal metastasis prediction and has good clinical translation prospects.
| 1. | Lin JL, Lin JX, Lin GT, Huang CM, Zheng CH, Xie JW, Wang JB, Lu J, Chen QY, Li P. Global incidence and mortality trends of gastric cancer and predicted mortality of gastric cancer by 2035. BMC Public Health. 2024;24:1763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 2. | Rijken A, Pape M, Simkens GA, de Hingh IHJT, Luyer MDP, van Sandick JW, van Laarhoven HWM, Verhoeven RHA, van Erning FN. Peritoneal metastases from gastric cancer in a nationwide cohort: Incidence, treatment and survival. Int J Cancer. 2024;154:992-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 3. | Jiang Y, Liang X, Wang W, Chen C, Yuan Q, Zhang X, Li N, Chen H, Yu J, Xie Y, Xu Y, Zhou Z, Li G, Li R. Noninvasive Prediction of Occult Peritoneal Metastasis in Gastric Cancer Using Deep Learning. JAMA Netw Open. 2021;4:e2032269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 4. | Liu P, Ding P, Wu H, Wu J, Yang P, Tian Y, Guo H, Zhao Q. Prediction of occult peritoneal metastases or positive cytology using CT in gastric cancer. Eur Radiol. 2023;33:9275-9285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10:2248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 6. | McCague C, Ramlee S, Reinius M, Selby I, Hulse D, Piyatissa P, Bura V, Crispin-Ortuzar M, Sala E, Woitek R. Introduction to radiomics for a clinical audience. Clin Radiol. 2023;78:83-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 7. | Li Q, Feng QX, Qi L, Liu C, Zhang J, Yang G, Zhang YD, Liu XS. Prognostic aspects of lymphovascular invasion in localized gastric cancer: new insights into the radiomics and deep transfer learning from contrast-enhanced CT imaging. Abdom Radiol (NY). 2022;47:496-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 8. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11:1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 9. | Liu Y, He C, Fang W, Peng L, Shi F, Xia Y, Zhou Q, Zhang R, Li C. Prediction of Ki-67 expression in gastrointestinal stromal tumors using radiomics of plain and multiphase contrast-enhanced CT. Eur Radiol. 2023;33:7609-7617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Zhang YP, Zhang XY, Cheng YT, Li B, Teng XZ, Zhang J, Lam S, Zhou T, Ma ZR, Sheng JB, Tam VCW, Lee SWY, Ge H, Cai J. Artificial intelligence-driven radiomics study in cancer: the role of feature engineering and modeling. Mil Med Res. 2023;10:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 82] [Reference Citation Analysis (0)] |
| 11. | Lin Q, Choyke PL, Sato N. Visualizing vasculature and its response to therapy in the tumor microenvironment. Theranostics. 2023;13:5223-5246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 12. | Wu A, Wu C, Zeng Q, Cao Y, Shu X, Luo L, Feng Z, Tu Y, Jie Z, Zhu Y, Zhou F, Huang Y, Li Z. Development and validation of a CT radiomics and clinical feature model to predict omental metastases for locally advanced gastric cancer. Sci Rep. 2023;13:8442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 13. | HajiEsmailPoor Z, Tabnak P, Baradaran B, Pashazadeh F, Aghebati-Maleki L. Diagnostic performance of CT scan-based radiomics for prediction of lymph node metastasis in gastric cancer: a systematic review and meta-analysis. Front Oncol. 2023;13:1185663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 14. | Zhou YH, Chen XL, Zhang X, Pu H, Li H. Dual-phase contrast-enhanced CT-based intratumoral and peritumoral radiomics for preoperative prediction of lymph node metastasis in gastric cancer. BMC Gastroenterol. 2025;25:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs. 2022;33:e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | Li LM, Feng LY, Liu CC, Huang WP, Yu Y, Cheng PY, Gao JB. Can visceral fat parameters based on computed tomography be used to predict occult peritoneal metastasis in gastric cancer? World J Gastroenterol. 2023;29:2310-2321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (2)] |
| 17. | Mondal D, Vanbelle S, Cassese A, Candel MJ. Review of sample size determination methods for the intraclass correlation coefficient in the one-way analysis of variance model. Stat Methods Med Res. 2024;33:532-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Lu J, Lu X, Wang Y, Zhang H, Han L, Zhu B, Wang B. Comparison between logistic regression and machine learning algorithms on prediction of noise-induced hearing loss and investigation of SNP loci. Sci Rep. 2025;15:15361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Hodneland E, Andersen E, Wagner-Larsen KS, Dybvik JA, Lura N, Fasmer KE, Halle MK, Krakstad C, Haldorsen I. Impact of MRI radiomic feature normalization for prognostic modelling in uterine endometrial and cervical cancers. Sci Rep. 2024;14:16826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 21. | Surjanovic N, Lockhart RA, Loughin TM. A generalized Hosmer-Lemeshow goodness-of-fit test for a family of generalized linear models. Test (Madr). 2024;33:589-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Ho SYA, Tay KV. Systematic review of diagnostic tools for peritoneal metastasis in gastric cancer-staging laparoscopy and its alternatives. World J Gastrointest Surg. 2023;15:2280-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 23. | Kerr KF, Brown MD, Zhu K, Janes H. Assessing the Clinical Impact of Risk Prediction Models With Decision Curves: Guidance for Correct Interpretation and Appropriate Use. J Clin Oncol. 2016;34:2534-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 494] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 24. | Qiu B, Zheng Y, Liu S, Song R, Wu L, Lu C, Yang X, Wang W, Liu Z, Cui Y. Multitask Deep Learning Based on Longitudinal CT Images Facilitates Prediction of Lymph Node Metastasis and Survival in Chemotherapy-Treated Gastric Cancer. Cancer Res. 2025;85:2527-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Xie K, Cui Y, Zhang D, He W, He Y, Gao D, Zhang Z, Dong X, Yang G, Dai Y, Li Z. Pretreatment Contrast-Enhanced Computed Tomography Radiomics for Prediction of Pathological Regression Following Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer: A Preliminary Multicenter Study. Front Oncol. 2021;11:770758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Huang ZN, Zhang HX, Sun YQ, Zhang XQ, Lin YF, Weng CM, Zheng CH, Ping-Li, Wang JB, Chen QY, Cao LL, Lin M, Tu RH, Huang CM, Lin JX, Xie JW. Multi-cohort study in gastric cancer to develop CT-based radiomic models to predict pathological response to neoadjuvant immunotherapy. J Transl Med. 2025;23:362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 27. | Song R, Chen W, Zhang J, Zhang J, Du Y, Ren J, Shi L, Cui Y, Yang X. Multiparametric MRI-based Radiomics Analysis for Prediction of Lymph Node Metastasis and Survival Outcome in Gastric Cancer: A Dual-center Study. Acad Radiol. 2024;31:4900-4911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12:186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 29. | Yang H, Yang M, Chen J, Yao G, Zou Q, Jia L. Multimodal deep learning approaches for precision oncology: a comprehensive review. Brief Bioinform. 2024;26:bbae699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/