Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.112981
Revised: August 30, 2025
Accepted: October 11, 2025
Published online: November 15, 2025
Processing time: 94 Days and 16.2 Hours
Gastric cancer is one of the most common malignant tumors of the digestive system globally, with a generally poor prognosis for patients with advanced dis
To develop and validate a novel survival prediction model for assessing the survival risk of advanced HER-2 negative gastric cancer patients receiving immunotherapy combined with chemotherapy, thereby enhancing the accuracy of prognostic evaluation and its clinical guidance value.
This retrospective study included 200 advanced HER-2 negative gastric cancer patients who received programmed cell death protein 1 inhibitors combined with chemotherapy. Independent prognostic factors for progression-free survival (PFS) and overall survival (OS) were identified using multivariable Cox regression analysis, and a nomogram model was constructed based on these factors. The variables included in the regression analysis were selected based on their clinical relevance, routine application in gastric cancer evaluation, and availability within our dataset. The model’s discrimination and calibration were assessed using the concordance index (C-index), the area under the receiver operating characteristic curve (AUC), and calibration plots.
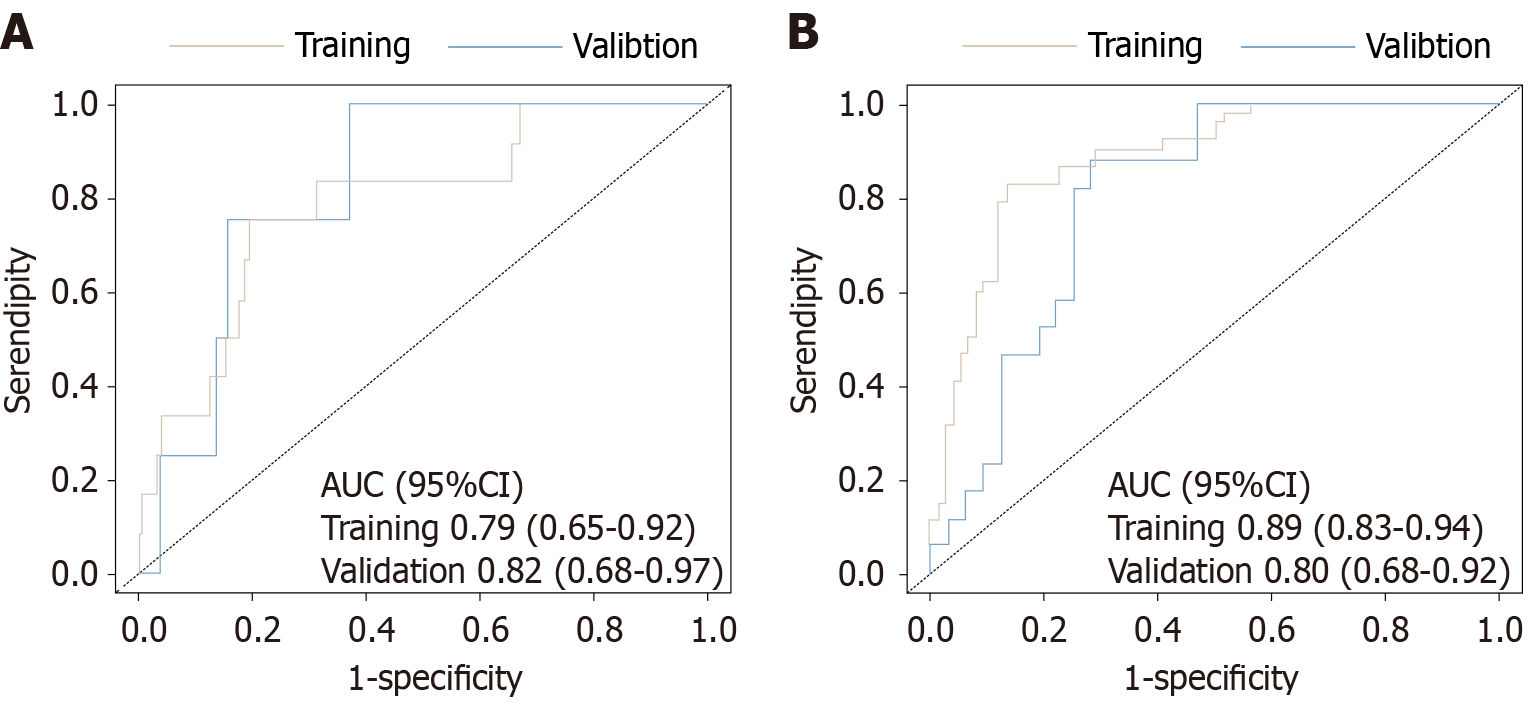
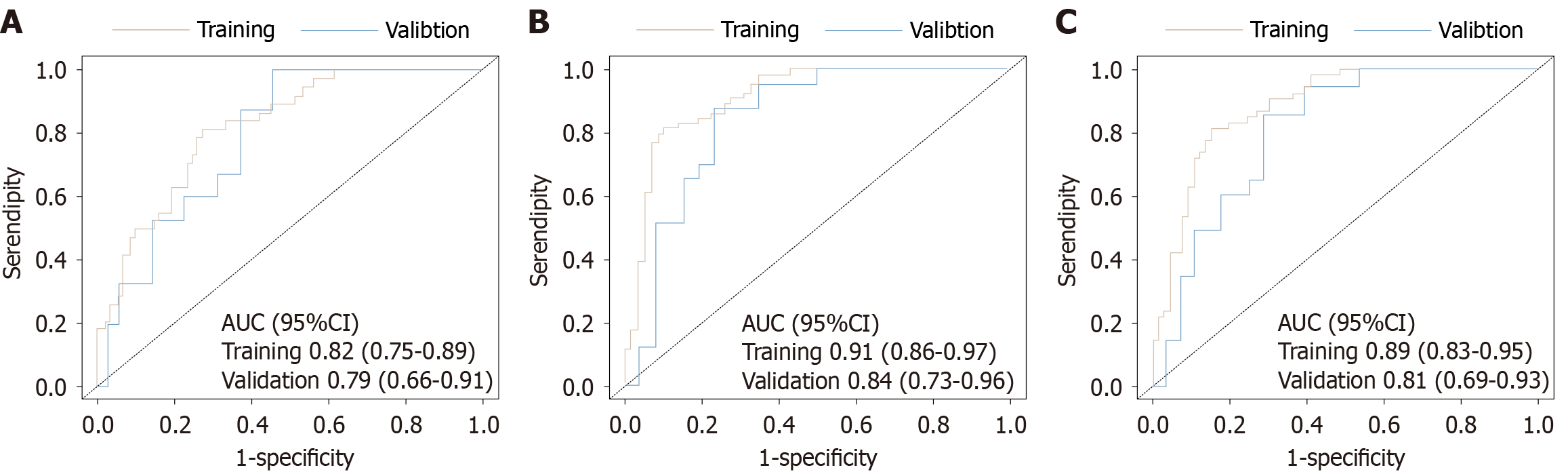
Among the 200 advanced HER-2 negative gastric cancer patients, multivariable Cox regression analysis identified programmed death-ligand 1 expression level, microsatellite status, tumor-node-metastasis stage, tumor differentiation, neutrophil-to-lymphocyte ratio, and C-reactive protein-albumin-lymphocyte index as independent prognostic factors for PFS and OS (all P values < 0.05). Based on these variables, nomogram models for PFS and OS were constructed. In the training set, the C-index for the PFS model was 0.82 [95% confidence interval (CI): 0.77-0.87], and in the internal validation set, it was 0.78 (95%CI: 0.70-0.87), indicating good discrimination ability. For AUC evaluation, the PFS model’s 3-month and 6-month prediction AUCs in the training set were 0.79 (95%CI: 0.65-0.92) and 0.89 (95%CI: 0.83-0.94), respectively. In the validation set, they were 0.82 (95%CI: 0.68-0.97) and 0.80 (95%CI: 0.68-0.92), respectively. For OS prediction, the C-index in the training set and validation set were 0.81 (95%CI: 0.76-0.86) and 0.78 (95%CI: 0.69-0.87), respectively. The nomogram also showed high accuracy in predicting OS at 12, 15, and 18 months. In the training set, the AUCs were 0.82 (95%CI: 0.75-0.89), 0.91 (95%CI: 0.86-0.97), and 0.89 (95%CI: 0.83-0.95), respectively. In the validation set, they were 0.79 (95%CI: 0.66-0.91), 0.84 (95%CI: 0.73-0.96), and 0.81 (95%CI: 0.69-0.93), respectively. Furthermore, calibration curves demonstrated that the predicted probabilities of the model were highly consistent with the actual observed values at different time points, suggesting that the model has good reliability and adaptability for clinical application.
