Published online Jan 15, 2026. doi: 10.4251/wjgo.v18.i1.111144
Revised: July 25, 2025
Accepted: November 18, 2025
Published online: January 15, 2026
Processing time: 199 Days and 4.7 Hours
The treatment technology of liver cancer is progressing. In addition to traditional surgical resection, combined therapies of immunotherapy based on immune checkpoint inhibitors, chemotherapy, and transcatheter arterial chemoembolization for hepatocellular carcinoma are more and more widely used. Accurate preoperative diagnosis of liver cancer will provide important information for comprehensive treatment and prognosis evaluation of liver cancer. Sonazoid-contrast-enhanced ultrasound is not only helpful for the qualitative diagnosis of liver lesions, but also has great potential in the diagnosis of histological differentiation of liver cancer.
To assess the differentiation of hepatocellular carcinoma (HCC) by utilizing the parameters and imaging features of Sonazoid-contrast-enhanced ultrasound (CEUS).
A retrospective analysis was conducted on the CEUS data of 239 lesions through case-control study. These patients received Sonazoid-CEUS within one week before surgery and were confirmed as HCC by postoperative pathology. Within the cases, patients were further categorized into well-differentiated and poorly-differentiated group. Time-intensity curves of the region of interest in both arterial and Kupffer phases were generated, allowing for the acquisition of quantitative parameters to assess the diagnostic efficacy in distinguishing lesions between these two groups and determining an appropriate cut-off value.
Univariate analysis showed that the absolute value of enhancement intensity (EIAV), intensity ratio (IR) and intensity difference (ID) in Kupffer phase were statistically different between the groups with different degree (P = 0.015, P = 0.000, P = 0.000). The sensitivity and specificity were 40.2%, 82.4%, 80.4% and 78.1%, 86.9% and 74.5%, respectively, for differentiating HCC lesions with EIAV ≥ 56.384 dB, IR ≥ 1.215 and ID ≥ 9.184 dB. The area under the receiver operating characteristic curve were 0.590, 0.877, 0.815. There was no significant difference in the parameters of arterial phase, including peak time, initial growth time, rise time and the absolute value of peak intensity of lesions between the two groups (P > 0.05). Multivariate analysis showed that the level of alpha-fetoprotein (AFP) and IR were risk factors for poor differentiation (P = 0.001).
Among the parameters of Sonazoid-CEUS, IR in Kupffer phase exhibits superior diagnostic efficacy with high sensitivity and specificity in the diagnose of pathological differentiation of HCC. Combined with preoperative AFP level, a more accurate diagnosis will be obtained. Compared with portal vein phase, Kupffer phase showed the ability to identify HCC lesions more sensitive. These findings hold significant guiding implications and reference value for clinical practice.
Core Tip: This study used quantitative parameters and imaging characteristics from Sonazoid-contrast-enhanced ultrasound using perfluorobutane microspheres to evaluate the histological differentiation of hepatocellular carcinoma (HCC). Both univariate and multivariate analyses indicated that Kupffer phase parameters, particularly the intensity ratio (IR), outperformed conventional clinical markers. IR proved to be a valuable, non-invasive predictor of tumor differentiation, supporting more informed preoperative decision-making in HCC management. It is simple to calculate, relatively unaffected by variations in hepatic perfusion or fibrosis, and well-suited for routine clinical application. Receiver operating characteristic analysis identified an optimal cut-off value of 1.215 for IR.
- Citation: Liu RB, Xin JY, Huang Z, Li KY. Sonazoid-contrast-enhanced ultrasound for the histological diagnosis of hepatocellular carcinoma. World J Gastrointest Oncol 2026; 18(1): 111144
- URL: https://www.wjgnet.com/1948-5204/full/v18/i1/111144.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i1.111144
Liver cancer remains one of the most prevalent and fatal malignancies globally[1]. In China, liver cancer ranks as the fifth most commonly diagnosed malignancy, with an estimated 410000 new cases and 390000 cancer-related deaths reported in 2020, representing a substantial burden on the healthcare system. Hepatocellular carcinoma (HCC) constitutes approximately 85% of these cases[2]. Extensive research has shown a strong correlation between the histological grade of HCC and its biological behavior and clinical prognosis. Poorly differentiated (P-D) HCC is more prone to microvascular invasion and is linked to poor outcomes, including a 5-year recurrence rate as high as 75% and a post-transplant re
Accurate preoperative imaging-based evaluation of the histological grade of HCC is essential for informing treatment strategies, predicting prognosis, and optimizing postoperative care. Histopathological assessment through needle biopsy or surgical excision remains the gold standard for determining tumor differentiation. However, this method has several important limitations. A key issue is intratumoral heterogeneity, particularly in larger lesions, which can result in biopsy samples failing to accurately reflect the tumor's overall pathology. Further, biopsies carry procedural risks. Right upper quadrant pain is reported in 0.05% to 84% of cases, with severe pain requiring further evaluation in up to 2.3%. He
Non-invasive imaging approaches for preoperative assessment of HCC histological grade are gaining recognition as valuable tools in clinical practice. They provide clinicians with important supplementary information to support more accurate and personalized treatment planning. Differences in tumor differentiation are closely linked to alterations in intratumoral blood flow and cellular architecture, which are reflected in imaging features such as growth patterns, tissue interface characteristics, echogenicity, and perfusion behavior. Several imaging modalities have been investigated for their potential to predict HCC differentiation, including contrast-enhanced ultrasonography (CEUS)[10,11], super-paramagnetic iron oxide-enhanced magnetic resonance imaging (SPIO-MRI)[12,13], Gd-EOB-DTPA enhanced magnetic resonance imaging (MRI)[14], and multidetector computed tomography. However, each imaging modality has its limitations. Gd-EOB-DTPA-enhanced MRI, which employs a hepatocyte-specific contrast agent taken up by functional hepatocytes and excreted through renal and biliary pathways, has shown promise in assessing tumor differentiation. Yet, in patients with impaired liver function, especially those with poor Child-Pugh classifications, the sensitivity for detecting well-differentiated (W-D) HCC is reduced, complicating accurate imaging-based diagnosis[15,16].
Recently, CEUS has become increasingly used in diagnosing liver tumors[17]. Sonazoid is an advanced contrast agent composed of perfluorobutane gas microbubbles encased in a phospholipid monolayer shell, offering enhanced stability and functioning as a blood pool agent. It enables high-resolution imaging of vascular phases, including the arterial, portal, and delayed phases. In addition to real-time vascular assessment, Sonazoid-enhanced CEUS uniquely provides a post-vascular, or Kupffer phase (KP), due to the agent's strong affinity for the reticuloendothelial system. This is reflected in the liver's selective uptake by Kupffer cells, allowing for targeted imaging of hepatic parenchyma[18-20]. In vitro studies have shown that up to 99% of Sonazoid microbubbles can be phagocytosed by Kupffer cells. Within approximately one minute of exposure to perfluorobutane microspheres, Kupffer cells adhere to the microbubbles and extend pseudopods to envelop them. By around five minutes, progressive phagocytosis and internalization of the microspheres are observed, with subsequent migration of the internalized particles toward the cell nucleus[19]. In clinical practice, the KP generally begins around 10 minutes after intravenous injection and remains stable for at least 60 minutes, often lasting even longer[17,21].
