Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.109127
Revised: June 6, 2025
Accepted: July 23, 2025
Published online: September 15, 2025
Processing time: 128 Days and 15.2 Hours
Hepatocellular carcinoma (HCC) is a significant global health challenge with rising incidence rates and poor prognoses. Aminoacyl-tRNA synthetases (ARSs) are important regulators implicated in the occurrence and progression of several cancers. However, their specific function in HCC remains unclear, and ARSs-related prognostic factors for patient stratification are lacking.
To investigate the ARSs-related mechanisms of HCC and establish an effective prognostic risk model for stratifying patients with HCC.
We screened ARSs genes of interest using differential gene expression, mutation, and survival analysis. Western blot and Immunohistochemistry were used to analyze MARS1 expression in the liver tissues of patients with HCC. Functional studies, including CCK-8 cell viability assay, EdU cell proliferation assay, cell cycle assays, Transwell migration and invasion assays, and in vivo tumor xenograft models, were conducted to comprehensively elucidate the specific role of MARS1 in HCC. Moreover, the MARS1-related prognostic score (MRPS) was established by LASSO regression and Cox regression analysis in The Cancer Genome Atlas-HCC and GSE14520 cohorts. Patients’ immunotherapy and chemotherapy responses were predicted by immunomicroenvironment and drug susceptibility analysis in both subgroups.
MARS1 was selected as a target gene from a series of ARSs genes, with markedly higher expression observed in HCC tissues compared to adjacent non-cancerous tissues. Silencing MARS1 considerably impeded the proliferation, migration, invasion, and tumorigenic abilities of HCC cells in vitro and in vivo. Moreover, high MRPSs were associated with poor overall survival, altered infiltration of T cells, macrophages, monocytes, elevated immune checkpoint expression (PD-L1, CTLA4, LAG3), and reduced drug sensitivity in HCC.
MARS1 promotes HCC development and represents a potential therapeutic target for HCC management. Fur
Core Tip: Aminoacyl-tRNA synthetases (ARSs) are important regulators implicated in the occurrence and progression of several cancers. However, their specific function in hepatocellular carcinoma (HCC) remains unclear, and effective ARSs-related prognostic risk factors for stratifying patients are lacking. In this study, we identified that Methionyl-tRNA synthetase 1 (MARS1) promotes the proliferation, migration, invasion, and tumor formation of hepatoma cells in vitro and in vivo, highlighting its potential as a therapeutic target for HCC. Moreover, we established MARS1-related prognostic score as an independent prognostic factor for patient survival and a predictor of tumor treatment response, contributing valuable insights into the development of personalized treatment strategies.
- Citation: Bu HY, Huang H, Li J, Huang ZB, Huang Y, Huang YK, Wang AM, Wu L, Yuan J, Wang RJ, Lu M, Yu SM, Yi PP, Chen YY, Jiang YP, Hu XW. Methionyl-tRNA synthetase 1 participates in hepatocellular carcinoma and its regulated gene profile possesses potent prognostic ability. World J Gastrointest Oncol 2025; 17(9): 109127
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/109127.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.109127
Primary liver cancer, ranked as the sixth most prevalent malignancy globally and the third leading cause of oncological mortality, poses a considerable health challenge, with hepatocellular carcinoma (HCC) representing the predominant subtype. Given the high incidence rate and adverse outcomes associated with HCC[1], the efficacy of known clinicopathological risk factors for stratifying patients remains inadequate. Consequently, the search for novel prognostic biomarkers has become essential for advancing strategies and improving therapeutic outcomes for HCC.
Current investigations into HCC pathogenesis have predominantly focused on epigenetic regulation, viral etiology, and oxidative stress[2-4]; however, the underlying mechanisms remain incompletely understood. Protein biosynthesis is essential to the survival, growth, and functional integrity of cancerous cells, and contributes to the complex etiology of HCC. However, the role of tRNA in mediating amino acid incorporation during protein translation has received comparatively lesser attention. Previous studies on protein synthesis have largely concentrated on RNA modification, elongation factors, amino acid metabolism, and post-translational modifications.
Aminoacyl-tRNA synthetases (ARSs), a cohort of enzymes performing an indispensable function in the process of mRNA translation, play an instrumental role. They accurately recognize and catalytically ligate specific amino acids to their corresponding tRNAs, thereby facilitating the assembly of aminoacyl-tRNAs[5]. The charged tRNAs are sub
The pivotal contribution of ARSs to the development of tumors highlights their potential as both therapeutic and prognostic biomarkers in oncology. Currently, only a few studies on ARSs in HCC have been published, with some focusing on GARS[13] and AARS2[14].
Here, we identify Methionyl-tRNA synthetase 1 (MARS1), a critical ARS gene, that holds promise as a therapeutic and prognostic marker in HCC pathogenesis. Furthermore, we develop risk models capable of providing prognostic pre
Transcriptome data from 424 patients with HCC were retrieved from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/projects/TCGA-LIHC), comprising 50 healthy and 374 tumor samples. Following the exclusion of samples with normal or lacking survival data, 371 tumor samples were retained for the analysis. The validation cohort included dataset GSE14520 data from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14520), in which samples lacking survival data were excluded, resulting in 242 tumor samples.
Somatic mutation data from 371 patients with HCC in the TCGA-HCC cohort were downloaded from the TCGA database and analyzed using the "maftools" R package.
Genes associated with the 41 ARSs were extracted from the Molecular Signature Database (https://www.gsea-msigdb.
Using the differential genes extracted in the merged cohort, the TCGA and GSE14520 cohorts were used as the training and validation groups, respectively. Univariate Cox analysis was conducted to screen genes (P < 0.05). Subsequently, LASSO Cox regression was used to select genes, minimizing the risk of overfitting, and the risk prediction model was established through multivariate Cox regression. The risk score formula is as follows:
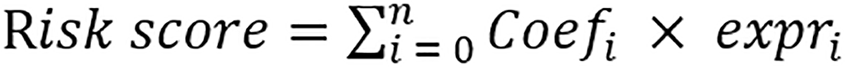 .
.
Where Coefi represents the corresponding coefficient of each gene, while expri represents the expression. The optimal cutoff value of MARS1-related prognostic score (MRPS) was calculated by the “survminer” package, and the samples were categorized into high- and low-MRPS groups. The R packages “survminer” and “survival” were used to plot the survival curves for both groups. Stability analysis of MRPS was performed using data from the validation group (GSE14520), and the “survival receiver operating characteristic (ROC)” was used in a time-dependent ROC analysis to assess the model's performance. Furthermore, based on clinical data from TCGA-HCC patients, univariate and multivariate Cox regression analyses were conducted to assess whether MRPS is an independent prognostic factor of overall survival (OS). The “rms” package was utilized to combine clinical factors with the MRPS of individual patients to create the nomogram. The predictive accuracy between different models was compared using ROC curves by the “survivalROC” R package.
Using the “survival” and “survminer” packages, according to model grouping, the Kaplan-Meier curves for each clinical characteristic were plotted.
Differential gene expression analysis based on the model groupings were conducted on TCGA-HCC tumor samples using the “limma” R package, with a P < 0.05 and |log2FC| > 1 indicating significant differential regulation. The “clusterProfiler” package was used to identify significantly enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and Gene Ontology (GO; P < 0.05).
With the Cell-type Identification by Estimating Relative Subsets of RNA Transcripts method, the “e1071”, “preprocessCor” as well as “limma” R packages were used to obtain 22 immune cell infiltration results of the TCGA cohort. These results were subsequently visualized utilizing the “ggplot2” and "tidyr” packages. Furthermore, a comparison of immune checkpoint and PD-L1 expression between high- and low-MRPS groups was conducted using the “ggpubr” package. Microsatellite instability (MSI) values were compared and visualized using the “ggpubr” and “ggplot2” packages.
Half-maximal inhibitory concentration (IC50) values for chemotherapeutic drugs in both high and low MRPS groups were predicted using the “pRRophetic” package.
Between January 2021 and December 2023, HCC tissue and adjacent non-tumor tissue specimens were collected from 12 patients with HCC who underwent hepatectomy at Xiangya Hospital Central South University (Changsha, China). All tissue samples were fixed in 4% paraformaldehyde and subsequently used to construct liver tissue microarrays for immunohistochemistry analysis. For five of the patients, portions of the liver tissue were immediately snap-frozen in liquid nitrogen after resection and stored at -80 °C for Western blot. The experiments using liver tissues collected from patients with HCC were approved by the Ethics Committee of Xiangya Hospital (No. 202009881).
HCC-LM3 and HCC-Huh7 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and incubated in Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA, United States) containing 10% FBS (NEWZERUM, Christchurch, New Zealand) and 1% penicillin/streptomycin (DMEM; Gibco). Using Lipo
Cells (1 × 107) were subcutaneously injected into BALB/c 4w nude mice (five per group; randomisation) (Vital River Laboratories, Beijing, China). The nude mice used in this experiment were housed under specific pathogen-free conditions, with controlled temperature and humidity. Every three days, the tumor volume was calculated using the formula length × width2 × 0.5 and recorded. After 2-3 weeks, the mice were humanely euthanized. Tumors were surgically excised and their weights were recorded. Animal experiments were conducted strictly in compliance with the NIH laboratory animal care and use guidelines. Animal experiments were approved by the Experimental Animal Ethics Review Committee of Xiangya Hospital (No. 202310052).
To lyse cells or homogenized tissues, RIPA buffer (Beyotime Biotechnology, Shanghai, China) was utilized together with phosphatase and protein inhibitors. The lysates were centrifuged at 14000 × g for 15 minutes, and then the BCA Protein Assay Kit (Thermo Scientific, MA, United States) was used to determine the protein concentration. PVDF membranes (0.22 µm; Millipore, Billerica, MA, United States) were used to transfer the separated proteins under low-temperature conditions after they were separated using 10% SDS PAGE. After a 2-hour blocking phase, primary antibodies from Proteintech (MARS1, mouse monoclonal, 1:20000, 67739-1-Ig; GAPDH, rabbit monoclonal, 1:20000, 10494-1-AP) were applied and left to incubate at 4 °C for the entire night. Afterward, the membranes were incubated with secondary antibodies tagged with horseradish peroxidase (FuShen, Goat Anti-Mouse IgG-HRP, 1:5000, FSM0056; Proteintech, Goat Anti-Rabbit IgG-HRP, 1:5000, SA00001-2) at room temperature for an hour. The blotted proteins expression was assessed utilizing a Bio-Rad ChemiDoc MP platform and an ECL kit (UElandy, Suzhou, China).
