Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.108277
Revised: June 4, 2025
Accepted: July 18, 2025
Published online: September 15, 2025
Processing time: 159 Days and 0.6 Hours
Perineural invasion (PNI) is common in pancreatic cancer (PC) and is associated with poor prognosis.
To investigate the correlation between PNI and clinical pathological features in PC.
Patients were retrospectively divided into non-neural invasion and neural inva
The neural invasion group had a higher proportion of tumors in the head, larger size, higher CA19-9 levels, lower survival rates, more abdominal pain, and more lymph node invasion. Pancreatic ductal adenocarcinoma and higher differentiation were more common in the neural invasion group. Tumor location, sur
Tumor location, size, CA19-9 level, abdominal pain, differentiation, and lymph node invasion are important factors in neural invasion and tumor progression in PC.
Core Tip: This study investigates the association between perineural invasion (PNI) and clinicopathological features in pancreatic cancer (PC). A total of 112 PC cases were analyzed and categorized into PNI and non-PNI groups. Tumor location, size, carbohydrate antigen 19-9 levels, abdominal pain, differentiation, and lymph node invasion were identified as independent risk factors for PNI. A predictive model based on these variables demonstrated high accuracy and consistency. These findings offer valuable insight into the pathological mechanisms of PNI and may aid in early diagnosis and individualized treatment strategies for PC patients.
- Citation: Lu XL, Ge C, Wang RC, Zang H. Correlation between perineural invasion and clinicopathological characteristics in pancreatic cancer. World J Gastrointest Oncol 2025; 17(9): 108277
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/108277.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.108277
Pancreatic cancer (PC) is a malignant disease with a significantly increased incidence in recent years and a high mortality rate[1]. Due to the lack of specific tumor markers, the diagnosis rate is very low, and only less than 20% of patients can be cured[2,3]. Currently, surgical resection is the main treatment for PC[4], but 40% of patients are already in the advanced stage with metastasis at the time of diagnosis, missing the best treatment opportunity[5,6]. Moreover, PC is largely resistant to both radiotherapy and chemotherapy, and the overall resection rate is low. As a result, the 5-year survival rate remains below 5%. Studies suggest that the aggressive nature of PC is largely attributed to its propensity for early invasion and metastasis[7,8].
Among its invasive patterns, perineural invasion (PNI) is a characteristic pattern of local spread in PC[9]. PNI is highly prevalent in PC and has been strongly associated with poor prognosis and decreased overall survival[10,11]. Mechanistically, neural invasion may occur via direct infiltration or along lymphatic and vascular structures surrounding the tumor[12]. PNI has been reported in 50% to 100% of PC cases and is now recognized as a hallmark of PC progression and a critical prognostic indicator[13]. It is also regarded as an independent risk factor for unfavorable outcomes in PC patients[14].
Given its high incidence and prognostic importance, investigating the clinicopathological factors associated with PNI may improve early identification of aggressive disease and inform individualized treatment strategies. Therefore, the aim of this study was to evaluate the correlation between PNI and various clinicopathological characteristics in patients with PC, and to explore its potential diagnostic and prognostic value.
Clinical data from our hospital., according to the presence or absence of PNI, the patients were divided into the non-PNI group (n = 37) and the PNI group (n = 75). The non-PNI group consisted of 22 males and 15 females, with an average age of (60.3 ± 4.33) years. The PNI group consisted of 38 males and 37 females, with an average age of (59.92 ± 3.25) years.
This study has been approved by the Institutional Review Board and Ethics Committee of our hospital, approval No. 2021KT179. The Institutional Review Board and Ethics Committee of our hospital waived the requirement for informed consent for this retrospective study, as it only utilized unidentified patient data and did not pose any potential harm or impact on patient care.
Inclusion criteria: Patients with PC who were first diagnosed and treated in our hospital; Patients with complete medical records; Patients or their families signed the informed consent form for this study.
Exclusion criteria: Patients who received other treatments such as chemotherapy and immunotherapy before surgery; Patients with other malignant tumors and other internal and surgical diseases such as hypertension, coronary heart disease, liver disease, and diabetes; Patients with incomplete medical records.