The prolonged enhancement observed during the KP reflects the distribution of Kupffer cells within the liver parenchyma, providing valuable information about the presence and characteristics of hepatic tumors. Studies have demonstrated that the number of Kupffer cells in HCC correlates with tumor differentiation, decreasing as the degree of differentiation worsens. A pathological analysis of liver tissue samples from HCC patients reported average Kupffer cell counts of 12.7 ± 6.8 in tumor tissue, 18.1 ± 8.2 in peritumoral tissue, and 18.9 ± 7.9 in adjacent normal liver, with a significantly lower count in cancerous tissue compared to surrounding areas (P < 0.05). Further stratification by his
These findings support the hypothesis that the degree of enhancement in the post-vascular (Kupffer) phase on Sonazoid-enhanced CEUS may serve as a surrogate marker for tumor differentiation. In a study conducted by Ohama et al[23], CEUS using perfluorobutane microbubbles was performed on 33 W-D HCC lesions, 40 moderately to P-D HCC lesions, and 9 cases of atypical hyperplasia. None of the atypical hyperplasia cases and only 3 (9%) of the W-D HCC lesions exhibited hypoenhancement during the post-vascular phase. Hypoenhancement was observed in most mode
While previous studies have demonstrated the clinical utility of CEUS with perfluorobutane microspheres in diag
The primary aim of this study was to evaluate the utility of preoperative B-mode ultrasound features combined with quantitative parameters from Sonazoid-enhanced CEUS in predicting the histological differentiation of HCC. The study aimed to establish a reliable, non-invasive diagnostic strategy for preoperative tumor grading by analyzing a large cohort of surgically and pathologically confirmed HCC lesions. This is of considerable clinical importance, as accurate assessment of tumor differentiation is essential for prognostication, selecting appropriate treatment modalities such as resection, transplantation, or ablation, and guiding personalized therapeutic plans. The study also aimed to determine optimal cut-off values for key CEUS-derived quantitative parameters that could serve as standardized reference points for non-invasive grading. The results of this study may support earlier risk stratification, reduce the need for invasive biopsies, and enhance the precision of HCC management in clinical settings.
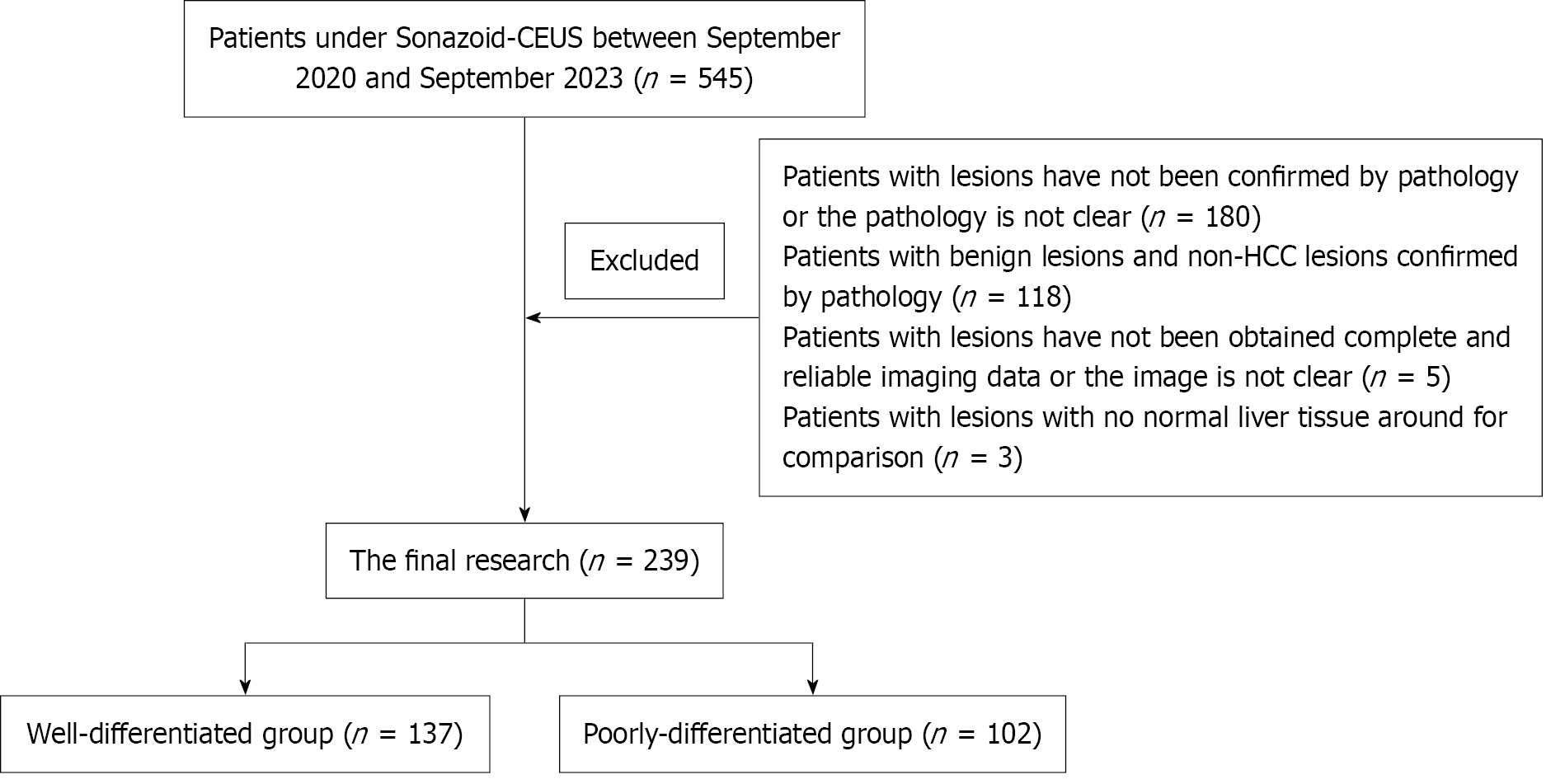
From September 2020 to September 2023, a total of 239 liver lesions pathologically confirmed as HCC were included in the final analysis. The inclusion criteria were: (1) Identification of intrahepatic lesions via routine B-mode ultrasound; (2) Completion of Sonazoid-enhanced CEUS within one week before surgery, with high-quality imaging data acquired across all phases arterial phase (AP), PVP, delayed/Late phase (DP/LP), and KP ensuring clear visualization with stable respiration and minimal pulmonary interference; and (3) Surgical resection followed by histopathological confirmation of HCC, including evaluation of tumor differentiation. Exclusion criteria are detailed in Figure 1. Furthermore, relevant clinical and laboratory data, including age, sex, lesion size, hepatitis B history, and serum alpha-fetoprotein (AFP) levels, were collected for subsequent analysis.
Imaging was performed using a GE color Doppler ultrasound system equipped with a convex array probe (model C1-6T), operating within a frequency range of 2.5-6 MHz. For CEUS, the mechanical index (MI) was maintained between 0.18 and 0.22. The contrast agent administered was perfluorobutane microspheres (Sonazoid, GE Healthcare, United States).