Microarrays containing HCC and adjacent normal tissues were dewaxed twice (30 minutes each) in a dewaxing solution (Servicebio, Wuhan, China) and rehydrated with graded ethanol (100%-100%-95%-80%-75%-50%). Antigen extraction from the paraffin sections was performed by high-pressure boiling for 10 minutes in EDTA solution. The sections were then incubated at room temperature for 15 minutes with endogenous peroxidase and for 30 minutes with goat serum (Zsbio, Beijing, China). Thereafter, sections were incubated with MARS1 polyclonal antibody (1:50; Proteintech, 14829-1-AP, Wuhan, China) at 4 °C overnight. Following washing, the sections were exposed to 3,3'-diaminobenzidine tetrahydrochloride (DAB) enhancement solution, added dropwise, and incubated for 20 minutes at room temperature. Following another wash, sections were incubated with a secondary antibody (Zsbio, Beijing, China) for 30 minutes, and subsequently underwent treatment with a freshly prepared solution of DAB (Zsbio, Beijing, China) in a dark environment. Hematoxylin solution (Servicebio, Wuhan, China) was used for counterstaining, and neutral resin was used to seal the sections.
Cells were cultured in 100 μL of medium suspension. They were then seeded into 96-well plates (HCC-LM3: 4000 cells/well, HCC-Huh7: 1500 cells/well). The next day, 10 μL CCK-8 (Ecotop Scientific, Guangzhou, China) was added to each well and incubated for 2 hours. Using a spectrophotometer (PerkinElmer, MA, United States), the absorbance was measured at 450 nm.
Cells were cultured in 1 mL of medium suspension and seeded into 12-well plates until the cells reached the appropriate growth state. After removing the initial medium, each well received the addition of EdU culture medium (EdU Cell Proliferation Kit, C0075S, Beyotime Biotechnology, Shanghai, China) to achieve a final concentration of 10 μM EdU. LM3 and Huh7 cells were then incubated for 3 hours and 2.5 hours, respectively. The EdU cultures were discarded and fixed with 4% paraformaldehyde (Biosharp, Anhui, China) for 15 minutes at room temperature. Subsequently, the cultures were rinsed three times with a washing solution (5 minutes/time) and permeabilized for 15 minutes with a permeabilizing solution containing 0.5% Triton X-100 and rinsed twice with a washing solution. The cells were treated with the prepared click reaction mixture at room temperature for 30 minutes, protected from light, and rinsed three times with washing solution. Nuclear staining was performed, and the results were examined under an AXIO SCOPE A1 microscope (Zeiss, Oberkochen, Germany).
After collection, the cells were washed twice with PBS, fixed in anhydrous ethanol for 12-24 hours and centrifuged before the assay. The ethanol was discarded, and the precipitate was loosened by gently flicking the walls of the tube. The cells were rehydrated by adding 2-5 mL of room-temperature PBS for 15 minutes. Following a centrifugation and disposal of the supernatant, 1 mL DNA staining solution (Cell Cycle Staining Kit, A3065, Liankebio, Zhejiang, China) was added and mixed by vortexing for 5-10 seconds. The lowest sampling rate was selected. Cell cycle was determined using FACS Canto II (BD Immunocytometry Systems, CA, United States), and the results were analyzed via Flow Jo software (BD Biosciences, OR, United States).
Migration and Matrigel invasion assays employed Transwell chambers (8 μm membrane pore size, 6.5 mm diameter) (Corning Costar, Corning, NY, United States). In Migration assays, LM3 cells (1 × 105 cells/well) or Huh7 cells (3 × 104 cells/well) were seeded into the upper chamber with serum-free medium, while migration-inducing medium (containing 10% FBS) was supplied to the lower chamber for incubation at 37 °C. The cells attached to the membrane's upper surface were carefully removed using a cotton swab following fixation with 4% paraformaldehyde, and the cells on the lower surface were then stained with crystal violet. Membranes were rinsed with PBS, air-dried, and imaged. Prior to the addition of cells, the top chamber was coated with Matrigel for the Matrigel invasion assays.
Survival analysis was performed using the R software (version 4.2.2). The Wilcoxon signed-rank test was applied to examine variations in the composition of immune-infiltrating cells, and the log-rank test was employed to assess survival disparities via Kaplan-Meier analysis. We employed two-tailed tests for all statistical analyses, setting the significance level at P < 0.05. Student’s t-test was used to compare differences between two groups, while one-way analysis of variance was applied for comparisons involving more than two groups. Mean ± SEM was used to record all quantitative data. Statistical analyses were conducted, and graphs were created using Prism version 8 (GraphPad Software, San Diego, CA, United States).
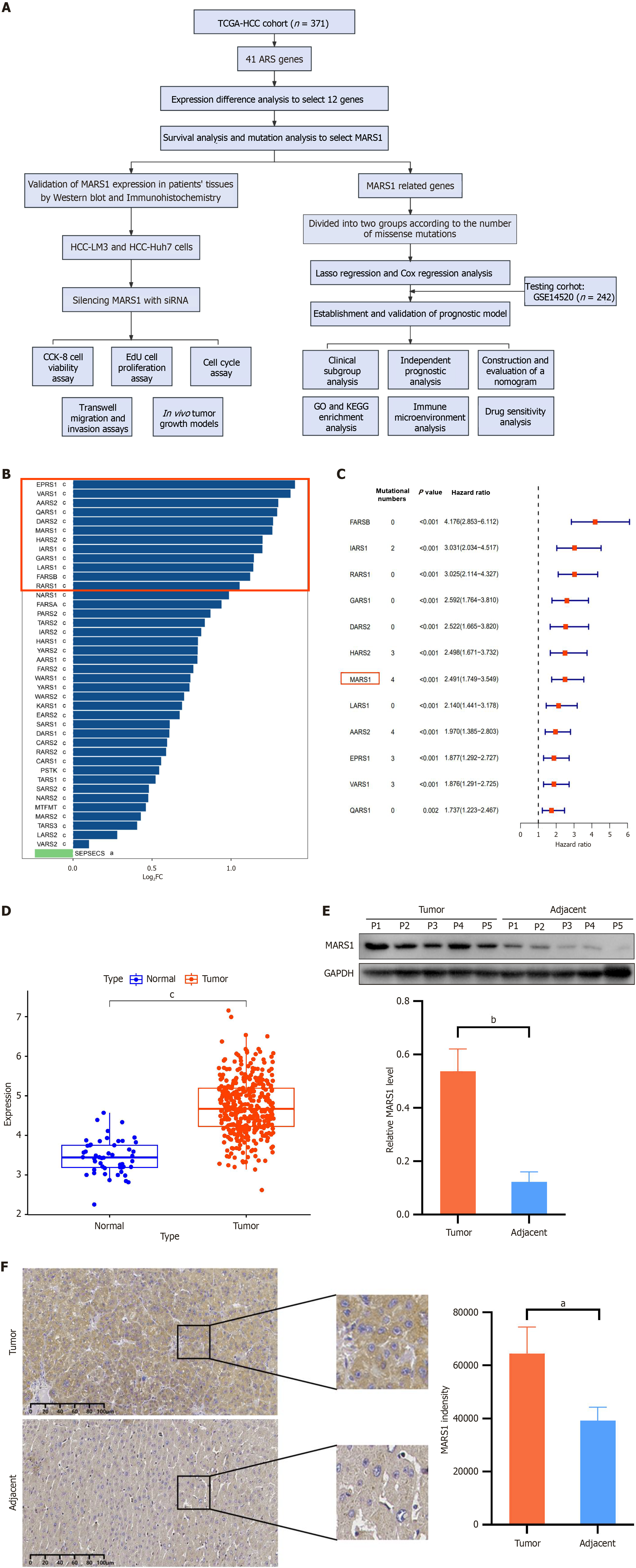
Although ARSs are essential for protein synthesis, their roles in HCC progression have scarcely been reported. To explore this, ARS genes responsible for accurate and efficient translation of the genetic code into functional proteins were analyzed using datasets from the KEGG database, and key DEGs were selected for further study (Figure 1A).
Overall, 41 ARS genes were analyzed for expression in HCC tissues and compared to adjacent normal tissues (|log2FC| > 1, P < 0.05). Twelve genes exhibited significant differential expression (Figure 1B). In addition, mutation analysis in these genes revealed that MARS1 and AARS2 exhibited the highest mutation rates. Survival analysis was constructed to elucidate the prognostic implications of these DEGs. MARS1 and AARS2 had hazard ratio of 2.491 and 1.970, respectively (Figure 1C). Accordingly, we selected MARS1 as the gene of interest.
The expression of MARS1 was further verified in human liver tissue samples collected at Xiangya Hospital, Central South University. According to KEGG data, MARS1 expression in 376 cancer tissues was 1.37 (representing 4.719/3.457) times higher than that in 50 adjacent normal tissues (Figure 1D). Western blot results showed that the expression of MARS1 in five patients with HCC was 4.40 times higher than that in adjacent normal tissues (0.5369 vs 0.1221; Figure 1E). Subsequently, immunohistochemistry analysis was performed on the liver tissue samples from 12 patients with HCC, revealing that the expression of MARS1 was 1.64 times higher in tumor tissues than that in adjacent normal tissues (64476 vs 39232; Figure 1F). These findings indicate that MARS1 expression is markedly upregulated in HCC and may serve as a potential biomarker or therapeutic target.