The nomogram model developed in this study effectively predicts the survival outcomes of advanced HER-2 negative gastric cancer patients receiving immunotherapy combined with chemotherapy, demonstrating good discrimination and consistency, and providing robust support for personalized clinical treatment decisions.
Core Tip: This study developed and validated a novel survival prediction model for advanced HER-2 negative gastric cancer patients receiving immunotherapy combined with chemotherapy. A retrospective analysis of 200 patients identified programmed death ligand 1 expression, microsatellite instability, tumor-node-metastasis stage, tumor differentiation, neutrophil-to-lymphocyte ratio, and C-reactive protein-albumin-lymphocyte index as independent prognostic factors. Based on these factors, nomogram models for progression-free survival and overall survival were constructed and validated using the concordance index (C-index) and area under the curve. The results showed good discrimination ability in both the training and validation sets, indicating that the model effectively predicts patient survival outcomes and provides strong support for personalized treatment decisions.
- Citation: Yao ZY, Bao G, Li GC, Hao QL, Ma LJ, Rao YX, Xu K, Ma X, Han ZX. Survival prognosis in advanced HER-2 negative gastric cancer treated with immunochemotherapy: A novel model. World J Gastrointest Oncol 2025; 17(11): 112981
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/112981.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.112981
Gastric cancer is the fifth most common malignancy worldwide and the third leading cause of cancer-related death[1]. The incidence of this disease is particularly high in East Asia, posing a significant threat to public health[2]. As a result of advancements in endoscopic screening, surgical techniques, and adjuvant therapies, the early diagnosis and cure rates of gastric cancer have improved in certain populations[3]. Nevertheless, a substantial proportion of patients are still diagnosed at an advanced stage, including cases with local progression or distant metastasis. While some patients with locally advanced disease may still benefit from curative-intent treatment, the overall prognosis remains poor due to tumor heterogeneity and treatment resistance[4]. For HER-2 negative, unresectable advanced gastric cancer patients, standard first-line treatment primarily relies on platinum-based and fluoropyrimidine-based chemotherapy regimens, with a median overall survival (OS) of less than one year, necessitating more effective therapeutic strategies[5].
In recent years, with the advancement of tumor immunology, immune checkpoint inhibitors (ICIs) have shown new prospects in gastric cancer treatment, especially targeting the programmed cell death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) pathway. This approach has provided some HER-2 negative patients with the possibility of long-term survival. Several phase III clinical trials have demonstrated that PD-1 inhibitors combined with chemotherapy as a first-line treatment can significantly extend progression-free survival (PFS) and OS in advanced gastric cancer patients, and it has gradually been incorporated into international clinical treatment guidelines[6,7]. However, this treatment strategy is not suitable for all patients, as a substantial number of individuals exhibit poor response to immunotherapy, and even rapid disease progression is observed, suggesting significant therapeutic heterogeneity in this population[8].
The individual variability in immune therapy response is a key challenge in the management of gastric cancer. Cur
Despite numerous studies on independent prognostic factors, there remains a lack of an integrated, personalized survival prediction tool that combines clinical, molecular, and inflammatory indicators to assess the survival prognosis of HER-2 negative advanced gastric cancer patients undergoing immunotherapy combined with chemotherapy. Nomo
Although some efforts have been made to predict outcomes in advanced gastric cancer, most existing approaches are limited by narrow applicability or lack of integration with immunotherapy-specific clinical parameters. Therefore, this study aims to construct and validate a multi-factorial nomogram model that integrates clinical and biological parameters, such as PD-L1 expression, MSI status, TNM stage, tumor differentiation, NLR, and CALLY index, to predict the PFS and OS risk in HER-2 negative advanced gastric cancer patients receiving PD-1 inhibitors combined with chemotherapy, improving the accuracy of personalized prognostic assessments and providing decision support for precision therapy.
This study is a single-center retrospective study, including advanced HER-2 negative gastric cancer patients who received treatment at the Affiliated Hospital of Xuzhou Medical University from January 2021 to December 2024. All patients received sintilimab combined with chemotherapy and completed the full follow-up data collection. Inclusion criteria were as follows: (1) Age ≥ 18 years; (2) Pathologically and radiologically confirmed gastric cancer; (3) HER-2 negative (IHC 0/1+ or IHC 2+ but FISH negative); (4) Unresectable or metastatic advanced disease; (5) Received at least two cycles of PD-1 inhibitor combined chemotherapy; and (6) Complete follow-up data, including survival outcomes. Exclusion criteria were: (1) Presence of other primary malignancies; (2) Concurrent radiation therapy or other immunotherapy; (3) Interruption of follow-up or loss to follow-up for more than 6 months during treatment; (4) Presence of severe infections or systemic inflammation; and (5) History of autoimmune disease or long-term use of immunosuppressive agents. A total of 200 patients were included in this retrospective study. This study was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (approval No. XYFY2023-KL277-01). Due to the retrospective nature of this study, the Ethics Committee waived the requirement for informed consent.
Routine clinical information for each patient, including gender, age, body mass index, and Eastern Cooperative Oncology Group performance status, was retrieved from the electronic medical records. Peripheral blood biomarkers were collected one week before the initiation of anti-tumor treatment, including carcinoembryonic antigen, carbohydrate antigen 199, NLR, systemic immune-inflammation index (SII), and CALLY index. Specifically, NLR = absolute neutrophil count/absolute lymphocyte count; SII = platelet count × neutrophil count/Lymphocyte count; CALLY = albumin × lymphocyte count/[C-reactive protein (CRP) × 104]. Tumor characteristics included PD-L1 expression, microsatellite status, Epstein-Barr virus (EBV) status, tumor location, tumor stage, and the presence or absence of liver or peritoneal metastasis.