Specimen processing: The specimens used in the study were collected immediately after surgical resection, fixed in 10% neutral formalin solution, embedded in paraffin, and subjected to S-100 protein immunohistochemical staining for detection. Phosphate-buffered saline was used as a negative control instead of the primary antibody, and S-100 protein was diluted at 1:1000. Electron microscopy observation: After fixation with 2.5% glutaraldehyde, the specimens were further fixed with osmium tetroxide, dehydrated with ethanol, and embedded in epoxy resin Epon812. Semi-thin sections were made before electron microscopy observation, stained with methylene blue, and located under an optical microscope (DM400M, LEICA, Germany), and then ultra-thin sections were made and stained with uranyl acetate-lead citrate for observation under an electron microscope (H-600, HITACHI, Japan) to determine the presence of PNI. According to the results of electron microscope section, the patients were divided into two groups: Non-PNI group and PNI group.
Baseline data: Including age, sex, body mass index, course of disease, smoking history, drinking history, family history of PC, etc. The clinicopathological characteristics were detected by B-ultrasound, computed tomography, magnetic resonance imaging, and other imaging examinations, the tumor location, tumor size, pathological type, differentiation degree, lymph node invasion, and other indicators were recorded. The abdominal pain status and overall survival of the two groups of patients were recorded. 3 mL of fasting venous blood was collected from all patients in the morning, and the serum was separated by centrifugation. The serum carbohydrate antigen 19-9 (CA19-9) level was detected using an automated chemiluminescence method.
Statistical analyses were performed using SPSS version 26.0 (IBM Corp., NY, United States). Categorical variables were expressed as counts and percentages, n (%), and the χ2 test was used when the sample size was ≥ 40 and the expected frequency (T) in all cells was ≥ 5. When 1 ≤ T < 5, the χ2 test with continuity correction was applied; for sample sizes < 40 or T < 1, Fisher’s exact test was used. Continuous variables following a normal distribution were presented as mean ± SD and compared using Student’s t test. Non-normally distributed data were expressed as median values and analyzed accordingly. A P value < 0.05 was considered statistically significant. Spearman correlation analysis was performed to assess associations between categorical variables, with neural infiltration coded as 1 (present) and 0 (absent). Variables showing significant differences in both univariate comparisons and correlation analysis were included as covariates in logistic regression analysis.
We analyzed the clinical data of 82 patients with PC. Patients were divided into non-invasive group (n = 37) and invasive group (n = 75). As shown in Table 1, the age (P = 0.641), body mass index (P = 0.384) and Course of disease (P = 0.938) were compared with those in the non-invasive group and invasive group, smoking history (P = 0.748), drinking histor9 (P = 0.746), family history of PC (P = 0.766), there was no significant difference in several aspects.
| Items | Non-PNI group (n = 17) | PNI group (n = 65) | t | P value |
| Age (years), mean ± SD | 60.42 ± 4.36 | 59.87 ± 3.25 | 0.485 | 0.633 |
| Gender (male/female) | 10 (58.82)/7(41.18) | 33 (50.77)/32 (49.23) | 0.102 | 0.749 |
| BMI (kg/cm2), mean ± SD | 25.64 ± 2.98 | 24.85 ± 2.67 | 0.987 | 0.334 |
| Course of disease (months), mean ± SD | 5.46 ± 1.87 | 5.67 ± 2.01 | 0.411 | 0.684 |
| Smoking history (yes/no) | 7 (41.18) | 24 (36.92) | 0.002 | 0.967 |
| Drinking history (yes/no) | 8 (47.06) | 28 (43.08) | 0.000 | 0.984 |
| Family history of pancreatic cancer (yes/no) | 3 (17.65) | 14 (21.54) | 0.000 | 0.987 |
As shown in Table 2, 7 cases in the non-PNI group had tumors in the head, 6 cases in the body, and 4 cases in the tail. In the PNI group, 48 cases had tumors in the head, 10 cases in the body, and 7 cases in the tail. The differences between the two groups were statistically significant (P < 0.05). Tumor size, CA19-9 Level, and the incidence of abdominal pain were significantly lower in the non-PNI group than in the PNI group, while overall survival was significantly higher (P < 0.05).