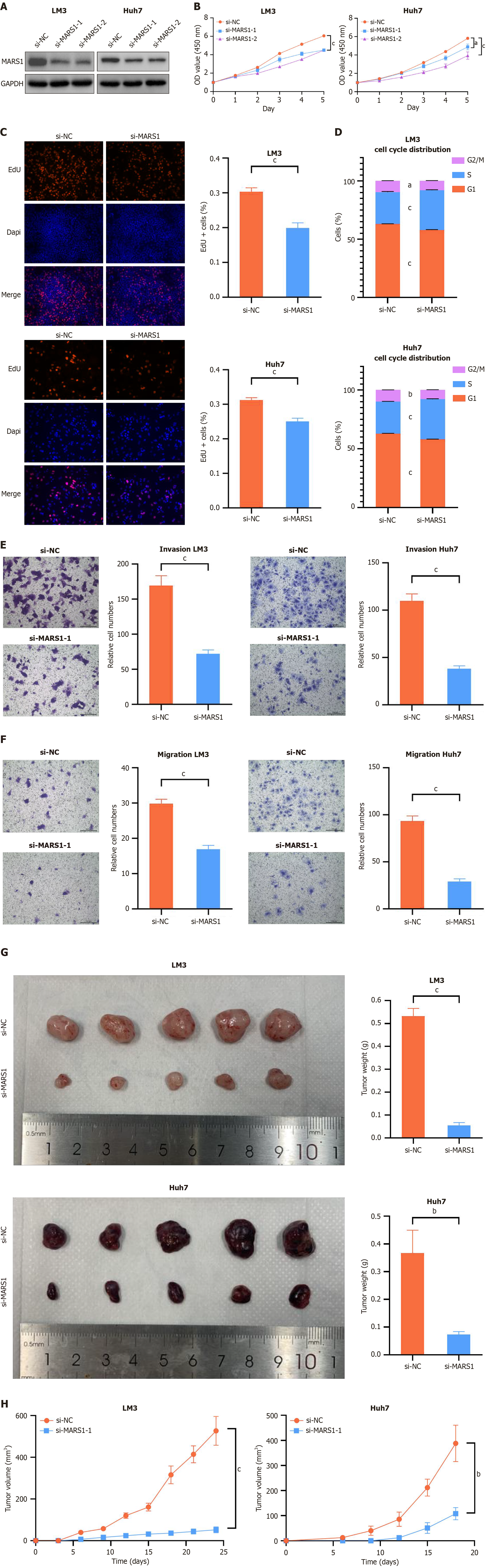
To elucidate the specific role of MARS1 in HCC, we used HCC-LM3 and HCC-Huh7 cells for experimental validation. RNA-silencing techniques were used to suppress MARS1 expression. Western blot confirmed the efficacy of both si-MARS1-1 and si-MARS1-2 in reducing MARS1 Levels in LM3 and Huh7 cells (Figure 2A). Subsequent CCK-8 assay revealed that the decrease in MARS1 expression markedly impeded the growth of these cells (Figure 2B). Given its superior inhibitory effect, si-MARS1-2 was selected for subsequent experiments. Further investigation using EdU assay reinforced the finding that silencing the expression of MARS1 inhibited the proliferative capacity of these cells (Figure 2C). Cell cycle analysis revealed that reduced MARS1 expression disrupted the progression from the S phase to the G2/M phase, indicating potential cell cycle arrest (Figure 2D). Transwell assays provided evidence that diminished MARS1 expression attenuated the migratory and invasive abilities of HCC cells (Figure 2E and F). Moreover, we observed that in vivo MARS1 depletion in both LM3 and Huh7 cells markedly reduced the volume and weight of mouse subcutaneous xenografted tumors (Figure 2G and H). Collectively, these results compellingly provide strong evidence that MARS1 is essential for promoting the proliferation, migration, invasion, and tumor formation of liver cancer cells, thereby underscoring its significance in the pathogenesis and progression of HCC.
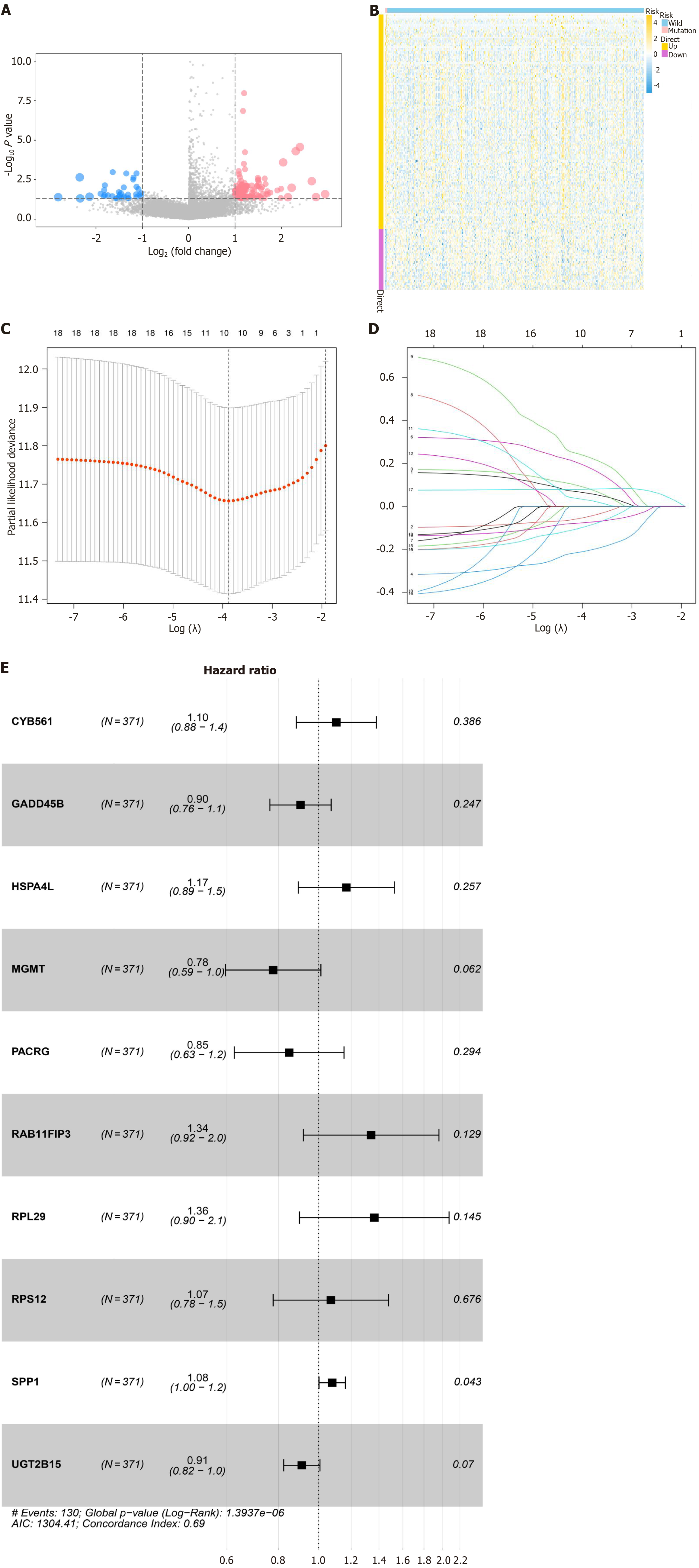
Considering the critical role of MARS1 in HCC, we utilized bioinformatics analyses to identify its associated key genes. Missense mutations in a protein disrupt its function as alteration of the amino acid sequence may substantially compromise its structure or catalytic activity. Given the role of ARS in protein synthesis, the effect of MARS1 missense mutations on the gene expression profile of HCC cells was explored. TCGA-HCC cohort were categorized into two according to whether or not they harbor MARS1 missense mutations. DEGs between four MARS1-mutated and 367 wild-type patients with HCC were screened. Gene expression differences between the two groups are presented as volcano plots (Figure 3A) and a heatmap (Figure 3B). We applied univariate Cox regression to examine the correlation between the 172 MARS1-related DEGs and the prognosis of patients with HCC. To further explore the impact of these DEGs on HCC occurrence and prognosis, 66 genes were identified as prognostic factors for OS in patients with HCC (P < 0.05). LASSO regression and multivariate Cox regression analysis were conducted to establish a prognostic model based on 10 MARS1-related genes in the training cohort (TCGA-HCC, n = 371) (Figure 3C and D). A forest plot displays the outcomes of the multivariate Cox regression analysis of these genes from the LASSO regression analysis (Figure 3E). To compute the MRPS of each sample, the following formula was employed: MRPS = [0.099 × expri (expression value) of CYB561] +
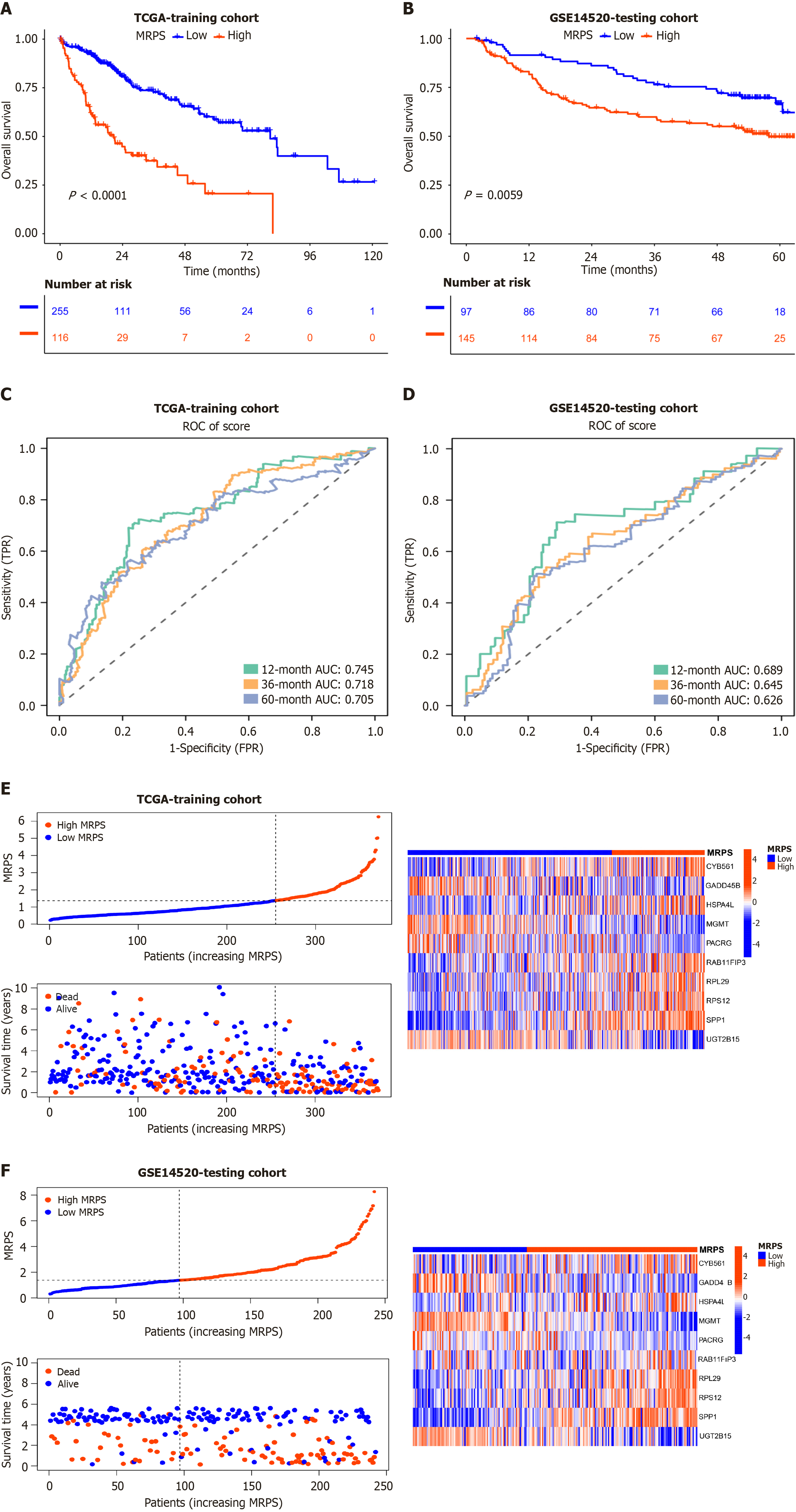
This risk model's predictive value was validated in a testing cohort (GSE14520; n = 242). Across the training and testing cohorts, the prognosis outcomes were considerably poorer in the high-MRPS group than in low-MRPS group, according to Kaplan-Meier survival analysis (P < 0.001, P = 0.0059; Figure 4A and B). In the training cohort, the area under the curve (AUC) values of the ROC curves for the high-MRPS group at the 1-, 3-, and 5-year intervals were 0.745, 0.718, and 0.705, respectively (Figure 4C). Meanwhile, the AUC values at the 1-, 3-, and 5-year intervals in the testing cohort were 0.689, 0.645, and 0.626, respectively (Figure 4D). The performance of the ROC curves of this model implied higher accuracy in the 1-year subgroup in the training and testing cohorts.