All patients received first-line treatment with sintilimab combined with chemotherapy. Sintilimab was administered intravenously at a dose of 200 mg every 3 weeks, in combination with a standard double-agent chemotherapy regimen. The standard chemotherapy regimens included the SOX or XELOX protocols. In the SOX regimen (S-1 + oxaliplatin), oxaliplatin 130 mg/m2 was administered intravenously on day 1; S-1 (tegafur) was given based on body surface area (BSA): BSA < 1.25 m2, 80 mg/day; BSA 1.25-1.5 m2, 100 mg/day; BSA ≥ 1.5 m2, 120 mg/day, divided into two doses (mor
The efficacy of all patients was assessed according to the Response Evaluation Criteria in Solid Tumors 1.1. Baseline imaging assessments contrast-enhanced computed tomography or magnetic resonance imaging were performed for all patients before the initiation of treatment. Subsequent imaging assessments were conducted every two treatment cycles (approximately every 6 weeks) to evaluate tumor load changes and determine the treatment response. The evaluation results were classified as: Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). To ensure consistency in imaging assessments, all imaging data were independently reviewed by two radiologists with over 3 years of experience in oncological imaging. In case of discrepancies, a third radiologist independently reviewed the images to reach a consensus. The primary endpoint of this study was OS, defined as the time from the initiation of sintilimab combined with chemotherapy to death or the last follow-up. The secondary endpoint was PFS, defined as the time from the start of treatment until the first imaging progression or clinical confirmation of disease progression. Additionally, the objective response rate (ORR), defined as the proportion of patients achieving CR or PR, and the disease control rate, including the proportion of patients with CR, PR, or SD, were also evaluated. Follow-up was mainly conducted through the electronic medical record system and telephone calls, with the last follow-up completed in December 2024. The median follow-up time for the entire study population was 14 months.
All data analyses were performed using R software (version 4.2.2). All statistical tests were two-sided, and a P value of < 0.05 was considered statistically significant. For continuous variables, the Shapiro-Wilk test was used to confirm non-normal distribution, and the median (interquartile range) was used for representation. Group comparisons were performed using the Mann-Whitney U test. Categorical variables were expressed as frequencies (percentages), and comparisons between groups were conducted using the χ2 test or Fisher’s exact test. Survival analysis was performed using the Kaplan-Meier method to plot PFS and OS curves, with the Log-rank test used to compare survival differences between groups. Univariate and multivariate analyses were performed using the Cox proportional hazards regression model to identify independent prognostic factors for PFS and OS. Variables with P < 0.05 in univariate analysis were included in the multivariate model. Significant variables were used to construct the nomogram model, and Harrell’s C-index was employed to assess the model’s discrimination ability. The area under the receiver operating characteristic curve (AUC) was used to assess prediction accuracy at different time points. Bootstrap resampling (1000 iterations) was used for internal validation, and calibration plots were generated to assess the consistency between predicted and actual observed values. Additionally, a logistic regression model was used to evaluate the independent factors affecting the ORR, with CR and PR as the responder group, and SD and PD as the non-responder group. Results were reported as odds ratios with 95% confidence intervals (CIs).
A total of 200 advanced HER-2 negative gastric cancer patients receiving first-line treatment with sintilimab combined with chemotherapy were included in this study. Of these, 125 were male (62.5%) and 75 were female (37.5%). The ma
| Characteristics | Total (n = 200) | Text (n = 60) | Train (n = 140) | Statistic | P value |
| Sex | χ2 = 0.63 | 0.426 | |||
| Male | 75 (37.50) | 25 (41.67) | 50 (35.71) | ||
| Female | 125 (62.50) | 35 (58.33) | 90 (64.29) | ||
| Age | χ2 =0.02 | 0.899 | |||
| < 60 | 78 (39.00) | 23 (38.33) | 55 (39.29) | ||
| ≥ 60 | 122 (61.00) | 37 (61.67) | 85 (60.71) | ||
| BMI, kg/m2 | χ2 =0.11 | 0.742 | |||
| < 25 | 166 (83.00) | 49 (81.67) | 117 (83.57) | ||
| ≥ 25 | 34 (17.00) | 11 (18.33) | 23 (16.43) | ||
| CEA, ng/mL | χ2 =0.01 | 0.925 | |||
| < 3 | 121 (60.50) | 36 (60.00) | 85 (60.71) | ||
| ≥ 3 | 79 (39.50) | 24 (40.00) | 55 (39.29) | ||
| CA199, U/mL | χ2 =0.60 | 0.440 | |||
| < 37 | 128 (64.00) | 36 (60.00) | 92 (65.71) | ||
| ≥ 37 | 72 (36.00) | 24 (40.00) | 48 (34.29) | ||
| ECOG PS | χ2 =0.20 | 0.653 | |||
| 0 | 72 (36.00) | 23 (39.33) | 49 (35.00) | ||
| 1 | 128 (64.00) | 37 (61.67) | 91 (65.00) | ||
| Staging | χ2 =0.28 | 0.598 | |||
| 3 | 89 (44.50) | 25 (41.67) | 64 (45.71) | ||
| 4 | 111 (55.50) | 35 (58.33) | 76 (54.29) | ||
| Site | χ2 =0.23 | 0.634 | |||
| Stomach | 142 (71.00) | 98 (70.00) | 44 (73.33) | ||
| Gastric and esophageal binding | 58 (29.00) | 72 (30.00) | 16 (26.67) | ||
| Differentiation | χ2 =1.06 | 0.304 | |||
| Low | 180 (90.00) | 56 (93.33) | 124 (88.57) | ||
| Medium-high differentiation | 20 (10.00) | 4 (6.67) | 16 (11.43) | ||
| Peritoneal metastasis | χ2 =0.81 | 0.367 | |||
| No | 113 (56.50) | 31 (51.67) | 82 (58.57) | ||
| Yes | 87 (43.50) | 29 (48.33) | 58 (41.43) | ||