All patients fasted for at least 8 hours before the examination. An 18-G indwelling needle was placed in the antecubital vein for contrast administration. Initial imaging began with a standard two-dimensional ultrasound scan to evaluate the lesion's location, size, shape, echogenicity, internal vascularity, and any signs of liver cirrhosis. The optimal imaging plane, typically the largest and clearest cross-section of the lesion with minimal respiratory movement and minimal interference from lung gas, was then selected to ensure image stability and quality. The ultrasound system was subsequently switched to contrast mode, with all parameters adjusted appropriately. The contrast agent, Sonazoid, was prepared according to the manufacturer's guidelines and administered intravenously at a dose of 0.015 mL/kg via the antecubital vein, followed by a 10 mL flush of sterile normal saline to ensure complete delivery.
Following contrast injection, lesion enhancement was continuously monitored. The AP commenced with the arrival of microbubbles and typically lasted over 45 seconds. This was followed by dynamic observation through the PVP, occurring between approximately 30-45 seconds and 2 minutes post-injection, then the LP from 4 minutes to 6 minutes. The KP was assessed around 12 minutes post-injection, with dynamic lesion imaging recorded for at least 5 seconds. A comprehensive post-vascular liver scan was also performed to identify additional hypoenhanced areas. If such areas were detected, the examination was temporarily halted to allow contrast clearance, after which CEUS was repeated. If no hypoenhancement was observed, the examination was concluded. Throughout image acquisition at each phase, patients were instructed to hold their breath to minimize motion artifacts caused by respiration.
Image analysis and data collection were independently performed by two experienced sonographers, each with over five years of expertise in ultrasound diagnostics and formal training in contrast-enhanced imaging. Using the ultrasound system's integrated software, time-intensity curves for the AP were generated within the defined region of interest (ROI) to measure key parameters, including onset time, peak time (PT), rise time (RT), and peak intensity absolute value. During the KP, time-intensity curves were similarly obtained for the lesion and adjacent normal liver parenchyma at equivalent depths. Three time points were randomly selected from each curve to record corresponding enhancement intensity (EIAV) values. Based on these values, the lesion's absolute EIAV, intensity difference (ID), and intensity ratio (IR) were calculated using standardized formulas. The final values used for statistical analysis represented the average of the measurements recorded by the two sonographers.
Selection of target area: The enhancement pattern of the lesion during the AP was carefully assessed. A 2 mm diameter sampling frame was placed over the region exhibiting the highest enhancement to serve as the measurement area. For the post-vascular phase, the sampling frame was adjusted to cover as much of the lesion as possible. Regions that showed no enhancement in any phase were considered necrotic and excluded from analysis. During the post-vascular phase, an additional 5 mm diameter sampling area was selected in the adjacent normal liver parenchyma at the same depth, approximately 1 cm from the lesion margin, while avoiding intrahepatic blood vessels.
Pathological results: All cases were pathologically confirmed as HCC. Lesions with high or moderate differentiation were classified as W-D, while those with low or poorly moderate differentiation were grouped as P-D. Written informed consent was not necessary obtained from all participants, and the Institutional Ethics Committee approved the study protocol (Approval No. TJ-IRB20210130).
The formula used for calculating the parameters of the posterior vascular phase was as follows:
Absolute value of lesion EIAV = |a1 + a2 + a3|/3.
a1: Lesion EIAV 1; a2: Lesion EIAV 2; a3: Lesion EIAV 3.
IR = (a1/b1 + a2/b2 + a3/b3)/3.
b1: Peripheral liver tissue intensification 1; b2: Peripheral liver tissue intensification 2; b3: Peripheral liver tissue intensification 3.
ID = (a1 − b1 + a2 − b2 + a3 − b3)/3.
Statistical analyses were performed using SPSS version 26.0. Continuous variables were reported as mean ± SD and compared between groups using the independent samples t-test if conformed to a normal distribution, if not, non-parametric tests are employed. Categorical variables were expressed as frequencies and analyzed using the χ2 test. Variables found statistically significant in univariate analysis were entered into a binary logistic regression model for multivariate analysis. Receiver operating characteristic (ROC) curve analysis was conducted on preoperative diagnostic parameters significant in univariate or multivariate analysis to determine optimal cut-off values, sensitivity, specificity, and the area under the ROC curve (AUC) for each quantitative metric in predicting HCC differentiation. A two-tailed P value of < 0.05 was considered indicative of statistical significance.
A total of 239 hepatic lesions from 237 patients were pathologically confirmed as HCC following surgical resection, including 137 W-D and 102 P-D tumors. Univariate analysis demonstrated significant differences between the W-D and P-D groups concerning lesion size, preoperative serum AFP levels, Ki-67 expression, and the presence of vascular invasion on postoperative pathology (P = 0.003, < 0.001, < 0.001, and 0.001, respectively). No significant differences were found between groups regarding sex, age, hepatitis B status, or presence of liver cirrhosis (P > 0.05). Multivariate logistic regression identified elevated AFP levels (≥ 7 ng/mL) as an independent predictor of poor tumor differentiation (P = 0.009), suggesting that higher preoperative AFP levels are significantly associated with lower histological grades. Details are presented in Table 1.
| Variable | Univariate analysis | Multivariate analysis | ||||
| Well-differentiation | Poor differentiation | P value | OR | 95%CI | P value | |
| Clinicopathologic features | ||||||
| Males | 126 (92.0) | 86 (84.3) | 0.064 | |||
| Age, year | 56 ± 11 | 54 ± 12 | 0.138 | |||
| HBsAg-positive | 113 (82.5) | 87 (85.3) | 0.447 | |||
| AFP ≥ 7 ng/mL | 67 (48.9) | 81 (79.4) | 0.000 | 2.799 | 1.334-5.871 | 0.006 |
| Ki-67 expression level | 73 (53.3) | 83 (81.4) | 0.000 | |||
| Vascular invasion | 34 (24.8) | 48 (47.1) | 0.001 | |||
| US features | ||||||
| Tumor size (cm) | 4.3 ± 2.5 | 5.4 ± 2.9 | 0.003 | 1.055 | 0.931-1.196 | 0.397 |
| Cirrhosis | 84 (59.2) | 58 (40.8) | 0.488 | |||
| AP (hyper, iso, hypo-enhancement) | 134, 3, 0 | 98, 3, 1 | 0.474 | |||
| PVP (hyper, iso, hypo-enhancement) | 10, 84.43 | 6, 69, 27 | 0.599 | |||
| DP (hyper, iso, hypo-enhancement) | 1, 16, 120 | 0, 11, 91 | 0.669 | |||
| KP (hyper, iso, hypo-enhancement) | 1, 2, 134 | 0, 2, 100 | 0.660 | |||
| CEUS | ||||||
| Arterial phase | ||||||
| OT (second) | 15.0 ± 3.0 | 15.2 ± 3.8 | 0.660 | |||
| PT (second) | 24.9 ± 5.4 | 24.7 ± 6.6 | 0.835 | |||
| RT (second) | 9.9 ± 3.6 | 9.5 ± 4.3 | 0.424 | |||
| PIAV (second) | 34.2 ± 5.8 | 33.0 ± 6.7 | 0.151 | |||
| Kupffer phase | ||||||
| EIAV (dB) | 52.4 ± 5.3 | 54.1 ± 5.3 | 0.015 | 0.945 | 0.869-1.027 | 0.183 |
| ID (dB) | 6.9 ± 4.3 | 12.2 ± 4.8 | 0.000 | 1.048 | 0.862-1.273 | 0.636 |
| IR | 1.127 ± 0.114 | 1.299 ± 0.137 | 0.000 | 110887.3081 | 121-101163217 | 0.001 |
No statistically significant differences (P > 0.05) were observed between the W-D and P-D groups in AP parameters, including IT, PT, RT, and absolute peak intensity value (AVI). These results indicate that early enhancement kinetics during the AP are not substantially affected by the histological grade of HCC (Table 1).
Univariate analysis showed that KP parameters EIAV, IR, and ID were significantly lower in W-D HCC lesions than P-D ones, reflecting reduced Kupffer cell uptake in less differentiated tumors. Multivariate analysis identified IR as an independent predictor of poor differentiation (P = 0.001). A higher IR denotes a more pronounced contrast enhancement difference between the lesion and adjacent liver parenchyma, suggesting impaired reticuloendothelial function in P-D tumors. These results underscore the potential of IR as a useful non-invasive imaging biomarker for tumor grading (Table 1 and Supplementary material).
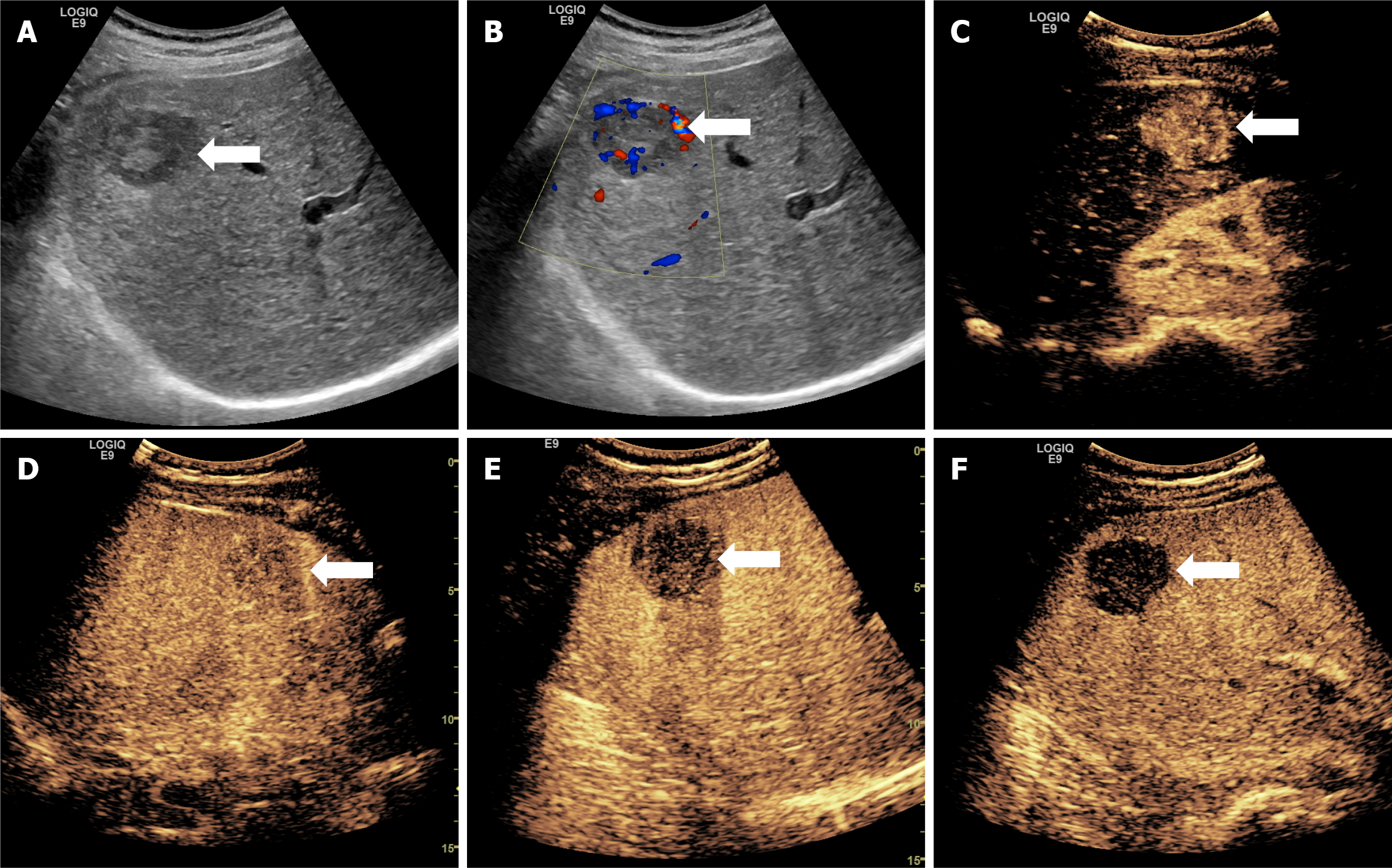
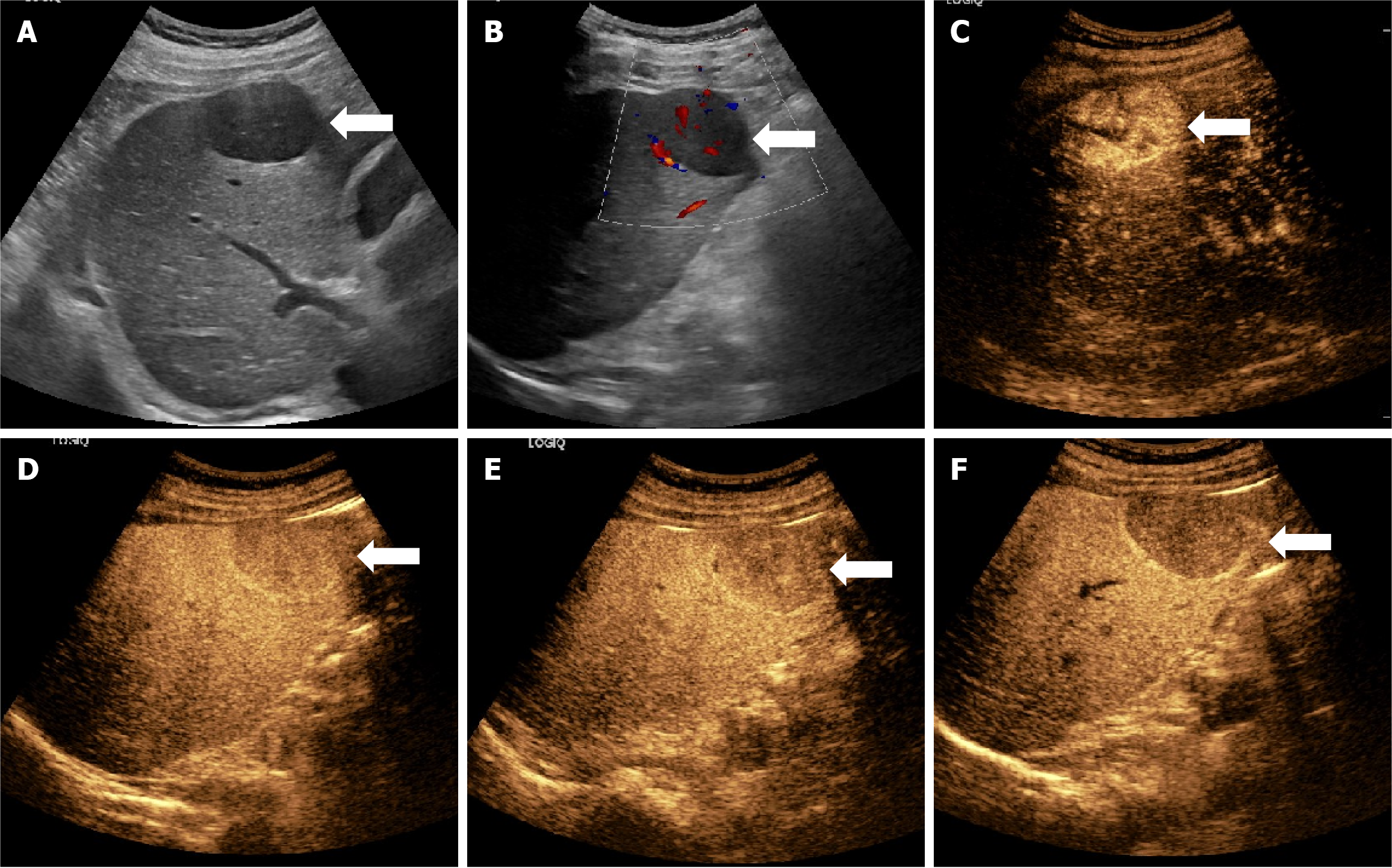
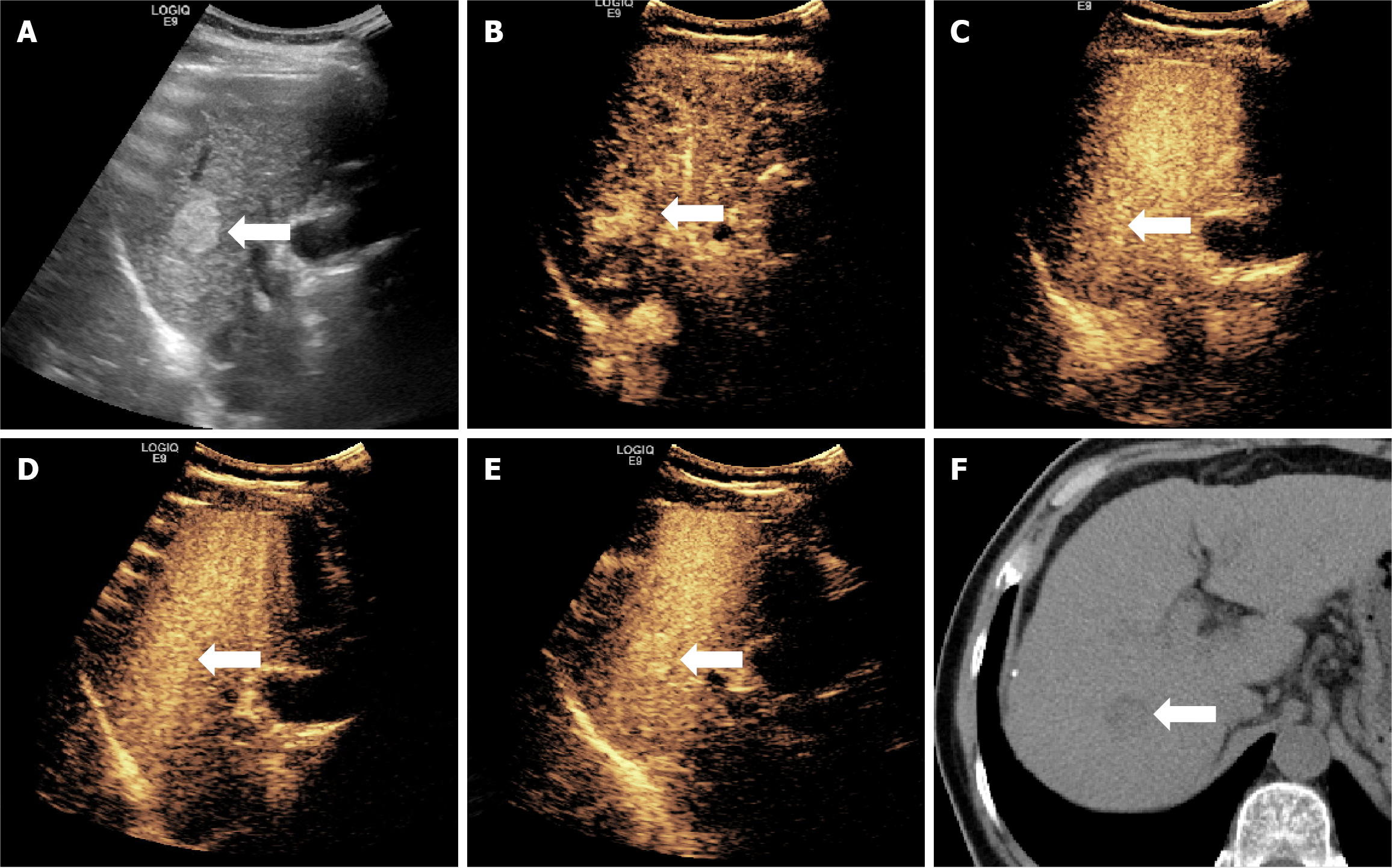
Almost all lesions displayed hyperenhancement in the arterial phase (97.1%) and hypoenhancement in the KP (97.9%), aligning with characteristic CEUS patterns of HCC. However, no significant differences in these enhancement patterns were found between the W-D and P-D groups across the imaging phases. This suggests that although phase-based qualitative enhancement is effective for diagnosing HCC, it alone is inadequate for differentiating tumor grade (Figures 2 and 3).