The distribution of survival status, risk scores, and heatmaps are shown in Figure 4E and F. Heatmaps of the two cohorts revealed that patients with high-MRPS exhibited higher expression levels of key genes, such as CYB561, HSPA4 L, RAB11FIP3, RPL29, RPS12, and SPP1, and had a higher number of mortality cases. Similar results were obtained for the external validation set (GSE14520), indicating the high usability and accuracy of our predictive model.
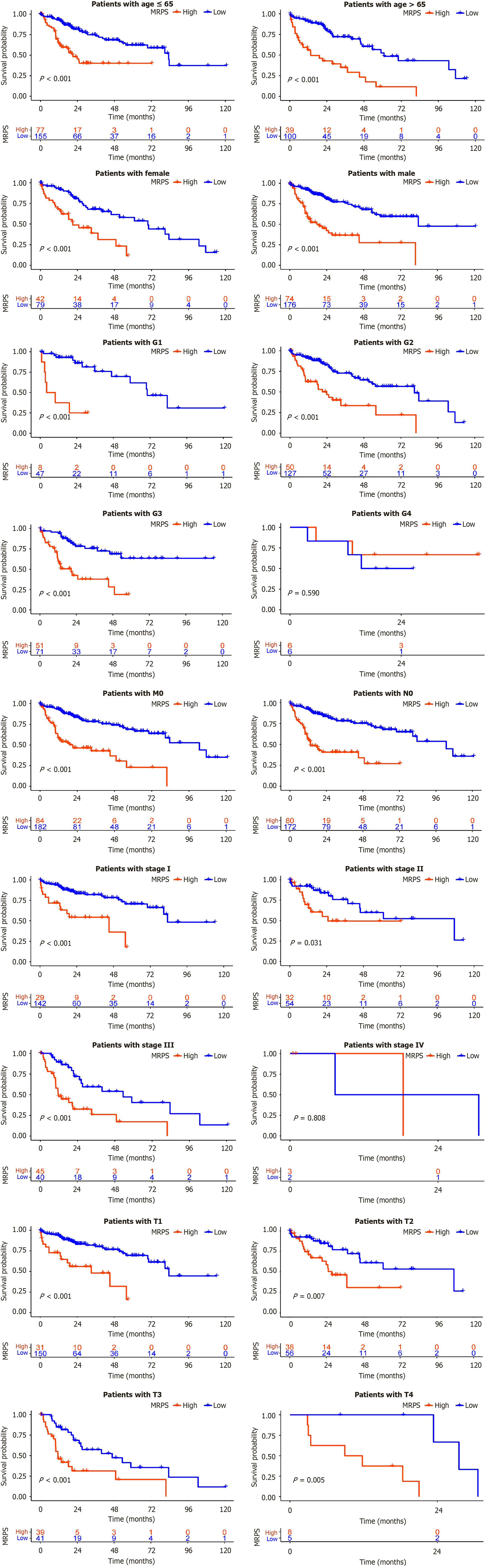
Patients with HCC were categorized into distinct subgroups according to age (≤ 65 and > 65 years), sex (male and female), tumor grade (G1, G2, G3, and G4), pathological M stage (M0), pathological N stage (N0), pathological TNM stage (I, II, III, IV), and pathological T stage (T1, T2, T3, and T4). Survival analysis results from a plot of Kaplan-Meier curves for each subgroup are shown in Figure 5. Survival rates were markedly reduced in the high-MRPS group compared to those in the low-MRPS group across subgroups, except for G4, IV, and T4, underscoring the potent predictive capacity of the model in most subgroups.
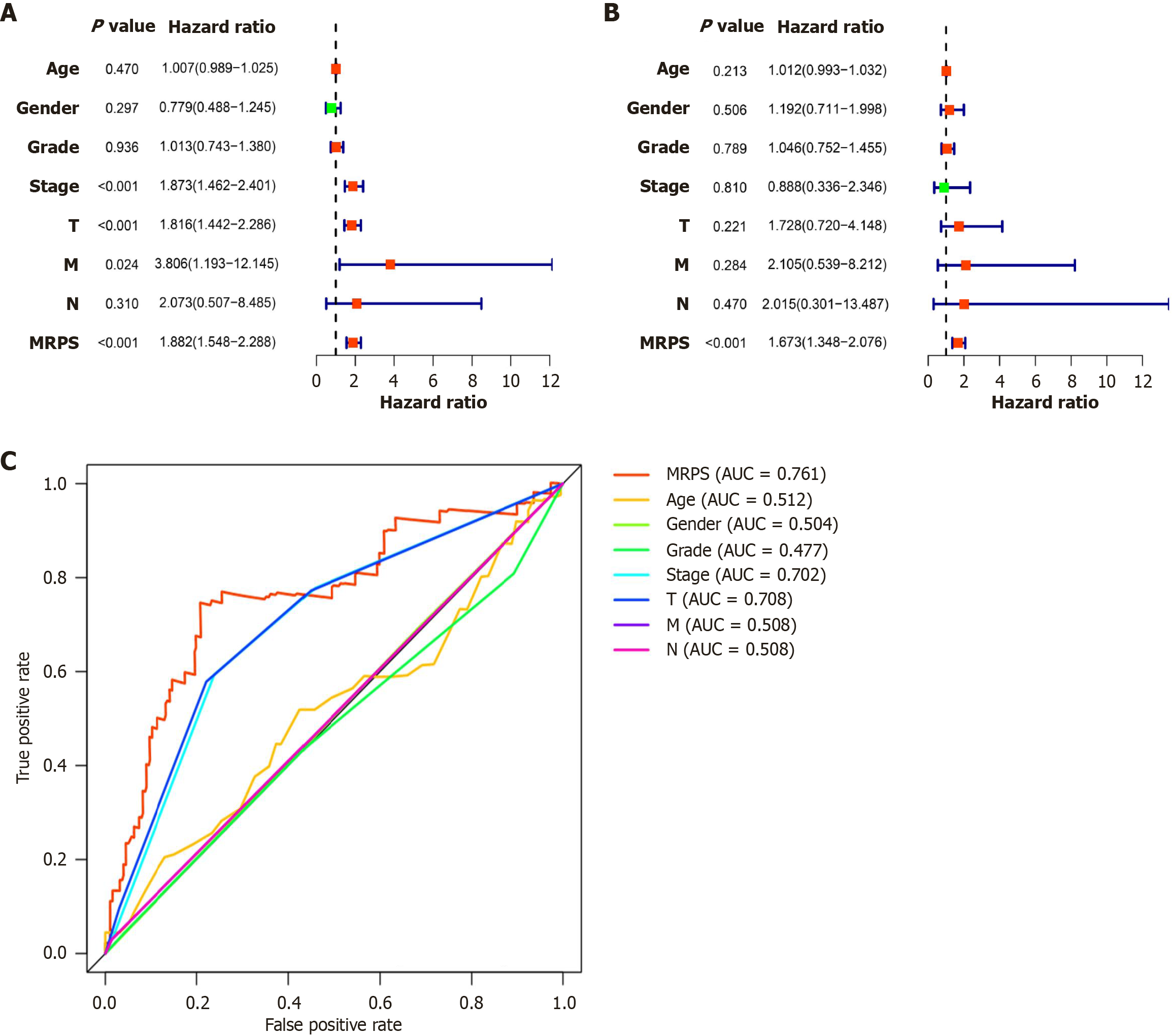
This study analyzed the relationship between the clinical parameters (age, sex, tumor grade, pathological stage, T stage, N stage, and M stage), MRPS, and OS by performing univariate and multivariate Cox regression analyses. The prognosis of patients with HCC was significantly associated with the pathological stage, T stage, and MRPS, but not with age, sex, grade, or N stage, according to univariate Cox regression analysis (Figure 6A). Multifactor Cox regression analysis confirmed MRPS as an independent prognostic factor (Figure 6B). ROC curves were employed to evaluated the predictive accuracies of these different models, which underscored the better predictive value of our risk model over traditional pathological staging in predicting disease prognosis (Figure 6C).
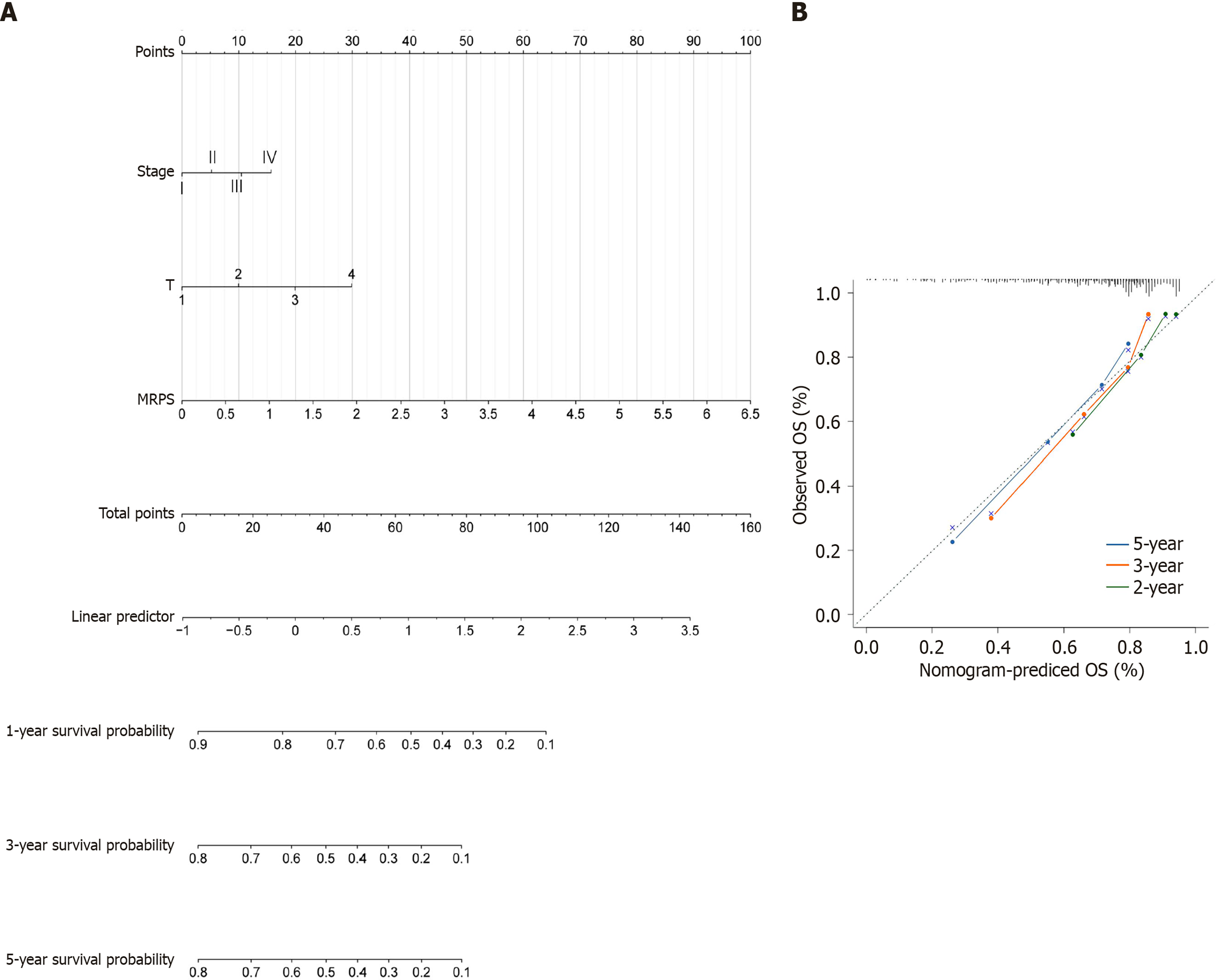
To facilitate individualized prognostic assessment, a prognostic nomogram was constructed using the MRPS value, pathological stage, and T stage (Figure 7A). Calibration curves produced with the “survival ROC” R package were used to verify the prediction accuracy (Figure 7B). Consistency between the predicted and actual observations was demonstrated by the calibration curves for the 1-, 3-, and 5-year OS nomogram models (C-index = 0.722).
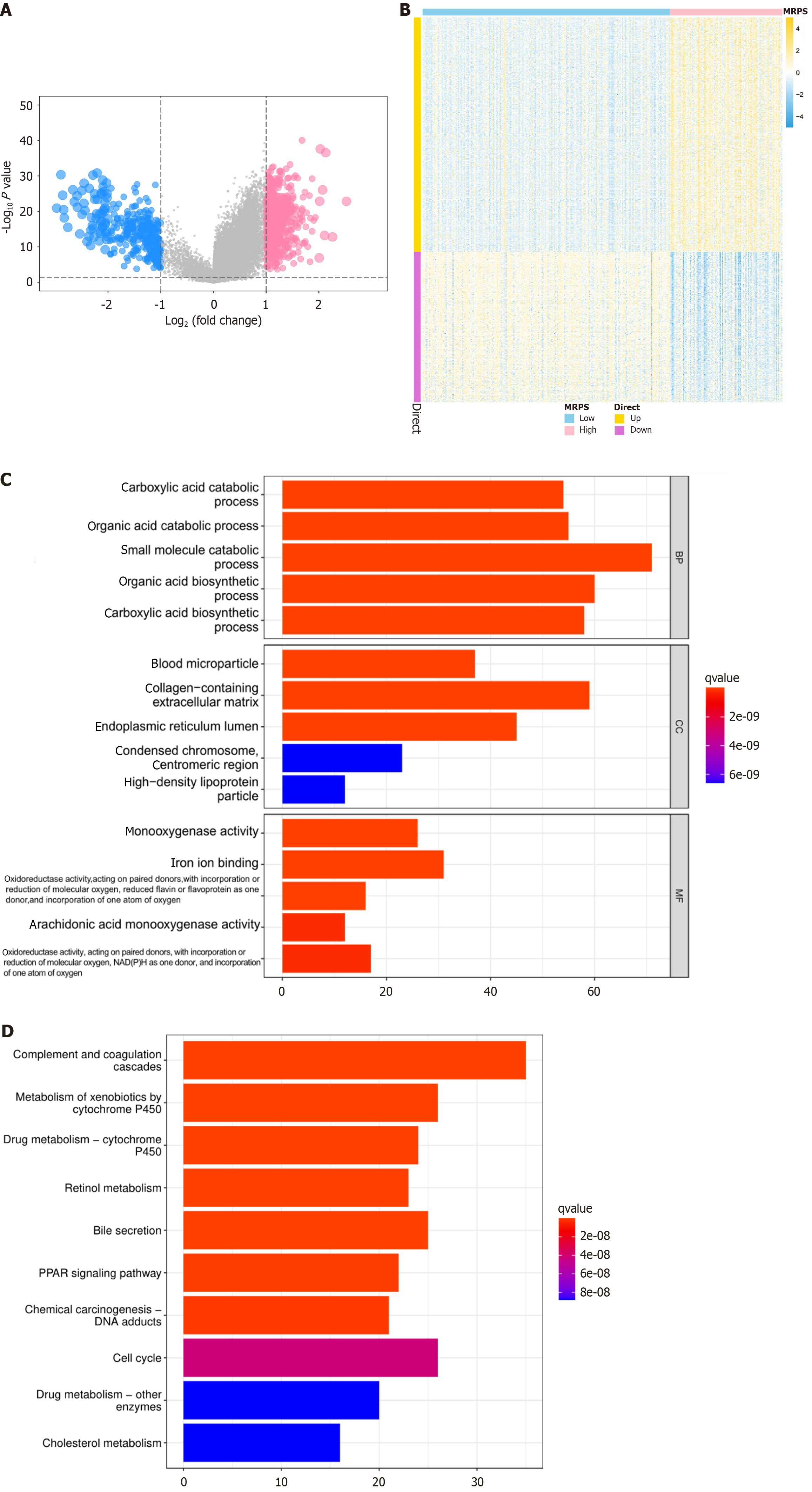
Differential gene expression analysis revealed 492 upregulated and 316 downregulated genes (Figure 8A and B). Subsequently, GO and KEGG analyses were performed on these DEGs. GO analysis demonstrated the involvement of MARS1-related genes in biological processes, including catabolism of organic and carboxylic acids, and small molecules; biosynthesis of organic and carboxylic acids; and cellular components, including blood microparticles, collagen-containing extracellular matrix, and endoplasmic reticulum lumen. Molecular functions were involved in processes, such as monooxygenase activity and iron ion binding (Figure 8C). The KEGG analysis showed that MARS1-related genes were mainly involved in complement and coagulation cascades, cell cycle, xenobiotic metabolism by cytochrome P450, and bile secretion (Figure 8D).