| Clinical characteristics | Non-PNI group (n = 17) | PNI group (n = 65) | t | P value |
| Tumor location | ||||
| Head | 7 (41.18) | 48 (73.85) | - | 0.033 |
| Body | 6 (35.29) | 10 (15.38) | ||
| Tail | 4 (23.53) | 7 (10.77) | ||
| Tumor size (cm), mean ± SD | 4.06 ± 0.89 | 5.37 ± 1.85 | 4.158 | < 0.001 |
| CA19-9 Level, mean ± SD | 260.67 ± 48.92 | 312.54 ± 57.62 | 3.745 | < 0.001 |
| Overall survival (months), mean ± SD | 12.76 ± 3.21 | 9.85 ± 2.18 | 3.531 | 0.002 |
| Abdominal pain | 3 (17.65) | 32 (49.23) | 4.279 | 0.039 |
| Clinical characteristics | Non-PNI group (n = 17) | PNI group (n = 65) | t/χ2 | P value |
| Pathological types | - | 0.019 | ||
| Ductal adenocarcinoma | 9 (52.94) | 42 (64.62) | - | - |
| Mucinous adenocarcinoma | 5 (29.41) | 23 (35.38) | ||
| Adenosquamous carcinoma | 2 (11.76) | 0 (0.00) | ||
| Small cell carcinoma | 1 (5.88) | 0 (0.00) | ||
| Differentiation degree | - | < 0.001 | ||
| Low | 2 (11.76) | 45 (69.23) | - | - |
| Medium | 3 (17.65) | 14 (21.54) | ||
| High | 12 (70.59) | 6 (9.23) | ||
| Lymph node infiltration | 32.024 | < 0.001 | ||
| Yes | 0 (0.00) | 51 (78.46) | - | - |
| No | 17 (100.00) | 14 (21.54) | ||
| Items | r | P value |
| Tumor location | -0.275 | 0.012 |
| Tumor size (cm) | 0.351 | 0.001 |
| CA19-9 Level | 0.376 | < 0.001 |
| Overall survival (months) | -0.392 | < 0.001 |
| Abdominal pain | 0.259 | 0.019 |
| Differentiation degree | 0.656 | < 0.001 |
| Lymph node infiltration | -0.275 | 0.012 |
As shown in Table 3, 9 cases of pancreatic ductal adenocarcinoma (PDAC), 5 cases of mucinous adenocarcinoma, 2 cases of adenosquamous carcinoma, and 1 case of small cell carcinoma were found in the non-PNI group. In the PNI group, there were 42 cases of PDAC and 23 cases of mucinous adenocarcinoma, with no cases of adenosquamous carcinoma or small cell carcinoma. The differences between the two groups were statistically significant (P < 0.05). The proportion of well-differentiated tumors was higher in the non-PNI group, while the proportion of poorly differentiated tumors was higher in the PNI group. There was no lymph node invasion in the non-PNI group, while the proportion of lymph node invasion was higher in the PNI group, and the difference was statistically significant (P < 0.05).
As shown in Table 4, correlation analysis revealed a negative correlation between tumor location, overall survival, and differentiation degree, and the occurrence of neural infiltration in PC (r = -0.294, -0.474, -0.648; P = 0.002, < 0.001, < 0.001, respectively). On the other hand, tumor size, CA19-9 Levels, abdominal pain, and lymphatic node infiltration showed a positive correlation with the occurrence of neural infiltration in PC (r = 0.362, 0.409, 0.293, 0.741; P < 0.001, < 0.001,
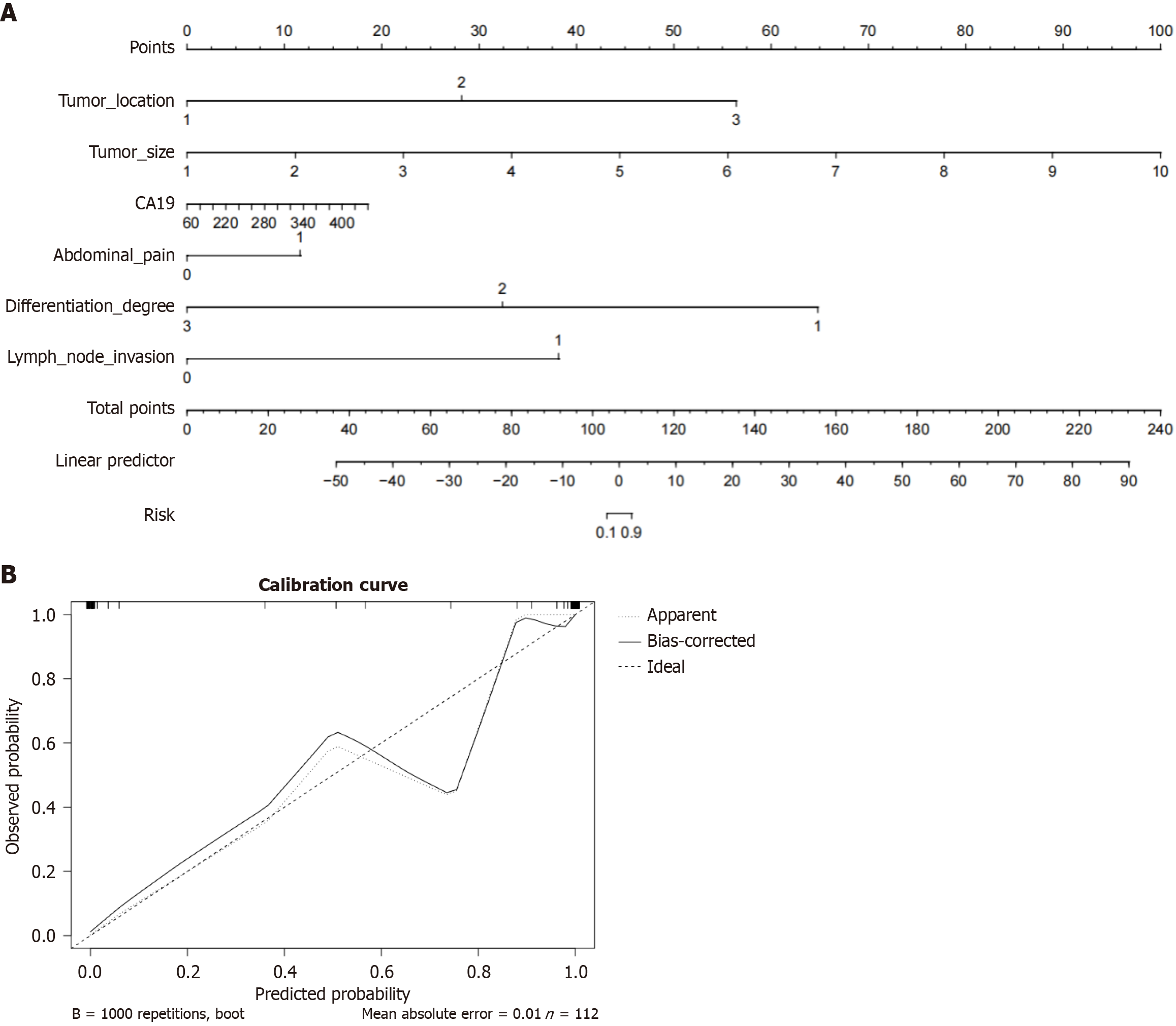
Based on the results of logistic regression analysis, 6 variables (tumor location, tumor size, CA19-9 Level, abdominal pain, differentiation degree, lymph node invasion) were selected to construct a line chart predictive model for the occurrence rate of neuroinvasion in PC (Figure 1A). Each patient’s pathological characteristics correspond to a separate score, and the total score is calculated by summing the scores of different factors. The higher the score, the higher the occurrence rate of neuroinvasion in PC.
The calibration curve shows the deviation between the predicted probability and the actual probability. The average absolute error between the actual occurrence rate and the predicted occurrence rate of the model is 0.01, and the calibration curve is repeated 1000 times (Figure 1B), indicating good consistency between the actual occurrence rate and the predicted results.
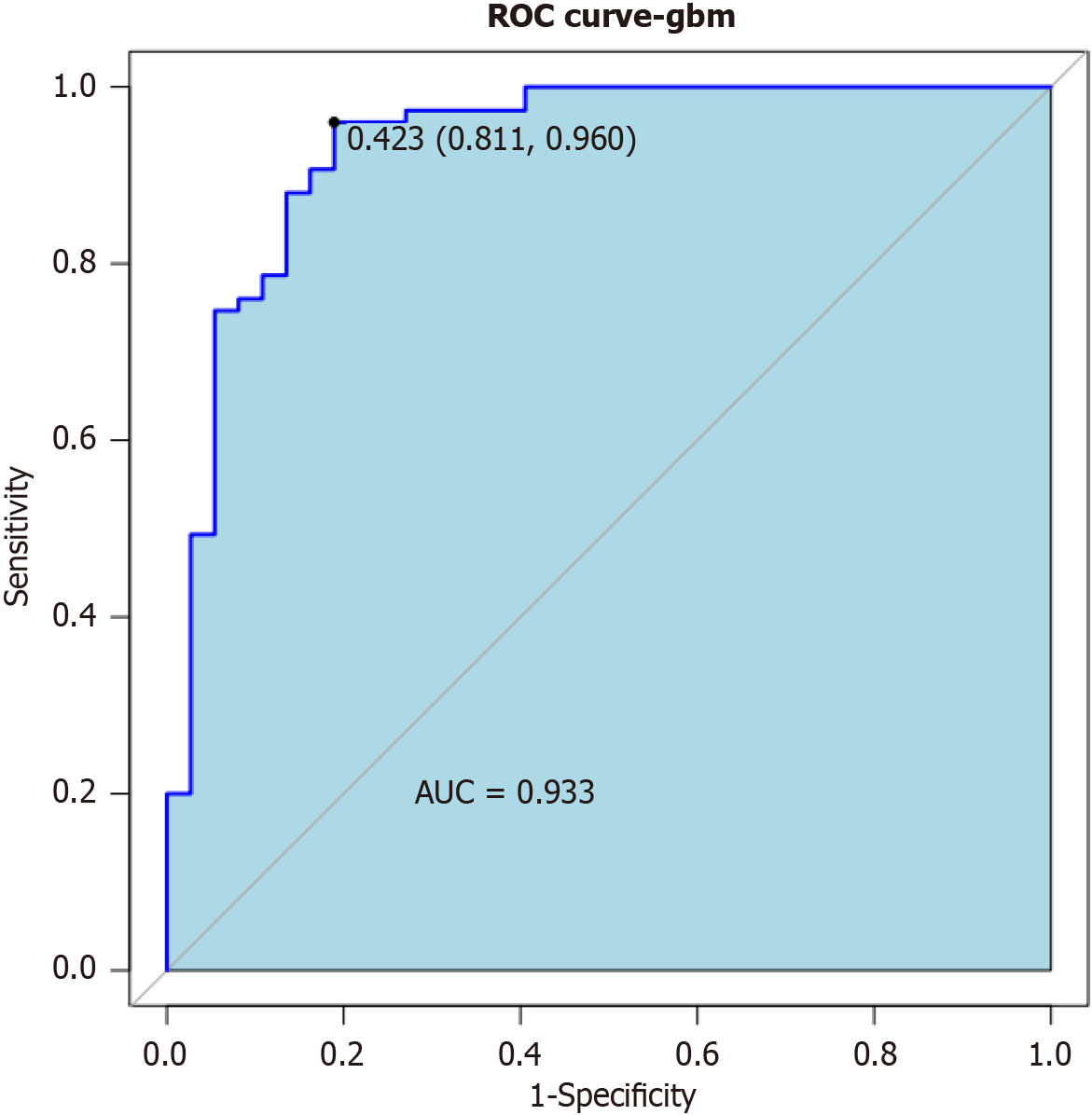
In this study, a combination model was developed by combining pathological feature parameters to predict the probability of neuroinvasion in PC patients. The results showed that the area under curve value of the combined model was 0.933 (Figure 2), indicating a significantly high predictive value in identifying the occurrence rate of neuroinvasion in PC patients using relevant pathological features.
