Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.112089
Revised: August 25, 2025
Accepted: October 22, 2025
Published online: November 15, 2025
Processing time: 107 Days and 17.3 Hours
Primary gastrointestinal lymphoma (PGIL) is a relatively uncommon clinical entity, exhibiting distinctive features including occult primary sites, nonspecific clinical presentations, and considerable diagnostic and therapeutic difficulties. Consequently, comprehensive clinical investigations into its clinicopathological characteristics and surgical intervention value are warranted to enhance dia
To investigate the clinicopathological characteristics and surgical significance of PGIL from a surgical perspective, providing a theoretical basis for optimizing diagnostic and therapeutic strategies.
This study included 50 cases of PGIL treated by the General Surgery Department of the Chinese PLA Air Force Medical Center from June 2001 to March 2025. Data were extracted from the Electronic Medical Record system for retrospective analysis. A retrospective analysis was conducted on their epidemiological, clinical manifestations, imaging, pathological features, and treatment outcomes. Descriptive statistics were applied for data summarization, with continuous variables presented as frequencies and percentages. Correlations between variables were assessed using the Spearman rank correlation coefficient.
All cases had the gastrointestinal tract as the primary site. Abdominal pain was the most common initial symptom (52.0%), with 80.0% of patients experiencing pain during the course of the disease, and 38.0% experiencing hema
Diffuse large B-cell lymphoma is the primary PGIL subtype. Imaging and endoscopic biopsy are diagnostic es
Core Tip: Primary gastrointestinal lymphoma predominantly manifests as diffuse large B-cell lymphoma, with abdominal pain as the leading symptom. Comprehensive diagnosis relies on imaging (computed tomography sensitivity: 94.3%) and endoscopic biopsy (detection rate: 91.5%). Surgery is pivotal for definitive diagnosis (via complete specimen acquisition), emergency management (e.g., obstruction/perforation), and primary lesion resection, particularly for tumors > 5 cm (26.5% complication rate). While combined surgery and chemotherapy showed higher improvement rates than chemotherapy alone, statistical significance was not reached. Integration of surgical strategies into multimodal therapy may optimize outcomes, emphasizing individualized approaches based on tumor size, stage, and complications.
- Citation: Yang CX, Xu LX, Liu J, Qiao HL, Dong ZW, Jiang D, Gu GL. Clinicopathological characteristics and surgical value of primary gastrointestinal lymphoma. World J Gastrointest Oncol 2025; 17(11): 112089
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/112089.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.112089
Lymphoma is a malignant tumor originating from the lymphohematopoietic system, posing a substantial global health burden. Among its subtypes, primary gastrointestinal lymphoma (PGIL) refers to lymphoma arising from the gastrointestinal tract and underlying mucosa-associated lymphoid tissue (MALT). In recent years, the incidence of PGIL has been rising. As one of the most common types of extranodal lymphomas, PGIL accounts for 30%-40% of all extranodal lymphomas and 1%-4% of all gastrointestinal malignancies. The stomach is the most frequently involved site, followed by the small intestine and colorectum[1]. The etiology and pathogenesis of PGIL remain incompletely understood. Current evidence suggests that multiple factors may contribute to its development, including radiation exposure, autoimmune diseases, microenvironmental dysregulation, and adverse lifestyle factors[2]. Furthermore, PGIL is relatively uncommon in clinical practice. Definitive diagnosis and subtyping often require surgical biopsy to obtain sufficient tissue samples, highlighting the critical role of surgical intervention. Moreover, the disease is prone to severe complications such as gastrointestinal bleeding, obstruction, and perforation. Therefore, a systematic analysis of its clinical characteristics and surgical value from a surgical perspective is of considerable importance.
Based on data from 50 patients with PGIL, a retrospective study was conducted to provide clinical insights for further optimizing the comprehensive management strategy of this disease.
Patients diagnosed with PGIL treated by the General Surgery Department of the Chinese People’s Liberation Army Air Force Medical Center (Beijing, China) from June 2001 to March 2025 were selected. This was a single-center retrospective study. The medical record data were cases with relatively complete traceable medical records and examination data in our hospital, and the earliest medical records that met the requirements were June 2001 to the time of the study, that is March 2025. Inclusion criteria were: (1) Confirmation of diagnosis by histomorphology and/or immunohistochemistry; and (2) Completion of at least one full treatment course at our institution. Exclusion criteria comprised: (1) Presence of concurrent other malignant tumors; and (2) Cases with in complete clinical information. These criteria were applied to minimize potential confounding effects from major comorbidities and inadequately documented cases. Ultimately, 50 patients were included in the study. Among them, 34 patients who underwent surgical resection with chemotherapy were assigned to the experimental group, whereas 16 patients receiving chemotherapy alone served as the control group.