| Liver metastasis | χ2 =2.15 | 0.143 | |||
| No | 157 (78.50) | 51 (85.00) | 106 (75.71) | ||
| Yes | 43 (21.50) | 9 (15.00) | 34 (24.29) | ||
| EBV status | χ2 =0.06 | 0.802 | |||
| No-infect | 187 (93.50) | 57 (95.00) | 130 (92.86) | ||
| Infect | 13 (6.50) | 3 (5.00) | 10 (7.14) | ||
| PD-L1 expression | χ2 =0.65 | 0.419 | |||
| CPS < 5 | 88 (44.00) | 29 (48.33) | 59 (42.14) | ||
| CPS ≥ 5 | 112 (56.00) | 31 (51.67) | 81 (57.86) | ||
| MMR status | χ2 =0.31 | 0.579 | |||
| pMMR | 169 (84.50) | 52 (86.67) | 117 (83.57) | ||
| dMMR | 31 (15.50) | 8 (13.33) | 23 (16.43) | ||
| CALLY index, median (Q1, Q3) | 1.13 (0.18, 4.99) | 1.25 (0.17, 3.15) | 1.03 (0.18, 5.19) | Z = -0.42 | 0.674 |
| NLR, median (Q1, Q3) | 2.58 (1.81, 4.17) | 2.61 (1.88, 4.04) | 2.57 (1.72, 4.30) | Z = -0.30 | 0.762 |
| SII, median (Q1, Q3) | 627.11 (339.65, 939.08) | 661.80 (361.13, 883.91) | 613.29 (325.57, 998.95) | Z = -0.53 | 0.598 |
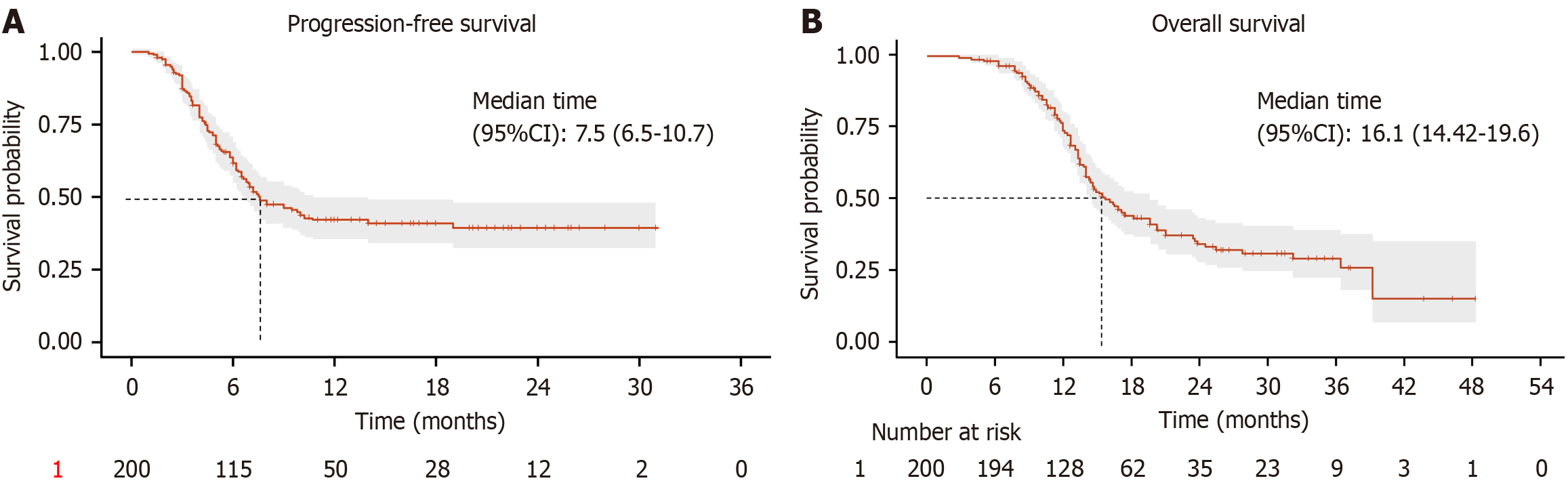
Among the 200 advanced HER-2 negative gastric cancer patients receiving sintilimab combined with chemotherapy, 1 patient (0.5%) achieved CR, 32 patients (16.0%) achieved PR, 59 patients (29.5%) had SD, and 108 patients (54.0%) had PD. The overall ORR was 16.5%, and the disease control rate was 46.0% (Table 2). Survival analysis showed that the median PFS was 7.5 months (95%CI: 6.5-10.7) (Figure 1A), and the median OS was 16.1 months (95%CI: 14.42-19.6) (Figure 1B).
| Parameter | ||
| Over best response | CR | 1 (0.5) |
| PR | 32 (16.0) | |
| SD | 59 (29.5) | |
| PD | 108 (54.0) | |
| ORR | CR + PR | 33 (16.5) |
| DCR | CR + PR + SD | 92 (46.0) |
To investigate the impact of different clinical variables on PFS, Cox proportional hazards regression analysis was performed on the relevant factors (Table 3). In the univariate Cox regression analysis, PD-L1 expression level, micro
| Factors | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (female vs male) | 1.08 (0.67-1.74) | 0.757 | ||
| Age (< 60 vs ≥ 60), years | 0.89 (0.56-1.42) | 0.629 | ||
| BMI (≥ 25 vs < 25), kg/m2 | 1.28 (0.73-2.26) | 0.386 | ||
| CEA (< 3 vs ≥ 3), ng/mL | 1.45 (0.92-2.29) | 0.107 | ||
| CA199 (< 37 vs ≥ 37), U/mL | 1.29 (0.81-2.06) | 0.278 | ||
| ECOG (0 vs 1) | 1.62 (0.97-2.68) | 0.063 | ||
| Staging (3 vs 4) | 4.68 (2.71-8.11) | < 0.001 | 4.61 (2.53-8.41) | < 0.001 |
| Differentiation (low vs medium-high) | 0.14 (0.03-0.56) | 0.006 | 0.20 (0.05-0.83) | 0.027 |
| Site (stomach vs gastric and esophageal binding) | 1.36 (0.81-2.28) | 0.252 | ||
| Liver metastasis (no vs yes) | 1.61 (0.98-2.66) | 0.060 | ||
| Peritoneal metastasis (no vs yes) | 1.51 (0.96-2.37) | 0.076 | ||
| PD-L1 expression (CPS < 5 vs CPS ≥ 5) | 0.17 (0.10-0.29) | < 0.001 | 0.37 (0.22-0.64) | < 0.001 |
| MMR status (dMMR vs pMMR) | 0.12 (0.04-0.37) | < 0.001 | 0.28 (0.08-0.94) | 0.040 |
| EBV status (infect vs no-infect) | 0.57 (0.21-1.56) | 0.274 | ||
| CALLY index | 0.88 (0.82-0.94) | < 0.01 | 0.91 (0.85-0.99) | 0.020 |
| NLR | 1.14 (1.08-1.22) | < 0.001 | 1.13 (1.05-1.22) | < 0.001 |
| SII | 1.07 (1.00-1.14) | 0.052 | ||
Similarly, univariate Cox regression analysis for OS showed that PD-L1 expression level, microsatellite status, tumor stage, tumor differentiation, NLR, and CALLY index were all significantly associated with PFS (P < 0.05). Multivariate Cox regression analysis revealed that PD-L1 expression (HR = 0.45, 95%CI: 0.26-0.76), microsatellite status (HR = 0.20, 95%CI: 0.06-0.73), tumor stage (HR = 3.73, 95%CI: 2.05-6.81), tumor differentiation (HR = 0.18, 95%CI: 0.04-0.74), NLR (HR = 1.16, 95%CI: 1.08-1.25), and CALLY index (HR = 0.88, 95%CI: 0.81-0.95) were independent prognostic factors for OS (Table 4).
| Factors | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (female vs male) | 1.10 (0.68-1.77) | 0.704 | ||
| Age (< 60 vs ≥ 60), years | 0.90 (0.57-1.43) | 0.663 | ||
| BMI (≥ 25 vs < 25), kg/m2 | 1.17 (0.66-2.06) | 0.586 | ||
| CEA (< 3 vs ≥ 3), ng/mL | 1.39 (0.88-2.20) | 0.152 | ||
| CA199 (< 37 vs ≥ 37), U/mL | 1.43 (0.89-2.27) | 0.136 | ||
| ECOG PS (0 vs 1) | 1.63 (0.98-2.71) | 0.059 | ||
| Staging (3 vs 4) | 4.12 (2.38-7.13) | < 0.001 | 3.73 (2.05-6.81) | < 0.001 |
| Differentiation (low vs medium-high) | 0.13 (0.03-0.54) | 0.005 | 0.18 (0.04-0.74) | 0.018 |
| Site (stomach vs gastric and esophageal binding) | 1.30 (0.77-2.18) | 0.326 | ||
| Liver metastasis (no vs yes) | 1.58 (0.96-2.61) | 0.072 | ||
| Peritoneal metastasis (no vs yes) | 1.41 (0.90-2.22) | 0.136 | ||
| PD-L1 expression (CPS < 5 vs CPS ≥ 5) | 0.18 (0.11-0.31) | < 0.001 | 0.45 (0.26-0.76) | 0.003 |
| MMR status (dMMR vs pMMR) | 0.10 (0.03-0.32) | < 0.001 | 0.20 (0.06-0.73) | 0.015 |
| EBV status (infect vs no-infect) | 0.57 (0.21-1.57) | 0.279 | ||
| CALLY index | 0.86 (0.79-0.92) | < 0.001 | 0.88 (0.81-0.95) | 0.002 |
| NLR | 1.18 (1.11-1.26) | < 0.001 | 1.16 (1.08-1.25) | < 0.001 |
| SII | 1.04 (0.97-1.11) | 0.275 | ||
To investigate the impact of different clinical variables on the ORR, logistic regression analysis was performed on the relevant factors. Univariate logistic regression analysis showed that PD-L1 expression level, microsatellite status, tumor stage, tumor differentiation, peritoneal metastasis, EBV infection, SII, and CALLY index were all significantly associated with PFS (P < 0.05). Multivariate logistic regression analysis revealed that PD-L1 expression (HR = 0.13, 95%CI: 0.02-0.66), MSI-H status (HR = 0.04, 95%CI: 0.04-0.61), tumor differentiation (HR = 0.11, 95%CI: 0.03-0.40), SII (HR = 1.33, 95%CI: 1.05-1.69), and CALLY index (HR = 0.90, 95%CI: 0.83-0.97) were independent prognostic factors for ORR (Table 5).
| Factors | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex (female vs male) | 0.62 (0.24-1.55) | 0.302 | ||
| Age (< 60 vs ≥ 60), years | 0.74 (0.30-1.85) | 0.520 | ||
| BMI (≥ 25 vs < 25), kg/m2 | 0.77 (0.21-2.87) | 0.701 | ||
| CEA (< 3 vs ≥ 3), ng/mL | 1.68 (0.67-4.20) | 0.266 | ||
| CA199 (< 37 vs ≥ 37), U/mL | 1.31 (0.55-3.11) | 0.543 | ||
| ECOG PS (0 vs 1) | 1.42 (0.54-3.74) | 0.480 | ||
| Staging (3 vs 4) | 2.49 (1.15-5.40) | 0.021 | 0.61 (0.15-2.45) | 0.483 |
| Differentiation (low vs medium-high) | 0.08 (0.03-0.22) | < 0.001 | 0.11 (0.03-0.40) | < 0.001 |
| Site (stomach vs gastric and esophageal binding) | 1.14 (0.50-2.58) | 0.760 | ||
| Liver metastasis (no vs yes) | 1.54 (0.59-3.98) | 0.376 | ||
| Peritoneal metastasis (no vs yes) | 2.66 (1.13-6.26) | 0.025 | 2.13 (0.54-8.44) | 0.284 |
| PD-L1 expression (CPS < 5 vs CPS ≥ 5) | 0.07 (0.02-0.28) | < 0.001 | 0.13 (0.02-0.66) | 0.014 |
| MMR status (dMMR vs pMMR) | 0.11 (0.04-0.31) | < 0.001 | 0.15 (0.04-0.61) | 0.008 |
| EBV status (infect vs no-infect) | 0.27 (0.08-0.89) | 0.031 | 0.32 (0.05-2.14) | 0.242 |
| CALLY index | 0.86 (0.79-0.92) | < 0.001 | 0.88 (0.81-0.95) | 0.002 |
| NLR | 1.16 (0.95-1.41) | 0.144 | ||
| SII | 1.28 (1.10-1.48) | 0.001 | 1.33 (1.05-1.69) | 0.017 |
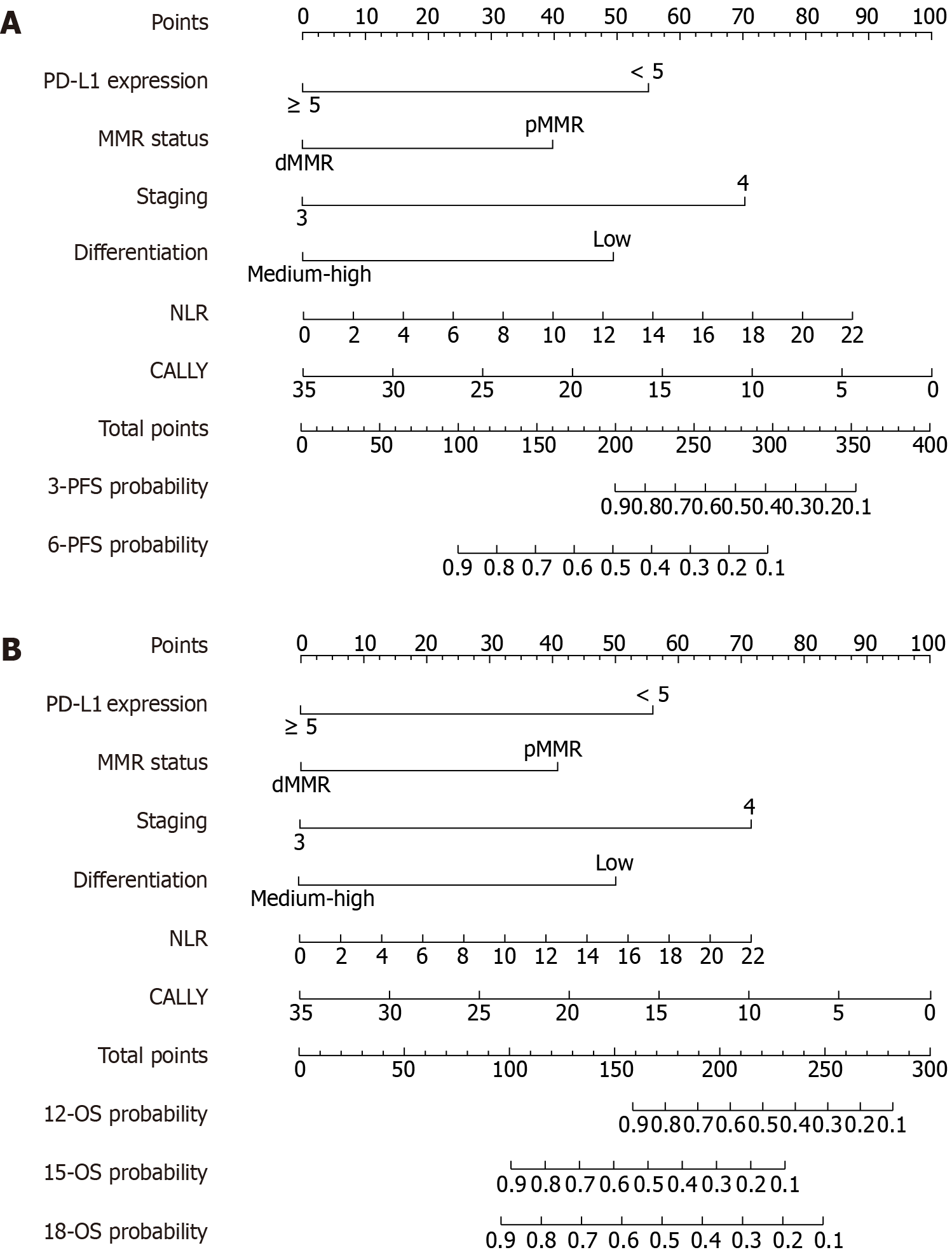
In the multivariate Cox regression analysis for PFS, PD-L1 expression level, microsatellite status, tumor stage, tumor differentiation, NLR, and CALLY index were identified as independent prognostic factors for PFS. Based on these six va
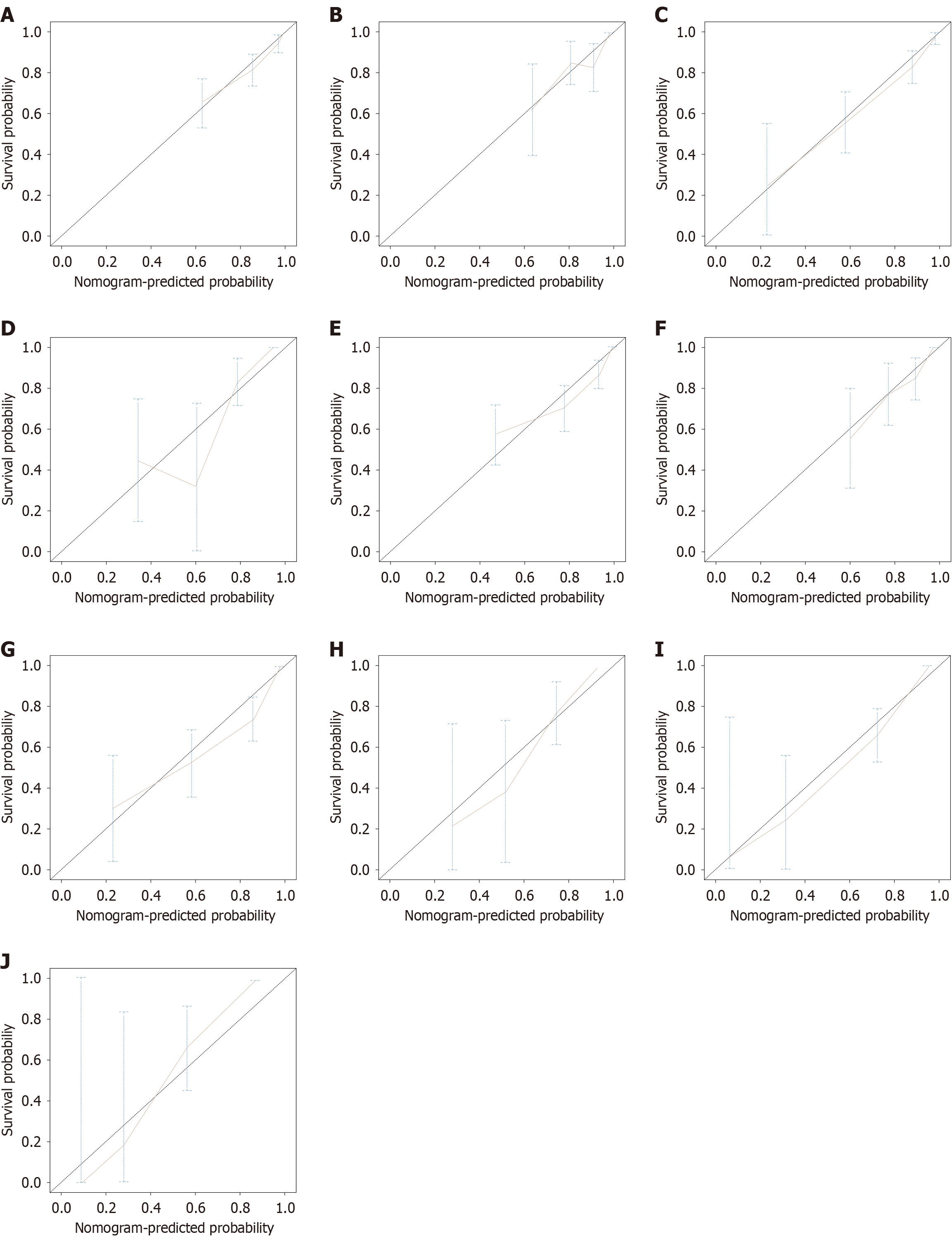
The study population was randomly divided into a training set (n = 140) and an internal validation set (n = 60) at a 7:3 ratio. The randomization was performed using computer-generated random numbers to avoid manual allocation bias. No stratification was applied, but the baseline characteristics were compared between the two cohorts to ensure balance and representativeness. The performance of the nomogram models for predicting PFS and OS was evaluated, including a comprehensive analysis of discrimination and calibration. For the PFS prediction model, the C-index in the training set was 0.82 (95%CI: 0.77-0.87), and in the validation set it was 0.78 (95%CI: 0.70-0.87), showing good model discrimination. Receiver operating characteristic curve analysis further validated the predictive accuracy of the model: In the training set, the AUC for 3-month and 6-month PFS were 0.79 (95%CI: 0.65-0.92) and 0.89 (95%CI: 0.83-0.94), respectively; in the validation set, the AUCs were 0.82 (95%CI: 0.68-0.97) and 0.80 (95%CI: 0.68-0.92), indicating good stability and reliability in the short- and medium-term PFS prediction (Figure 3). The AUC values reflect the model’s ability to distinguish patients with different survival risks; higher AUCs indicate stronger discriminative performance at each time point. For the OS prediction model, the C-index in the training set was 0.81 (95%CI: 0.76-0.86), and in the validation set it was 0.78 (95%CI: 0.69-0.87), also showing good discrimination. AUC analysis showed that in the training set, the AUCs for 12-month, 15-month, and 18-month OS predictions were 0.82 (95%CI: 0.75-0.89), 0.91 (95%CI: 0.86-0.97), and 0.89 (95%CI: 0.83-0.95), respectively; in the validation set, they were 0.79 (95%CI: 0.66-0.91), 0.84 (95%CI: 0.73-0.96), and 0.81 (95%CI: 0.69-0.93), respectively (Figure 4). These results indicate that the OS model exhibits good predictive performance for the medium and long term and has high stability and potential for generalization in both the training and validation populations. Furthermore, calibration plots showed that the predicted survival probabilities for both the PFS and OS models were highly consistent with the actual observed values at different time points, indicating good calibration (Figure 5).
This study constructed and validated a multi-parameter nomogram model based on real-world data from 200 advanced HER-2 negative gastric cancer patients receiving first-line treatment with sintilimab combined with chemotherapy. We identified PD-L1 expression, microsatellite status, TNM stage, tumor differentiation, NLR, and the CALLY index as independent prognostic factors for PFS and OS. The prediction models based on these variables demonstrated good discrimination and calibration during internal validation, providing strong clinical utility.
In recent years, the use of ICIs in advanced gastric cancer has become a research hotspot. Phase III studies such as CheckMate-649 and ATTRACTION-4 have confirmed the efficacy of PD-1 inhibitors combined with chemotherapy as a first-line treatment, particularly in patients with PD-L1 positive tumors, who benefit more significantly from this approach[6,15,16]. However, immune therapy in gastric cancer demonstrates significant individual variability, with some patients showing limited short-term benefits and even immune-related progression[17]. Therefore, identifying potential immune therapy benefit populations has become a critical issue in clinical practice.
A notable innovation in our study was the integration of immune molecular markers (such as PD-L1 and MSI status), traditional clinicopathological features (such as TNM stage and tumor differentiation), and inflammation-nutrition indices (such as NLR and CALLY) into the model. This integration not only enhanced the predictive performance of the model but also better reflected the biological heterogeneity of gastric cancer patients. PD-L1 expression and MSI status have been widely recognized as key predictive factors for immunotherapy, with high PD-L1 expression enhancing the response rate to immunotherapy[18,19], and MSI-H status typically associated with high mutation burden, promoting neoantigen generation and immune system recognition[20].
TNM stage and tumor differentiation, as traditional prognostic factors, retain their independent predictive value in this study, emphasizing that tumor burden and biological behavior remain fundamental factors influencing the efficacy of ICIs. The inclusion of NLR and CALLY index highlights the regulatory role of systemic inflammation and host nutritional status on the response to immunotherapy. NLR reflects the ratio of neutrophils to lymphocytes in peripheral blood, with high NLR values typically indicating enhanced pro-inflammatory states and immune suppression[21]. On one hand, neutrophils can promote tumor growth and immune evasion by secreting pro-inflammatory cytokines such as inter
The CALLY index is a composite score that reflects both systemic inflammation and nutritional status, consisting of albumin, lymphocyte count, and CRP[27]. Low albumin levels suggest malnutrition or liver dysfunction, while high CRP indicates an enhanced systemic inflammatory response; lymphocyte count is closely associated with anti-tumor immunity. Thus, a low CALLY index often indicates the combined presence of immune suppression, malnutrition, and systemic inflammation, suggesting limited benefits from immunotherapy. Recent studies have shown that CRP can activate the signal transducer and activator of transcription 3 signaling pathway, promoting regulatory T cell expansion and the release of suppressive cytokines, which indirectly inhibits effector T-cell function[28,29]. Furthermore, low albumin levels not only reflect insufficient nutritional support but also likely lead to dysfunction in various immune cells, such as downregulation of major histocompatibility complex expression on antigen-presenting cells and impaired dendritic cell migration[30,31]. Additionally, lymphocyte reduction is closely associated with impaired tumor immune clearance. These mechanisms work together in the tumor immune microenvironment, leading to an immune-suppressive state, thus reducing the response rate and therapeutic durability of immunotherapy. Our study shows that the CALLY index is an independent predictor of PFS, OS, and ORR, consistent with findings from recent studies on gastric cancer and non-small cell lung cancer treated with ICIs[27,32], supporting its use as a repeatable, simple, and clinically accessible biomarker for risk stratification.
Compared to previous studies, our research has several innovative aspects. First, while most existing studies focus on single factors or individual immune markers in predicting the outcomes of immunotherapy, our study integrates clinical, molecular, and inflammation-nutrition parameters to establish a multivariate survival model, which demonstrates greater practicality and interpretability. Second, the model was internally validated, showing good C-index values (PFS model: Training set 0.82, validation set 0.78; OS model: Training set 0.81, validation set 0.78) and high AUC values, indicating its stable predictive ability at multiple time points. Additionally, logistic regression for ORR further revealed the impact of PD-L1, MSI, SII, and other markers on the ORR, providing reference for short-term efficacy prediction.
It is important to emphasize that the model has broad potential for clinical application. By quantifying scores, the model allows for the rapid assessment of individual patient risks, aiding clinical decision-making. For example, in patients with high tumor burden but good inflammatory status, an aggressive immunotherapy combination strategy could be considered; whereas in high-risk populations with poor differentiation, high NLR, and low CALLY index, the treatment expectations and toxicity risks should be carefully weighed. Furthermore, the model could also serve as a dynamic monitoring tool during patient follow-up, reassessing risk based on trends in blood biomarkers.
Of course, there are certain limitations to this study. First, being a single-center retrospective study, it is limited by study design and sample sources, which may introduce selection and information biases, and the relatively small sample size could affect the generalizability of the model. Second, we only included patients who received sintilimab combined with chemotherapy, excluding those who received other PD-1 inhibitors (e.g., nivolumab, pembrolizumab) or combined with other targeted or local therapies, which limits the applicability of the model to broader ICI strategies. Another limitation is that we grouped unresectable locally advanced and metastatic gastric cancer patients together under the umbrella of “advanced gastric cancer”. While both subtypes share similar treatment strategies and lack indications for curative surgery, they may differ in disease biology and prognosis. Due to sample size limitations, we were unable to perform a subgroup analysis to assess whether the prognostic model applies equally to both subgroups. This aspect warrants further validation in future studies with larger, stratified cohorts. Furthermore, some molecular markers, such as PD-L1 and MSI status, have inconsistent detection platforms, scoring methods, and interpretation standards, which may affect data stability and cross-center reproducibility. Additionally, our model did not include newer immune microenvironment-related biomarkers such as tumor-infiltrating lymphocytes, immune phenotypes, or T-cell exhaustion markers (e.g., T cell immunoglobulin and mucin-domain-containing-3, lymphocyte activation gene-3), which have shown important value in predicting immune therapy efficacy. These could be integrated into the predictive tool in future studies using techniques like tissue immunohistochemistry, multi-omics, or spatial transcriptomics. Finally, this study was only internally validated, lacking external independent cohorts for model validation and calibration. Future research should further test the model’s stability and clinical applicability in multi-center, prospective, large-sample studies.
In conclusion, the nomogram model developed in this study, based on PD-L1, MSI status, TNM stage, tumor differentiation, NLR, and CALLY index, effectively predicts the PFS and OS of advanced HER-2 negative gastric cancer patients receiving sintilimab combined with chemotherapy. The model demonstrates good predictive performance and clinical utility. It is expected to provide a quantitative support tool for precision immunotherapy, facilitating the individualization and standardization of treatment. Further validation and optimization in multi-center, prospective studies are needed in the future.
This study, based on real-world data, constructed and validated a nomogram prediction model for evaluating the survival outcomes of HER-2 negative advanced gastric cancer patients receiving sintilimab combined with chemotherapy. The model integrates multiple parameters, including immune, biological, and inflammation-nutrition factors, and demonstrates good predictive performance and clinical applicability. The CALLY index and NLR, as important indicators reflecting systemic immune status, play a crucial role in the model, further enhancing its potential value in the individualized management of immunotherapy. This tool is expected to provide valuable reference for clinical precision decision-making, and further validation in multi-center prospective cohorts is still needed in the future.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68587] [Article Influence: 13717.4] [Reference Citation Analysis (201)] |
| 2. | Yan C, Shan F, Ying X, Li Z. Global burden prediction of gastric cancer during demographic transition from 2020 to 2040. Chin Med J (Engl). 2023;136:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3303] [Article Influence: 550.5] [Reference Citation Analysis (6)] |
| 4. | Gong Y, Wang P, Zhu Z, Zhang J, Huang J, Xu H. Clinicopathological Characteristics and Prognosis of Upper Gastric Cancer Patients in China: A 32-Year Single-Center Retrospective Clinical Study. Gastroenterol Res Pract. 2019;2019:9248394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Qin S, Bai Y, Li J, Pan H, Luo S, Qu Y, Ye F, Yang L, Liu T, Li W, Chen X, Yang J, Ying J, Lin X, Zhao L, Liang X, Mao Y, Guo R, Zuo Y, Bordia S, Li S. First-Line Pembrolizumab Plus Chemotherapy for HER2-Negative Advanced Gastric Cancer: China Subgroup Analysis of the Randomized Phase 3 KEYNOTE-859 Study. Adv Ther. 2025;42:1892-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 2224] [Article Influence: 444.8] [Reference Citation Analysis (1)] |
| 7. | Janjigian YY, Ajani JA, Moehler M, Shen L, Garrido M, Gallardo C, Wyrwicz L, Yamaguchi K, Cleary JM, Elimova E, Karamouzis M, Bruges R, Skoczylas T, Bragagnoli A, Liu T, Tehfe M, Zander T, Kowalyszyn R, Pazo-Cid R, Schenker M, Feeny K, Wang R, Lei M, Chen C, Nathani R, Shitara K. First-Line Nivolumab Plus Chemotherapy for Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: 3-Year Follow-Up of the Phase III CheckMate 649 Trial. J Clin Oncol. 2024;42:2012-2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 152] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 8. | Wang MX, Gao SY, Yang F, Fan RJ, Yang QN, Zhang TL, Qian NS, Dai GH. Hyperprogression under treatment with immune-checkpoint inhibitors in patients with gastrointestinal cancer: A natural process of advanced tumor progression? World J Clin Oncol. 2022;13:729-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Hou W, Zhao Y, Zhu H. Predictive Biomarkers for Immunotherapy in Gastric Cancer: Current Status and Emerging Prospects. Int J Mol Sci. 2023;24:15321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Pietrantonio F, Randon G, Di Bartolomeo M, Luciani A, Chao J, Smyth EC, Petrelli F. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open. 2021;6:100036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 11. | Zhang AZ, Yuan X, Liang WH, Zhang HJ, Li Y, Xie YF, Li JF, Jiang CH, Li FP, Shen XH, Pang LJ, Zou H, Zhou WH, Li F, Hu JM. Immune Infiltration in Gastric Cancer Microenvironment and Its Clinical Significance. Front Cell Dev Biol. 2021;9:762029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Mashukov A, Shapochka D, Seleznov O, Kobyliak N, Falalyeyeva T, Kirkilevsky S, Yarema R, Sulaieva O. Histological differentiation impacts the tumor immune microenvironment in gastric carcinoma: Relation to the immune cycle. World J Gastroenterol. 2021;27:5259-5271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Zhang S, Qiu C, Yu H, Xu Y, Xu X. Prognostic value of neutrophil to lymphocyte ratio in gastric cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol. 2023;13:1070019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 14. | Aoyama T, Maezawa Y, Hashimoto I, Hara K, Tamagawa A, Kazama K, Kato A, Cho H, Nakazono M, Numata M, Kawahara S, Tanabe M, Morita J, Oshima T, Saito A, Yukawa N, Rino Y. The CRP-albumin-lymphocyte (CALLY) Index Is an Independent Prognostic Factor for Gastric Cancer Patients who Receive Curative Treatment. Anticancer Res. 2024;44:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 15. | Boku N, Omori T, Shitara K, Sakuramoto S, Yamaguchi K, Kato K, Kadowaki S, Tsuji K, Ryu MH, Oh DY, Oh SC, Rha SY, Lee KW, Chung IJ, Sym SJ, Chen LT, Chen JS, Bai LY, Nakada T, Hagihara S, Makino R, Nishiyama E, Kang YK. Nivolumab plus chemotherapy in patients with HER2-negative, previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: 3-year follow-up of the ATTRACTION-4 randomized, double-blind, placebo-controlled, phase 3 trial. Gastric Cancer. 2024;27:1287-1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 16. | Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 591] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 17. | Yasuda T, Wang YA. Gastric cancer immunosuppressive microenvironment heterogeneity: implications for therapy development. Trends Cancer. 2024;10:627-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 123] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 18. | Li JB, Lai MY, Lin ZC, Guan WL, Sun YT, Yang J, Wang WX, Yang ZR, Qiu MZ. The optimal threshold of PD-L1 combined positive score to predict the benefit of PD-1 antibody plus chemotherapy for patients with HER2-negative gastric adenocarcinoma: a meta-analysis. Cancer Immunol Immunother. 2024;73:132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 19. | Yang J, Luo W, Ma X, Cui Y, Xie J, Pan C, Chen Z, Yang S. Meta-Analysis of the Efficacy and Safety of Pembrolizumab in the Treatment of Advanced Gastric Cancer and Gastroesophageal Junction Cancer. Chemotherapy. 2025;70:37-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Ooki A, Osumi H, Yoshino K, Yamaguchi K. Potent therapeutic strategy in gastric cancer with microsatellite instability-high and/or deficient mismatch repair. Gastric Cancer. 2024;27:907-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Zhang W, Tan Y, Li Y, Liu J. Neutrophil to Lymphocyte ratio as a predictor for immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. 2023;14:1234142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 22. | Tobin RP, Jordan KR, Kapoor P, Spongberg E, Davis D, Vorwald VM, Couts KL, Gao D, Smith DE, Borgers JSW, Robinson S, Amato C, Gonzalez R, Lewis KD, Robinson WA, Borges VF, McCarter MD. IL-6 and IL-8 Are Linked With Myeloid-Derived Suppressor Cell Accumulation and Correlate With Poor Clinical Outcomes in Melanoma Patients. Front Oncol. 2019;9:1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 23. | Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, Walsh AM, Baxi V, Pandya D, Baradet T, Locke D, Wu Q, Reilly TP, Phillips P, Nagineni V, Gianino N, Gu J, Zhao H, Perez-Gracia JL, Sanmamed MF, Melero I. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 419] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 24. | Tan S, Zheng Q, Zhang W, Zhou M, Xia C, Feng W. Prognostic value of inflammatory markers NLR, PLR, and LMR in gastric cancer patients treated with immune checkpoint inhibitors: a meta-analysis and systematic review. Front Immunol. 2024;15:1408700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 25. | Knetki-Wróblewska M, Grzywna A, Krawczyk P, Wojas-Krawczyk K, Chmielewska I, Jankowski T, Milanowski J, Krzakowski M. Prognostic significance of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) in second-line immunotherapy for patients with non-small cell lung cancer. Transl Lung Cancer Res. 2025;14:749-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 26. | Gou M, Qu T, Wang Z, Yan H, Si Y, Zhang Y, Dai G. Neutrophil-to-Lymphocyte Ratio (NLR) Predicts PD-1 Inhibitor Survival in Patients with Metastatic Gastric Cancer. J Immunol Res. 2021;2021:2549295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Li J, Zhang S, Hu X, Huang T, Chen M. Correlation between the C-reactive protein (CRP)-albumin-lymphocyte (CALLY) index and the prognosis of gastric cancer patients after gastrectomy: a systematic review and meta-analysis. Surg Today. 2025;55:483-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Huang L, Zhao Y, Shan M, Wang S, Chen J, Liu Z, Xu Q. Targeting crosstalk of STAT3 between tumor-associated M2 macrophages and Tregs in colorectal cancer. Cancer Biol Ther. 2023;24:2226418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 29. | Bakrim S, Fessikh ME, Elhrech H, Omari NE, Amanullah M, Ming LC, Moshawih S, Bouyahya A. Targeting inflammation in cancer therapy: from mechanistic insights to emerging therapeutic approaches. J Transl Med. 2025;23:588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 30. | Worbs T, Hammerschmidt SI, Förster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17:30-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 638] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 31. | Ulfig A, Bader V, Varatnitskaya M, Lupilov N, Winklhofer KF, Leichert LI. Hypochlorous acid-modified human serum albumin suppresses MHC class II - dependent antigen presentation in pro-inflammatory macrophages. Redox Biol. 2021;43:101981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Liu XY, Zhang X, Zhang Q, Ruan GT, Liu T, Xie HL, Ge YZ, Song MM, Deng L, Shi HP. The value of CRP-albumin-lymphocyte index (CALLY index) as a prognostic biomarker in patients with non-small cell lung cancer. Support Care Cancer. 2023;31:533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/