PNI refers to the phenomenon of cancer cells growing around or inside nerve bundles. It is more common in squamous cell carcinoma and basal cell carcinoma in the head and neck region, and has a higher incidence in tumors such as prostate cancer and colon cancer, with an incidence rate of 60%-100%, which seriously affects prognosis[15-17]. PNI inside and outside the pancreas is a typical neurological symptom of PC and the main cause of local recurrence after surgery. It is an adverse prognostic indicator of PC, and pancreatic extra-PNI often coexists with pancreatic intra-PNI[18,19]. Under the light microscope, the number of infiltrated nerve bundles can be seen at 100 times magnification, and PC PNI can be divided into no infiltration (0 bundles), mild infiltration (1-5 bundles), moderate infiltration (6-10 bundles), and severe infiltration (more than 10 bundles). For PC, from a histological perspective, PNI can be divided into three types: Intratumoral, extratumoral within the pancreas, and extrapancreatic retroperitoneal[20-22]. Currently, the early diagnosis of PC PNI is limited to clinical data, and the indicators related to PNI are also the core content of this study.
In this study, we studied postoperative specimens of 112 patients with PC in our hospital, of which 75 cases had PNI, with an incidence rate of 66.96%, which is consistent with the incidence rate of PNI in PC. This study showed that the distribution of tumors in both groups was predominantly in the head, and there is evidence that there is a large amount of nerve tissue in the tail of the pancreatic body, which is more conducive to cancer cell metastasis and invasion[23]. In this study, the proportion of tumors in the head was large, and the correlation analysis found that the distribution of tumors was negatively correlated with the occurrence of PNI, which may also be a reason why the actual rate of nerve tissue invasion is not high. However, the distribution of tumors in the non-PNI group was more dispersed, which was significantly different from the PNI group (P < 0.05).
Tumor size, CA19-9 Level, and the incidence of abdominal pain were significantly lower in the non-PNI group than in the PNI group, while overall survival was significantly higher (P < 0.05). The correlation analysis found that overall survival was negatively correlated with the occurrence of PNI in PC, while tumor size, CA19-9 Level, and the abdominal pain were positively correlated. These findings suggest that PNI in PC is significantly associated with tumor burden, biomarker levels, and symptom presentation. Consistent with our results, Wang et al[24] reported a higher frequency of abdominal pain in patients with PNI, and Zhao et al[25] demonstrated a strong association between elevated CA19-9 Levels and PNI.
The proportion of PDAC was the highest in both groups, reflecting the fact that PDAC accounts for approximately 90% of all PCs and is known for its strong neurotropic behavior. This neurotropism is believed to contribute to the high incidence of PNI and tumor recurrence in PDAC. Schorn et al[26] conducted a study using consecutive histological sections of PDAC specimens and found that all patients exhibited PNI, emphasizing the strong association between PDAC and neural invasion. However, their study had a relatively small sample size, which may have led to an overestimation of PNI prevalence due to a high proportion of PDAC cases. In our study, we also observed a significantly higher proportion of PDAC in the PNI group, supporting the neuroinvasive tendency of this histological subtype. In contrast, the non-PNI group showed greater heterogeneity in pathological types, including mucinous adenocarcinoma, adenosquamous carcinoma, and small cell carcinoma. These subtypes differ in biological characteristics and clinical outcomes. For instance, mucinous adenocarcinomas may present with lower invasiveness[27], while adenosquamous and small cell carcinomas are typically more aggressive but their association with PNI remains unclear due to their rarity[28,29]. These differences are not only histological but also prognostic - different subtypes are associated with varying survival outcomes and patterns of progression. Therefore, the observed disparity in pathological subtype distribution (P < 0.05) between groups may influence both the risk of PNI and patient prognosis. While the diversity of subtypes in the non-PNI group may introduce confounding effects when comparing clinical outcomes such as overall survival, it also reinforces the strong association between PNI and PDAC. This emphasizes the need to consider tumor histology when interpreting PNI-related findings and suggests that future studies should stratify analyses by subtype to reduce heterogeneity and enhance interpretability.
Regarding the relationship between lymph node metastasis and PNI, previous studies have suggested that tumors with a higher tendency to invade perineural spaces are also more likely to exhibit lymphatic spread[30]. Consistent with this, our study found that none of the patients in the non-PNI group had lymph node metastasis, whereas a significantly higher proportion of patients in the PNI group did (P < 0.05). Shen et al[31] further demonstrated that patients without PNI or lymph node metastasis had markedly better 5-year survival outcomes compared to those with both features, reinforcing the prognostic implications of their co-occurrence.
Similarly, with respect to histological differentiation, our findings revealed that well-differentiated tumors were more common in the non-PNI group, while poorly differentiated tumors predominated in the PNI group (P < 0.05). Although literature on this relationship remains mixed[32], our results support the hypothesis that poor differentiation is more frequently associated with neural invasion.
It is important to highlight that both lymph node metastasis and poor tumor differentiation are established markers of aggressive tumor biology and independently associated with worse overall survival in PC. Their higher prevalence in the PNI group suggests they may act as potential confounding factors influencing the observed survival differences between groups. Therefore, although PNI was associated with shorter overall survival in our cohort, part of this effect may be mediated or amplified by these co-existing adverse pathological features. We acknowledge this as a limitation and recommend that future studies employ multivariate analyses to better isolate the independent prognostic contribution of PNI.
Relevance analysis revealed a negative correlation between pathological type and differentiation degree of PC and the occurrence of neural invasion, while lymph node infiltration showed a positive correlation with neural invasion. These findings indicate a significant correlation between the occurrence of neural invasion in PC and pathological type, differentiation degree, and lymph node infiltration.
The logistic regression analysis shows that tumor location, tumor size, CA19-9 Level, abdominal pain, differentiation degree, and lymph node infiltration are independent risk factors for neuroinvasion in PC. The constructed column line chart prediction model and calibration curve chart show good consistency between the actual incidence and the predicted results. At the same time, the combined model of pathological features has an area under curve value of 0.933, indicating that identifying the incidence of neuroinvasion in PC patients through relevant pathological features has significant predictive value.
In recent years, artificial intelligence (AI) has shown great promise in medical diagnostics, including its application in oncology. While not the direct focus of this study, our findings on the clinicopathological predictors of PNI provide a foundation that could be further developed using AI-driven models. For example, machine learning algorithms trained on imaging features, tumor biomarkers, and clinical variables could be used to preoperatively predict the likelihood of PNI. Such models would enable clinicians to identify high-risk patients who might benefit from neoadjuvant chemotherapy, even if the tumor appears technically resectable[33,34]. Furthermore, postoperative confirmation of PNI status could serve as a criterion for stratifying adjuvant therapy intensity or guiding surveillance frequency. In this context, AI does not merely represent a general diagnostic aid, but a potential tool to support individualized treatment planning based on predicted or confirmed PNI risk. Future studies integrating AI with clinicopathological data and imaging could significantly advance precision medicine in PC management.
This study identified key clinicopathological factors - such as tumor location, larger size, elevated CA19-9, poor differentiation, abdominal pain, and lymph node invasion - as significantly associated with PNI in PC. Recognizing these factors can help predict PNI risk preoperatively, guiding the use of neoadjuvant therapy and personalized treatment planning. Postoperative confirmation of PNI may also support tailored adjuvant strategies and closer surveillance. These findings highlight the clinical relevance of PNI in risk stratification and decision-making.
| 1. | Moore A, Donahue T. Pancreatic Cancer. JAMA. 2019;322:1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 2. | Zhao Z, Liu W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol Cancer Res Treat. 2020;19:1533033820962117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 242] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 3. | Pawlik TM. Pancreatic Cancer. Surg Oncol Clin N Am. 2021;30:xiii-xixv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Kolbeinsson HM, Chandana S, Wright GP, Chung M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J Invest Surg. 2023;36:2129884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 251] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 5. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 862] [Article Influence: 172.4] [Reference Citation Analysis (0)] |
| 6. | Loveday BPT, Lipton L, Thomson BN. Pancreatic cancer: An update on diagnosis and management. Aust J Gen Pract. 2019;48:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, Zhang T, Dai M, Zhao Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 8. | Stoffel EM, Brand RE, Goggins M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology. 2023;164:752-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 325] [Reference Citation Analysis (1)] |
| 9. | Miura T, Mitsunaga S, Ikeda M, Ohno I, Takahashi H, Kuwata T, Ochiai A. Neural Invasion Spreads Macrophage-Related Allodynia via Neural Root in Pancreatic Cancer. Anesth Analg. 2018;126:1729-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | You GH, Wang YJ, Zhang LY, Zhang M. [Research progression in neural invasion model of pancreatic cancer]. Zhonghua Zhong Liu Za Zhi. 2020;42:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Zhu M, Luo F, Xu B, Xu J. Research Progress of Neural Invasion in Pancreatic Cancer. Curr Cancer Drug Targets. 2024;24:397-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Wakiya T, Ishido K, Kimura N, Nagase H, Yoshizawa T, Morohashi S, Fujita H, Kanda T, Tatara Y, Saruwatari J, Kijima H, Hakamada K. Eukaryotic initiation factor 2 signaling behind neural invasion linked with lymphatic and vascular invasion in pancreatic cancer. Sci Rep. 2021;11:21197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Yi SQ, Miwa K, Ohta T, Kayahara M, Kitagawa H, Tanaka A, Shimokawa T, Akita K, Tanaka S. Innervation of the pancreas from the perspective of perineural invasion of pancreatic cancer. Pancreas. 2003;27:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Wang PH, Song N, Shi LB, Zhang QH, Chen ZY. The relationship between multiple clinicopathological features and nerve invasion in pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2013;12:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Miyai M, Iwama T, Hara A, Tomita H. Exploring the Vital Link Between Glioma, Neuron, and Neural Activity in the Context of Invasion. Am J Pathol. 