General information such as sex, age, and lifestyle habits of the enrolled patients were extracted from the electronic medical record system. The chief complaints, concurrent symptoms, duration and severity of symptoms at presentation, and detailed medical and family history, were systematically documented. Past medical history, including family history, was also documented. Additionally, images and reports of ultrasound, X-ray, computed tomography (CT), positron emission tomography (PET)/CT, magnetic resonance imaging, and other examinations were collected, recording the location, size, and invasion of the tumor. Endoscopic findings, including tumor size, biopsy site, and biopsy results, were also recorded. Pathological data included histological types and immunohistochemistry test results. Postoperative complications were statistically analyzed. This study only included patients who continued follow-up treatment at our hospital after surgery. The final treatment outcomes were categorized as improved, stable, or deteriorated based on clinical symptoms, physical signs, and imaging or PET/CT findings.
Clinical data were statistically organized and descriptively analyzed using Excel, SPSS 24.0, and Origin Pro 2024 software. Categorical variables are presented as the frequencies and percentages, either overall or stratified by group. Intergroup comparisons for count data were performed using the χ2 test or Fisher’s exact test, as appropriate. Associations between variables were assessed using Spearman’s rank correlation coefficient. P < 0.05 was considered statistically significant.
The age of onset of patients in this group ranged from 15 years to 89 years, with a median age of 59 years. Patients over 60 years of age accounted for a higher proportion (40.0%). Males comprised the majority of this group (64.0%). The most common primary site for the lymphoma was the intestine (70.0%). Abdominal pain was the most frequent initial symptom (52.0%) and concurrent symptom (80.0%). Tumors larger than 5 cm were found in 46.0% of the patients. Additional data are summarized in Table 1.
| Item | Category | Number of cases | Percentage |
| Basic characteristics | |||
| Sex | Male | 32 | 64% |
| Female | 18 | 36% | |
| Family history | Yes | 16 | 32% |
| Behavioral risk factors | Smoking | 5 | 10% |
| Alcohol consumption | 5 | 10% | |
| Tumor characteristics | |||
| Primary site | Stomach | 15 | 30% |
| Intestine | 35 | 70% | |
| Maximum tumor diameter | > 5 cm | 23 | 46% |
| ≤ 5 cm | 16 | 32% | |
| Infiltrative growth difficult to evaluate | 11 | 22% | |
| Clinical manifestations | |||
| Initial symptom | Abdominal pain | 26 | 52% |
| Abdominal mass | 8 | 16% | |
| Mass with pain | 2 | 4% | |
| Hematochezia or melena | 7 | 14% | |
| Others | 7 | 14% | |
| Concurrent symptoms | Pain | 40 | 80% |
| Hematochezia/melena | 19 | 38% | |
| Nausea and vomiting | 16 | 32% | |
| Weight loss | 18 | 36% | |
| Anemia | 19 | 38% | |
| Comorbidities | Cardiac disease | 8 | 16% |
| Chest disease | 20 | 40% | |
| Gastritis | 13 | 26% | |
| Liver disease | 17 | 34% | |
| Gallbladder disease | 8 | 16% | |
| Other abdominal diseases | 28 | 56% | |
| Central nervous system disease | 5 | 10% | |
| Hypertension | 10 | 20% | |
| Diabetes | 7 | 14% | |
| Metastasis to other sites | 30 | 60% | |
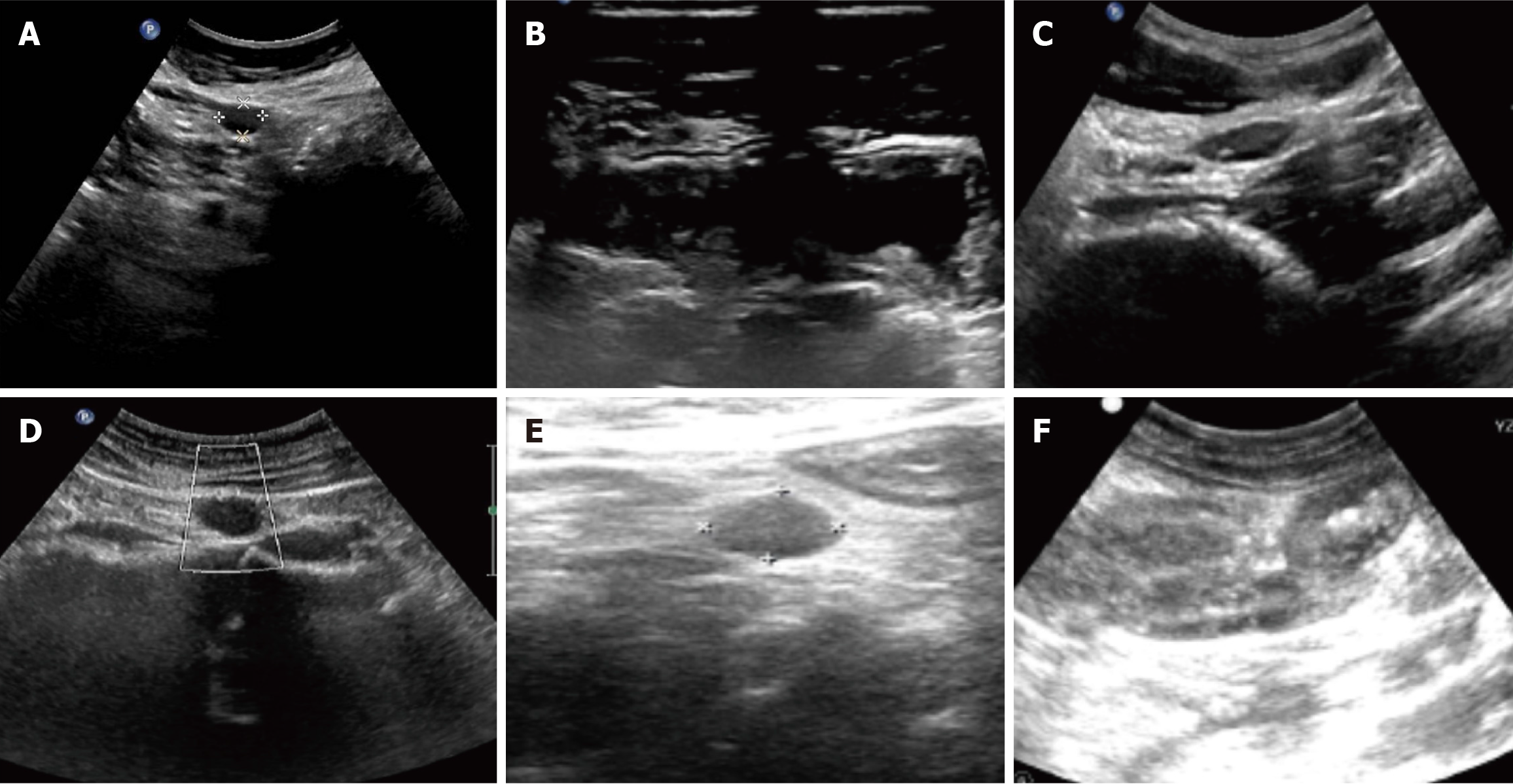
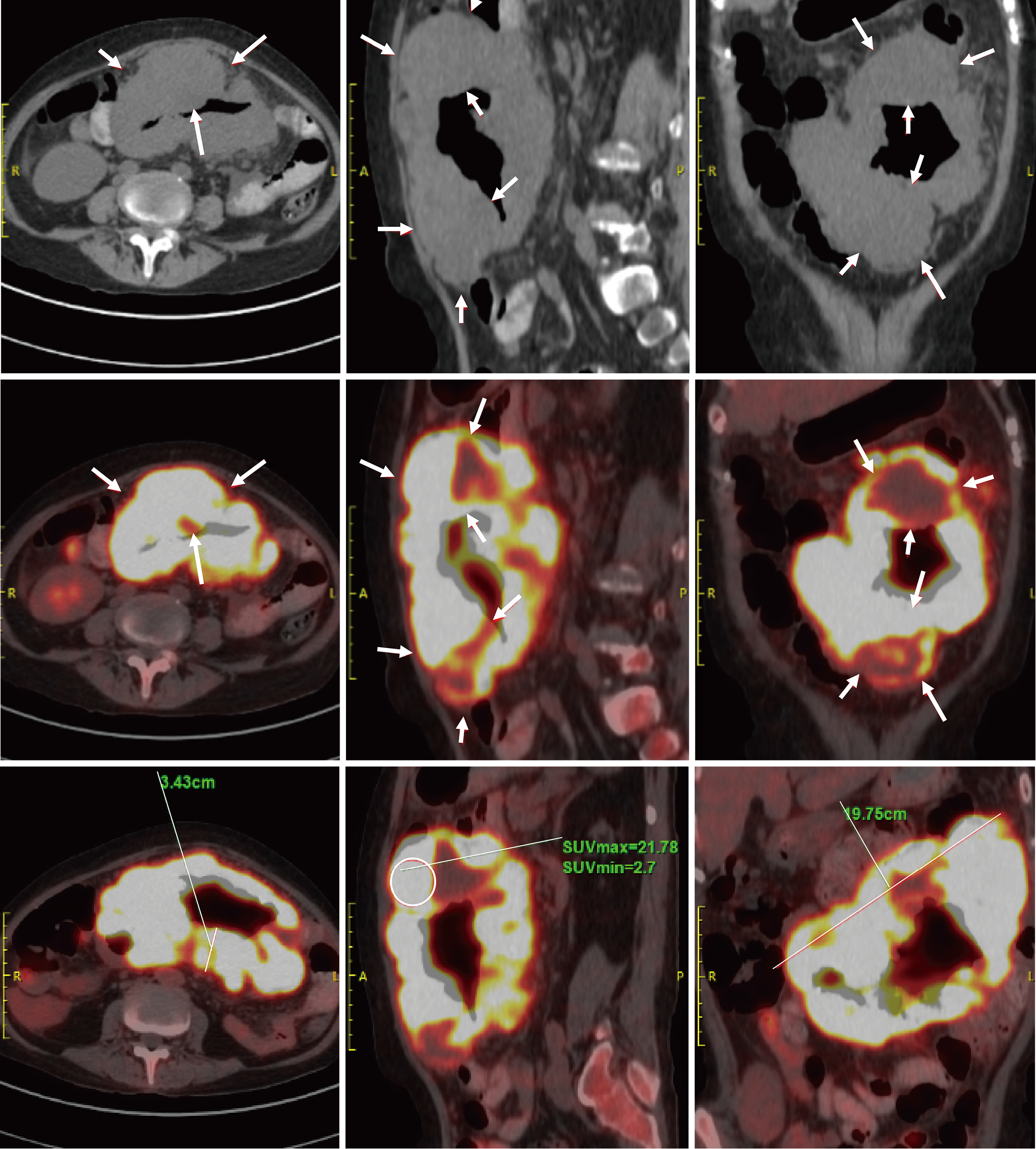
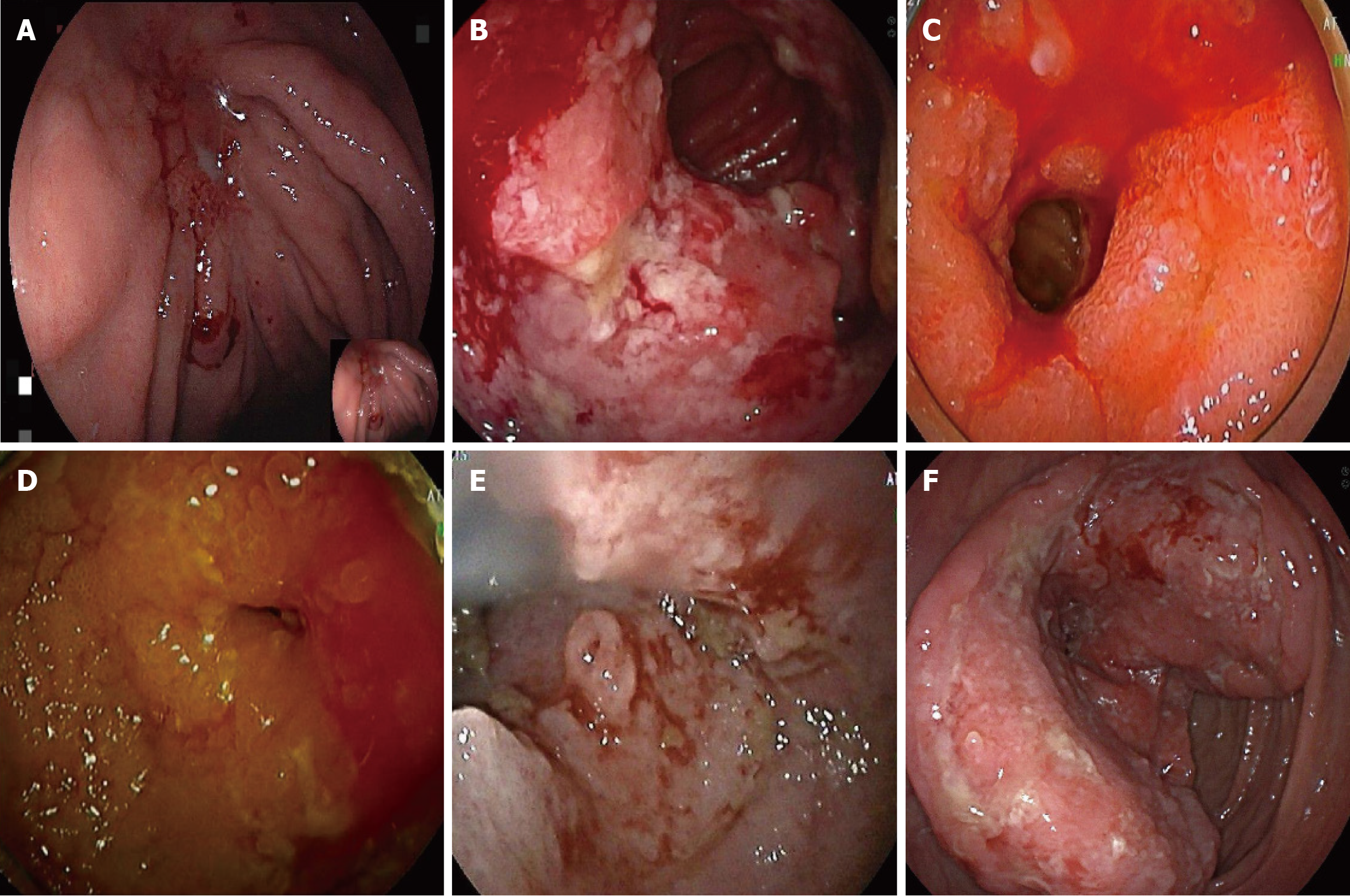
Ultrasonography is often less sensitive due to the varying primary locations and deep-seated nature of the lesions. Different tumor sites may exhibit distinct features. For example, lymphoma in the small intestine may present as thickening of the intestinal wall, which can extend to the mesentery, abdominal wall, or retroperitoneum (Figure 1). Most cases are also associated with abnormally enlarged lymph nodes. CT typically reveals masses closely related to the gastrointestinal tract or abnormal thickening of the intestinal wall, with the highest overall diagnostic rate of 94.3%. In cases of metastasis, PET/CT was employed for post-diagnostic assessment of metastasis, demonstrating lymph nodes with abnormal metabolic activity (Figure 2). The number of PET/CT-positive cases in Table 2 corresponds to the number of patients with confirmed metastasis. Endoscopic examinations commonly reveal gastrointestinal ulcers, intestinal stenosis, or space-occupying masses (Figure 3).