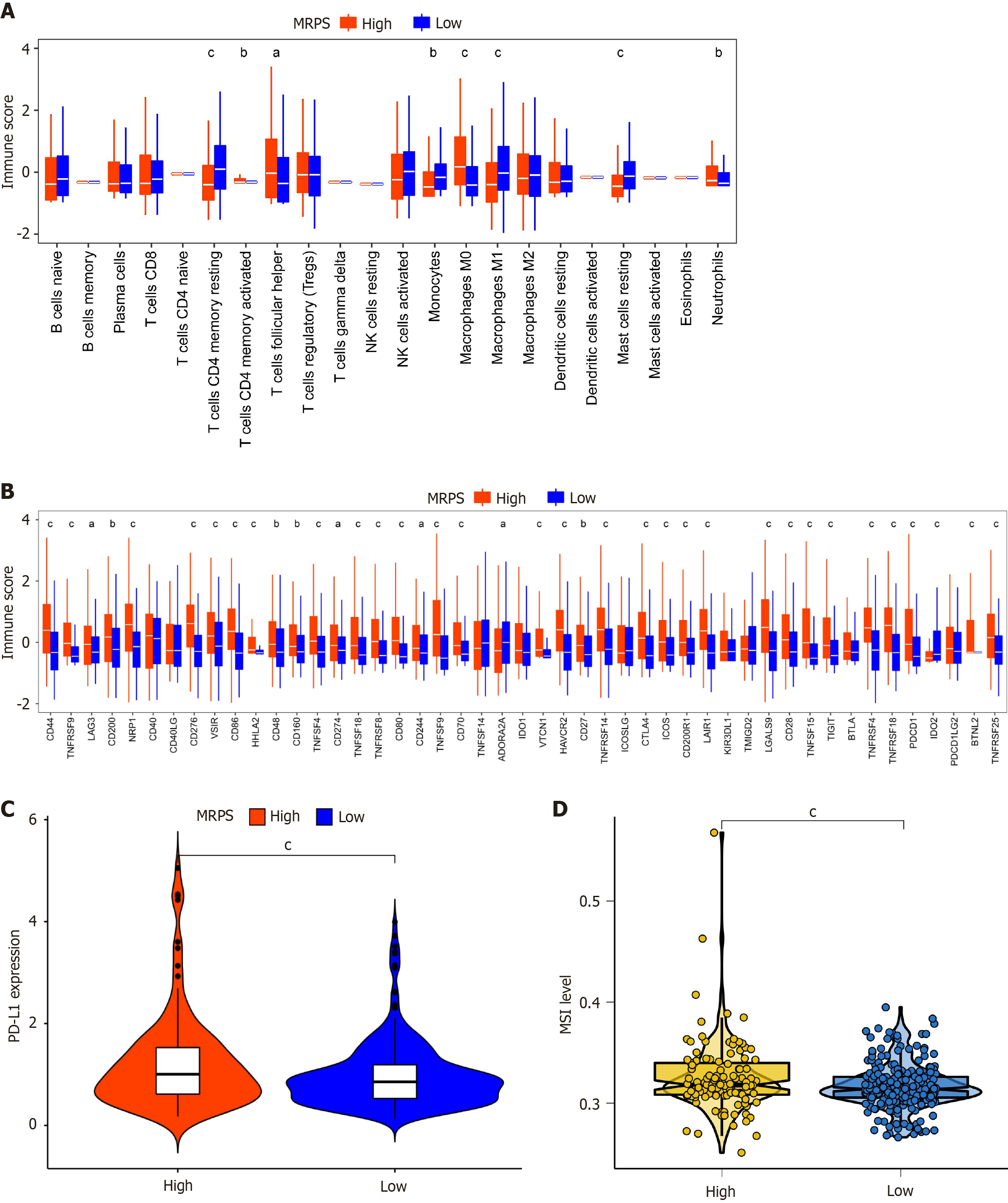
The identification of MARS1 as a key oncogene in HCC prompted the hypothesis that it may regulate the HCC immune microenvironment. Therefore, the infiltration levels of 22 immune cell types in the two MRPS groups were assessed (Figure 9A). The analysis showed lower infiltration of resting CD4+ memory T cells, monocytes, M1 macrophages, and resting mast cells in the tumor tissue of patients with high MRPS, while activated CD4+ memory T cells, follicular helper T cells, M0 macrophages, and neutrophils exhibited higher infiltration levels.
Immunological surveillance against cancer relies heavily on immunological checkpoints, which serve as critical control points that modulate the intensity and duration of immune responses. We investigated the immune checkpoints’ expression between both MRPS subgroups. Figure 9B shows significantly higher immune scores for CD44, TNFRSF9, LAG3, CD200, NRP1, CD276, VSIR, CD86, HHLA2, CD48, CD160, TNFSF4, CD274, TNFSF18, TNFRSF8, CD80, CD244, TNFSF9, CD70, VTCN1, HAVCR2, CD27, TNFRSF14, CTLA4, ICOS, CD200R1, LAIR1, LGALS9, CD28, TNFSF15, TIGIT, TNFRSF4, TNFRSF18, PDCD1, BTNL2, TNFRSF25 in the high-MRPS group. In contrast, the immune scores of ADORA2A and IDO2 were lower than those of the low-MRPS group.
Subsequently, PD-L1 expression levels and MSI values were analyzed within the TCGA cohort utilizing the “ggpubr” and “ggplot2” R packages. The high-MRPS group had considerably higher levels of both the PD-L1 expression and the MSI level in comparison to the low-MRPS group (Figure 9C and D).
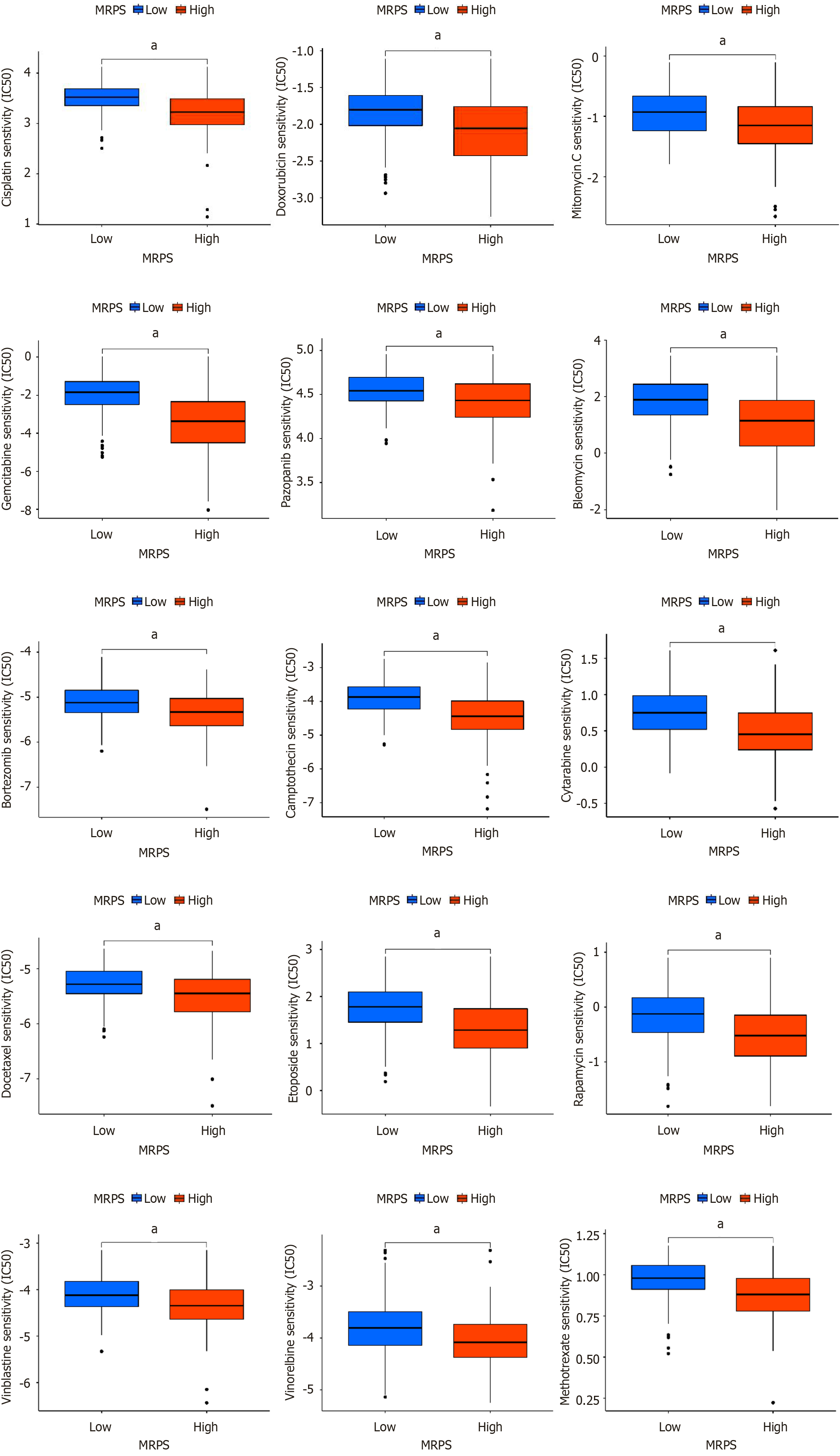
Considering the crucial role of chemotherapy in HCC clinical management, the relationship between MRPS and clinical response to chemotherapeutic drugs was investigated. Using the “pRRophetic” R package, we calculated the IC50 values for 15 anticancer drugs, including cisplatin, doxorubicin, mitomycin C, gemcitabine, pazopanib, bleomycin, bortezomib, camptothecin, cytarabine, docetaxel, etoposide, rapamycin, vinblastine, vinorelbine, and methotrexate, in different MRPS subgroups. The high-MRPS group exhibited reduced sensitivity to all drugs compared to that of the low-MRPS group (Figure 10).
HCC is characterized by pronounced heterogeneity, which complicates accurate prognostic predictions using traditional staging systems. Hence, an urgent need exists to identify novel prognostic markers and develop innovative assessment methods for HCC. In this study, we comprehensively analyzed the relationship between 41 ARS genes and their association with clinical profiles of patients with HCC using TCGA dataset. MARS1 emerged as our gene of interest due to its high association with the clinical outcome of patients with HCC, as well as its highest mutation rate among all ARSs. The mutated proteins are often considered potential therapeutic targets. For instance, in non-small cell lung cancer (NSCLC), targeted therapies based on tyrosine kinase inhibitors have reached phase III clinical trials for the management of patients with epidermal growth factor receptor mutations[15,16]. These therapies have demonstrated marked efficacy, considerably improving treatment outcomes and prolonging survival[17]. In the present study, we identified MARS1 as a critical biomarker and prognostic target for the management of HCC.
MARS1 is an enzyme that catalyzes the connection between methionine and its corresponding tRNA, forming methionyl-tRNA. Since methionyl-tRNA is the primary initiator molecule in protein biosynthesis, MARS1 Likely plays a key role in translational regulation. Previous studies have shown that MARS1 is a valuable marker for diagnosing lymph node metastasis in NSCLC[18] and cholangiocarcinoma[19]. Additionally, high MARS1 expression is associated with adverse clinical outcomes in patients with NSCLC[20] and breast cancer[21]. The dysregulated cell cycle is a hallmark characteristic of cancer cells[22]. In p16INK4a-negative tumors, MARS1 controls the cell cycle by stabilizing CDK4[23]. However, the clinical implications and biological functions of MARS1 in HCC remain largely unexplored. To address this knowledge gap, this study comprehensively evaluates these aspects.
Our investigation revealed that MARS1 expression was considerably increased in tumor tissues compared with adjacent normal tissues of patients with HCC, as evidenced by both database analyses and clinical specimen evaluations. Further corroborated by cellular and animal model studies, our findings demonstrate that MARS1 significantly affects the biological functions of HCC cells, including enhancing their proliferation, migration, and invasion capabilities, as well as oncogenicity. These insights highlight the potential of MARS1 as a novel therapeutic and prognostic target for HCC management.
Several studies have suggested associations between MARS1 and various tumor types. However, a direct assessment of its impact on prognosis and treatment responses in patients with HCC remains unexplored. Thus, we established a prognostic signature composed of 10 MARS1-associated genes using data from the TCGA and GEO databases. These genes include RPL29 and RPS12 (involved in protein synthesis), RAB11FIP3 (intracellular vesicle transport), MGMT (DNA damage repair), GADD45B and CYB561 (cell senescence), and SPP1 (cell migration/invasion/adhesion).
Among the ten genes examined in this study, RPL29, RAB11FIP3, and MGMT had the highest absolute coefficient values. RPL29 is a component of the 60S subunit of eukaryotic ribosomes, essential for protein synthesis. Dysregulation of ribosomal proteins has been implicated in several human diseases. Previous studies have suggested that RPL29 may serve as a prognostic marker for unresectable pancreatic cancer, squamous cell carcinoma, and NSCLC[24-26]. RAB11FIP3 interacts with members of the RAB11 family of small GTPases involved in intracellular vesicular trafficking, thus playing key roles in regulating various cellular processes. RAB11FIP3 may influence tumor progression by affecting the vesicular transport pathway. MGMT (O6-methylguanine-DNA methyltransferase) is a DNA repair protein important for protecting cells from alkylating agents’ deleterious effects. MGMT not only protects normal cells from tumor invasion but also protects tumor cells from the influence of chemotherapy[27]. High MGMT expression may limit liver tissue susceptibility to alkylated carcinogens, thereby conferring protection against malignant transformation[28]. RPS12 is another component of eukaryotic ribosomal[29]. Increased RPS12 expression has been described in gastric and cervical cancers[30,31]. Katanaev et al[32] identified RPS12 as a regulator of Wnt secretion and activity, suggesting its potential involvement in the tumorigenesis of Wnt-dependent cancers, such as HCC. CYB561 is a diheme transmembrane protein integral to intracellular redox reactions, iron metabolism, and cellular senescence[33-35]. Recent findings have shown elevated CYB561 expression in breast cancer, and its increased levels directly correlate with reduced OS among patients[36]. Ge et al[37] revealed that CYB561 knockdown considerably inhibits human acute myeloid leukemia cell growth, suggesting its potential as an important prognostic biomarker for cancer. However, further investigation is needed to determine the precise functions these genes play in HCC, as they have not yet been identified. The functional relationship between these genes and MARS1 has not yet been clarified at present.
Currently, some reports have indicated the roles of GADD45B and SPP1 in HCC. GADD45B is critical for its involvement in cell cycle regulation, survival, apoptosis, senescence as well as DNA repair[38] and plays a pivotal role in the pathogenesis of HCC, where it is considerably under-expressed compared to non-tumorigenic liver tissues[39]. Similarly, SPP1 is a soluble cytokine and matrix-binding protein essential to the regulation of cell migration, extracellular matrix invasion, and cell adhesion[40]. Several studies have reported an increase in SPP1 expression during tumorigenesis, inflammation, cellular stress, and injury response[41,42]. SPP1 is markedly upregulated in liver injury[43,44] and is a key factor mediating liver inflammation and HCC[45]. However, reports on the relationship between these two genes and the prognosis of patients with HCC are lacking, thereby warranting further studies.
The tumor immune microenvironment encompasses a complex array of cells and molecules surrounding the tumor[46], and its state may remarkably influence tumor progression and treatment response[47,48]. Our study revealed significantly altered infiltration of T cells, monocytes, macrophages, and other cells in the high-MRPS group, indicating that MARS1-related genes markedly influence the immune landscape within the tumor microenvironment. Although immunotherapies hold promise for specific subsets of patients with HCC, discriminating patients who are likely to respond to this approach remains considerably challenging. Here, we demonstrated that the high-MRPS group possessed high levels of CTLA4, LAG3, PD-L1, and other immune checkpoints, along with high MSI, suggesting a greater potential for benefiting from immunotherapy in this subgroup. In addition, reports suggest that SPP1 and RAB11FIP3 expression may affect PD-L1 blockade immunotherapy by disrupting the tumor immune barrier[49] and facilitating PD-L1 intranuclear somatic recycling[50]. Thus, MRPS could improve treatment response by distinguishing patients sensitive to immunotherapy. Targeting MARS1 and related genes is a potential therapeutic strategy for treating HCC.
Furthermore, our analysis of the chemotherapy sensitivity revealed that patients in the high-MRPS group were less susceptible to therapeutic drugs commonly used in clinical setting. Previous studies have elucidated the role of MARS1 in tumor suppression via its interaction with AIMP3 (ARS-Interacting Multifunctional Protein-3)[51]. Following DNA damage, MARS1 releases bound AIMP3, which moves to the nucleus to facilitate DNA repair. The MARS1-related gene, MGMT, is reported to regulate DNA damage repair and could serve as a druggable target for cancer treatment, especially in those with high drug resistance[52,53]. Therefore, the increased DNA repair ability of cancer cells may be partially responsible for the reduced drug sensitivity in the high-MRPS group.
Our study preliminarily explored the impact of MARS1 on HCC progression, confirming its marked potential in prognostic evaluation for patients with HCC, thereby paving the way for future research on MARS1. Nevertheless, some limitations of this study must be addressed. First, although patients with HCC were categorized based on MARS1 missense mutations, the precise biological implications of these mutations remain unclear. Second, the limited number of individuals in the HCC patient cohort with MARS1 missense mutations highlights the need for a larger cohort to enable more detailed analyses. Third, although MARS1 promotes the proliferation, migration, invasion, and tumor formation of HCC cells, the underlying mechanisms regulating MARS1-related genes remain unclear, and require further study.
MARS1 is significantly upregulated in HCC and drives tumor progression, highlighting its potential as a therapeutic target. MRPS is a valuable tool for patient stratification and may guide individualized treatment strategies in HCC.
| 1. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1413] [Article Influence: 353.3] [Reference Citation Analysis (1)] |
| 2. | Yang L, Zou T, Chen Y, Zhao Y, Wu X, Li M, Du F, Chen Y, Xiao Z, Shen J. Hepatitis B virus X protein mediated epigenetic alterations in the pathogenesis of hepatocellular carcinoma. Hepatol Int. 2022;16:741-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Ivanov AV, Valuev-Elliston VT, Tyurina DA, Ivanova ON, Kochetkov SN, Bartosch B, Isaguliants MG. Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget. 2017;8:3895-3932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Liu M, Jiang L, Guan XY. The genetic and epigenetic alterations in human hepatocellular carcinoma: a recent update. Protein Cell. 2014;5:673-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Zou Y, Yang Y, Fu X, He X, Liu M, Zong T, Li X, Htet Aung L, Wang Z, Yu T. The regulatory roles of aminoacyl-tRNA synthetase in cardiovascular disease. Mol Ther Nucleic Acids. 2021;25:372-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Sung Y, Yoon I, Han JM, Kim S. Functional and pathologic association of aminoacyl-tRNA synthetases with cancer. Exp Mol Med. 2022;54:553-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 7. | Jiang L, Wang J, Liu Z, Zhang Q, Yang XL. Seryl-tRNA synthetase inhibits Wnt signaling and breast cancer progression and metastasis. FASEB J. 2025;39:e70294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Ju J, Zhang H, Lin M, Yan Z, An L, Cao Z, Geng D, Yue J, Tang Y, Tian L, Chen F, Han Y, Wang W, Zhao S, Jiao S, Zhou Z. The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J Clin Invest. 2024;134:e174587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 182] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 9. | Nam SH, Kim D, Lee D, Lee HM, Song DG, Jung JW, Kim JE, Kim HJ, Kwon NH, Jo EK, Kim S, Lee JW. Lysyl-tRNA synthetase-expressing colon spheroids induce M2 macrophage polarization to promote metastasis. J Clin Invest. 2018;128:5034-5055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 581] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 11. | Passarelli MC, Pinzaru AM, Asgharian H, Liberti MV, Heissel S, Molina H, Goodarzi H, Tavazoie SF. Leucyl-tRNA synthetase is a tumour suppressor in breast cancer and regulates codon-dependent translation dynamics. Nat Cell Biol. 2022;24:307-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Nie J, Liu T, Mao T, Yang H, Deng W, Liu X, Fu B. Transcriptome sequencing and single-cell sequencing analysis identify GARS1 as a potential prognostic and immunotherapeutic biomarker for multiple cancers, including bladder cancer. Front Immunol. 2023;14:1169588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Wang J, Yang B, Wang D, Han R, Bi Z, Lin L. GARS is implicated in poor survival and immune infiltration of hepatocellular carcinoma. Cell Signal. 2022;94:110302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Liu L, Gao J, Liu X, Zhang F, Hu B, Zhang H, Wang Z, Tang H, Shi JH, Zhang S. AARS2 as a novel biomarker for prognosis and its molecular characterization in pan-cancer. Cancer Med. 2023;12:21531-21544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 15. | Lu S, Zhou J, Jian H, Wu L, Cheng Y, Fan Y, Fang J, Chen G, Zhang Z, Lv D, Jiang L, Wu R, Jin X, Zhang X, Zhang J, Xie C, Sun G, Huang D, Cui J, Guo R, Han Z, Chen Z, Liang J, Zhuang W, Hu X, Zang A, Zhang Y, Cang S, Lan Y, Chen X, Liu L, Li X, Chen J, Ma R, Guo Y, Sun P, Tian P, Pan Y, Liu Z, Cao P, Ding L, Wang Y, Yuan X, Wu P. Befotertinib (D-0316) versus icotinib as first-line therapy for patients with EGFR-mutated locally advanced or metastatic non-small-cell lung cancer: a multicentre, open-label, randomised phase 3 study. Lancet Respir Med. 2023;11:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Shi Y, Chen G, Wang X, Liu Y, Wu L, Hao Y, Liu C, Zhu S, Zhang X, Li Y, Liu J, Cao L, Cheng Y, Zhao H, Zhang S, Zang A, Cui J, Feng J, Yang N, Liu F, Jiang Y, Gu C; FURLONG investigators. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. 2022;10:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 17. | da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathol. 2011;6:49-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 672] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 18. | Lee JM, Kim T, Kim EY, Kim A, Lee DK, Kwon NH, Kim S, Chang YS. Methionyl-tRNA Synthetase is a Useful Diagnostic Marker for Lymph Node Metastasis in Non-Small Cell Lung Cancer. Yonsei Med J. 2019;60:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Jang SI, Kwon NH, Lim BJ, Nahm JH, Park JS, Kang CM, Park SR, Lee Sd SY, Kang BS, Kim S, Lee DK. New staining method using methionyl-tRNA synthetase 1 antibody for brushing cytology of bile duct cancer. Gastrointest Endosc. 2020;92:310-319.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Kim EY, Jung JY, Kim A, Kim K, Chang YS. Methionyl-tRNA synthetase overexpression is associated with poor clinical outcomes in non-small cell lung cancer. BMC Cancer. 2017;17:467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Jin Q, Liu G, Wang B, Li S, Ni K, Wang C, Ren J, Zhang S, Dai Y. High methionyl-tRNA synthetase expression predicts poor prognosis in patients with breast cancer. J Clin Pathol. 2020;73:803-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Liu J, Peng Y, Wei W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022;32:30-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 288] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 23. | Kwon NH, Lee JY, Ryu YL, Kim C, Kong J, Oh S, Kang BS, Ahn HW, Ahn SG, Jeong J, Kim HK, Kim JH, Han DY, Park MC, Kim D, Takase R, Masuda I, Hou YM, Jang SI, Chang YS, Lee DK, Kim Y, Wang MW, Basappa, Kim S. Stabilization of Cyclin-Dependent Kinase 4 by Methionyl-tRNA Synthetase in p16(INK4a)-Negative Cancer. ACS Pharmacol Transl Sci. 2018;1:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Yamamoto H, Takaki A, Hayashi T, Furukawa M, Tao H, Shien K, Soh J, Okabe K, Miyoshi S, Toyooka S. P2.01-032 Impact of Preoperative Serum Anti-60S Ribosomal Protein L29 Levels on Prognosis in Patients Who Underwent Surgery for Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12:S804. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Muro S, Miyake Y, Kato H, Tsutsumi K, Yamamoto K. Serum anti-60S ribosomal protein L29 antibody as a novel prognostic marker for unresectable pancreatic cancer. Digestion. 2015;91:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Li J, Peng Y. Knockdown RPL29 Gene Can Inhibit the Proliferation, Invasion of Squamous Cell Carcinomas. Ann Clin Lab Sci. 2019;49:763-769. [PubMed] |
| 27. | Bai P, Fan T, Sun G, Wang X, Zhao L, Zhong R. The dual role of DNA repair protein MGMT in cancer prevention and treatment. DNA Repair (Amst). 2023;123:103449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | Qin X, Zhang S, Matsukuma S, Zarkovic M, Shimizu S, Ishikawa T, Nakatsuru Y. Protection against malignant progression of spontaneously developing liver tumors in transgenic mice expressing O(6)-methylguanine-DNA methyltransferase. Jpn J Cancer Res. 2000;91:1085-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Lin A, Chan YL, Jones R, Wool IG. The primary structure of rat ribosomal protein S12. The relationship of rat S12 to other ribosomal proteins and a correlation of the amino acid sequences of rat and yeast ribosomal proteins. J Biol Chem. 1987;262:14343-14351. [PubMed] |
| 30. | Chen D, Zhang R, Shen W, Fu H, Liu S, Sun K, Sun X. RPS12-specific shRNA inhibits the proliferation, migration of BGC823 gastric cancer cells with S100A4 as a downstream effector. Int J Oncol. 2013;42:1763-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Cheng Q, Lau WM, Tay SK, Chew SH, Ho TH, Hui KM. Identification and characterization of genes involved in the carcinogenesis of human squamous cell cervical carcinoma. Int J Cancer. 2002;98:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Katanaev VL, Kryuchkov M, Averkov V, Savitsky M, Nikolaeva K, Klimova N, Khaustov S, Solis GP. HumanaFly: high-throughput transgenesis and expression of breast cancer transcripts in Drosophila eye discovers the RPS12-Wingless signaling axis. Sci Rep. 2020;10:21013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Asard H, Barbaro R, Trost P, Bérczi A. Cytochromes b561: ascorbate-mediated trans-membrane electron transport. Antioxid Redox Signal. 2013;19:1026-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Zhou X, Guo X, Han J, Wang M, Liu Z, Ren D, Zhao J, Li Z. Cytochrome b561 regulates iron metabolism by activating the Akt/mTOR pathway to promote Breast Cancer Cells proliferation. Exp Cell Res. 2023;431:113760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 35. | Kang MK, Kameta A, Shin KH, Baluda MA, Kim HR, Park NH. Senescence-associated genes in normal human oral keratinocytes. Exp Cell Res. 2003;287:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Zhou X, Shen G, Ren D, Guo X, Han J, Guo Q, Zhao F, Wang M, Dong Q, Li Z, Zhao J. Expression and clinical prognostic value of CYB561 in breast cancer. J Cancer Res Clin Oncol. 2022;148:1879-1892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Ge Y, Hong M, Zhang Y, Wang J, Li L, Zhu H, Sheng Y, Wu WS, Zhang Z. miR-30e-5p regulates leukemia stem cell self-renewal through the Cyb561/ROS signaling pathway. Haematologica. 2024;109:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Schäfer A. Gadd45 proteins: key players of repair-mediated DNA demethylation. Adv Exp Med Biol. 2013;793:35-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Qiu W, David D, Zhou B, Chu PG, Zhang B, Wu M, Xiao J, Han T, Zhu Z, Wang T, Liu X, Lopez R, Frankel P, Jong A, Yen Y. Down-regulation of growth arrest DNA damage-inducible gene 45beta expression is associated with human hepatocellular carcinoma. Am J Pathol. 2003;162:1961-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X, Ge X, Fiel MI, Nieto N. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin α(V)β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatology. 2012;55:594-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 41. | El-Tanani MK, Campbell FC, Kurisetty V, Jin D, McCann M, Rudland PS. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | Uede T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol Int. 2011;61:265-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 43. | Lorena D, Darby IA, Gadeau AP, Leen LL, Rittling S, Porto LC, Rosenbaum J, Desmoulière A. Osteopontin expression in normal and fibrotic liver. altered liver healing in osteopontin-deficient mice. J Hepatol. 2006;44:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH, Mi Z, Pereira TA, Zdanowicz M, Malladi P, Chen Y, Moylan C, Jung Y, Bhattacharya SD, Teaberry V, Omenetti A, Abdelmalek MF, Guy CD, Adams DH, Kuo PC, Michelotti GA, Whitington PF, Diehl AM. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 45. | Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicol Sci. 2008;103:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2346] [Reference Citation Analysis (0)] |
| 47. | Yang Y, Chen L, Zheng B, Zhou S. Metabolic hallmarks of natural killer cells in the tumor microenvironment and implications in cancer immunotherapy. Oncogene. 2023;42:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 48. | Bader JE, Voss K, Rathmell JC. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol Cell. 2020;78:1019-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 728] [Article Influence: 121.3] [Reference Citation Analysis (2)] |
| 49. | Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, Sun L, Liu Y, Du Y, Guo X, Cui T, Zhou H, Wang J, Yin D, Song R, Zhang S, Cai W, Meng F, Guo H, Zhang B, Yang D, Bao R, Hu Q, Wang J, Ye Y, Liu L. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. 2023;78:770-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 463] [Article Influence: 154.3] [Reference Citation Analysis (0)] |
| 50. | Weng N, Zhou C, Zhou Y, Zhong Y, Jia Z, Rao X, Qiu H, Zeng G, Jin X, Zhang J, Zhuang Z, Liang Z, Deng Y, Li Q, Yang S, Luo H, Wang H, Wu X. IKZF4/NONO-RAB11FIP3 axis promotes immune evasion in gastric cancer via facilitating PD-L1 endosome recycling. Cancer Lett. 2024;584:216618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 51. | Kwon NH, Kang T, Lee JY, Kim HH, Kim HR, Hong J, Oh YS, Han JM, Ku MJ, Lee SY, Kim S. Dual role of methionyl-tRNA synthetase in the regulation of translation and tumor suppressor activity of aminoacyl-tRNA synthetase-interacting multifunctional protein-3. Proc Natl Acad Sci U S A. 2011;108:19635-19640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Wickström M, Dyberg C, Milosevic J, Einvik C, Calero R, Sveinbjörnsson B, Sandén E, Darabi A, Siesjö P, Kool M, Kogner P, Baryawno N, Johnsen JI. Wnt/β-catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat Commun. 2015;6:8904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 53. | Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol. 2002;20:2388-2399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 332] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/