2023;193:669-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Wang L, Xia Y, Jiang T, Li Y, Shen Y, Lin J, Li F, Wang W, Zhang D, Xu H, Yang L, Xu Z. Neural Invasion is an Independent Prognostic Factor in Young and Lymph Node Negative Gastric Cancer Patients Underwent Curative Gastrectomy. J Invest Surg. 2023;36:2257785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Jiang P, Li X, Wang S, Liu Y. Prognostic Significance of PNI in Patients With Pancreatic Head Cancer Undergoing Laparoscopic Pancreaticoduodenectomy. Front Surg. 2022;9:897033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Zhang W, He R, Yang W, Zhang Y, Yuan Q, Wang J, Liu Y, Chen S, Zhang S, Zhang W, Zhu Z, Zhang J, Wang Z, Li J. Autophagic Schwann cells promote perineural invasion mediated by the NGF/ATG7 paracrine pathway in pancreatic cancer. J Exp Clin Cancer Res. 2022;41:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Li J, Kang R, Tang D. Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun (Lond). 2021;41:642-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 20. | Liu Q, Ma Z, Cao Q, Zhao H, Guo Y, Liu T, Li J. Perineural invasion-associated biomarkers for tumor development. Biomed Pharmacother. 2022;155:113691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 21. | Tian Z, Ou G, Su M, Li R, Pan L, Lin X, Zou J, Chen S, Li Y, Huang K, Chen Y. TIMP1 derived from pancreatic cancer cells stimulates Schwann cells and promotes the occurrence of perineural invasion. Cancer Lett. 2022;546:215863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 22. | Gasparini G, Pellegatta M, Crippa S, Lena MS, Belfiori G, Doglioni C, Taveggia C, Falconi M. Nerves and Pancreatic Cancer: New Insights into a Dangerous Relationship. Cancers (Basel). 2019;11:893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Liang L, Dong M, Cong K, Chen Y, Ma Z. Correlations of Moesin expression with the pathological stage, nerve infiltration, tumor location and pain severity in patients with pancreatic cancer. J BUON. 2019;24:1225-1232. [PubMed] |
| 24. | Wang J, Chen Y, Li X, Zou X. Perineural Invasion and Associated Pain Transmission in Pancreatic Cancer. Cancers (Basel). 2021;13:4594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Zhao Y, Wang C. Clinicopathological Features, Recurrence Patterns, and Prognosis of Pancreatic Adenocarcinoma with Normal Serum CA19-9. A Consecutive Series of 154 Cases from a Single Institute. J Gastrointest Surg. 2020;24:855-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Schorn S, Demir IE, Haller B, Scheufele F, Reyes CM, Tieftrunk E, Sargut M, Goess R, Friess H, Ceyhan GO. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma - A systematic review and meta-analysis. Surg Oncol. 2017;26:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 27. | Wang R, Su D, Liu Y, Qiu J, Cao Z, Yang G, Luo W, Tao J, Zhang T. Cancer-specific survival and metastasis in pancreatic mucinous cystadenocarcinoma: A SEER-based cohort study. Front Oncol. 2022;12:985184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Braun R, Klinkhammer-Schalke M, Zeissig SR, Kleihus van Tol K, Bolm L, Honselmann KC, Petrova E, Lapshyn H, Deichmann S, Abdalla TSA, Heckelmann B, Bronsert P, Zemskov S, Hummel R, Keck T, Wellner UF. Clinical Outcome and Prognostic Factors of Pancreatic Adenosquamous Carcinoma Compared to Ductal Adenocarcinoma-Results from the German Cancer Registry Group. Cancers (Basel). 2022;14:3946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Elzein S, Bao F, Lin R, Schnickel G, Lowy AM, Botta GP. Tri-modal management of primary small cell carcinoma of the pancreas (SCCP): a rare neuroendocrine carcinoma (NEC). BMC Gastroenterol. 2021;21:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Jurcak N, Zheng L. Signaling in the microenvironment of pancreatic cancer: Transmitting along the nerve. Pharmacol Ther. 2019;200:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Shen S, Wang Q, Wang X, Ding J, Chen F, Xiao Y, Qin T, Qian W, Li J, Ma Q, Ma J. Nodal Enhances Perineural Invasion in Pancreatic Cancer by Promoting Tumor-Nerve Convergence. J Healthc Eng. 2022;2022:9658890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Biankin AV, Morey AL, Lee CS, Kench JG, Biankin SA, Hook HC, Head DR, Hugh TB, Sutherland RL, Henshall SM. DPC4/Smad4 expression and outcome in pancreatic ductal adenocarcinoma. J Clin Oncol. 2002;20:4531-4542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Hafizovic L, Causevic A, Deumic A, Becirovic LS, Pokvic LG, Badnjevic A. The Use of Artificial Intelligence in Diagnostic Medical Imaging: Systematic Literature Review. 2021 IEEE 21st International Conference on Bioinformatics and Bioengineering (BIBE); 2021 Oct 25-27; Kragujevac, Serbia. New York: IEEE, 2021: 1-6. |
| 34. | Hrvat F, Spahic L, Pokvic LG, Badnjevic A. Artificial Neural Networks for Prediction of Medical Device Performance based on Conformity Assessment Data: Infusion and perfusor pumps case study. 2020 9th Mediterranean Conference on Embedded Computing (MECO); Jue 8-11; Budva, Montenegro. 2020 New York: IEEE, 2020: 1-4. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/