| Characteristics | Number of examinations | Positive diagnoses | Percentage |
| Ultrasound | 49 | 18 | 36.7% |
| CT | 35 | 33 | 94.3% |
| PET-CT | 36 | 24 | 66.7% |
| Endoscopy | 47 | 43 | 91.5% |
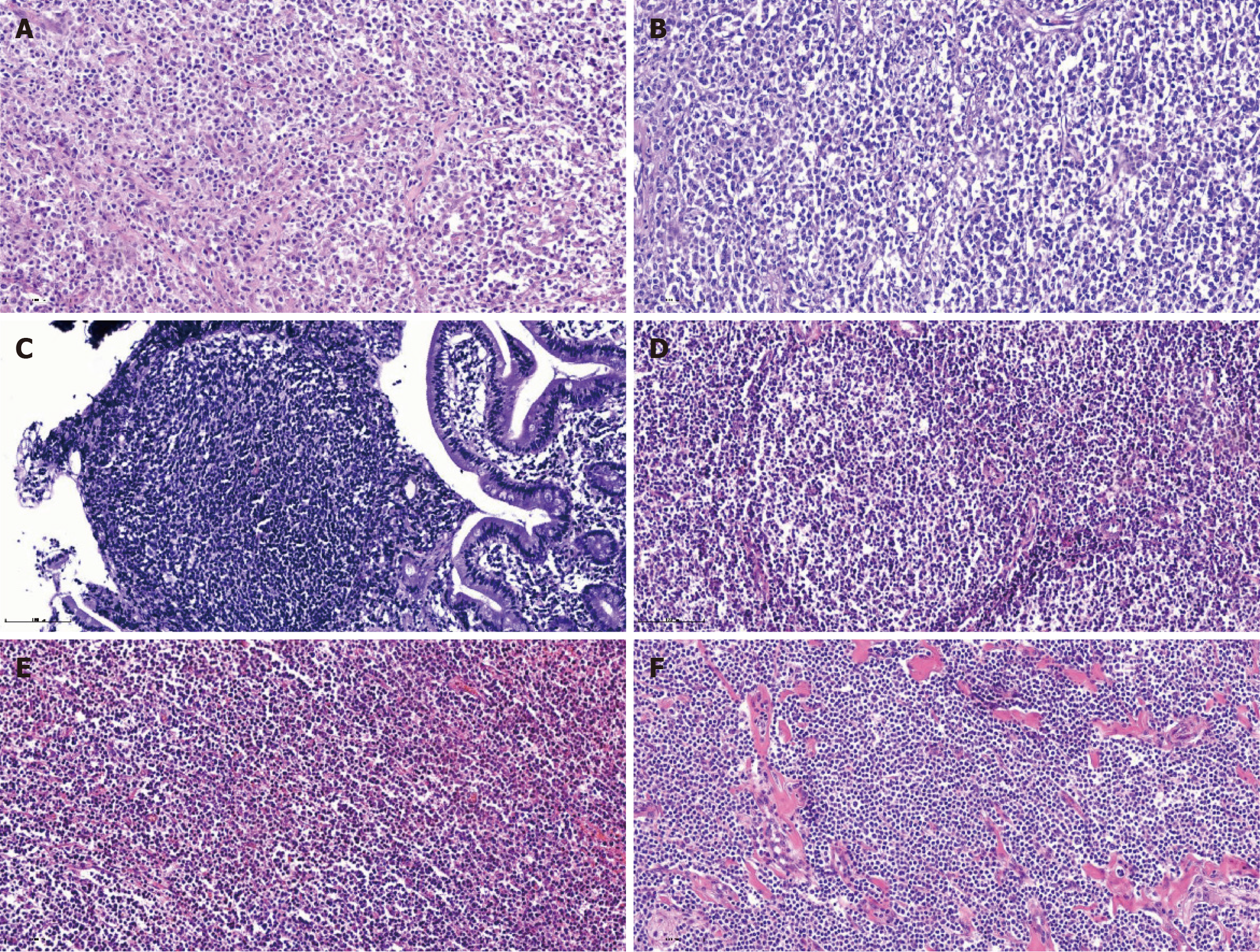
Diffuse large B-cell lymphoma (DLBCL) represents the most prevalent histologic subtype of PGIL. In this study, DLBCL constituted the highest proportion (52.0%) of cases, which is consistent with established literature. Immunophenotypic positivity rate serves as a critical indicator for determining cellular origin and differentiation, and reliably reflects both proliferative activity and malignant potential. The immunophenotypic positive rates of various subtypes were as follows: (1) DLBCL (26 cases), with high positive rates for cluster of differentiation 20 (CD20) (84.6%, 22/26), paired box protein 5 (80.7%, 21/26), and multiple myeloma protein 1 (65.4%, 17/26); (2) MALT lymphoma (12 cases), with high positive rates for CD20 (100%, 12/12), B-cell lymphoma 2 (Bcl-2) (75%, 9/12), and CD79a (66.7%, 8/12); (3) T-cell lymphoma (TCL) (5 cases), with high positive rates for Bcl-2 (100%, 5/5), CD3, and CD5 (80%, 4/5), as well as CD8, CD20, and CD56 (60.0%, 3/5); (4) Follicular lymphoma (FL) (5 cases), with high positive rates for CD20, CD21, and Bcl-2 (80%, 4/5); and (5) Transformed lymphoma (MALT to DLBCL) (1 case), positive for CD3, CD5, CD10, CD20, CD23, CD79a, Bcl-2, and Bcl-6 (Figure 4).
The results in this group showed that B-cell-derived lymphomas (DLBCL, FL, MALT) shared the common feature of CD20 positivity, whereas TCL was mainly characterized by Bcl-2 positivity. Transformed lymphoma exhibited over
The Lugano staging system was adopted in this study to guide treatment selection and prognostic evaluation, based primarily on the extent of disease involvement (localized gastrointestinal tract, regional lymph nodes, or disseminated spread) and the presence of systemic “B symptoms”. In our cohort, stage II disease accounted for the largest proportion (44.0%), with the median stage being II. The International Prognostic Index was applied as one of the most widely utilized and critical prognostic tools in lymphoma. It integrates clinical features reflective of the patient’s overall condition and tumor biological behavior to accurately predict survival outcomes and treatment response. According to the International Prognostic Index scoring, the low-risk category represented the largest subgroup (58.0%), as detailed in Table 3.
| Characteristics | Classification | Number of cases | Percentage |
| Lugano stage | I | 10 | 20% |
| II | 22 | 44% | |
| III | 6 | 12% | |
| IV | 12 | 24% | |
| IPI risk | Low | 29 | 58% |
| Low-intermediate | 10 | 20% | |
| High-intermediate | 9 | 18% | |
| High | 2 | 4% |
Among the surgery group, 9 cases (22.5%) developed postoperative complications, including poor incision healing, infection, and fever. The occurrence of complications was not significantly correlated with staging or risk. However, all tumors in these cases were larger than 5 cm. Prognostic data were available for 22 cases in the surgical group. Prognostic analysis showed no statistically significant difference in outcomes between the surgery plus chemotherapy group and the chemotherapy only group, although the improvement rate was higher in the surgical group (Table 4). Correlation analysis of outcomes revealed no significant correlations, suggesting the need for further analysis with a larger sample size (Table 4).
| Characteristics | Classification | Number of cases | Percentage |
| Surgery group | Improvement | 9 | 40.9% |
| Stable | 5 | 22.7% | |
| Deterioration | 8 | 36.3% | |
| Non-surgery group | Improvement | 4 | 25.0% |
| Stable | 6 | 37.5% | |
| Deterioration | 6 | 37.5% |
This study was designed to analyze the clinicopathological characteristics of PGIL and offer clinical insights for its management. PGIL is a common site for extranodal lymphoma[3-6]. Lymphoma involving the gastrointestinal tract not only compromises nutritional status but may also lead to life-threatening complications such as hemorrhage or obstruction as the disease progresses. Therefore, elucidating the distribution of involved sites, histologic subtypes, and clinical manifestations of PGIL is critical for guiding clinical decision-making, facilitating early diagnosis, and improving treatment outcomes. In the present case series, intestinal involvement was more frequently observed. The distribution of gastrointestinal pathological subtypes is site-specific, with MALT and DLBCL predominantly occurring in the stomach, while FL and intestinal disease-associated TCL are more common in the small intestine[4,7]. In this group, abdominal pain was the predominant initial and concurrent symptom. This was often accompanied by nonspecific manifestations such as abdominal distension, nausea, vomiting, gastrointestinal bleeding, weight loss, and anemia. From a clinical perspective, patients often present with diverse and nonspecific symptoms due to the deep anatomical location and the lack of characteristic manifestations. Abdominal pain was the most common initial and concomitant symptom in this cohort, accompanied by nonspecific presentations such as bloating, nausea, vomiting, gastrointestinal bleeding, weight loss, and anemia. These nonspecific manifestations frequently contribute to diagnostic challenges, including misdiagnosis or delayed detection. Therefore, recognizing these symptom patterns may enhance clinical vigilance toward this disease and help reduce diagnostic delays.
Given the diagnostic challenges associated with PGIL, its accurate identification should be guided by the principle of multi-site integrated and complementary evaluation. Ultrasound has a low sensitivity for PGIL and should be combined with CT and other examinations such as endoscopic biopsy. In this group, the mass detection rate by CT was 94.3%, while the diagnostic confirmation rate by endoscopy was 91.5%. In particular, lesions causing unexplained gastrointestinal bleeding are frequently found in the small intestine, and comprehensive oral and anal enteroscopy is essential to assess the entire digestive tract. Direct endoscopic biopsy can enhance the diagnostic accuracy. However, there remains a risk of missed diagnosis in clinical practice. Some patients with small intestinal lymphoma in this group underwent only gastroscopy or colonoscopy, while others with colonic lymphoma only received gastroscopy. Therefore, in patients with unexplained gastrointestinal bleeding but negative initial examinations, the possibility of tumors in occult locations should always be considered. Comprehensive multi-site examination should be supplemented. Additionally, PET/CT provides critical guidance in evaluating metastasis, determining surgical feasibility, and restaging following treatment[8]. It frequently reveals occult metastatic lesions, thereby helping to compensate for the limitations of preoperative mono-imaging assessment[8].
Histological biopsy remains the gold standard for the diagnosis of PGIL[9]. The primary methods for obtaining tissue specimens include puncture, endoscopic sampling, and surgical resection biopsy[10]. Surgery offers the advantage of fully preserving the lesion’s original appearance, providing ample tissue for histological examination, which is a si
Regarding histopathological subtypes, all cases in this group were non-Hodgkin's lymphoma, with DLBCL accounting for up to 52%. DLBCL is typically the most common pathological type of gastrointestinal lymphoma[12], with a minority originating from MALT transformation[14]. FL and MALT transformations were also observed in this study. Similarly, primary thyroid lymphoma predominantly consists of DLBCL (50%-70%) and MALT lymphoma (10%-50%)[15]. These findings further confirm the dominant role of these histopathological subtypes in gastrointestinal lymphoma, which enables more tailored therapeutic strategies depending on the specific subtype. In the pathological findings, this study showed that B-cell-derived lymphomas (DLBCL, FL, MALT) share the common feature of CD20 positivity, which is consistent with findings from previous literature[12]. Genetic testing and molecular pathology are driving the ad
Regarding therapeutic strategies, preoperative surgery for PGIL is demonstrated to be safe and feasible[14]. Fur
In summary, through a systematic analysis of clinical data from patients with PGIL, this study has delineated the clinical characteristics, diagnostic approaches, treatment strategies, and prognostic factors associated with this disease, thereby providing valuable insights for clinical practice. Looking forward, advances in precision medicine - incorporating molecular profiling, genetic testing, and artificial intelligence - are expected to facilitate more individualized management of PGIL, ultimately leading to improved diagnostic accuracy and patient outcomes. However, as a retrospective study with a limited sample size from a single center, the present findings may be subject to potential regional and selection biases. Therefore, future large-scale, randomized, prospective studies are warranted to validate and extend these observations.
PGIL is commonly associated with abdominal pain as the main symptom, with DLBCL being the most prevalent subtype. Diagnosis requires a combination of imaging techniques, including ultrasound, CT, and endoscopic biopsy, with PET/CT playing a crucial role in evaluating metastasis. Surgery is essential for diagnostic sampling, lesion resection, symptom relief, and the management of life-threatening complications, and can improve the prognosis in combination with radiotherapy and chemotherapy.
| 1. | Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17:697-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 245] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (3)] |
| 2. | Filip PV, Vladareanu AM, Diaconu LS, Cuciureanu D, Tomescu A, Pop CS. Primary Gastrointestinal Lymphoma: A Prospective Unicentric Study on a Romanian Cohort. J Gastrointestin Liver Dis. 2025;34:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Li M, Zhang S, Gu F, Xiao W, Yao J, Chao K, Chen M, Li J, Zhong B. Clinicopathological characteristics and prognostic factors of primary gastrointestinal lymphoma: a 22-year experience from South China. Int J Clin Exp Pathol. 2014;7:2718-2728. [PubMed] |
| 4. | Alvarez-Lesmes J, Chapman JR, Cassidy D, Zhou Y, Garcia-Buitrago M, Montgomery EA, Lossos IS, Sussman D, Poveda J. Gastrointestinal Tract Lymphomas. Arch Pathol Lab Med. 2021;145:1585-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Wang W, Lin P, Yao H, Jia X, Sun J. Clinical analysis of Primary Gastrointestinal Non-Hodgkin's Lymphoma. Pak J Med Sci. 2017;33:1406-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Lin H, Zhou K, Peng Z, Liang L, Cao J, Mei J. Surgery and chemotherapy cannot improve the survival of patients with early-stage mucosa-associated lymphoid tissue derived primary pulmonary lymphoma. Front Oncol. 2022;12:965727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Derringer GA, Thompson LD, Frommelt RA, Bijwaard KE, Heffess CS, Abbondanzo SL. Malignant lymphoma of the thyroid gland: a clinicopathologic study of 108 cases. Am J Surg Pathol. 2000;24:623-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 239] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Zanoni L, Bezzi D, Nanni C, Paccagnella A, Farina A, Broccoli A, Casadei B, Zinzani PL, Fanti S. PET/CT in Non-Hodgkin Lymphoma: An Update. Semin Nucl Med. 2023;53:320-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 9. | Eichenauer DA, Aleman BMP, André M, Federico M, Hutchings M, Illidge T, Engert A, Ladetto M; ESMO Guidelines Committee. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv19-iv29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 10. | Ansell SM. Hodgkin lymphoma: 2025 update on diagnosis, risk-stratification, and management. Am J Hematol. 2024;99:2367-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 11. | Connors JM, Cozen W, Steidl C, Carbone A, Hoppe RT, Flechtner HH, Bartlett NL. Hodgkin lymphoma. Nat Rev Dis Primers. 2020;6:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 12. | Sehn LH, Salles G. Diffuse Large B-Cell Lymphoma. N Engl J Med. 2021;384:842-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 759] [Article Influence: 151.8] [Reference Citation Analysis (0)] |
| 13. | Li SE, Cheng LL, Zhu WD. [Comparison of next-generation sequencing quality control parameters between core needle biopsy and surgical specimen paraffin-embedded tissues in lymphoma diagnosis]. Linchuang Yu Shiyan Binglixue Zazhi. 2024;40:1111-1112. [DOI] [Full Text] |
| 14. | Matysiak-Budnik T, Fabiani B, Hennequin C, Thieblemont C, Malamut G, Cadiot G, Bouché O, Ruskoné-Fourmestraux A. Gastrointestinal lymphomas: French Intergroup clinical practice recommendations for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFH). Dig Liver Dis. 2018;50:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Saito Y, Watanabe N, Suzuki N, Saito N, Narimatsu H, Takami H, Kameyama K, Yoshioka K, Masaki C, Akaishi J, Hames KY, Matsumoto M, Fukushita M, Yoshihara A, Okamura R, Tomoda C, Suzuki A, Matsuzu K, Kitagawa W, Nagahama M, Noh JY, Sugino K, Ito K. Role of Surgery in Patients with Stage IE Primary Thyroid MALT Lymphoma Staged by a Modified Classification System: The Tokyo Classification. Cancers (Basel). 2023;15:1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Hwang HS, Yoon DH, Suh C, Park CS, Huh J. Prognostic value of immunohistochemical algorithms in gastrointestinal diffuse large B-cell lymphoma. Blood Res. 2013;48:266-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Qiao HH, Wang YL, Su LP, Bai M, Gao X, Ma RJ, Zhang DD, Zhang MZ, Zhu LN, Zhang XD. [Value of CD30 detection in the diagnosis and treatment of lymphoma: a multi-center retrospective study]. Zhonghua Zhongliu Fangzhi Zazhi. 2024;31:569-574. [DOI] [Full Text] |
| 18. | Nomura E, Uchimi K, Abue M, Kon H, Noguchi T, Suzuki S, Suzuki M, Onodera H, Tateno H, Ota Y. [Regression of MALT lymphoma of the rectum after Helicobacter pylori eradication therapy in a patient negative for Helicobacter pylori]. Nihon Shokakibyo Gakkai Zasshi. 2010;107:1466-1473. [PubMed] |
| 19. | Chai YJ, Hong JH, Koo do H, Yu HW, Lee JH, Kwon H, Kim SJ, Choi JY, Lee KE. Clinicopathological characteristics and treatment outcomes of 38 cases of primary thyroid lymphoma: a multicenter study. Ann Surg Treat Res. 2015;89:295-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Lai YL, Lin JK, Liang WY, Huang YC, Chang SC. Surgical resection combined with chemotherapy can help achieve better outcomes in patients with primary colonic lymphoma. J Surg Oncol. 2011;104:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Su PF. [Clinical efficacy of radiotherapy and chemotherapy combined with surgical treatment for the patients with primary mediastinal B-cell lymphoma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015;23:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Zhu Y, Yang S, He X. Prognostic evaluation models for primary thyroid lymphoma, based on the SEER database and an external validation cohort. J Endocrinol Invest. 2022;45:815-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Wang S, Han JL, Hu QY, Fu ZM. [Effect of surgery on advanced stage diffuse large B-cell lymphoma patients: A cohort study of propensity matched populations]. Wuhan Daxue Xuebao. 2022;43:56-62. [DOI] [Full Text] |
| 24. | Smith S. Transformed lymphoma: what should I do now? Hematology Am Soc Hematol Educ Program. 2020;2020:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Pan Z, Huang Z, Xing Z, Yang J, Huang S, Zhang Y. Prognostic factors and surgical approaches in the analysis of primary central nervous system diffuse large B-cell lymphoma: a large population-based cohort study and external validation. Front Neurol. 2024;15:1431614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |