Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.108470
Revised: May 26, 2025
Accepted: July 25, 2025
Published online: September 15, 2025
Processing time: 153 Days and 17 Hours
The National Comprehensive Cancer Network guidelines recommend adjuvant chemotherapy (ACT) for patients with stage II colon cancer who have undergone curative surgery when fewer than 12 lymph nodes (LNs) are retrieved. This study seeks to further examine the requirement for ACT in individuals who had 12 or more LNs harvested.
To investigate if stage II colon cancer patients with 12 or more LNs retrieved bene
This retrospective cohort study included individuals diagnosed with stage II colon cancer who underwent surgery between 2008 and 2017 from the Surveillance, Epidemiology, and End Results (SEER) registry and a Chinese multicenter database. All patients had at least 12 LNs retrieved. The key endpoint was overall survival (OS). Cox regression analysis was performed to assess independent OS predictors. Propensity score matching controlled for confounders, and Kaplan-Meier analysis evaluated the impact of ACT on survival.
A total of 32742 patients with stage II colon cancer from the SEER cohort and 3153 patients from the Chinese cohort were included. The average number of LNs retrieved was 20.0 (15.0, 26.0) in the SEER cohort and 18.0 (15.0, 22.0) in the Chinese cohort. No-ACT remained an independent risk factor in both cohorts (hazard ratio = 1.589, 95% confidence interval: 1.485-1.700 and hazard ratio = 1.865, 95% confidence interval: 1.465-2.375, respectively). In the SEER cohort, patients in the ACT group consistently demonstrated better 5-year OS rates both before and after propensity score matching (79.4% vs 66.1% and 79.4% vs 69.4%, both P < 0.0001). Similarly, these findings were further validated in the Chinese cohort (91.2% vs 82.1% and 90.0% vs 82.8%, both P < 0.0001). ACT improved prognosis even in T3 and grade 1/2 patients.
This research, based on two large population-based cohorts, demonstrates that stage II colon cancer patients with 12 or more LNs retrieved can still benefit from ACT.
Core Tip: No adjuvant chemotherapy (ACT) continues to be a significant independent predictor for patients with stage II colon cancer who have 12 or more lymph nodes (LNs) removed. Even among these patients with T3 stage and grade 1/2, ACT still leads to improved prognosis. Based on two large-scale population-based cohorts and the propensity score matching method, we validated the generalizability and reliability of these findings. This research confirms that patients with adequate LNs retrieval (≥ 12 LNs) can still benefit from ACT, which is expected to provide valuable insights and further guide clinical practice in the management of colorectal carcinoma.
- Citation: Huo Y, Zhang YY, Jiao S, Bai ZY, Bai WQ, Zhou HT, Guan X. Efficacy of adjuvant chemotherapy in stage II colon cancer patients with twelve or more lymph nodes retrieve. World J Gastrointest Oncol 2025; 17(9): 108470
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/108470.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.108470
Colorectal cancer (CRC) is the second most common cause of cancer-related deaths globally[1]. Within the group of stage II CRC patients, the 5-year overall survival (OS) rate following surgery ranges from approximately 68% to 83%, with recurrence rates particularly high in high-risk stage II cases, reaching as high as 40%-50%[2,3]. While surgery remains the cornerstone of treatment, postoperative adjuvant therapy is critical in enhancing outcomes and lowering the risk of recurrence. Given the distinct pathophysiological differences between colon and rectal cancers, treatment guidelines, such as those from the National Comprehensive Cancer Network, recommend adjuvant chemotherapy (ACT) for stage II colon cancer and postoperative radiotherapy for stage II rectal cancer. The decision to administer ACT in stage II colon cancer is generally based on the occurrence of high-risk attributes associated with recurrence, such as poor histological differentiation (grade III or IV), T4 tumors, and inadequate lymph node (LN) retrieval (fewer than 12 nodes).
However, the survival advantages associated with ACT in patients diagnosed with stage II colon cancer, particularly among high-risk patients, remain a point of continued debate. Babcock et al[4] proposed that the survival benefit of ACT varies depending on specific high-risk characteristics, suggesting a need for refined risk stratification. Booth et al[5] found that ACT does not contribute to survival benefits, even for patients with high-risk stage II colon cancer. In contrast, Baxter et al[3] offered novel insights into the criteria for selecting patients for ACT. As a result, uncertainty persists regarding the appropriate indications for ACT, warranting a re-evaluation of existing guidelines. LN retrieval is one of the most pivotal factors in ACT decision-making. The National Comprehensive Cancer Network guidelines suggest that individuals with stage II CRC who have had fewer than 12 LNs assessed should undergo ACT. This suggestion stems from the risk of stage migration due to inadequate LN retrieval, which can impact prognosis and influence treatment decisions. Xie et al[6] argued that inadequate LN examination may result in downstaging of stage II colon cancer, potentially compromising prognosis. Conversely, adequate LN assessment improves staging accuracy and supports more informed decisions regarding the necessity of ACT[7,8]. Furthermore, Chang et al[9] found that the evaluation of an increased number of LNs was positively correlated with improved survival rates in stage II CRC patients. This finding suggests that patients with a greater number of LNs examined may have stronger immune competence and could potentially experience enhanced benefit from ACT. To address these issues, our study aimed to assess whether patients with stage II colon cancer who had 12 or more LNs examined continue to derive benefits from ACT. This research may contribute to more tailored and evidence-based treatment guidelines.
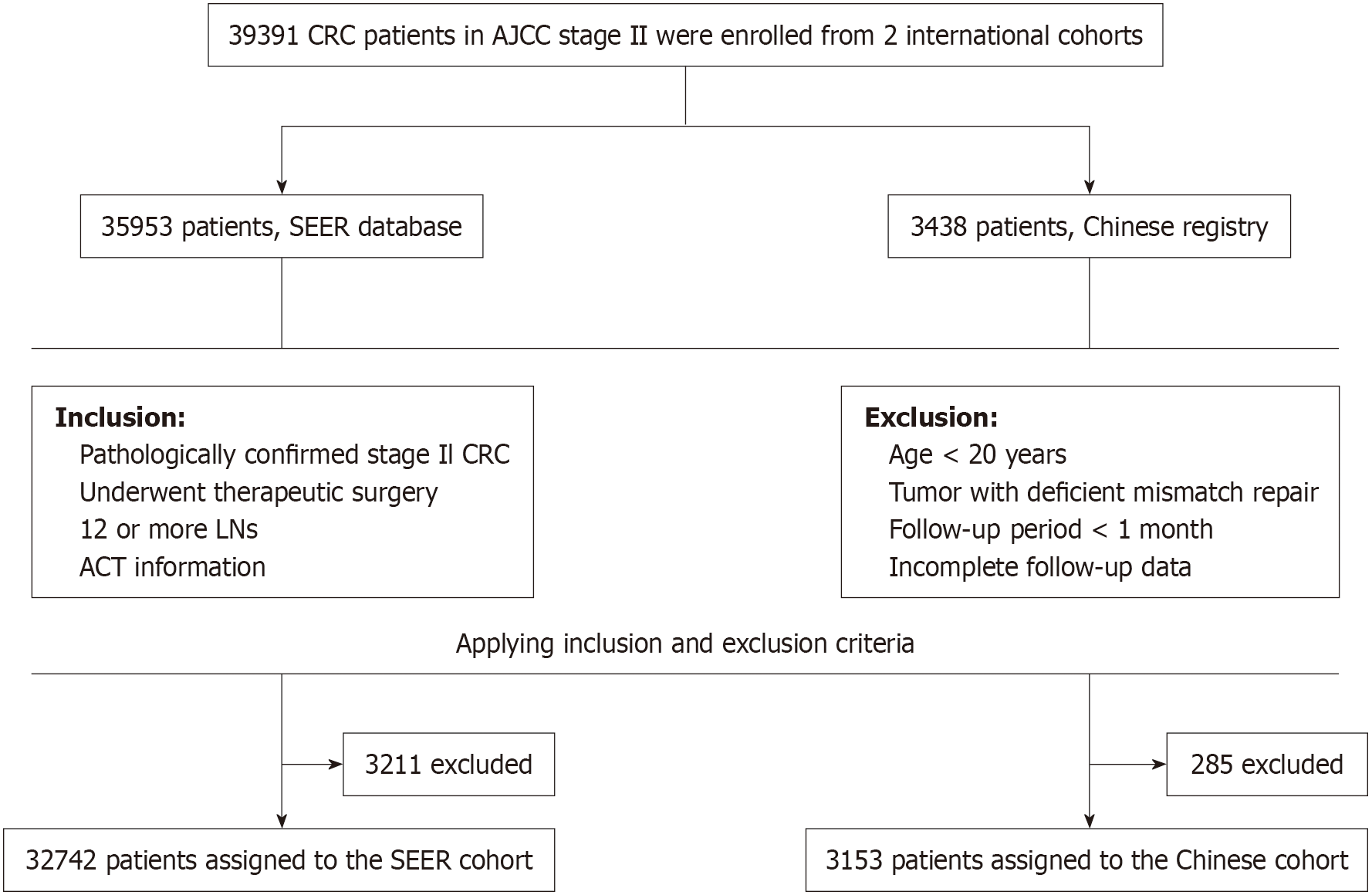
Figure 1 provides a schematic overview of the study. To assess whether individuals with stage II CRC who had at least 12 LNs assessed derive survival benefits from ACT, we analyzed data from two cohorts: The Surveillance, Epidemiology, and End Results (SEER) cohort (2008-2017) and a Chinese cohort (2009-2018). The SEER cohort was used as the training cohort, while the Chinese cohort served as the validation cohort. All information was retrieved from electronic medical records and telephone follow-ups. Given that this was a retrospective analysis, informed consent was not necessary, and all patient data were anonymized.
The criteria for inclusion in the study were as follows: (1) Pathologically confirmed diagnosis of stage II CRC; (2) Underwent curative-intent surgery; (3) 12 or more LNs were removed; (4) T3 or T4 tumor stage; (5) Available tumor grade classification; (6) Documented histological subtype; (7) Available information on ACT; and (8) Complete follow-up data. Exclusion criteria were as follows: (1) Age < 20 years; (2) Presence of tumors with deficient mismatch repair; (3) Follow-up duration less than 1 month; (4) History of other malignancies; and (5) Incomplete follow-up data.
The clinical variables included gender, age, tumor histological differentiation, tumor size, LN count, T stage, histological subtype, and ACT records. The diagnosis was established through comprehensive preoperative and postoperative evaluations supported by clinical evidence. The primary outcome was OS, which was defined as the time from the pathological diagnosis of CRC to either death from any cause or the last follow-up. The Chinese cohort comprised multicenter data collected from five hospitals, while survival data for the SEER cohort were derived from the SEER database[10].
The calculation of propensity scores was performed using a logistic regression model that incorporated variables such as age, gender, clinical T stage, tumor histological differentiation, and tumor size. Baseline characteristics before and after propensity score matching (PSM) were summarized, with a caliper width of 0.02 used for nearest-neighbor matching between groups, implemented via the “MatchIt” package in R.
The prognostic significance of ACT was assessed in both the ACT and no-ACT groups using the Kaplan-Meier method, and comparisons were performed with the log-rank test. The prognostic influence of ACT was assessed in patients classified as T3 stage and with grade 1/2 tumors.
Categorical variables are presented as counts and proportions, and group differences were evaluated using Fisher’s exact test. For continuous variables with a non-normal distribution, the median and interquartile range are reported, and group differences were evaluated with the Mann-Whitney U test. Cox proportional hazards regression analyses, both univariate and multivariate, were used to identify factors predicting OS. The threshold for inclusion was set at P < 0.05. A P value below 0.05 was regarded as a statistically significant difference. All statistical computations and data visualizations were conducted using appropriate R software packages. Statistical analyses were conducted utilizing R software for all computations (R version 4.3.1).
The SEER cohort consisted of 32742 patients and the Chinese cohort included 3153 patients. Table 1 outlines the baseline clinical characteristics of the two cohorts. The mean number of LNs collected was 20.0 (15.0, 26.0) in the SEER cohort and 18.0 (15.0, 22.0) in the Chinese cohort. A total of 28228 patients (86.2%) in the SEER cohort did not receive ACT, compared to 1429 patients (45.3%) in the Chinese cohort. Regarding gender distribution, a greater proportion of males was noted in the SEER cohort (52.4%), whereas a higher percentage of females was observed in the Chinese cohort (57.3%). The two cohorts demonstrated comparable distributions in T stage (T3: 85.3% vs 70.9%; T4: 14.7% vs 29.1%) and histological differentiation (grade 1/2: 80.1% vs 87.5%; grade 3/4: 19.9% vs 12.5%). Supplementary Table 1 shows the results of the analysis of heterogeneity between the two cohorts. The results indicate significant differences in examined LNs (ELNs) (P < 0.001), chemotherapy (P < 0.001), age (P < 0.001), gender (P < 0.001), grade (P < 0.001), T stage (P < 0.001), and tumor size (P = 0.003) between the two cohorts. However, histology (P = 0.057) was consistent across the groups. Among the subgroups, there were 22700 patients with T3 stage and grade 1/2 tumors in the SEER cohort, compared to 1966 in the Chinese cohort.
| Characteristics | SEER database | Chinese registry |
| Total | 32742 | 3153 |
| Examined lymph node, median (IQR) | 20.00 (15.00, 26.00) | 18.00 (15.00, 22.00) |
| Chemotherapy | ||
| No | 28228 (86.2) | 1429 (45.3) |
| Yes | 4514 (13.8) | 1724 (54.7) |
| Size, median (IQR) | 5.00 (3.80, 7.00) | 5.00 (4.00, 6.50) |
| Age | ||
| ≥ 65 | 22829 (69.7) | 1269 (40.2) |
| 20-64 | 9913 (30.3) | 1884 (59.8) |
| Sex | ||
| Female | 17156 (52.4) | 1345 (42.7) |
| Male | 15586 (47.6) | 1808 (57.3) |
| Grade | ||
| Grade 1/2 | 26221 (80.1) | 2758 (87.5) |
| Grade 3/4 | 6521 (19.9) | 395 (12.5) |
| Histology | ||
| Adenocarcinoma | 27996 (85.5) | 2683 (85.1) |
| Mucinous adenocarcinoma | 4222 (12.9) | 425 (13.5) |
| Others | 524 (1.6) | 45 (1.4) |
| T stage | ||
| T3 | 27920 (85.3) | 2234(70.9) |
| T4 | 4822 (14.7) | 919 (29.1) |
All variables related to clinicopathological characteristics and surgical factors were entered into the univariate analysis. In the SEER cohort, univariate analysis showed that ELNs (P < 0.001), tumor size (P = 0.030), age (P < 0.001), gender (P = 0.039), histological differentiation (P < 0.001), T stage (P < 0.001), and ACT (P < 0.001) were significantly associated with OS. Variables with a P value < 0.05 in the univariate analysis were included in the multivariate Cox regression model. After adjusting for additional prognostic variables, ACT continued to be independently linked to improved OS (hazard ratio = 1.589, 95% confidence interval: 1.485-1.700, P < 0.001; Table 2). In the Chinese cohort, similar results were observed, with ACT also identified as a singular predictor of OS (hazard ratio = 1.865, 95% confidence interval: 1.465-2.375, P < 0.001; Table 2).
| Characteristics | Univariate analysis1 | Multivariate analysis1 | Univariate analysis2 | Multivariate analysis2 | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| ELN, median (IQR) | 0.978 (0.976, 0.980) | < 0.001 | 0.984 (0.982, 0.986) | < 0.001 | 0.958 (0.940, 0.976) | < 0.001 | 0.962 (0.944, 0.979) | < 0.001 |
| Size, median (IQR) | 1.005 (1.000, 1.010) | 0.03 | 1.004 (1.000, 1.009) | 0.057 | 0.996 (0.946, 1.048) | 0.87 | ||
| Age | ||||||||
| 20-64 | 0.302 (0.286-0.318) | < 0.001 | 0.333 (0.315-0.351) | < 0.001 | 0.346 (0.275-0.437) | < 0.001 | 0.414 (0.326-0.526) | < 0.001 |
| Sex | ||||||||
| Male | 1.039 (1.002-1.078) | 0.039 | 1.160 (1.118-1.203) | < 0.001 | 1.196 (0.951-1.505) | 0.126 | ||
| Grade | ||||||||
| Grade 3/4 | 1.214 (1.162-1.268) | < 0.001 | 1.154 (1.103-1.208) | < 0.001 | 1.212 (0.886-1.657) | 0.23 | ||
| Histology | ||||||||
| Adenocarcinoma | ||||||||
| Mucinous adenocarcinoma | 1.045 (0.991-1.103) | 0.104 | 1.011 (0.958-1.067) | 0.695 | 1.086 (0.786-1.500) | 0.616 | ||
| Others | 1.251 (1.088-1.440) | 0.002 | 1.104 (0.956-1.274) | 0.178 | 0.721 (0.269-1.936) | 0.516 | ||
| T stage | ||||||||
| T4 | 1.568 (1.495-1.644) | < 0.001 | 1.795 (1.709-1.886) | < 0.001 | 1.497 (1.192-1.879) | 0.001 | 1.569 (1.249-1.970) | < 0.001 |
| Chemotherapy | ||||||||
| Yes | 1.885 (1.768-2.009) | < 0.001 | 1.589 (1.485-1.700) | < 0.001 | 2.246 (1.775-2.841) | < 0.001 | 1.865 (1.465-2.375) | < 0.001 |
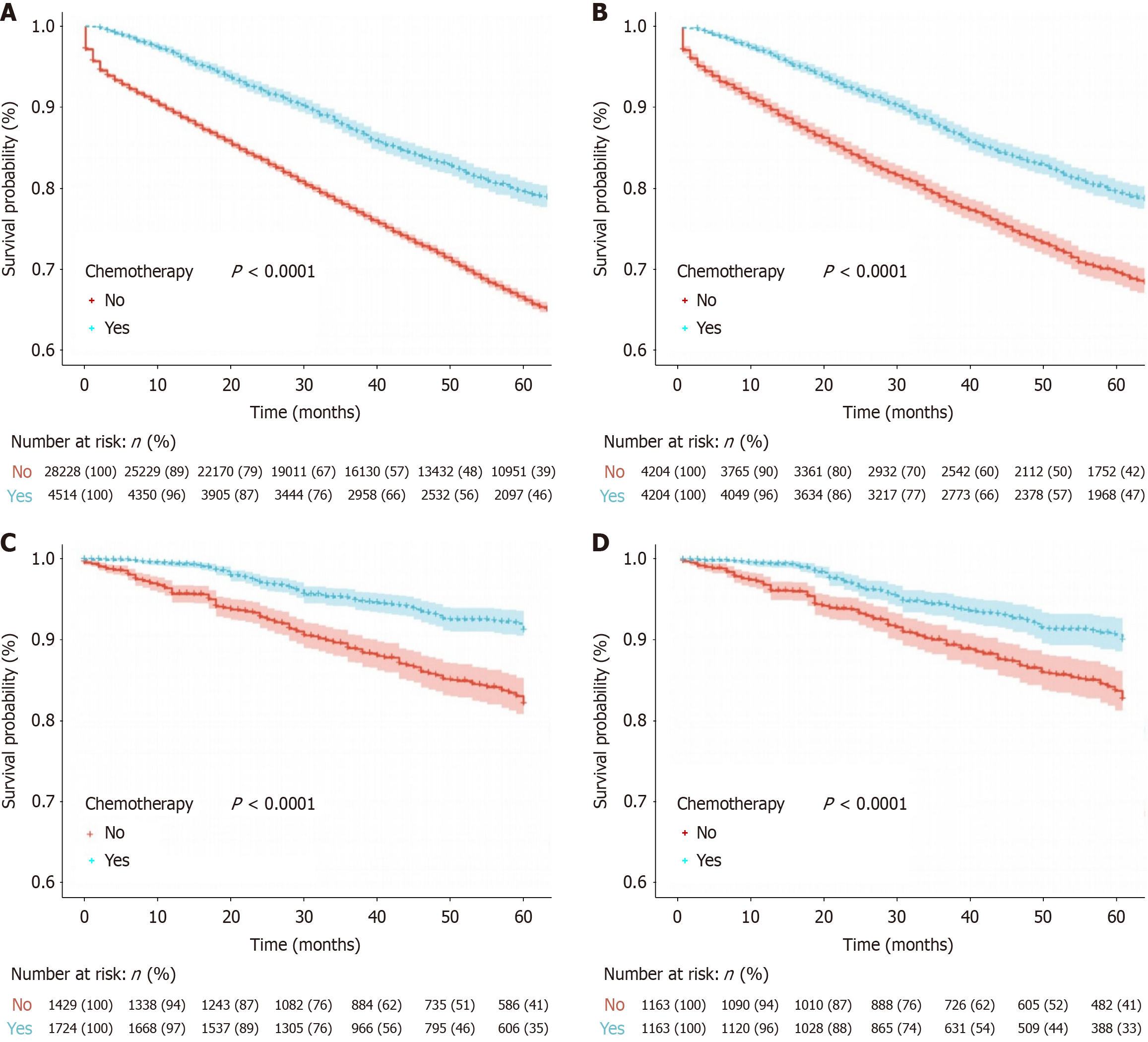
Survival analyses were conducted in both cohorts by comparing outcomes between the ACT and no-ACT groups. PSM was employed to account for differences at baseline and the matched baseline characteristics are outlined in Table 3. In the SEER cohort, prior to PSM, 32742 patients were analyzed (ACT group vs no-ACT group: 4514 vs 28228). After PSM, 8408 patients remained (4204 in each group). In the Chinese cohort, 3153 patients were analyzed prior to PSM (ACT group vs no-ACT group: 1724 vs 1429), and 2326 patients remained after matching (1163 in each group). The matching process significantly reduced baseline differences, thereby enhancing the comparability of the two groups. Survival data from the SEER cohort, both pre-and post-PSM, indicated a significant association between ACT and improved OS (Figure 2A and B, all log-rank P < 0.001). The 5-year OS rates were 79.4% vs 66.1% before PSM and 79.4% vs 69.4% after PSM for the ACT and no-ACT groups, respectively. Similarly, in the Chinese cohort, the ACT group also showed superior 5-year OS rates and improved prognosis compared to the no-ACT group (Figure 2C and D, all log-rank P < 0.001). The 5-year OS rates were 91.2% vs 82.1% before PSM and 90.0% vs 82.8% after PSM.
| Characteristics | Before PSM1 | After PSM1 | Before PSM2 | After PSM2 | ||||||||
| No | Yes | P value | No | Yes | P value | No | Yes | P value | No | Yes | P value | |
| Total | 28228 | 4514 | 4204 | 4204 | 1429 | 1724 | 1163 | 1163 | ||||
| ELN, mean ± SD | 22.18 ± 9.92 | 23.36 ± 10.69 | < 0.001 | 22.52 ± 10.15 | 23.40 ± 10.72 | < 0.001 | 19.98 ± 8.28 | 19.79 ± 7.59 | 0.502 | 19.68 ± 7.83 | 19.97 ± 7.64 | 0.368 |
| Size, mean ± SD | 5.55 ± 3.50 | 6.13 ± 3.66 | < 0.001 | 6.08 ± 3.88 | 6.13 ± 3.52 | 0.568 | 5.21 ± 2.15 | 5.46 ± 2.23 | 0.002 | 5.23 ± 2.15 | 5.28 ± 2.08 | 0.58 |
| Age | < 0.001 | 0.658 | < 0.001 | 0.834 | ||||||||
| 20-64 | 7144 (25.3) | 2769 (61.3) | 2438 (58.0) | 2459 (58.5) | 681 (47.7) | 1203 (69.8) | 666 (57.3) | 660 (56.7) | ||||
| Sex | < 0.001 | 0.169 | 0.097 | 0.474 | ||||||||
| Male | 13274 (47.0) | 2312 (51.2) | 2128 (50.6) | 2192 (52.1) | 796 (55.7) | 1012 (58.7) | 673 (57.9) | 691 (59.4) | ||||
| Grade | < 0.001 | 0.96 | 1 | 0.9 | ||||||||
| Grade 3/4 | 5395 (19.1) | 1126 (24.9) | 1025 (24.4) | 1028 (24.5) | 179 (12.5) | 216 (12.5) | 145 (12.5) | 142 (12.2) | ||||
| Histology | 0.255 | 0.436 | 0.002 | 0.164 | ||||||||
| Adenocarcinoma | 24161 (85.6) | 3835 (85.0) | 3610 (85.9) | 3591 (85.4) | 1238 (86.6) | 1445 (83.8) | 1011 (86.9) | 981 (84.4) | ||||
| Mucinous adenocarcinoma | 3627 (12.8) | 595 (13.2) | 531 (12.6) | 535 (12.7) | 164 (11.5) | 261 (15.1) | 138 (11.9) | 169 (14.5) | ||||
| Others | 440 (1.6) | 84 (1.9) | 63 (1.5) | 78 (1.9) | 27 (1.9) | 18 (1.0) | 14 (1.2) | 13 (1.1) | ||||
| T stage | < 0.001 | 0.641 | 0.27 | 0.525 | ||||||||
| T4 | 3167 (11.2) | 1655 (36.7) | 1369 (32.6) | 1348 (32.1) | 402 (28.1) | 517 (30.0) | 336 (28.9) | 351 (30.2) | ||||
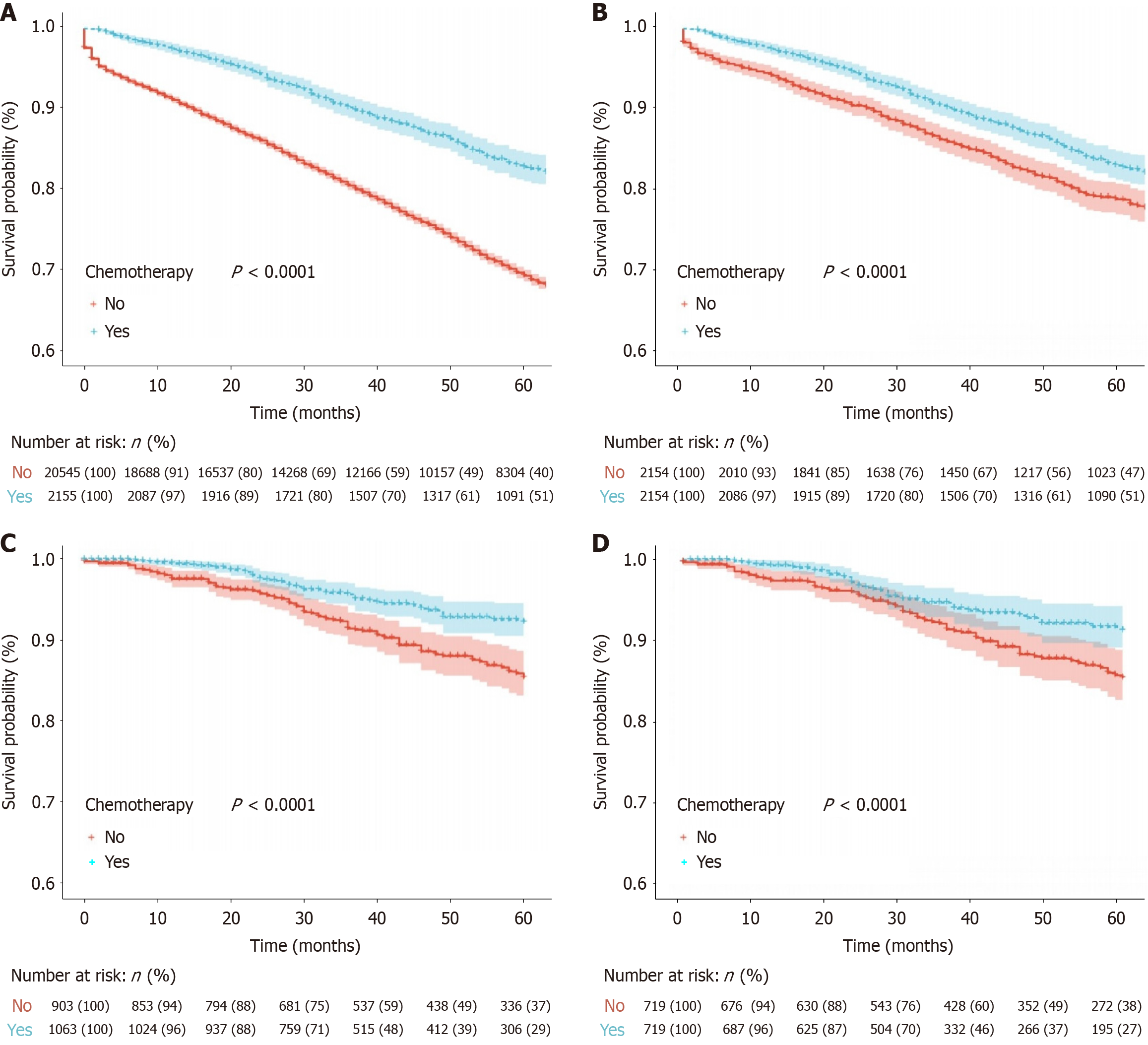
To mitigate the impact of risk factors, such as T4 stage and poor tumor differentiation, on the decision to administer ACT, we carried out a subgroup analysis, incorporating only patients from both cohorts with T3 tumors and grade 1 or 2 histology. The results remained consistent. In the SEER cohort, the 5-year OS rates for the ACT vs no-ACT groups were 82.9% vs 69.4% before PSM (P < 0.0001) and 82.9% vs 78.8% after PSM (P < 0.0001). Similarly, in the Chinese cohort, the 5-year OS rates were 92.3% vs 85.4% before PSM (P < 0.0001) and 91.2% vs 85.4% after PSM (P = 0.0083; Figure 3).
Survival outcomes after surgery for stage II colon cancer vary widely, highlighting the importance of evaluating adjuvant therapy in patients at elevated risk of recurrence. However, substantial uncertainty persists regarding the consistent application of ACT in this setting[3,4]. The extent of LN retrieval remains a critical factor in the decision-making process for ACT. According to current clinical guidelines, fewer than 12 examined LNs is considered a high-risk feature warranting chemotherapy. Notably, our study revealed that the lack of ACT is independently linked to a worse prognosis, even in patients with stage II CRC and ELNs ≥ 12. After controlling for confounding variables, these patients still benefit from ACT. Even among these T3 stage grade 1/2 patients, ACT can still improve prognosis. These findings are based on analyses of both the SEER cohort and a Chinese cohort, which represent distinct demographic and healthcare environments. Marked differences were noted between the two cohorts in terms of ELNs, chemotherapy use, age, gender, tumor grade, and T stage. To account for these disparities, an inter-cohort heterogeneity analysis was performed, confirming substantial differences across several variables. Nevertheless, both cohorts produced consistent outcomes, underscoring the robustness and external validity of our clinical conclusions.
Recent studies have indicated that substantial controversy persists regarding the specific survival advantages of ACT for stage II colon cancer patients, especially in terms of accurately identifying individuals who would most benefit[3,11]. For example, Verhoeff et al[12] propose that ACT is appropriate for patients with stage II colon carcinoma, although uncertainties remain concerning the association between high-risk factors, excluding T4, and survival benefit. These authors further advocate a revision of current high-risk criteria to better define subgroups within stage II colon cancer patients who could derive benefit from ACT. At present, most research focuses on pathological T4 stages, with fewer studies on LNs[13]. Our study provided a comprehensive evaluation of LN assessment in the context of ACT. In clinical practice, the implementation of chemotherapy regimens relies on accurate disease staging to guide treatment decisions. However, despite standardized staging systems, numerous factors, such as clinical variability, genetic predispositions, and lifestyle influences, can compromise staging accuracy.
Specifically in terms of LN retrieval, inadequate sampling may lead to stage migration, thereby affecting treatment decisions and outcomes. For example, Zhang et al[14] demonstrated that a reduced number of ELNs is a critical risk factor for disease recurrence in stage II CRC patients. Other studies have similarly found that the number of LNs retrieved is strongly associated with prognostic evaluation in colon cancer[15,16]. Conversely, adequate LN retrieval can significantly reduce the incidence of stage migration, thereby informing more precise chemotherapy planning. Vather et al[17] also reported that an increased number of LNs examined is linked to better 5-year survival outcomes in patients with stage II and III colon cancer. Based on our findings, refining patient selection criteria for ACT helped mitigate the influence of stage migration on treatment planning in specific subgroups. However, it is important to note that even after adjusting for potential risk factors, such as LNs, patients with well-defined staging still exhibited the concept of “residual risk”. This underscores the need for future multidimensional evaluations to support the development of personalized treatment strategies.
Adequate LN retrieval may also indicate a stronger immune response, potentially enhancing the benefit derived from ACT. Therefore, it is essential to assess whether patients with ELNs ≥ 12 can derive a prognostic benefit from ACT, which was a key focus of our study. Zhang et al[18] underlined the gap in predictive models for accurately identifying stage II colon cancer patients who are at high risk and would benefit from routine ACT. Their study proposed a risk scoring system, incorporating LN count, as a clinical tool to guide ACT decision-making. Our study found that ACT can confer a prognostic advantage in this group. These results support the development of more individualized clinical treatment plans and align with previous findings. For instance, Schrag et al[19] suggested that higher LN counts in colon cancer may reflect greater use of ACT. Conversely, insufficient LN retrieval (< 12) in early-stage colon cancer is often considered an indication for ACT, an observation consistent with aspects of our analysis. However, our results have not yet been directly compared with existing prognostic models, which limits their immediate clinical applicability, particularly in the context of individualized treatment planning. Future research should aim to integrate our findings with established predictive frameworks to develop more comprehensive models for identifying stage II colon cancer patients most likely to benefit from ACT.
There are several limitations in this study. First, it is retrospective and observational in design, making it inherently susceptible to potential selection bias. Second, although efforts were made to minimize confounding variables, unmeasured factors may still have influenced the association between ACT and prognosis. These include variability in LN examination practices across surgeons, pathologists, and healthcare institutions[20]. Moreover, factors such as comorbidities like diabetes, hypertension, and bowel obstruction may shorten survival time, while conditions like tumor obstruction/perforation could increase the likelihood of infection. Variations in R0 resection status and treatment adherence may also influence disease progression, which in turn could affect treatment outcomes. In future research, as the database continues to improve, it will be essential to incorporate more potential risk factors to refine and enhance the research findings. Third, both the SEER database and the Chinese multicenter dataset lacked granular molecular and genomic information. Specific molecular subtypes, such as deficient mismatch repair, microsatellite instability-high, or mutations in B-Raf proto-oncogene V600E, human epidermal growth factor receptor 2, Kirsten rat sarcoma viral oncogene homolog G12C, or DNA polymerase epsilon/DNA polymerase delta 1, were not available[21]. Furthermore, detailed chemotherapy regimen data (including type, dosage intensity, and cycle number) were not captured. This study used a binary classification of chemotherapy (yes vs no), limiting the granularity and clinical applicability of our findings. Different ACT regimens can have varied effects on outcomes, and the absence of this information constrains the ability to tailor treatment recommendations based on individual patient profiles. These limitations reduce the generalizability of our results in the context of personalized medicine. Fourth, although our results provide important insights into treatment strategies, practical clinical applications still require further exploration. Further studies should prioritize integrating these findings into existing prognostic models to develop more comprehensive tools for identifying stage II colon cancer patients who stand to receive the highest benefit from ACT. Nevertheless, our study provides valuable evidence for guiding treatment choices in patients with stage II colon cancer and supports the refinement of clinical guidelines toward more individualized care.
For individuals with stage II CRC and at least 12 LNs assessed, ACT is associated with improved prognosis. These findings offer valuable insights to inform clinical decision-making for this patient group. However, additional prospective studies are needed to validate these results and facilitate their incorporation into personalized treatment strategies.
We wish to sincerely thank the students and professors whose contributions have significantly enhanced this article.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56675] [Article Influence: 7084.4] [Reference Citation Analysis (135)] |
| 2. | Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev. 2008;2008:CD005390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Baxter NN, Kennedy EB, Bergsland E, Berlin J, George TJ, Gill S, Gold PJ, Hantel A, Jones L, Lieu C, Mahmoud N, Morris AM, Ruiz-Garcia E, You YN, Meyerhardt JA. Adjuvant Therapy for Stage II Colon Cancer: ASCO Guideline Update. J Clin Oncol. 2022;40:892-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 210] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 4. | Babcock BD, Aljehani MA, Jabo B, Choi AH, Morgan JW, Selleck MJ, Luca F, Raskin E, Reeves ME, Garberoglio CA, Lum SS, Senthil M. High-Risk Stage II Colon Cancer: Not All Risks Are Created Equal. Ann Surg Oncol. 2018;25:1980-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Booth CM, Nanji S, Wei X, Peng Y, Biagi JJ, Hanna TP, Krzyzanowska MK, Mackillop WJ. Adjuvant Chemotherapy for Stage II Colon Cancer: Practice Patterns and Effectiveness in the General Population. Clin Oncol (R Coll Radiol). 2017;29:e29-e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Xie D, Song X, Tong L. Stage migration resulting from inadequate number of examined lymph nodes impacts prognosis in stage II colon cancer after radical surgery. Int J Colorectal Dis. 2021;36:959-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Qiao Y, Zhu J, Han T, Jiang X, Wang K, Chen R, Du Y, Li J, Sun L. Finding the minimum number of retrieved lymph nodes in node-negative colorectal cancer using Real-world Data and the SEER database. Int J Surg. 2023;109:4173-4184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Kotake K, Honjo S, Sugihara K, Hashiguchi Y, Kato T, Kodaira S, Muto T, Koyama Y. Number of lymph nodes retrieved is an important determinant of survival of patients with stage II and stage III colorectal cancer. Jpn J Clin Oncol. 2012;42:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 794] [Article Influence: 41.8] [Reference Citation Analysis (1)] |
| 10. | Doll KM, Rademaker A, Sosa JA. Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg. 2018;153:588-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 324] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 11. | Giannakis M, Ng K. To Treat or Not to Treat: Adjuvant Therapy for Stage II Colon Cancer in the Era of Precision Oncology. J Oncol Pract. 2017;13:242-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Verhoeff SR, van Erning FN, Lemmens VE, de Wilt JH, Pruijt JF. Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer. 2016;139:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Lindskog EB, Gunnarsdóttir KÁ, Derwinger K, Wettergren Y, Glimelius B, Kodeda K. A population-based cohort study on adherence to practice guidelines for adjuvant chemotherapy in colorectal cancer. BMC Cancer. 2014;14:948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Zhang C, Zhao S, Wang X. Analysis of the risk factor of insufficient examined lymph nodes in stage II colon cancer from the perspective of stage migration: A retrospective study combined with external validation. Int J Surg. 2022;101:106628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 15. | Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, Brouwers M, Charette M, Haller DG. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408-3419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1017] [Cited by in RCA: 1078] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 16. | Martínez Ortega P, Cienfuegos JA, Baixauli J, Sánchez Justicia C, Abengózar M, Pastor Idoate C, Hernández Lizoáin JL. Prognostic significance of lymph node count in high-risk node-negative colon carcinoma. Rev Esp Enferm Dig. 2020;112:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Vather R, Sammour T, Kahokehr A, Connolly AB, Hill AG. Lymph node evaluation and long-term survival in Stage II and Stage III colon cancer: a national study. Ann Surg Oncol. 2009;16:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Zhang CD, Wang JN, Sui BQ, Zeng YJ, Chen JQ, Dai DQ. Prognostic and Predictive Model for Stage II Colon Cancer Patients With Nonemergent Surgery: Who Should Receive Adjuvant Chemotherapy? Medicine (Baltimore). 2016;95:e2190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Schrag D, Rifas-Shiman S, Saltz L, Bach PB, Begg CB. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20:3999-4005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 182] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Becerra AZ, Aquina CT, Berho M, Boscoe FP, Schymura MJ, Noyes K, Monson JR, Fleming FJ. Surgeon-, pathologist-, and hospital-level variation in suboptimal lymph node examination after colectomy: Compartmentalizing quality improvement strategies. Surgery. 2017;161:1299-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Fariña-Sarasqueta A, van Lijnschoten G, Moerland E, Creemers GJ, Lemmens VEPP, Rutten HJT, van den Brule AJC. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol. 2010;21:2396-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/