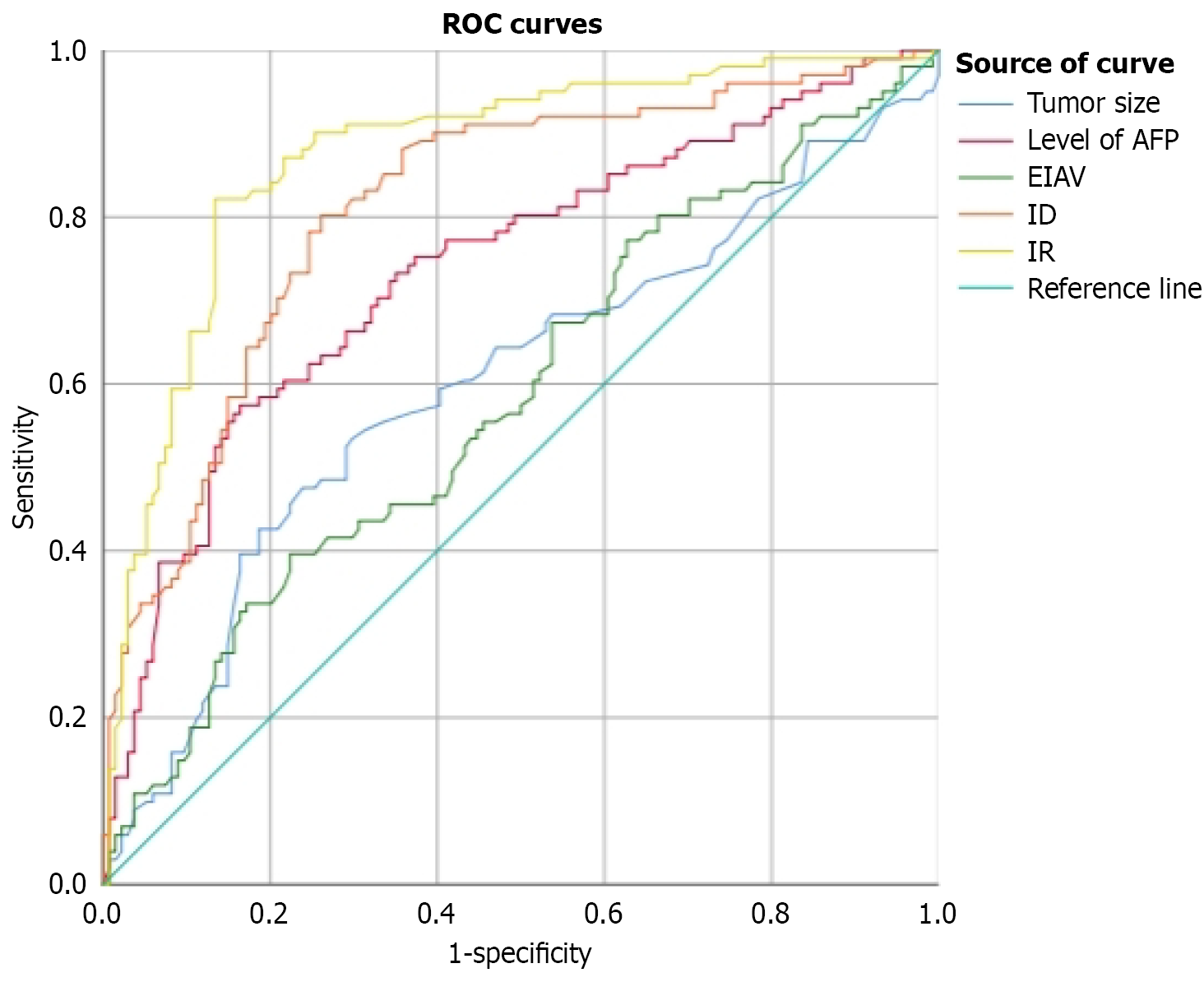
The ROC curve analysis was conducted for two preoperative clinical indicators, lesion size and AFP level, yielding AUC values of 0.610 and 0.739, respectively, indicating modest diagnostic performance. In comparison, quantitative parame
| Variable | Cut-off value | Sensitivity (%) | Specificity (%) | AUC |
| AFP (ng/mL) | 97.69 | 57.4 | 83.6 | 0.739 |
| Tumor size (cm) | 4.65 | 53.9 | 70.8 | 0.610 |
| EIAV (dB) | 56.384 | 40.2 | 78.1 | 0.590 |
| ID (dB) | 9.184 | 80.4 | 74.5 | 0.815 |
| IR | 1.215 | 82.4 | 86.9 | 0.877 |
Univariate analysis revealed significant differences in EIAV, IR, and ID across HCC lesions with different degrees of differentiation. When applying thresholds of EIAV ≥ 56.384 dB, IR ≥ 1.215, and ID ≥ 9.184 dB to identify P-D tumors, the corresponding sensitivities were 40.2%, 80.4%, and 86.9%, while specificities were 82.4%, 78.1%, and 74.5%, respectively. The respective AUC values were 0.590 for EIAV, 0.877 for IR, and 0.815 for ID. These results suggest that lower tumor differentiation is associated with increased EIAV, IR, and ID values. IR demonstrated the highest diagnostic performance among the three, followed by ID. Although EIAV showed high specificity, its limited sensitivity implies that values above 56.384 dB strongly suggest poor differentiation, but lower values cannot reliably indicate W-D status. Therefore, combining EIAV with IR and ID enhances diagnostic accuracy. Multivariate analysis further identified IR as an in
Compared to the diagnostic performance of MRI in evaluating the histological grade of HCC, the IR and ID parameters in the KP of Sonazoid-CEUS demonstrated comparable efficacy. For instance, Yan et al[15] developed a combined model integrating a Gd-EOB-DTPA-enhanced MRI radiomics signature with clinical predictors. This achieved excellent performance in predicting HCC grade in the test cohort (AUC: 0.801). Similarly, Mao et al[14] reported that a radiomics signature based on multiphase MRI exhibited strong calibration and clinical utility for grading solitary HCC. In their test set, the AUCs for various models were as follows: ANN using arterial phase (ANN-AP) 0.889, logistic regression for arterial phase (LR-AP) 0.777, ANN for hepatobiliary phase (ANN-HBP) 0.941, LR-HBP 0.819, ANN combining AP and HBP 0.944, and LR combining AP and HBP 0.792.
Furthermore, SPIO-MRI studies have shown that the ratio of Kupffer cells in tumor tissue relative to surrounding liver parenchyma declines with decreasing tumor differentiation, indicating reduced SPIO uptake in P-D HCC[12,13]. A noteworthy advantage of contrast-enhanced ultrasound is its capacity for real-time, continuous dynamic observation of lesion perfusion and vascularity. This feature minimizes the risk of missing key enhancement patterns due to suboptimal timing during MRI acquisition. Furthermore, CEUS remains effective even in patients with impaired liver function (e.g., low Child-Pugh scores), and its diagnostic accuracy is less influenced by hepatic or renal dysfunction, making it a valuable tool in clinical practice.
In this study, five lesions exhibited hyperenhancement or isoenhancement during the KP, while the remaining lesions showed hypoenhancement (Figure 5). Of these five atypical cases, four (80.0%) were classified as W-D and one (20.0%) as P-D. This pattern may be explained by the structural resemblance of W-D lesions to normal hepatic tissue, which likely supports the survival and function of Kupffer cells. Therefore, some Kupffer cells may remain active within these lesions, enabling uptake of the contrast agent and resulting in higher or equivalent enhancement during the KP.
Moreover, four of these five lesions (80.0%) appeared hyperechoic on gray-scale ultrasound. Since the MI used in fluorobutane contrast imaging is higher than that used in other CEUS modalities, background tissue suppression is reduced, potentially affecting the visual appearance of lesions in the post-vascular phase. Despite these exceptions, the vast majority of lesions in this study demonstrated hypoenhancement during the KP, consistent with diminished Kupffer cell activity in HCC.
Some studies have proposed that changes in Kupffer cell density occur later than alterations in intratumoral venous blood flow during tumor differentiation. As a result, imaging based on portal vein perfusion may detect histological changes earlier than the PVP alone[26,27]. However, our findings challenge this conventional view. In the W-D group, the numbers of lesions showing hyperenhancement, isoenhancement, and hypoenhancement in the PVP were 10, 84, and 43, respectively, compared to 1, 2, and 134 in the KP. For the P-D group, the corresponding numbers were 6, 69, and 27 in the PVP, and 0, 2, and 100 in the KP. Further, 66.4% (91/137) of W-D lesions and 71.6% (73/102) of P-D lesions did not demonstrate obvious washout during the PVP but did exhibit clear washout in the KP.
However, there were no significant differences in enhancement regression patterns between the two differentiation groups across the imaging phases. These findings suggest that the KP may provide greater sensitivity in identifying histological differences compared to earlier vascular phases. As HCC progresses through different differentiation stages, the reduction in echo intensity during the KP appears more pronounced than changes in vascular density observed in the PVP. This implies that the depletion of Kupffer cells may occur earlier in tumor development than vascular remodeling, making KP imaging a more sensitive marker for grading differentiation.
Therefore, reliance on contrast clearance patterns during the portal phase may not reliably reflect tumor grade. In comparison, the degree of hypoenhancement in the KP, driven by Kupffer cell loss, offers a more meaningful and earlier indication of histological progression. In essence, KP hypoenhancement could serve as an early and sensitive imaging biomarker of tumor differentiation. Supporting this, previous studies have reported that 10%-33% of HCC lesions exhibit washout exclusively in the KP, without corresponding washout in the delayed phase[28-30]. Our study identified this pattern in 10.2% (14/137) of W-D and 13.7% (14/102) of P-D lesions, reinforcing that Kupffer cell depletion is a genuine and progressive feature of HCC development. These results further emphasize the KP as a more sensitive window for detecting differentiation-related changes[31]. Some studies have suggested that in chronic liver disease, regenerative nodules may progressively enlarge, transition into dysplastic nodules, and eventually develop into HCC. Throughout this process, the nodules exhibit characteristic changes in blood supply: A gradual decline in portal venous perfusion, accompanied by an initial decrease and subsequent increase in arterial blood flow as differentiation progresses[32]. On CEUS imaging, washout during the PVP can be observed. However, the decline in portal vessel density and blood supply is not linear; instead, it follows a gradual decline followed by a more rapid decrease.
Washout in the portal phase may be influenced by other contributing factors beyond vascular density alone[33]. The reduction in Kupffer cell numbers in HCC is driven by alterations in the tumor microenvironment, which persist throughout the entire course of nodular progression and are both a cause and consequence of tumor evolution. As such, Kupffer cell depletion may precede the reduction in portal blood supply. This could explain our findings and suggests that integrating Kupffer-phase imaging and vascular assessment may enhance the accuracy of histological grading in HCC lesions.
The results of this study suggest that quantitative analysis of the AP using fluorobutane-enhanced contrast ultrasound is ineffective for distinguishing HCC lesions with varying degrees of differentiation. Although previous studies have indicated that vascular patterns in the AP, contrast washout rates in the portal phase, and combined arterial and early portal enhancement evaluations may offer clues about tumor grade[26,34], these methods present significant practical limitations. Assessing vascular distribution in the AP is highly subjective, and accurately identifying the transition between arterial and early portal phases is technically challenging, which hinders consistency and reproducibility in clinical settings. Furthermore, the wide inter-lesional variability in AP enhancement patterns may compromise the reliability of quantitative assessments, limiting the diagnostic utility of AP-derived parameters in this context. However, the KP offers a well-defined and prolonged observation window (approximately 12 minutes), which enhances measure
This study highlights the value of KP imaging features in the histological assessment of HCC. It introduces novel quantitative parameters, EIAV, IR, and ID, with IR showing the strongest diagnostic performance. These indicators surpass traditional clinical markers and offer a non-invasive, reliable means for predicting tumor differentiation, supporting more informed preoperative decision-making in HCC management. Furthermore, the study's robustness is reinforced because all cases were pathologically confirmed through surgical resection. The comprehensive analysis of clinical data alongside multimodal ultrasound imaging further underscores the feasibility and credibility of the findings.
This study has several limitations that should be acknowledged. First, although a correlation was established between AFP levels and tumor differentiation, and a cut-off value was identified via ROC curve analysis, extreme AFP values in certain patients (e.g., diluted levels exceeding 60500 ng/mL) may have skewed the group averages and influenced the overall results. Second, due to the limited availability of cases with purely defined histological grades, tumors were categorized into two broader groups for analysis: W-D (comprising well-, high-moderate-, and moderate-differentiated HCC) and P-D (including moderate-low and P-D HCC). Third, ROIs were selected based on the visually most enhanced areas during the arterial phase, a subjective method intended to capture peak vascularity. Although two blinded resear
In conclusion, the IR, a quantitative parameter derived from the KP of fluorobutane-enhanced contrast ultrasound, demonstrated excellent diagnostic performance in distinguishing HCC lesions according to their histological differentiation. Among all the evaluated indicators, IR exhibited the highest sensitivity, specificity, and overall accuracy, making it the most reliable predictor of poor differentiation. Its biological relevance further supports its diagnostic value, as it reflects the early decline in Kupffer cell activity during tumor progression. IR is straightforward to calculate, shows minimal susceptibility to individual variability in hepatic perfusion or fibrosis, and is highly applicable in clinical settings. Unlike qualitative imaging features, IR offers an objective and reproducible metric for preoperative evaluation. These findings suggest that IR holds promise as a non-invasive imaging biomarker for early risk stratification and therapeutic decision-making in HCC. Further validation in larger, multicenter studies is warranted to confirm its clinical utility.
| 1. | Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep. 2019;6:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4298] [Article Influence: 226.2] [Reference Citation Analysis (2)] |
| 3. | Tamura S, Kato T, Berho M, Misiakos EP, O'Brien C, Reddy KR, Nery JR, Burke GW, Schiff ER, Miller J, Tzakis AG. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136:25-30; discussion 31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 192] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M, Sassa T. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Oishi K, Itamoto T, Amano H, Fukuda S, Ohdan H, Tashiro H, Shimamoto F, Asahara T. Clinicopathologic features of poorly differentiated hepatocellular carcinoma. J Surg Oncol. 2007;95:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Li X, Huang L, Leng X. Analysis of prognostic factors of more/equal to10 years of survival for liver cancer patients after liver transplantation. J Cancer Res Clin Oncol. 2018;144:2465-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2025;83:502-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 112] [Article Influence: 112.0] [Reference Citation Analysis (1)] |
| 8. | Wen N, Cai Y, Li F, Ye H, Tang W, Song P, Cheng N. The clinical management of hepatocellular carcinoma worldwide: A concise review and comparison of current guidelines: 2022 update. Biosci Trends. 2022;16:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 9. | Chen VL, Sharma P. Role of Biomarkers and Biopsy in Hepatocellular Carcinoma. Clin Liver Dis. 2020;24:577-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Wang F, Numata K, Okada M, Chuma M, Nihonmatsu H, Moriya S, Nozaki A, Ogushi K, Luo W, Ruan L, Nakano M, Otani M, Inayama Y, Maeda S. Comparison of Sonazoid contrast-enhanced ultrasound and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid MRI for the histological diagnosis of hepatocellular carcinoma. Quant Imaging Med Surg. 2021;11:2521-2540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Tanaka H, Iijima H, Higashiura A, Yoh K, Ishii A, Takashima T, Sakai Y, Aizawa N, Iwata K, Ikeda N, Iwata Y, Enomoto H, Saito M, Imanishi H, Hirota S, Fujimoto J, Nishiguchi S. New malignant grading system for hepatocellular carcinoma using the Sonazoid contrast agent for ultrasonography. J Gastroenterol. 2014;49:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Li YW, Chen ZG, Wang JC, Zhang ZM. Superparamagnetic iron oxide-enhanced magnetic resonance imaging for focal hepatic lesions: systematic review and meta-analysis. World J Gastroenterol. 2015;21:4334-4344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 13. | Saito K, Ledsam J, Sourbron S, Araki Y. Validation study of perfusion parameter in hypervascular hepatocellular carcinoma and focal nodular hyperplasia using dynamic susceptibility magnetic resonance imaging with super-paramagnetic iron oxide: comparison with single level dynamic CT arteriography. Quant Imaging Med Surg. 2020;10:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Mao Y, Wang J, Zhu Y, Chen J, Mao L, Kong W, Qiu Y, Wu X, Guan Y, He J. Gd-EOB-DTPA-enhanced MRI radiomic features for predicting histological grade of hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2022;11:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Yan Y, Si Z, Chun C, Chao-Qun P, Ke M, Dong Z, Li W. Multiphase MRI-Based Radiomics for Predicting Histological Grade of Hepatocellular Carcinoma. J Magn Reson Imaging. 2024;60:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Choi JW, Lee JM, Kim SJ, Yoon JH, Baek JH, Han JK, Choi BI. Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR Images and their value as an imaging biomarker. Radiology. 2013;267:776-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, Chammas MC, Chaubal N, Choi BI, Clevert DA, Cui X, Dong Y, D'Onofrio M, Fowlkes JB, Gilja OH, Huang P, Ignee A, Jenssen C, Kono Y, Kudo M, Lassau N, Lee WJ, Lee JY, Liang P, Lim A, Lyshchik A, Meloni MF, Correas JM, Minami Y, Moriyasu F, Nicolau C, Piscaglia F, Saftoiu A, Sidhu PS, Sporea I, Torzilli G, Xie X, Zheng R. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579-2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 18. | Watanabe R, Matsumura M, Munemasa T, Fujimaki M, Suematsu M. Mechanism of hepatic parenchyma-specific contrast of microbubble-based contrast agent for ultrasonography: microscopic studies in rat liver. Invest Radiol. 2007;42:643-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol. 2007;33:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 20. | Liu K, He X, Lei XZ, Zhao LS, Tang H, Liu L, Lei BJ. Pathomorphological study on location and distribution of Kupffer cells in hepatocellular carcinoma. World J Gastroenterol. 2003;9:1946-1949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Lee JY, Minami Y, Choi BI, Lee WJ, Chou YH, Jeong WK, Park MS, Kudo N, Lee MW, Kamata K, Iijima H, Kim SY, Numata K, Sugimoto K, Maruyama H, Sumino Y, Ogawa C, Kitano M, Joo I, Arita J, Liang JD, Lin HM, Nolsoe C, Gilja OH, Kudo M. The AFSUMB Consensus Statements and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound using Sonazoid. J Med Ultrasound. 2020;28:59-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Arita J, Hasegawa K, Takahashi M, Hata S, Shindoh J, Sugawara Y, Kokudo N. Correlation between contrast-enhanced intraoperative ultrasound using Sonazoid and histologic grade of resected hepatocellular carcinoma. AJR Am J Roentgenol. 2011;196:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Ohama H, Imai Y, Nakashima O, Kogita S, Takamura M, Hori M, Seki Y, Sawai Y, Igura T, Fukuda K, Makino Y, Morimoto O, Ohsawa M, Sakamoto M, Murakami T. Images of Sonazoid-enhanced ultrasonography in multistep hepatocarcinogenesis: comparison with Gd-EOB-DTPA-enhanced MRI. J Gastroenterol. 2014;49:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Korenaga K, Korenaga M, Furukawa M, Yamasaki T, Sakaida I. Usefulness of Sonazoid contrast-enhanced ultrasonography for hepatocellular carcinoma: comparison with pathological diagnosis and superparamagnetic iron oxide magnetic resonance images. J Gastroenterol. 2009;44:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Kudo M, Ueshima K, Osaki Y, Hirooka M, Imai Y, Aso K, Numata K, Kitano M, Kumada T, Izumi N, Sumino Y, Ogawa C, Akazawa K. B-Mode Ultrasonography versus Contrast-Enhanced Ultrasonography for Surveillance of Hepatocellular Carcinoma: A Prospective Multicenter Randomized Controlled Trial. Liver Cancer. 2019;8:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 26. | Saito A, Yamamoto M, Katagiri S, Yamashita S, Nakano M, Morizane T. Early hemodynamics of hepatocellular carcinoma using contrast-enhanced ultrasound with Sonazoid: focus on the pure arterial and early portal phases. Glob Health Med. 2020;2:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Maruyama H, Takahashi M, Ishibashi H, Okabe S, Yoshikawa M, Yokosuka O. Changes in tumor vascularity precede microbubble contrast accumulation deficit in the process of dedifferentiation of hepatocellular carcinoma. Eur J Radiol. 2010;75:e102-e106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Kudo M, Hatanaka K, Inoue T, Maekawa K. Depiction of portal supply in early hepatocellular carcinoma and dysplastic nodule: value of pure arterial ultrasound imaging in hepatocellular carcinoma. Oncology. 2010;78 Suppl 1:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Takahashi M, Maruyama H, Ishibashi H, Yoshikawa M, Yokosuka O. Contrast-enhanced ultrasound with perflubutane microbubble agent: evaluation of differentiation of hepatocellular carcinoma. AJR Am J Roentgenol. 2011;196:W123-W131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Inoue T, Kudo M, Maenishi O, Komuta M, Nakashima O, Kojiro M, Maekawa K. Value of liver parenchymal phase contrast-enhanced sonography to diagnose premalignant and borderline lesions and overt hepatocellular carcinoma. AJR Am J Roentgenol. 2009;192:698-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Hwang JA, Jeong WK, Kang HJ, Lee ES, Park HJ, Lee JM. Perfluorobutane-enhanced ultrasonography with a Kupffer phase: improved diagnostic sensitivity for hepatocellular carcinoma. Eur Radiol. 2022;32:8507-8517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272:635-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 369] [Article Influence: 30.8] [Reference Citation Analysis (1)] |
| 33. | Nguyen SA, Merrill CD, Burrowes DP, Medellin GA, Wilson SR. Hepatocellular Carcinoma in Evolution: Correlation with CEUS LI-RADS. Radiographics. 2022;42:1028-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 34. | Tada T, Kumada T, Toyoda H, Ito T, Sone Y, Kaneoka Y, Maeda A, Okuda S, Otobe K, Takahashi K. Utility of Contrast-enhanced Ultrasonography with Perflubutane for Determining Histologic Grade in Hepatocellular Carcinoma. Ultrasound Med Biol. 2015;41:3070-3078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |