Published online Jan 15, 2026. doi: 10.4251/wjgo.v18.i1.114648
Revised: October 31, 2025
Accepted: December 4, 2025
Published online: January 15, 2026
Processing time: 109 Days and 9.6 Hours
Well-differentiated small bowel mesenteric liposarcoma (LPS) is rare, with high malignancy, poor prognosis, and high preponderance to local recurrence.
Here we described a 71-year-old male, who complains of persistent abdominal distension for a month. The clinical manifestation is a huge abdominal mass oc
Completed surgical resection was cornerstone, and histopathological and mole
Core Tip: Well-differentiated small bowel mesenteric liposarcoma (LPS) is rare, with high malignancy, poor prognosis, and high preponderance to local recurrence. The patient underwent complete surgical resection of the tumor, and the weight of the tumor was approximately 11 kg. Both histopathological and fluorescence in situ hybridization examination confirmed that MDM2 amplification, and the small intestine mesenteric well-differentiated LPS was accurate diagnosed.
- Citation: Tian Y, Liu GQ, Li CF, Tian QM, Qiao S. Colossal well-differentiated liposarcoma of the small bowel mesentery: A case report. World J Gastrointest Oncol 2026; 18(1): 114648
- URL: https://www.wjgnet.com/1948-5204/full/v18/i1/114648.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i1.114648
Liposarcomas (LPS) are rare malignant tumors of adipocytic differentiation, they are commonly found soft tissue sarcomas, but their occurrence in the small intestinal mesentery are extraordinarily rare. LPS are most detected in the soft tissues of the extremities and retroperitoneum, and less commonly in the mesentery, spermatic cord, mediastinum, head, and neck[1]. It presents as an abdominal enormous mass of indeterminate origin with vague abdominal distension; the size of the small bowel mesentery mass is approximately 25 cm × 23 cm × 15 cm. In general, small bowel mesenteric LPS occurs between 50 years and 70 years with a higher incidence in males.
Well-differentiated LPS (WDLPS) occurring in the mesentery of the small and large intestine is extremely uncommon. It usually presents as a large painless mass, which is incidentally found[2]. WDLPS/de-differentiated LPS (DDLPS) is a major subtype of LPS, and it accounts for approximately 40%-50% of all LPS, with an anatomical predilection for the retroperitoneum, and carrying the MDM2 and CDK4 genes[3]. MDM2 amplification can provide strong evidence for the correct diagnosis of WDLPS.
This case report aimed to present a rare case of a giant WDLPS, which originated from the small bowel mesentery treated successfully by complete surgical excision. The patient was a 71-year-old male who suffered from abdominal tremendous mass and was treated in Tongren City People’s Hospital in January 2025. Here the medical history, clinical symptoms, signs, laboratory results, imaging data, and histopathological examination results were reported. The patient was informed that the data from his case would be submitted for publication, and he agreed.
A 71-year-old male patient was presented to the hospital with primary complaint of "palpable abdominal mass for a month".
One month ago, the patient suddenly developed an abdominal mass without any obvious cause, the mass gradually en
The patient had no history of any diseases, family history of hereditary diseases, digestive diseases, hepatitis, tu
The patient had no personal or family history of tumours.
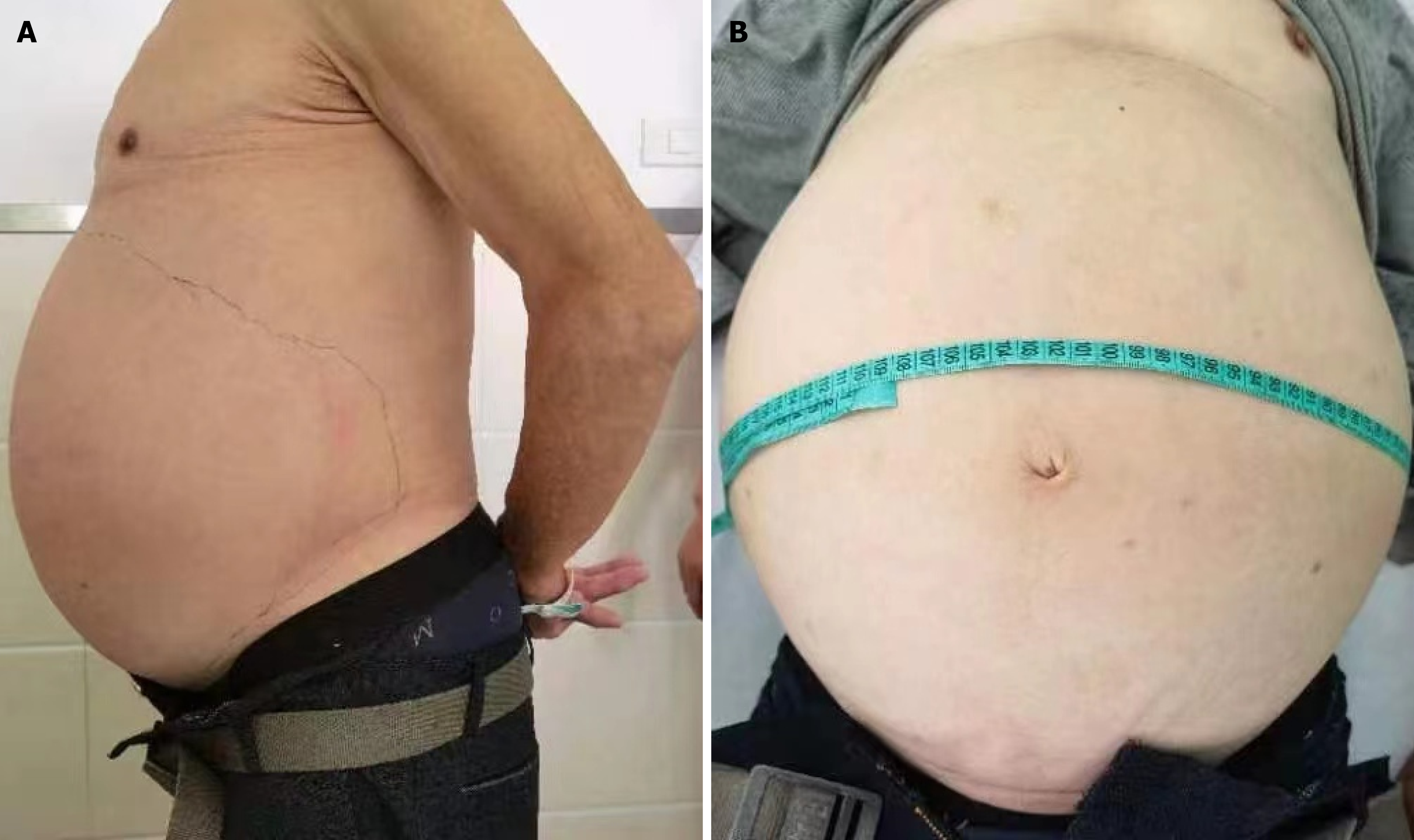
A clinical examination revealed a temperature of 36.7 °C, respiratory rate of 20 breaths per minute, pulse rate of 78 beats per minute, and blood pressure level of 145/98 mmHg. His height is 165 cm, weight is 69 kg, body mass index is 26.08 kg/m2. Abdominal physical examination revealed abdominal distention (frog belly). The mass occupied the entire ab
The routine blood results, cancer antigen 19-9, carcinoembryonic antigen, alpha fetoprotein, liver function tests, and renal function were normal. The patient was negative for hepatitis B, syphilis, and human immunodeficiency virus. The electrocardiogram was also normal.
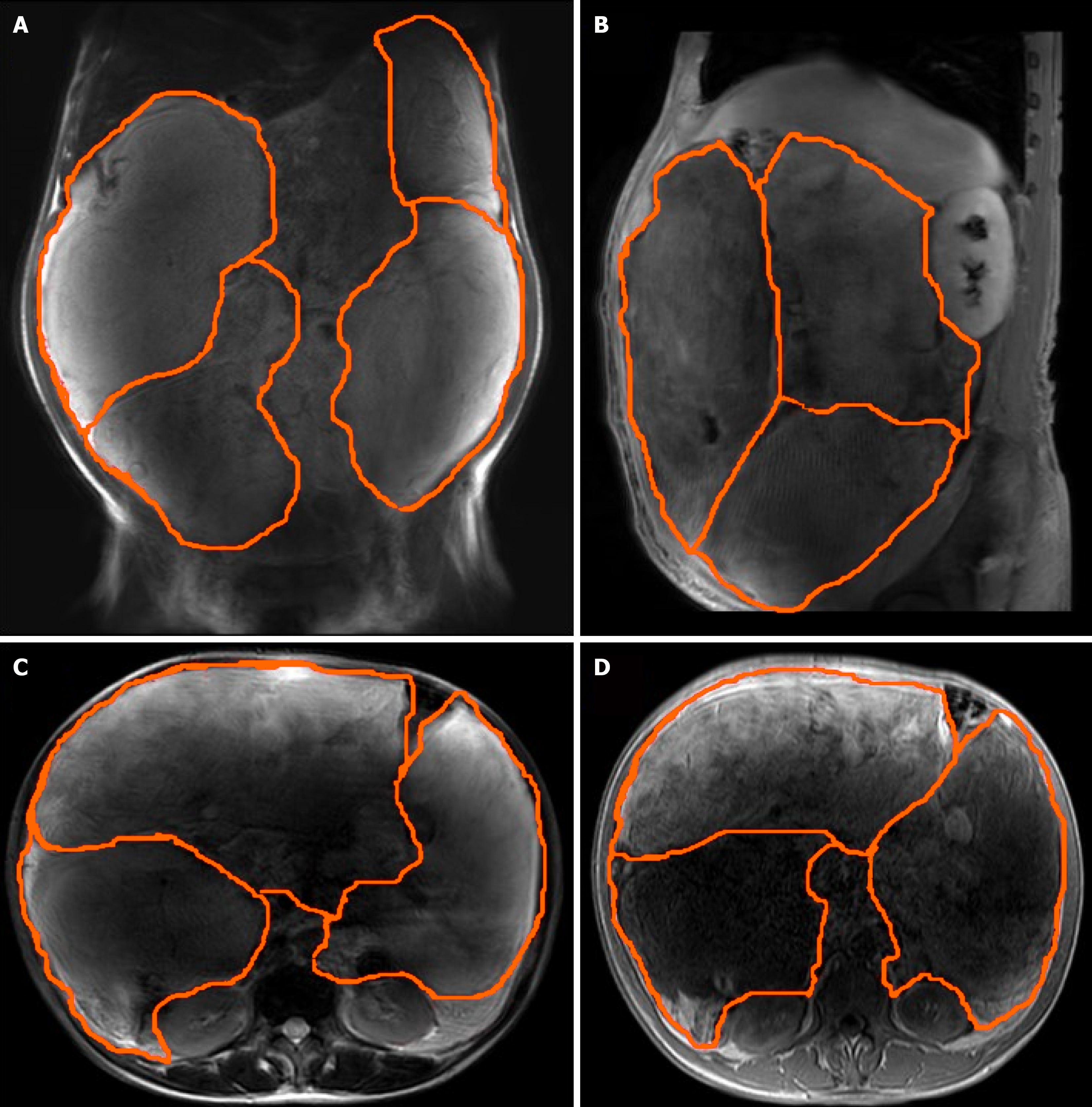
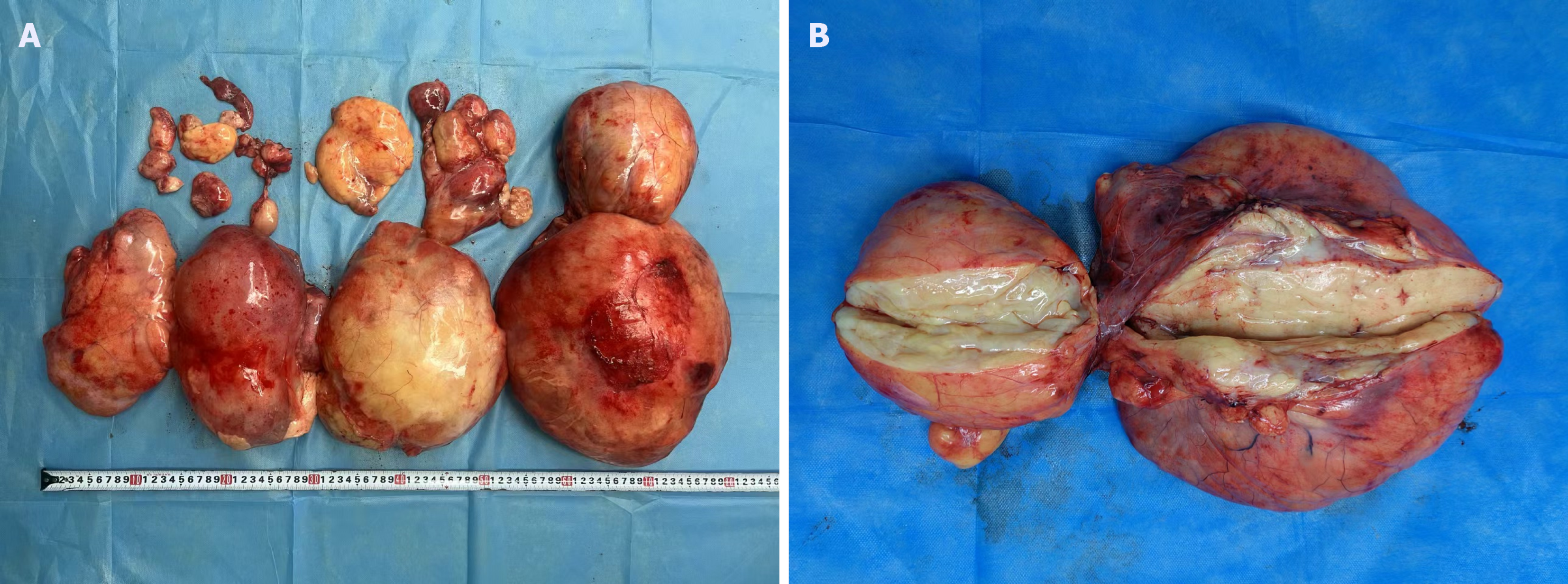
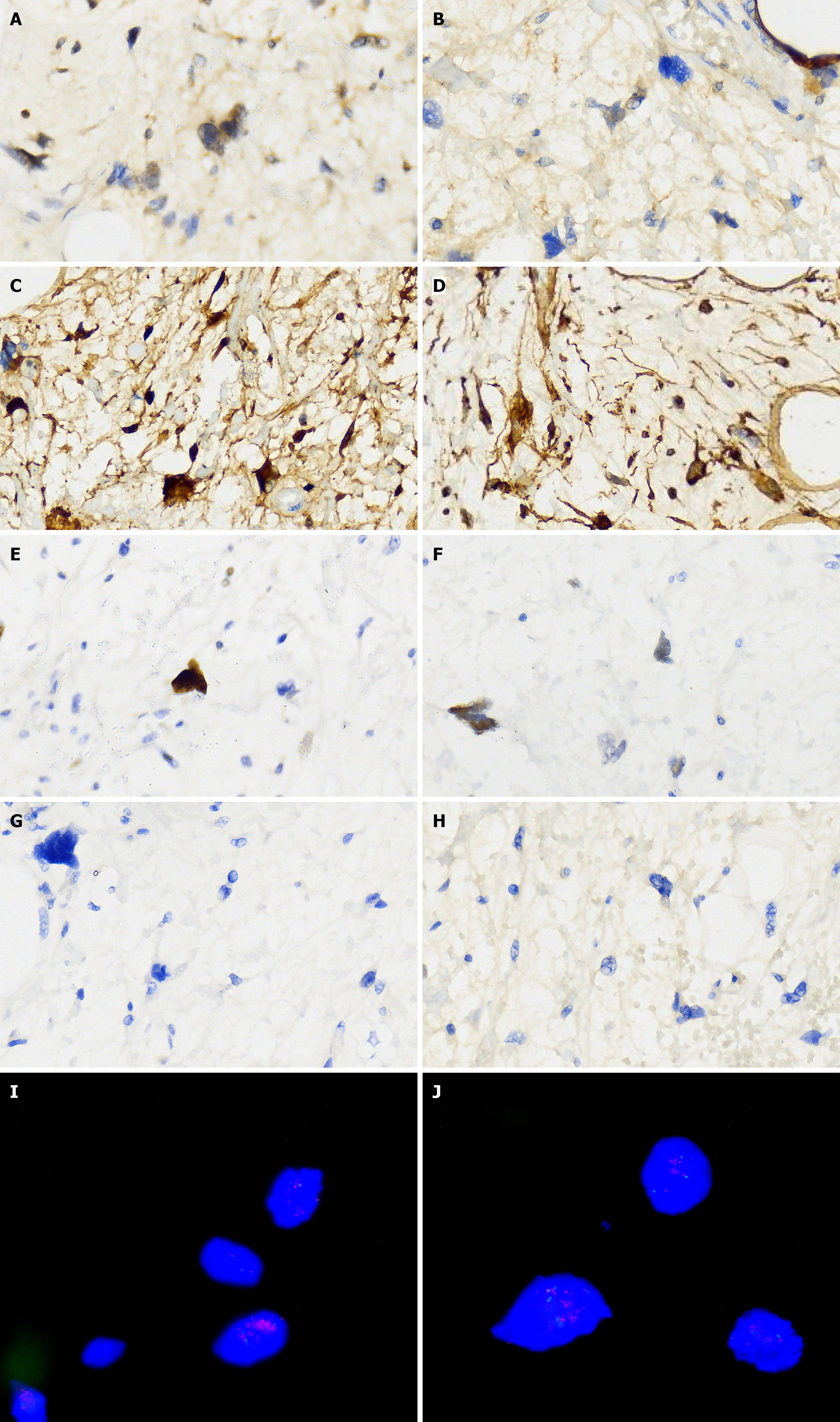
Abdominal ultrasound at another hospital manifested an abdominal giant mass in the abdomen in which the origin is unknown. CT scan of the chest at our hospital indicated that no lesions of metastasis were found. Abdominal enhanced CT examination at another hospital detected a large, solid abdominal mass that almost entirely occupied the abdominal cavity. Abdominal and pelvic magnetic resonance imaging at our hospital was completed, which showed that multiple round masses were found in the abdominal cavity with an envelope. The mass originated from the mesentery. It was also well circumscribed, lobulated, heterogeneous, and compartmentalized, with a maximum size of 25 cm × 23 cm × 15 cm (Figure 2). The mass removed intraoperatively was isolated, with a maximum size of 25 cm × 23 cm × 15 cm, and all masses were approximately 11 kg in weight (Figure 3A). The mass cut surface was soft, with a white-to-yellow color (Figure 3B). The histopathological examination of the specimens revealed that the tumor composed of atypical cells. In the focal areas, WDLPS was observed. Immunohistochemistry staining showed that the leucocyte antigen was positive on MDM2, S-100, P53, P16, Vim, and Ki-67 (3%) tumor cells and negative on EMA and CK-pan (Figure 4). Given these fin
We organized a multidisciplinary team discussion preoperatively, the patient was preliminarily diagnosed with abdominal giant mass based on the physical examination and imaging report, and drew up an accurate surgical plan.
The final diagnosis was colossal WDLPS of the small bowel mesentery.
The patient’s family should be fully informed of the relevant surgical risks, and they should sign the surgical consent form preoperatively. After improving the relevant preoperative preparation, the entire abdominal heterogeneous mass was excised successfully on January 24, 2025. The whole surgery lasted for 2 hours, blood loss was approximately 50 mL, without blood transfusion and intraoperative complications.
The patient died 2 months after discharge, and the specific cause of death is unknown.
LPS frequently develop from fat, muscles, and other connective tissues of mesenchymal origin and are a rare class of extremely aggressive cancers, which often fatal disease due to their higher recurrence, poor prognosis, higher and significant mortality rate among the patients[4].
The World Health Organization classification of soft tissue and bone tumors recognizes four major LPS subtypes: (1) ALP/WDLPS; (2) DDLPS; (3) Myxoid LPS; and (4) Pleomorphic LPS[5]. These four main subgroups are characterized by distinctive morphologies, unique genetic findings, and distinct clinical behavior. Accurate classification requires the in
The clinical symptoms of intra-abdominal LPS are mainly painless palpable masses, which are presented with inherent characteristics in relation to deep localization and expansive growth. Clinically, the tumors tend to present with ab
Genetically, WDLPS is a genetically distinct group of lesions, represented by the presence of a unique ring and enor
MDM2 is a proto-oncogene which is involved in cell cycle regulation, approximately 7% of all human malignancies and one-third of all types of sarcomas involve MDM2 amplification, although the amplification rates are significantly higher in WDLPS and DDLPS. MDM2 is an E3 ubiquitin protein ligase whose expression products binds to the trans
Given the rarity of small bowel mesenteric LPS, the possibility of secondary DDLPS should be excluded, and molecular genetic validation and examinations must be performed to exclude metastasis to confirm whether it is of primary re
WDLPS arises at similar frequency in the retroperitoneum and limbs. Morphological variants of WDLPS tend to exhibit anatomical tropism, which can be helpful diagnostically. Moreover, the difference in MDM2 amplification between tumor and adipose tissue exterior the small bowel mesentery demonstrated that the tumor stemmed from the small bowel mesentery, and confirmed the accurate diagnosis of WDLPS.
WDLPS is a low-grade tumor characterized by malignant adipocytes, slow-growing masses with no metastatic po
At present, without validated guidelines have been established for the treatment of mesenteric LPS. Regardless of the type of LPS, provided that there is no organ infiltration or distant metastasis, surgical excision is the recommended pre
Clinically, the potential morbidity must be taken into account, while adhering to oncological principles (e.g., not severely damaging the integrity of the tumor), with the goal of at least complete resection. Surgery should remove as much of the tumour and surrounding adipose tissue as possible, or else the remaining adipose tissue could be a cause of recurrence. If the adjacent tissues and organs were invaded, the surrounding infiltrating tissues and organs should be removed to obtain a good prognosis. Regular follow-up and long-term monitoring of the surgical site are recommended for assessment of the local condition. However, regardless of the degree of the resection, the integrity of surgical resection is an independent prognostic factors of local recurrence and overall survival[20].
Due to the large size of the tumor, unclear defined disease scope, and its location in the pivotal structures of the small intestine mesentery, surgical resection becomes technically challenging. Textbook outcomes are complicated measures aimed at determining the gold standard surgical outcomes for complicated tumor resection, which include direct or alternative indicators of perioperative duration, technical proficiency, intraoperative complications, and short-term morbidity rate and mortality[21].
The accurate diagnosis of WDLPS demands an experienced pathologist and applies immunohistochemistry and cy
The amplification of MDM2 and CDK4 is the main molecular feature of WDLPS and DDLPS. For these cases arising from rare diseases, postoperative histological inspection and molecular detection can be valuable for making a correct diagnosis. Immunohistochemical and fluorescence in situ hybridization analysis of this case revealed that high am
Given the rarity of this disease, different LPS subtypes have varying clinical features and sensitivities to treatment re
At present, scholars focus is on defining sarcomas based on their histologic subtype, molecular profile and genetic al
Anatomical position is a significant prognostic factor for LPS, and retroperitoneal LPS ecumenically exhibits a poor clinical manifestation[24]. Tumor grading, subtype, surgical complete resection, metastasis, and tumor size are associated with the prognosis for LPS[25].
Despite the patient did not receive postoperative radiotherapy or chemotherapy on account of personal selection, but close and regular follow-up ensured prompt management of any tumor progression. It is worth noting that MDM2 amplification in this type of tumor is beneficial for patients to provide opportunities to benefit from targeted therapy. MDM2 is a paramount driver gene for ALT/WDLPS/DDLPS and even if it is remained in clinical trial stages, which ex
However, after the patient was discharged, we conducted close follow-up via phone two months later. We learned that the patient had passed away, but the specific cause of death is unknown. We didn't know whether it was due to pul
In clinical practice, when an abdominal mass is associated with a radiographical mass lesion, especially the mass is derived from the small bowel mesentery or retroperitoneal, LPS should be considered in differential diagnosis. In ad
In conclusion, complete surgical resection remains the primary curative treatment for colossal mesenteric WDLPS. Histopathological examination supplemented by MDM2 amplification analysis is crucial for a definitive diagnosis. The role of adjuvant therapy in this specific scenario remains unclear and requires further investigation. Despite successful resection, close postoperative monitoring is essential, acknowledging that patient comorbidities and compliance with follow-up significantly impact on overall outcomes.
| 1. | Kallen ME, Hornick JL. The 2020 WHO Classification: What's New in Soft Tissue Tumor Pathology? Am J Surg Pathol. 2021;45:e1-e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 2. | Thway K. Well-differentiated liposarcoma and dedifferentiated liposarcoma: An updated review. Semin Diagn Pathol. 2019;36:112-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 222] [Article Influence: 31.7] [Reference Citation Analysis (4)] |
| 3. | Lee ATJ, Thway K, Huang PH, Jones RL. Clinical and Molecular Spectrum of Liposarcoma. J Clin Oncol. 2018;36:151-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 264] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 4. | M S A, K C, Bhargavan RV, Somanathan T, Subhadradevi L. An overview on liposarcoma subtypes: Genetic alterations and recent advances in therapeutic strategies. J Mol Histol. 2024;55:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 5. | Anderson WJ, Doyle LA. Updates from the 2020 World Health Organization Classification of Soft Tissue and Bone Tumours. Histopathology. 2021;78:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 6. | Dei Tos AP. Liposarcomas: diagnostic pitfalls and new insights. Histopathology. 2014;64:38-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Yee EJ, Stewart CL, Clay MR, McCarter MM. Lipoma and Its Doppelganger: The Atypical Lipomatous Tumor/Well-Differentiated Liposarcoma. Surg Clin North Am. 2022;102:637-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 8. | Dal Cin P, Kools P, Sciot R, De Wever I, Van Damme B, Van de Ven W, Van den Berghe H. Cytogenetic and fluorescence in situ hybridization investigation of ring chromosomes characterizing a specific pathologic subgroup of adipose tissue tumors. Cancer Genet Cytogenet. 1993;68:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 126] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Jing W, Lan T, Qiu Y, Peng R, Lu Y, Chen H, Chen M, He X, Chen C, Zhang H. Expression of FRS2 in atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma: an immunohistochemical analysis of 182 cases with genetic data. Diagn Pathol. 2021;16:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Hung YP, Michal M, Dubuc AM, Rosenberg AE, Nielsen GP. Dysplastic lipoma: potential diagnostic pitfall of using MDM2 RNA in situ hybridization to distinguish between lipoma and atypical lipomatous tumor. Hum Pathol. 2020;101:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Assi T, Kattan J, Rassy E, Nassereddine H, Farhat F, Honore C, Le Cesne A, Adam J, Mir O. Targeting CDK4 (cyclin-dependent kinase) amplification in liposarcoma: A comprehensive review. Crit Rev Oncol Hematol. 2020;153:103029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Ray-Coquard I, Blay JY, Italiano A, Le Cesne A, Penel N, Zhi J, Heil F, Rueger R, Graves B, Ding M, Geho D, Middleton SA, Vassilev LT, Nichols GL, Bui BN. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. Lancet Oncol. 2012;13:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 473] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 13. | Tortorelli I, Napolitano A, Zhou Y, Huang P, Jones RL. MDM2 inhibitors in sarcomas: results and next steps. Curr Opin Oncol. 2025;37:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Munisamy M, Mukherjee N, Thomas L, Pham AT, Shakeri A, Zhao Y, Kolesar J, Rao PPN, Rangnekar VM, Rao M. Therapeutic opportunities in cancer therapy: targeting the p53-MDM2/MDMX interactions. Am J Cancer Res. 2021;11:5762-5781. [PubMed] |
| 15. | Konopleva M, Martinelli G, Daver N, Papayannidis C, Wei A, Higgins B, Ott M, Mascarenhas J, Andreeff M. MDM2 inhibition: an important step forward in cancer therapy. Leukemia. 2020;34:2858-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 16. | Gutierrez A, Snyder EL, Marino-Enriquez A, Zhang YX, Sioletic S, Kozakewich E, Grebliunaite R, Ou WB, Sicinska E, Raut CP, Demetri GD, Perez-Atayde AR, Wagner AJ, Fletcher JA, Fletcher CD, Look AT. Aberrant AKT activation drives well-differentiated liposarcoma. Proc Natl Acad Sci U S A. 2011;108:16386-16391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Choi KY, Jost E, Mack L, Bouchard-Fortier A. Surgical management of truncal and extremities atypical lipomatous tumors/well-differentiated liposarcoma: A systematic review of the literature. Am J Surg. 2020;219:823-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Watson S, Gruel N, Le Loarer F. New developments in the pathology and molecular biology of retroperitoneal sarcomas. Eur J Surg Oncol. 2023;49:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Cha EJ. Dedifferentiated liposarcoma of the small bowel mesentery presenting as a submucosal mass. World J Gastrointest Oncol. 2011;3:116-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, Van Coevorden F, Rutkowski P, Callegaro D, Hayes AJ, Honoré C, Fairweather M, Cannell A, Jakob J, Haas RL, Szacht M, Fiore M, Casali PG, Pollock RE, Raut CP. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma (RPS): A Report on 1007 Patients From the Multi-institutional Collaborative RPS Working Group. Ann Surg. 2016;263:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 413] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 21. | Murphy S, Allan C, Barbour A, Donoghue V, Smithers BM. Textbook Outcomes for Retroperitoneal Sarcoma Resection: A Multi-Centre Review. Curr Oncol. 2025;32:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | da Mota VHS, Freire de Melo F, de Brito BB, da Silva FAF, Teixeira KN. Molecular docking of DS-3032B, a mouse double minute 2 enzyme antagonist with potential for oncology treatment development. World J Clin Oncol. 2022;13:496-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Callegaro D, Sarre Lazcano C, Cardona K. ASO Practice Guidelines Series: Soft Tissue Sarcoma of the Extremities and Superficial Trunk. Ann Surg Oncol. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Gootee J, Aurit S, Curtin C, Silberstein P. Primary anatomical site, adjuvant therapy, and other prognostic variables for dedifferentiated liposarcoma. J Cancer Res Clin Oncol. 2019;145:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Knebel C, Lenze U, Pohlig F, Lenze F, Harrasser N, Suren C, Breitenbach J, Rechl H, von Eisenhart-Rothe R, Mühlhofer HML. Prognostic factors and outcome of Liposarcoma patients: a retrospective evaluation over 15 years. BMC Cancer. 2017;17:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Cassinelli G, Pasquali S, Lanzi C. Beyond targeting amplified MDM2 and CDK4 in well differentiated and dedifferentiated liposarcomas: From promise and clinical applications towards identification of progression drivers. Front Oncol. 2022;12:965261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/