Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.111142
Revised: August 15, 2025
Accepted: October 17, 2025
Published online: November 15, 2025
Processing time: 142 Days and 16.5 Hours
Hepatocellular carcinoma (HCC) is a globally prevalent malignancy associated with high morbidity and mortality. Transfer RNA (tRNA)-derived small RNAs (tsRNAs), a class of small non-coding RNAs originating from tRNA, have em
To investigate differentially expressed tsRNAs in HCC, identify potential bio
Differentially expressed tsRNAs in Barcelona Clinic Liver Cancer 0/A-stage HCC tissues were identified through high-throughput sequencing. Agarose gel electrophoresis, Sanger sequencing, and quantitative polymerase chain reaction were conducted to detect 5’-tRNA halve (tiRNA)-lysine (Lys)-CTT in tissues and serum samples. The diagnostic performance of 5’-tiRNA-Lys-CTT was evaluated using receiver operating characteristic analysis. HCC cell proliferation was examined using the Cell Counting Kit-8 assay, colony formation assay, and 5-ethynyl-2’-deoxyuridine staining. Additionally, the migratory capability of HCC cells was investigated using Transwell assays.
The 5’-tiRNA-Lys-CTT demonstrated excellent stability and can be easily detected. Its expression was significantly upregulated in 50 HCC tissues, 110 HCC serum samples, and 5 HCC cell lines vs control groups, and the di
The 5’-tiRNA-Lys-CTT levels were higher in early HCC patients. 5’-tiRNA-Lys-CTT is a promising diagnostic biomarker for early-stage HCC and may play an oncogenic role in HCC by interacting with downstream mRNA targets via specific pathways.
Core Tip: Through high-throughput sequencing, we found transfer RNA (tRNA)-derived small RNA differentially expressed in early-stage hepatocellular carcinoma (HCC) tissues. 5’-tRNA halve (tiRNA)-lysine (Lys)-CTT, one of the tRNA-derived small RNAs, had great diagnostic efficacy and potential as new “liquid biopsy” biomarkers for the diagnosis of early stage of HCC. We speculated that 5’-tiRNA-Lys-CTT may be involved in multiple cancer-related signaling pathways, and would like to investigate the specific mechanisms of 5’-tiRNA-Lys-CTT in HCC in future studies.
- Citation: Yuan J, Gu WC, Xu TX, Shen XJ, Li X, Shen L, Zhang Y, Ju SQ. 5’-transfer RNA halve-lysine-CTT as a promising biomarker for early detection of hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(11): 111142
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/111142.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.111142
Primary liver cancer, the sixth most commonly diagnosed type of cancer, is a leading cause of cancer-related mortality worldwide, with China being disproportionately affected. Hepatocellular carcinoma (HCC), accounting for 75%-85% of liver cancer cases, poses significant challenges due to high recurrence rates after surgical resection and late-stage diagnosis[1,2]. Currently, the diagnosis of HCC mostly depends on imaging diagnosis and the monitoring of blood indicators such as alpha-fetoprotein (AFP), abnormal prothrombinogen (APT), and viral copy number. Nevertheless, the ability of imaging to detect HCC in its early stage is only 47%, whereas the diagnostic sensitivity of AFP is 52.9%. Additionally, around 30% of patients with HCC do not show elevated levels of AFP[3,4]. Furthermore, elevated AFP levels may be observed in non-HCCs, such as cirrhosis and chronic hepatitis, resulting in a certain false-positive rate. Vitamin K deficiency or the administration of anticoagulant therapy has been demonstrated to result in false positives in APT, thereby affecting the interpretation of test results. Presently, among the diagnostic markers for HCC, none have sufficient sensitivity for extremely early-stage HCC (≤ 2 cm), making precise early screening difficult to achieve. Hence, it is imperative to discover novel early diagnostic indicators, understand the mechanisms driving HCC progression, and develop effective therapeutic strategies to improve outcomes. The presence of accumulated genetic and epigenetic alterations has been demonstrated to exert a significant influence on the expression of both messenger RNAs (mRNAs) and noncoding RNAs (ncRNAs). This influence contributes to the development of tumors and the aggressive behavior characteristic of HCC[5]. Further research into novel functional genes and the underlying molecular pathways is crucial for advancing drug development and enhancing the prognosis of HCC patients.
The ncRNAs are single-stranded RNA molecules that do not code for proteins. Next-generation sequencing technology has facilitated the identification of an increasing number of ncRNAs, including microRNAs, long non-coding RNAs, P-element-induced wimpy testis-interacting RNAs, and circular RNAs[6]. These ncRNAs are involved in various phy
In 1979, Speer et al[11] made a notable discovery regarding the levels of small regulatory molecules, termed “tsRNAs”, within the urinary samples of individuals diagnosed with urogenital malignancies. They found that these levels exhibited an increase and subsequently returned to normal post-chemotherapy[11]. Due to their substantial expression, scientists are developing a more precise comprehension of the functions of tsRNAs[12,13]. Recent studies have shown that some tsRNAs, recognized for their stability in the bloodstream, can serve as novel biomarkers for specific forms of cancer[14,15]. For instance, Wang et al[16] discovered that six tRFs derived from the 5’ ends of tRNAs showed a significant decrease in expression levels in plasma samples from patients with early-stage breast cancers. Additionally, Yang et al[17] were the first to analyze tsRNA patterns in the serum of individuals diagnosed with systemic lupus erythematosus. They found that the levels of tRF-histidine-GTG-1 were significantly higher in systemic lupus erythematosus patients’ serum. Furthermore, serum tRF-proline-AGG-004 and tRF-leucine-CAG-002 have demonstrated potential as diagnostic markers for pancreatic cancer and have been implicated in the promotion of its growth[18].
The tsRNAs play several biological roles, including in gene suppression, protein interaction, ribosome synthesis, RNA modification, and oncogenic development[9,19]. They are also linked to cancer-related activities such as cell growth, migration, and invasion[20-25]. Additionally, tsRNAs can serve as non-invasive biomarkers[16-18]. For example, Kim et al[25] demonstrated that the inhibition of 3’-tsRNA-leucine-CAG, which binds to at least two ribosomal protein mRNAs, induces programmed cell death both in vitro and in a mouse model of patient-derived HCC. Mo et al[21] discovered that 5’-tiRNA-valine has the capacity to function as a tumor suppressor by impeding the canonical Wnt signaling pathway. This finding indicates its promise as a promising diagnostic biomarker for breast cancer. In lung cancer, the expression of tsRNA-5001a was significantly upregulated and associated with a higher likelihood of postoperative recurrence and a poorer outcome[24]. These findings highlight the importance of tsRNAs in cancer biology and their potential as the
For all samples collected from January 2021 to October 2023 for this study, we followed the ethical rules of the World Medical Association. Serum samples for 110 preoperative HCC patients and 110 healthy donors were obtained from the clinical laboratory of Affiliated Hospital of Nantong University. For each participant, 3 mL of blood was extracted by venipuncture, and the serum was separated by centrifugation. Furthermore, we obtained 50 sets of HCC tissues and matched paracancerous tissues from our hospital’s operating room. These tissues were promptly frozen in liquid nitrogen and stored in -80 °C refrigerators for long-term preservation. Patient diagnoses were confirmed by pathology. Neoa
The isolation of total RNA was performed using TRIzol (Invitrogen, Thermo Fisher Scientific, MA, United States), and the evaluation of RNA quality and quantity was conducted using Qubit® 2.0 (Thermo Fisher Scientific, MA, United States) and the Agilent 2200 TapeStation (Agilent Technologies, CA, United States) instrument, respectively. The tsRNAs are heavily decorated by RNA modifications that interfere with small RNA sequencing library construction. We performed the following steps before library preparation for total RNA samples: 3’-aminoacyl (charged) deacylation to yield 3’-OH for 3’ adaptor ligation, 3’-cP (2’,3’-cyclic phosphate) removal to yield 3’-OH for 3’ adaptor ligation, 5’-OH (hydroxyl group) phosphorylation to yield 5’-P for 5’-adaptor ligations, and m1A and m3C demethylation for efficient reverse transcription. The NEBNext® Multiplex Small RNA Library Prep Set for Illumina (New England Biolabs, MA, United States) was utilized to create a small RNA library using 1 μg of total RNA from each sample, following the instructions provided by the manufacturer. The HiSeq 2500 SE50 (Illumina, Inc., CA, United States) sequencing machine was employed to sequence the library. The clean reads obtained were compared to the MINTbase database (http://cm.jefferson.edu/MINTbase/) using MINTmap software[26]. Subsequently, new predictions for tsRNA were made for reads that were not found in MINTbase. EdgeR software was utilized to examine alterations in tsRNAs across various groups[27].
The RNA was isolated from blood samples of HCC patients using a total RNA purification kit and a rotating column separation kit (BioTeke, Wuxi, China). For tissue and cell samples, RNA extraction was performed using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, MA, United States). Total RNA was reverse transcribed into complementary DNA using specific primers (Ribobio, Guangzhou, China) and a Revert Aid RT Reverse Transcription Kit (Thermo Fisher Scientific, MA, United States). The reaction was carried out in a 10-μL volume and incubated at 42 °C for 60 minutes, followed by inactivation at 70 °C for 5 minutes. Quantification was necessary before the reaction.
A quantitative polymerase chain reaction (qPCR) experiment was conducted using a Bio-Rad CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., CA, United States) with a total volume of 20 μL. The reaction mixture comprised 10 μL of ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China), 5 μL of complementary DNA, 1 μL each of tsRNA and RNU6B (U6) primers, and 3 μL of enzyme-free water. To normalize the relative expression of tsRNAs, U6 was employed as an internal control, while RNA taken from the pooled serum of 10 healthy donors was utilized as an external control. The synthesis of all primers was conducted by RiboBio (Guangzhou, China). The data were analyzed using the 2-ΔΔCt method, which involves calculating the ΔΔCt value using the formula: ΔΔCt = ΔCttarget (Cttarget - Ctreference) - ΔCtexternal control (Ctexternal control - Ctreference) during the reaction. The RT primer was 5’-GTCGTA TCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGTCTCA-3’, the forward primer was 5’-GGCTAGCTCAGTCGGTAGAGCA-3’, and the reverse primer was 5’-AGTGCAGGGTCCGAGGTATT-3’.
We employed 2% agarose gel electrophoresis was employed to separate either 6 μL of DNA marker or 1 μL of DNA samples that were mixed with 5 μL of DNA loading buffer (Beyotime Biotechnology, Shanghai, China). The DNA ladder (500 bp Plus Marker; Sangon Biotech, Shanghai, China) consisted of eight linear DNA bands with sizes between 50 and 500 base pairs, with the 250-bp band intensified to act as a reference point. Gel imaging was used to observe DNA fragments on the gel (ChemiDocTM MP; Bio-Rad Laboratories, Inc., CA, United States).
HCC cell lines (Hep3B, Huh-7, MHCC97-H, MHCC97-L, HCCLM3, and PLC-PRF-5) and a human normal hepatic cell line (QSG-7701) were acquired from the Institute of Biochemistry and Cell Biology (Shanghai, China) and Beyotime Biotechnology (Shanghai, China). Huh-7, MHCC97-H, MHCC97-L, HCCLM3, and PLC-PRF-5 cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium (Gibco, Thermo Fisher Scientific, MA, United States). Hep3B cells were grown in a minimum essential medium (Invitrogen, Thermo Fisher Scientific, MA, United States) supplemented with GlutaMAX, nonessential amino acids, and sodium pyruvate, while QSG-7701 cells were maintained in RPMI-1640 medium (Gibco, Thermo Fisher Scientific, MA, United States). The media were supplemented with 10% fetal bovine serum (FBS) from Gibco (Thermo Fisher Scientific, MA, United States) and a 1% penicillin-streptomycin mix from HyClone (UT, United States), and incubated at 37 °C with 5% CO2.
RNA was extracted from both the nucleus and cytoplasm of the MHCC97-H and Hep3B cell lines using the PARISTTM kit (Invitrogen, Thermo Fisher Scientific, MA, United States). Subsequently, quantification of tsRNA RNA expression in both the nucleus and cytoplasm was conducted using quantitative reverse transcription polymerase chain reaction. The nuclear reference U6 and the cytoplasmic reference 18S were utilized.
A 5’-tiRNA-Lys-CTT mimic, negative control (NC) mimic, inhibitor, and NC inhibitor were acquired from Ribobio (Guangzhou, China), transfected into 6-well plates using Lipofectamine 3000 (Invitrogen, Thermo Fisher Scientific, MA, United States) reagent, and allowed to grow overnight before transfection. The concentration of the 5’-tiRNA-Lys-CTT mimics used for transfection was 50 nM, whereas the concentration of the 5’-tiRNA-Lys-CTT inhibitor was 100 nM. Following transfection for 48 hours, the cells were gathered to evaluate the expression of 5’-tiRNA-Lys-CTT using quantitative reverse transcription polymerase chain reaction. Following transfection for one night, the cells underwent cellular phenotypic tests.
In a Cell Counting Kit-8 (CCK-8) experiment, cells that had been treated were placed in 96-well plates (density of 3 × 103 cells per well) and then cultivated for 1-5 days. Every well was treated with 100 μL of full culture media containing 10% FBS. Each well was supplemented with 10 μL of CCK-8 detection solution (Dojindo Laboratories, Japan) and incubated for 2 hours. Optical density at 450 nm was measured using a microplate reader (TECAN-Spark; Tecan, Männedorf, Switzerland).
In a colony formation experiment, 1 × 103 cells were placed in 2 mL of complete culture media on a 6-well plate and incubated for 14 days. After 2 weeks, the colonies were immobilized using 4% paraformaldehyde solution and subjected to staining using crystal violet. Quantification of cell clones was performed utilizing ImageJ software version 1.47 (National Institutes of Health, United States).
5-ethynyl-2’-deoxyuridine (EdU) staining assay was performed using the CellorLabTM EdU Cell Proliferation Kit (Epi
After 48 hours of transfection, the cells were treated with trypsin-ethylenediaminetetraacetic acid (Beyotime Biotechnology, Shanghai, China) to induce digestion. The cell suspension was introduced into the upper chamber of the Transwell system (Costar®; Corning Inc., NY, United States). Subsequently, 100 μL of a cell solution, with 5 × 104 cells in a medium lacking serum, were placed on the filter membrane in the upper chamber. Additionally, 500 μL of media containing 10% (v/v) FBS was added to the lower chamber. The cells were incubated at 37 °C with 5% CO2 for 24 hours. The next day, cells that hadn’t migrated in the upper chamber were removed with a cotton swab, and those on the lower side of the Transwell inserts were fixed and stained with a 0.2% crystal violet solution. The number of cells that migrated to the underside of the inserts was quantified with ImageJ software (National Institutes of Health, United States).
The naming conventions and compositions of tsRNAs can be found in MINTbase version 2.0 (https://cm.jefferson.edu/MINTbase/). tsRNAs and target genes were identified using the following tools: Miranda (http://www.microrna.org/), TargetScan (https://www.targetscan.org/vert_80/), RNAhybrid (https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid/), and RIsearch (https://rth.dk/resources/risearch/). Gene expression analysis was performed using Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses, and the Reactome (https://curator.reactome.org/) pathway database.
The high-throughput sequencing data were analyzed using the R package edgeR. A significance threshold of P < 0.05 and |Log2FC| > 1.5 was used. The data were analyzed using SPSS Statistics Version 20.0 (IBM SPSS Statistics, IL, United States) and GraphPad Prism 9.0 (GraphPad Software, CA, United States). The mean ± SD was calculated to report the relative expression of tsRNAs in each group. Prior to the study, the normality of the data was evaluated using normality and lognormality tests, which indicated that all the data had a normal distribution. The t-tests were used to assess differences between groups. The diagnostic performance of tsRNAs for HCC was evaluated using ROC curve and area under the curve (AUC) analyses. Prior to graphing the ROC curve, we conducted binomial logistic regression. Cox’s proportional hazards model was utilized for multivariate analysis. The risk ratio and its corresponding 95% confidence interval were documented for each marker. The tsRNA cut-off values were determined using the Youden index, while the cut-off values for AFP and APT were calculated based on the reference ranges of Affiliated Hospital of Nantong University (15 ng/mL and 40 mAU/mL, respectively). The experiments were conducted independently on at least three occasions, and statistical significance was established when the P-value was less than 0.05.
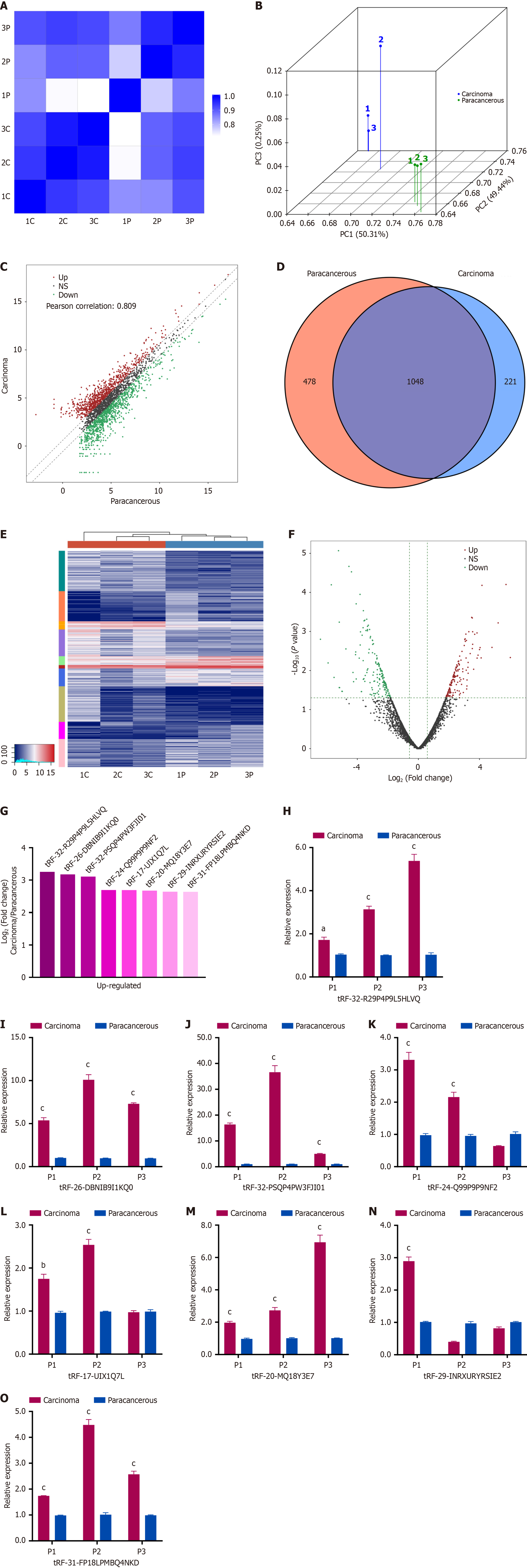
High-throughput sequencing was conducted on three paired cancerous and paracancerous tissues from HCC patients at the BCLC 0/A stage to identify differentially expressed tsRNAs (P < 0.05, |Log2FC| > 1.5). Correlation and principal component analyses were conducted to ensure sample consistency (Figure 1A and B). Scatter plots were generated displaying differential expression patterns, with up and down regulated tsRNAs shown separately (Figure 1C). A Venn diagram was established to illustrate commonly and specifically expressed tsRNAs (Figure 1D). Heatmap and volcano plots were generated to visualize overall expression changes (Figure 1E and F).
The statistical analysis identified 316 dysregulated tsRNAs, with 149 being upregulated and 167 downregulated. From among the top upregulated tsRNAs, eight candidates were selected based on the average expression level and fluo
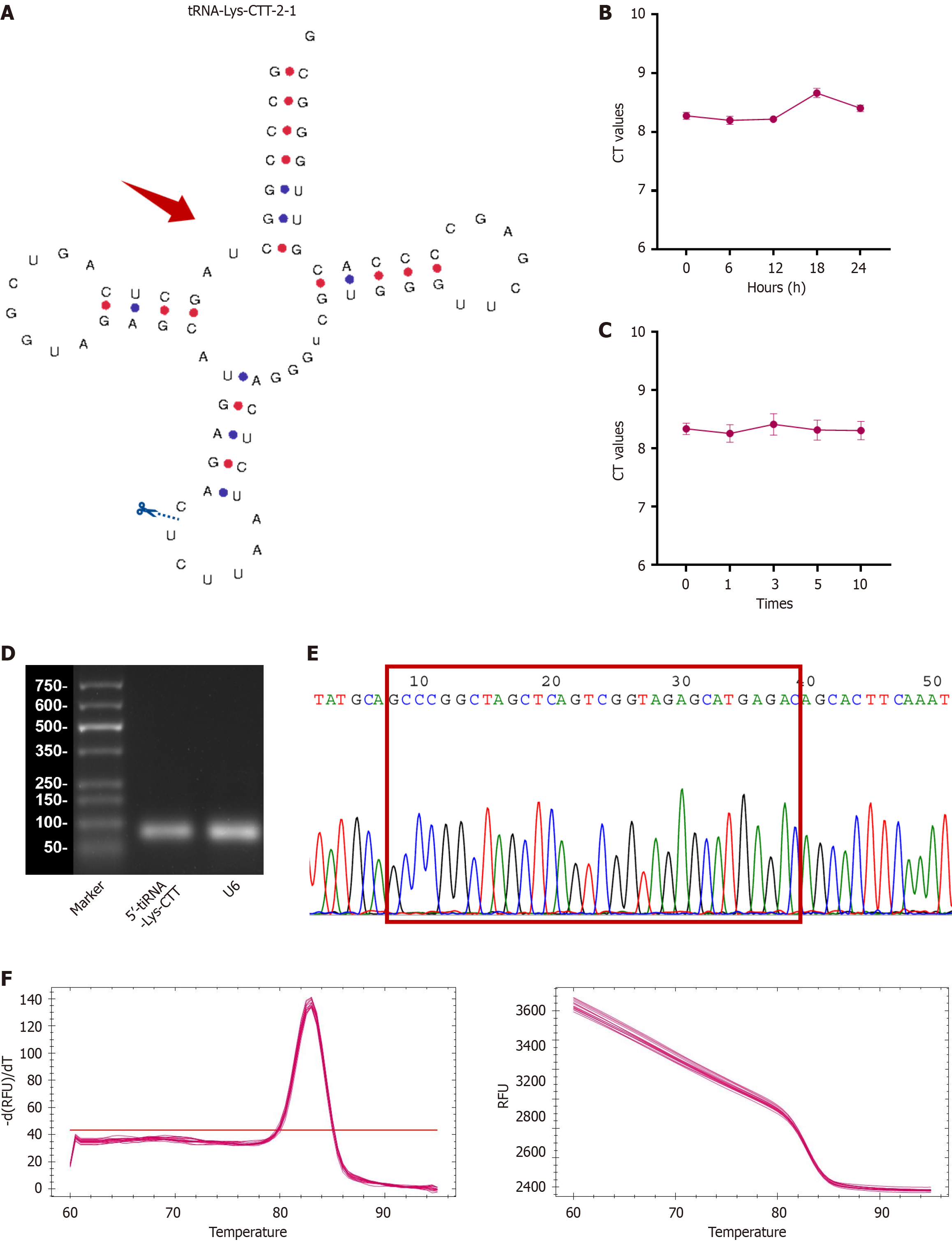
Currently, two primary categories of tsRNAs exist: The tiRNAs (specifically, 5’-tiRNA and 3’-tiRNA), and tRFs (including 1-tRF, 3-tRF, 5-tRF, and i-tRF). In the MINTbase version 2.0 database (http://cm.jefferson.edu/MINTbase/), the tRF-32-PSQP4PW3FJI01 molecule is 32 nucleotides in length (5’-GCCCGGCUAGCUCAGUCGGUAGAGCAUGAGAC-3’) and classified as a 5’-tiRNA (Supplementary Figure 1). This molecule corresponds to the 5’-half of the mature tRNA-Lys-CTT. Figure 2A illustrates the configuration of the tRNA and the locations where cleavage occurs. Based on this information, we have renamed tRF-32-PSQP4PW3FJI01 as 5’-tiRNA-Lys-CTT.
To assess the suitability of 5’-tiRNA-Lys-CTT, comprehensive validation experiments were conducted. Initially, serum samples were subjected to incubation at ambient temperature for varying durations (0 hour, 6 hours, 12 hours, 18 hours, and 24 hours; Figure 2B), followed by multiple cycles of freezing and thawing (0, 1, 3, 5, and 10 repetitions; Figure 2C). The results showed that the cycle threshold values for detecting 5’-tiRNA-Lys-CTT exhibit minimal fluctuation, suggesting that its detection would not be easily influenced. To confirm the specificity of the qPCR product, we con
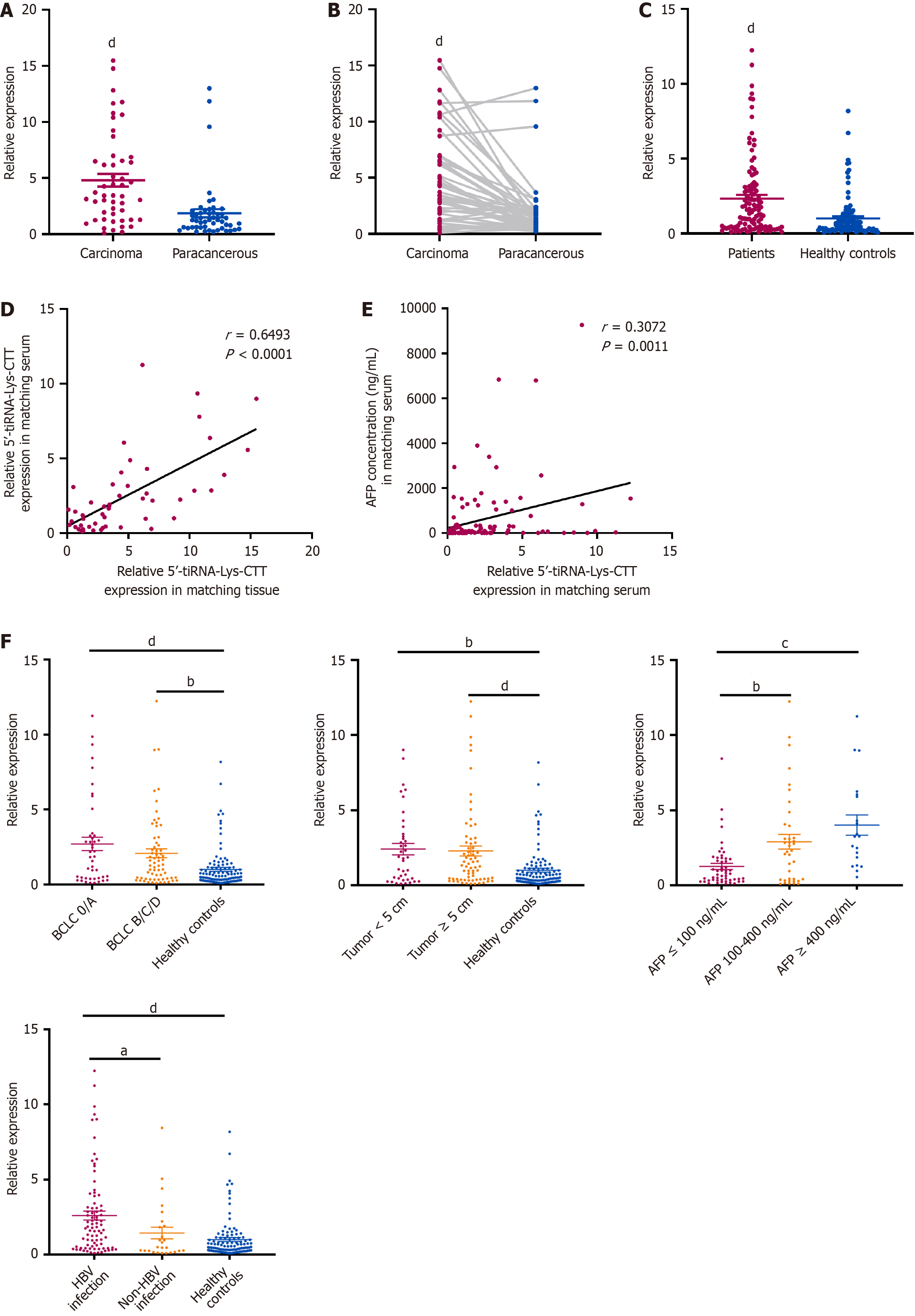
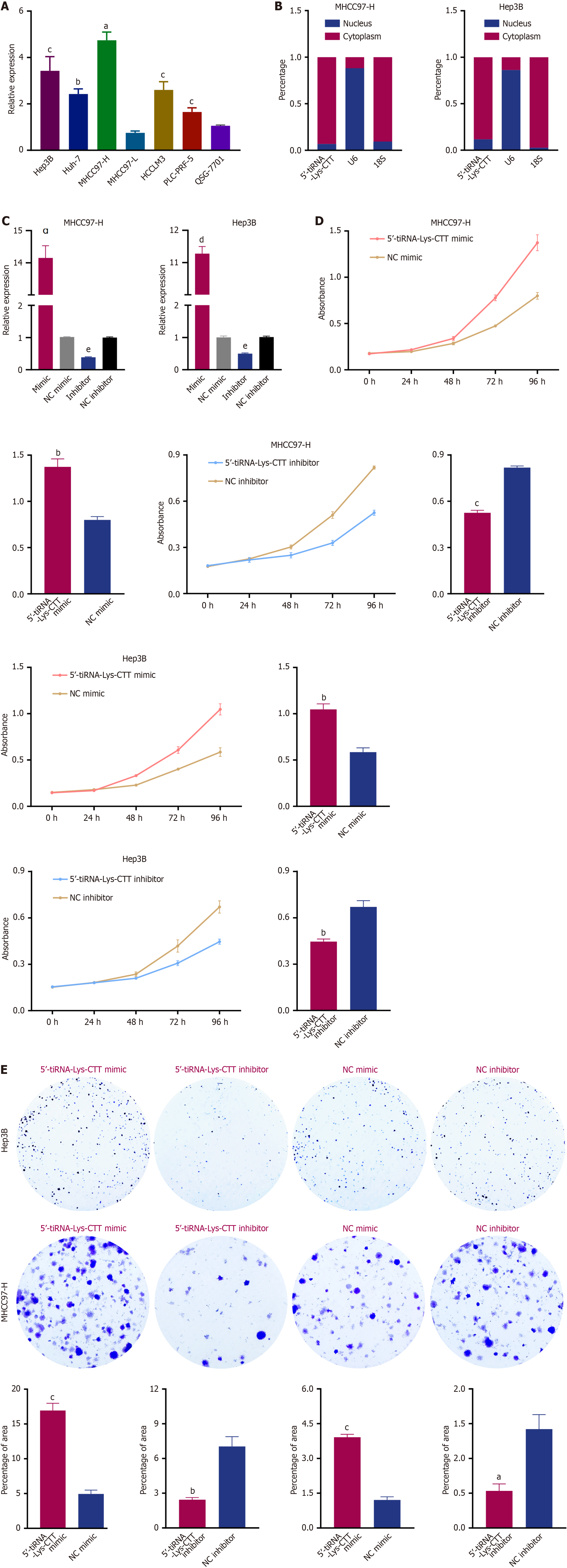
A total of 50 tissue samples from patients with HCC were collected to evaluate 5’-tiRNA-Lys-CTT expression levels. The findings indicated that 5’-tiRNA-Lys-CTT expression was significantly higher in tumor tissues compared to adjacent non-tumor tissues (Figure 3A). Furthermore, the results of unpaired comparisons were consistent with the paired comparisons in 50 pairs of tissue samples (Figure 3B). To investigate the level of 5’-tiRNA-Lys-CTT in the serum of patients with HCC and determine if it can be easily detected and provide an accurate diagnosis, we obtained 110 serum samples from HCC patients and 110 serum samples from healthy individuals. The expression of 5’-tiRNA-Lys-CTT was markedly elevated in HCC patients compared to healthy controls (Figure 3C). The expression of 5’-tiRNA-Lys-CTT in tissues was correlated with its levels in the corresponding patient’s serum (Figure 3D). χ2 analysis of clinicopathologic parameters was conducted on 110 HCC patients (Table 1). The findings indicated a significant correlation between 5’-tiRNA-Lys-CTT expression and tumor size (P = 0.0174), BCLC stage (P = 0.0064), and cirrhosis of the liver (P = 0.0161). However, there was no significant association between 5’-tiRNA-Lys-CTT expression and patient gender, age, tumor differentiation, or metastasis.
| Characteristics | Patients | 5’-tiRNA-Lys-CTT | P value | |
| High | Low | |||
| Number | 110 | 55 (50) | 55 (50) | |
| Gender | 0.2343 | |||
| Male | 70 (63.6) | 38 (34.5) | 32 (29.1) | |
| Female | 40 (36.4) | 17 (15.5) | 23 (20.9) | |
| Age (years) | 0.6884 | |||
| < 60 | 38 (34.5) | 18 (16.4) | 20 (18.2) | |
| ≥ 60 | 72 (65.5) | 37 (33.6) | 35 (31.8) | |
| Tumor size (cm) | 0.0174a | |||
| < 5 | 40 (36.4) | 26 (23.6) | 14 (12.7) | |
| ≥ 5 | 70 (63.6) | 29 (26.4) | 41 (37.3) | |
| BCLC stage | 0.0064b | |||
| 0/A | 44 (40.0) | 29 (26.4) | 15 (13.6) | |
| B/C/D | 66 (60.0) | 26 (23.6) | 40 (36.4) | |
| Tumor differentiation | 0.2290 | |||
| Well/moderate | 72 (65.5) | 39 (35.5) | 33 (30.0) | |
| Poor | 38 (34.5) | 16 (14.5) | 22 (20.0) | |
| Metastasis | 0.0348 | |||
| Negative | 49 (44.5) | 30 (27.3) | 19 (17.3) | |
| Positive | 61 (55.5) | 25 (22.7) | 36 (32.7) | |
| Cirrhosis of the liver | 0.0161a | |||
| Absence | 38 (34.5) | 13 (11.8) | 25 (22.7) | |
| Presence | 72 (65.5) | 42 (38.2) | 30 (27.3) | |
Then, the expression level of serum 5’-tiRNA-Lys-CTT was compared with that of AFP in these 110 patients. The results revealed a positive association between 5’-tiRNA-Lys-CTT expression and AFP concentration (Figure 3E). Furthermore, patients with a history of hepatitis B virus (HBV) infection had notably higher levels of 5’-tiRNA-Lys-CTT expression (Figure 3F). Given the lack of prior research on 5’-tiRNA-Lys-CTT and its association with both AFP and HBV infection, we proposed that 5’-tiRNA-Lys-CTT could potentially serve as a biomarker for the early detection of HCC. This could be advantageous for identifying patients at risk of developing liver cancer at an early stage.
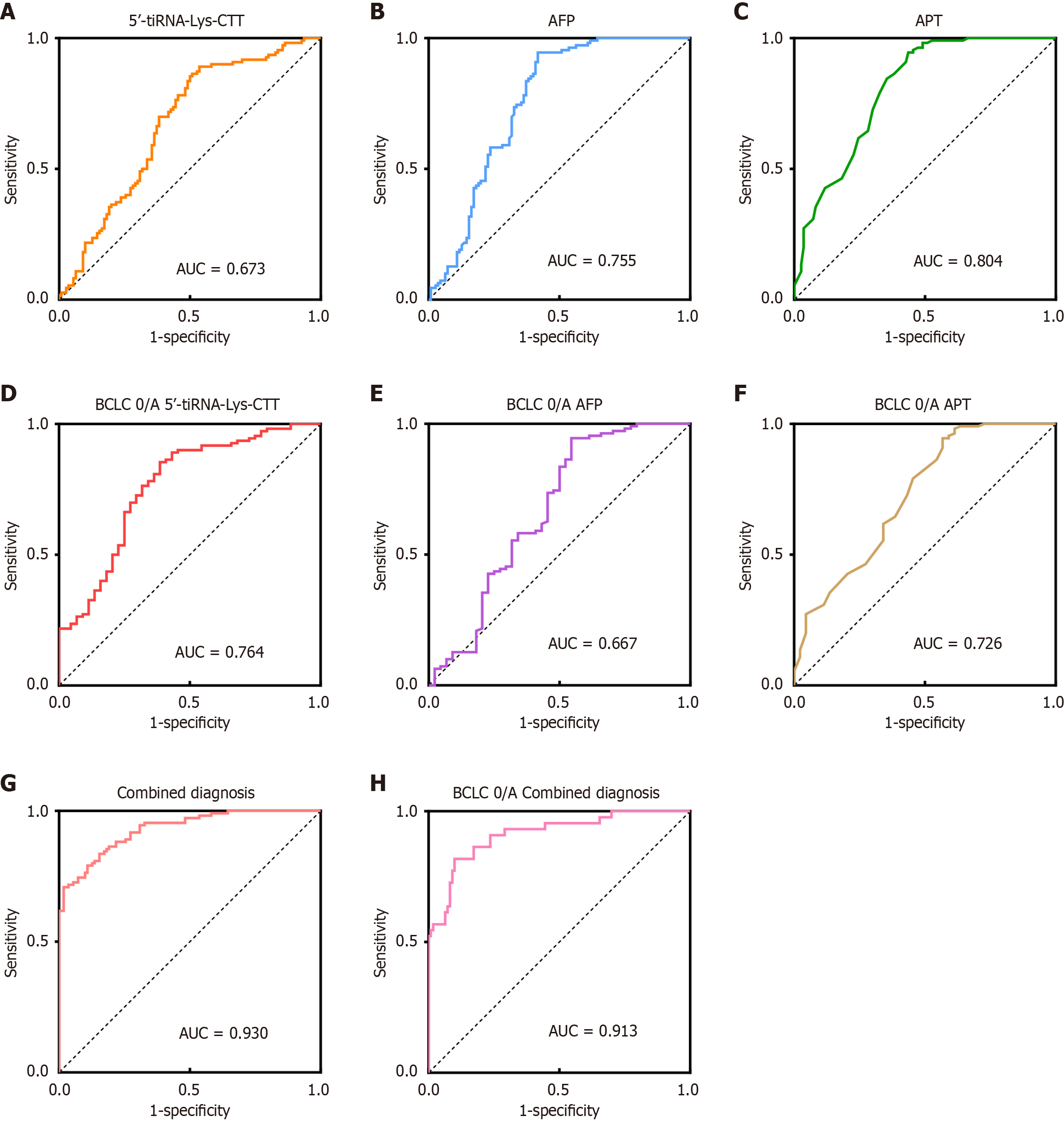
The diagnostic efficacy of 5’-tiRNA-Lys-CTT was further evaluated. ROC curve analysis indicated that the AUC for 5’-tiRNA-Lys-CTT was 0.673, lower than the AUC for AFP (0.755) and APT (0.804) (Figure 4A-C;Supplementary Table 2). In a subset analysis of patients at the BCLC 0/A stage, the AUC for 5’-tiRNA-Lys-CTT was 0.764, exceeding those for AFP (0.667) and APT (0.726) (Figure 4D-F;Supplementary Table 3). When compared to a healthy reference group, a combined model incorporating all three biomarkers demonstrated superior diagnostic performance, achieving an AUC of 0.930 for HCC patients and 0.913 for BCLC 0/A-stage patients (Figure 4G and H). This combined model outperformed any single biomarker in terms of diagnostic accuracy. These findings suggest that while 5’-tiRNA-Lys-CTT may not be as sensitive as AFP and APT in diagnosing HCC patients across all stages, it shows superior performance in the detection of early-stage HCC. Moreover, when used in conjunction with other established biomarkers, 5’-tiRNA-Lys-CTT significantly enhances overall diagnostic accuracy, highlighting its potential as a valuable addition to the existing diagnostic toolkit for HCC.
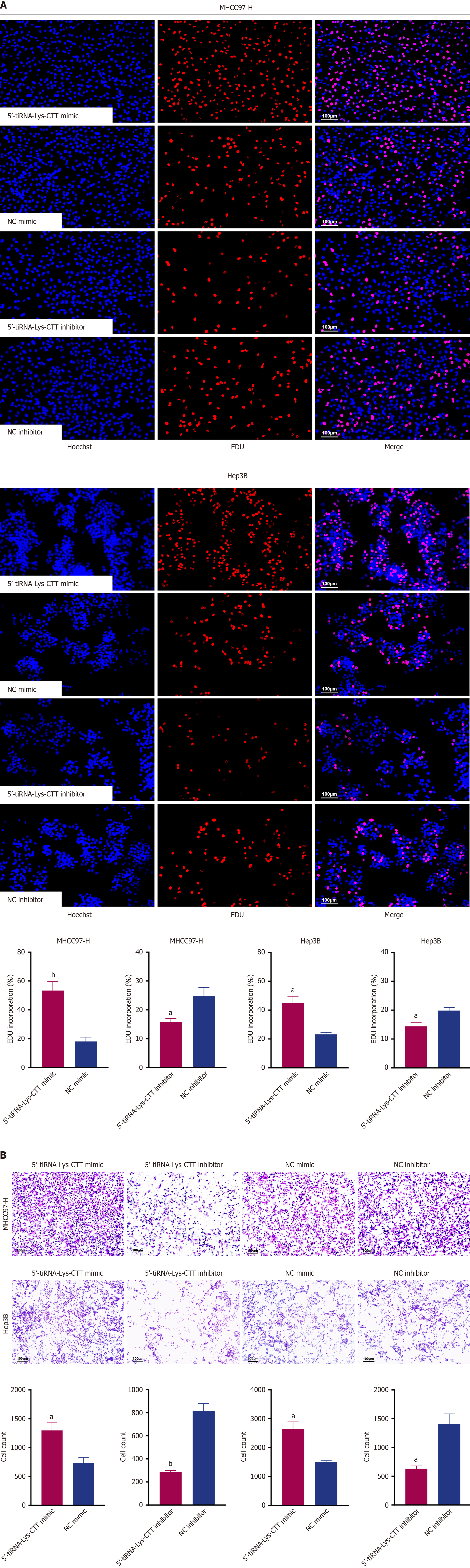
To investigate the biological function of 5’-tiRNA-Lys-CTT in HCC, we first analyzed its relative expression in several HCC cell lines. As shown in Figure 5A, MHCC97-H and Hep3B cells exhibited higher expression of 5’-tiRNA-Lys-CTT compared to a normal liver cell line (QSG-7701). Next, we examined the intracellular location of 5’-tiRNA-Lys-CTT in MHCC97-H and Hep3B cell lines. Nuclear-cytoplasmic fractionation experiments revealed that 5’-tiRNA-Lys-CTT is predominantly localized in the cytoplasm of these cells (Figure 5B). Subsequently, we performed transfection experiments utilizing a mimic of 5’-tiRNA-Lys-CTT and a negative control (NC) mimic, as well as an inhibitor of 5’-tiRNA-Lys-CTT and an NC inhibitor, in both MHCC97-H and Hep3B cells. The transfection efficiency of the 5’-tiRNA-Lys-CTT mimic and inhibitor was confirmed using qPCR (Figure 5C). To assess the impact of 5’-tiRNA-Lys-CTT on cell proliferation, CCK-8 assays were conducted. Overexpression of 5’-tiRNA-Lys-CTT significantly increased cell proliferation compared to the NC mimic group, whereas suppression of 5’-tiRNA-Lys-CTT resulted in a notable decrease in cell proliferation (Figure 5D). Colony formation assay and EdU staining yielded similar results (Figures 5E and 6A).
To investigate the potential role of 5’-tiRNA-Lys-CTT in tumor cell migration, Transwell assays were performed. Elevated expression of 5’-tiRNA-Lys-CTT in both MHCC97-H and Hep3B cells enhanced their ability to migrate compared to the NC mimic group. Conversely, reduced expression of 5’-tiRNA-Lys-CTT significantly impaired cell migration (Figure 6B). These results strongly suggest that 5’-tiRNA-Lys-CTT acts as an oncogenic driver in the de
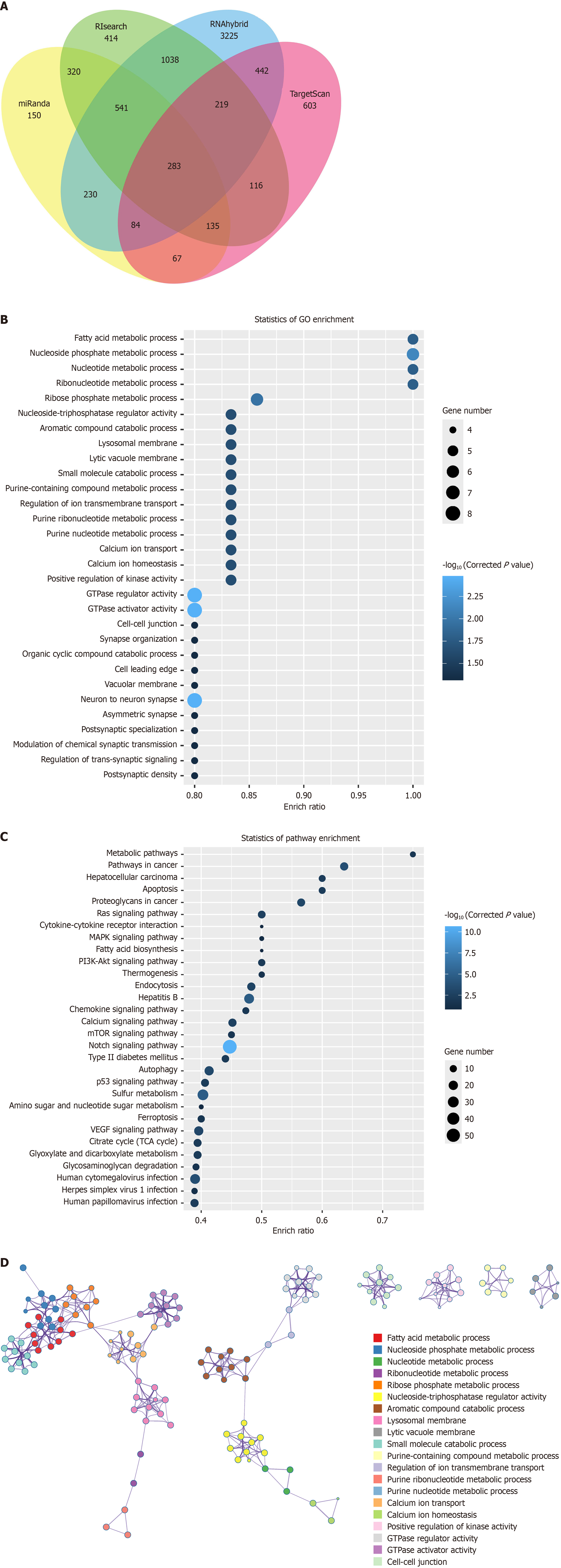
Furthermore, we utilized various bioinformatics tools, including miRanda, TargetScan, RIsearch, and RNAhybrid, to predict potential target genes of 5’-tiRNA-Lys-CTT. By analyzing the overlapping results from these four prediction tools, we identified a total of 283 potential target genes with a strong probability of binding to 5’-tiRNA-Lys-CTT (Figure 7A; Supplementary Table 4). GO functional enrichment analysis of these 283 target genes suggested that 5’-tiRNA-Lys-CTT may be involved in processes such as fatty acid metabolism, nucleoside phosphate metabolism, and nucleotide me
Despite significant advances in cancer diagnosis and treatment, HCC remains the most common type of liver cancer and a leading cause of cancer-related mortality worldwide. While progress has been made in surgical procedures, radio
Recent advances in next-generation sequencing technology have significantly enhanced our understanding of the molecular mechanisms underlying tumor formation. As technology progresses, various forms of short cancer-associated ncRNAs have been identified in different cells, serum, and tissues. tRNAs and their variants, which are highly prevalent small RNA molecules, have gained significant attention due to their involvement in cancer development[28]. Multiple studies have documented the role of tsRNAs in the onset and progression of various cancers, including pancreatic cancer[18], breast cancer[16,21,23], non-small cell lung cancer[29], and colorectal cancer[20]. Furthermore, numerous studies have demonstrated the potential of serum tsRNAs as novel biomarkers for diagnosing HCC[30]. For instance, Zhan et al[31] identified that serum mitochondrial tRF-glutamine-TTG-006 was significantly sensitive and specific for early detection of HCC. Similarly, Zuo et al[32] formulated a diagnostic model to construct a risk score signature aimed at predicting liver cancer prognosis through tsRNA analysis. According to the model, HCC tissue exhibited higher expression of tRF-20-HDK2RSI2, tRF-20-6S7P4PZ3, and tRF-18-8R1546D2, but lower expression of tRF-20-73VL4YMY and tRF-24-S3M8309N0Y[32]. Nonetheless, the thorough exploration of the basic mechanisms of tsRNAs in HCC is still in its infancy.
In this study, we leveraged high-throughput sequencing technology to examine the differential expression of tsRNAs between HCC tissue and adjacent normal tissues. Significantly, some tsRNAs were increased in HCC, suggesting they might contribute to HCC progression. Conversely, other tsRNAs exhibited decreased expression, suggesting a possible role in tumor suppression. The expression of tRF-32-PSQP4PW3FJI01 was significantly increased in three sets of HCC tissues, and further analysis revealed that tRF-32-PSQP4PW3FJI01 corresponds to the 5’-half of the mature tRNA-Lys-CTT. Based on this information, we renamed tRF-32-PSQP4PW3FJI01 as 5’-tiRNA-Lys-CTT. Investigations into the mo
The current study demonstrated that the expression of 5’-tiRNA-Lys-CTT in HCC tissues was markedly higher compared to paracancerous tissues. Additionally, its expression in HCC serum was statistically significantly elevated compared to the normal physical examination population. The qPCR results were consistent with the high-throughput sequencing data, suggesting a strong association between elevated 5’-tiRNA-Lys-CTT expression and tumor deve
We further investigated the biological roles of 5’-tiRNA-Lys-CTT in the development of HCC. Through CCK-8 assay, colony formation assay, and EdU staining, we explored its impact on the proliferation of MHCC97-H and Hep3B cells. Our results indicated that 5’-tiRNA-Lys-CTT overexpression led to a significant increase in cell proliferation, while decreased expression effectively suppressed tumor cell proliferation. In addition, overexpression of 5’-tiRNA-Lys-CTT also resulted in enhanced migratory capabilities. These findings collectively propose that 5’-tiRNA-Lys-CTT may function as an oncogene in HCC progression.
The microRNAs typically bind to the 3’-UTR of mRNAs, leading to mRNA degradation or blocking translation. Similarly, tsRNAs have the ability to interact with specific mRNA targets, thereby influencing their stability or tran
The 5’-tiRNA-Lys-CTT, a short ncRNA molecule, showed significantly increased expression in HCC cell lines as well as in the serum and tissues of HCC patients. Moreover, it possesses the essential characteristics necessary to function as a biomarker for HCC. Mechanistically, 5’-tiRNA-Lys-CTT may bind to downstream target genes and be involved in various cellular processes and signaling pathways directly implicated in the initiation and progression of HCC. The findings of this study have advanced our understanding of the molecular processes underlying HCC and underscored the potential significance of 5’-tiRNA-Lys-CTT as a therapeutic target in HCC treatment.
The authors would like to thank all the patients and donors involved for providing tissue and serum samples, and the Ethics Committee of Affiliated Hospital of Nantong University for supporting this study.
| 1. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1551] [Article Influence: 387.8] [Reference Citation Analysis (41)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68227] [Article Influence: 13645.4] [Reference Citation Analysis (201)] |
| 3. | Kim DY, Toan BN, Tan CK, Hasan I, Setiawan L, Yu ML, Izumi N, Huyen NN, Chow PK, Mohamed R, Chan SL, Tanwandee T, Lee TY, Hai TTN, Yang T, Lee WC, Chan HLY. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin Mol Hepatol. 2023;29:277-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 4. | Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, Chintharlapalli S, Abada PB, Sherman M, Zhu AX. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 5. | Nagaraju GP, Dariya B, Kasa P, Peela S, El-Rayes BF. Epigenetics in hepatocellular carcinoma. Semin Cancer Biol. 2022;86:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 217] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 6. | Pardini B, Sabo AA, Birolo G, Calin GA. Noncoding RNAs in Extracellular Fluids as Cancer Biomarkers: The New Frontier of Liquid Biopsies. Cancers (Basel). 2019;11:1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 7. | Slack FJ, Chinnaiyan AM. The Role of Non-coding RNAs in Oncology. Cell. 2019;179:1033-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 1205] [Article Influence: 200.8] [Reference Citation Analysis (0)] |
| 8. | Chen Q, Zhang X, Shi J, Yan M, Zhou T. Origins and evolving functionalities of tRNA-derived small RNAs. Trends Biochem Sci. 2021;46:790-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 9. | Xie Y, Yao L, Yu X, Ruan Y, Li Z, Guo J. Action mechanisms and research methods of tRNA-derived small RNAs. Signal Transduct Target Ther. 2020;5:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 10. | Lee YS, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs). Genes Dev. 2009;23:2639-2649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 895] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 11. | Speer J, Gehrke CW, Kuo KC, Waalkes TP, Borek E. tRNA breakdown products as markers for cancer. Cancer. 1979;44:2120-2123. [PubMed] [DOI] [Full Text] |
| 12. | Wang Y, Weng Q, Ge J, Zhang X, Guo J, Ye G. tRNA-derived small RNAs: Mechanisms and potential roles in cancers. Genes Dis. 2022;9:1431-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 13. | George S, Rafi M, Aldarmaki M, ElSiddig M, Al Nuaimi M, Amiri KMA. tRNA derived small RNAs-Small players with big roles. Front Genet. 2022;13:997780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 14. | Green D, Fraser WD, Dalmay T. Transfer RNA-derived small RNAs in the cancer transcriptome. Pflugers Arch. 2016;468:1041-1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Chu X, He C, Sang B, Yang C, Yin C, Ji M, Qian A, Tian Y. Transfer RNAs-derived small RNAs and their application potential in multiple diseases. Front Cell Dev Biol. 2022;10:954431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 16. | Wang J, Ma G, Li M, Han X, Xu J, Liang M, Mao X, Chen X, Xia T, Liu X, Wang S. Plasma tRNA Fragments Derived from 5' Ends as Novel Diagnostic Biomarkers for Early-Stage Breast Cancer. Mol Ther Nucleic Acids. 2020;21:954-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Yang P, Zhang X, Chen S, Tao Y, Ning M, Zhu Y, Liang J, Kong W, Shi B, Li Z, Shen H, Wang Y. A Novel Serum tsRNA for Diagnosis and Prediction of Nephritis in SLE. Front Immunol. 2021;12:735105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Jin F, Yang L, Wang W, Yuan N, Zhan S, Yang P, Chen X, Ma T, Wang Y. A novel class of tsRNA signatures as biomarkers for diagnosis and prognosis of pancreatic cancer. Mol Cancer. 2021;20:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 19. | Hu Y, Cai A, Xu J, Feng W, Wu A, Liu R, Cai W, Chen L, Wang F. An emerging role of the 5' termini of mature tRNAs in human diseases: Current situation and prospects. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Tao EW, Wang HL, Cheng WY, Liu QQ, Chen YX, Gao QY. A specific tRNA half, 5'tiRNA-His-GTG, responds to hypoxia via the HIF1α/ANG axis and promotes colorectal cancer progression by regulating LATS2. J Exp Clin Cancer Res. 2021;40:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Mo D, Jiang P, Yang Y, Mao X, Tan X, Tang X, Wei D, Li B, Wang X, Tang L, Yan F. A tRNA fragment, 5'-tiRNA(Val), suppresses the Wnt/β-catenin signaling pathway by targeting FZD3 in breast cancer. Cancer Lett. 2019;457:60-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Liu D, Wu C, Wang J, Zhang L, Sun Z, Chen S, Ding Y, Wang W. Transfer RNA-derived fragment 5'tRF-Gly promotes the development of hepatocellular carcinoma by direct targeting of carcinoembryonic antigen-related cell adhesion molecule 1. Cancer Sci. 2022;113:3476-3488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Goodarzi H, Liu X, Nguyen HC, Zhang S, Fish L, Tavazoie SF. Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell. 2015;161:790-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 701] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 24. | Hu F, Niu Y, Mao X, Cui J, Wu X, Simone CB 2nd, Kang HS, Qin W, Jiang L. tsRNA-5001a promotes proliferation of lung adenocarcinoma cells and is associated with postoperative recurrence in lung adenocarcinoma patients. Transl Lung Cancer Res. 2021;10:3957-3972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Kim HK, Fuchs G, Wang S, Wei W, Zhang Y, Park H, Roy-Chaudhuri B, Li P, Xu J, Chu K, Zhang F, Chua MS, So S, Zhang QC, Sarnow P, Kay MA. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature. 2017;552:57-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 434] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 26. | Loher P, Telonis AG, Rigoutsos I. MINTmap: fast and exhaustive profiling of nuclear and mitochondrial tRNA fragments from short RNA-seq data. Sci Rep. 2017;7:41184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22632] [Cited by in RCA: 30756] [Article Influence: 1809.2] [Reference Citation Analysis (0)] |
| 28. | Li J, Zhu L, Cheng J, Peng Y. Transfer RNA-derived small RNA: A rising star in oncology. Semin Cancer Biol. 2021;75:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Yang W, Gao K, Qian Y, Huang Y, Xiang Q, Chen C, Chen Q, Wang Y, Fang F, He Q, Chen S, Xiong J, Chen Y, Xie N, Zheng D, Zhai R. A novel tRNA-derived fragment AS-tDR-007333 promotes the malignancy of NSCLC via the HSPB1/MED29 and ELK4/MED29 axes. J Hematol Oncol. 2022;15:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 30. | Zhou Y, Hu J, Liu L, Yan M, Zhang Q, Song X, Lin Y, Zhu D, Wei Y, Fu Z, Hu L, Chen Y, Li X. Gly-tRF enhances LCSC-like properties and promotes HCC cells migration by targeting NDFIP2. Cancer Cell Int. 2021;21:502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Zhan S, Yang P, Zhou S, Xu Y, Xu R, Liang G, Zhang C, Chen X, Yang L, Jin F, Wang Y. Serum mitochondrial tsRNA serves as a novel biomarker for hepatocarcinoma diagnosis. Front Med. 2022;16:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Zuo Y, Chen S, Yan L, Hu L, Bowler S, Zitello E, Huang G, Deng Y. Development of a tRNA-derived small RNA diagnostic and prognostic signature in liver cancer. Genes Dis. 2022;9:393-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |